Abstract
Study Objectives:
The catalytic function of the enzyme carbonic anhydrase (CA) plays a fundamental role in carbon dioxide (CO2), proton (H+), and bicarbonate (HCO3-) homeostasis. Hypoxia and tissue acidosis have been proposed to increase physiological CA activity in various compartments of the body. We hypothesized that CA activity in blood is upregulated in patients with obstructive sleep apnea (OSA).
Design:
Cross-sectional analysis of a sleep clinic cohort.
Settings:
Sleep laboratory at a university hospital.
Participants:
Seventy referred patients with suspected OSA (48 males, age 54 ± 13 y, apnea-hypopnea index (AHI) median [interquartile range] 21 [8–41] n/h).
Interventions:
N/A.
Measurements and Results:
In-laboratory cardiorespiratory polygraphy was used to assess OSA. CA activity was determined by an in vitro assay that quantifies the pH change reflecting the conversion of CO2 and H2O to HCO3- and H+. CA activity was positively associated with AHI and 4% oxygen desaturation index (ODI4) (Spearman correlation r = 0.44 and 0.47, both P < 0.001). The associations (CA activity versus logAHI and CA versus logODI4) were independent of sex, age, body mass index, presleep oxygen saturation, nocturnal oxygen saturation, hypertension status, and use of diuretic medication in two generalized linear models (P = 0.007 and 0.011, respectively). Sitting diastolic blood pressure was associated with CA activity after adjustment of sex, age, body mass index, mean oxygen saturation, and AHI (P = 0.046).
Conclusions:
Carbonic anhydrase (CA) activity increased with apnea-hypopnea index and related nocturnal hypoxemia measures in patients with obstructive sleep apnea (OSA). Altered CA activity may constitute a component that modulates respiratory control and hemodynamic regulation in patients with OSA.
Citation:
Wang T, Eskandari D, Zou D, Grote L, Hedner J. Increased carbonic anhydrase activity is associated with sleep apnea severity and related hypoxemia. SLEEP 2015;38(7):1067–1073.
Keywords: blood pressure, carbonic anhydrase, intermittent hypoxia, obstructive sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA) characterized by repetitive upper airway collapse is associated with intermittent hypoxia, hyperventilation, and transient arousals from sleep. These consequences of OSA promote autonomic and neurohumoral activation, oxidative stress, and inflammation which contribute to increased cardiovascular and metabolic morbidities in OSA.1,2
The enzyme carbonic anhydrase (CA) catalyzes intercon-version of carbon dioxide (CO2) and water into carbonic acid, protons (H+), and bicarbonate (HCO3-). At least 16 CA isoenzymes have been characterized in mammals. A specific role of CA in respiration and ventilatory control is suggested by the presence of various CA isoforms in lung and brain capillary endothelium, kidney, muscle, carotid bodies, and central chemosensitive areas in the rostroventrolateral medulla oblongata.3,4 In the erythrocyte membrane and cytoplasm the prevailing isoforms are CA I and II and the majority of CO2 hydration is performed by CA II.5 Several physiological stressors such as acclimatization to chronic hypoxia at high altitude, intense physical exercise, and hyperventilation have been associated with altered levels of CA activity.6–8 Patients with OSA are exposed to numerous apnea/hypopnea episodes and cyclical changes of hypoxia/hyperoxia and hypocapnia/ hypercapnia during sleep. However, the potential effect of these repetitive changes on blood CA activity has not been studied. Moreover, in patients with OSA, pharmacological inhibition of CA has been shown to reduce apneic events both at low and high altitude.9–11
In the current study, we analyzed the association between whole blood CA activity and sleep apnea in a clinical population of patients with suspected OSA. We hypothesized that OSA is associated with increased CA activity in a manner proportional to the severity of the disease. The association between CA activity and blood pressure (BP) was also investigated.
METHODS
Study Population
Patients referred to the Department of Sleep Medicine at the Sahlgrenska University Hospital with suspected OSA were randomly recruited in the study (n = 74). Patients with a known history of chronic obstructive pulmonary disease or an obesity hypoventilation condition were excluded from the analysis (n = 4). All other relevant comorbidity was determined and medication records were reviewed and documented. The study protocol was approved by the Regional Ethics Committee for Human Research at the University of Gothenburg. Informed oral and written consent was obtained from all participants prior to study procedures.
Study Protocol
All patients in the current protocol had been referred to the sleep laboratory and all study-related investigations took place in association with a full night attended cardiorespira-tory polygraphic recording (Embletta, Embla, Buffalo, NY, USA). Sitting systolic/diastolic BP and heart rate were measured in the evening before sleep recording. A venous blood sample was obtained in the morning immediately following the sleep study.
Sleep Study
The polygraphic recording montage consisted of a nasal cannula, thorax and abdominal respiratory effort belts, and finger pulse oximeter. Apnea-hypopnea events were manually scored according to the American Academy of Sleep Medicine (AASM) criteria 2007.12 Apnea events were scored if there was a ≥ 90 % decrease in the amplitude of airflow. Hypopnea events were scored if there was a ≥ 30% reduction in airflow associated with a ≥ 3% oxygen desaturation. A minimum event duration of 10 sec was required. Apneahypopnea index (AHI) was defined by total number of events divided by analysis time (lights off to lights on during the recording session). Oxygen desaturation index (ODI4) was defined as total number of events with a ≥ 4% reduction of oxygen saturation per hour. Mean nocturnal oxygen saturation (SpO2) was calculated as the mean oxygen saturation measured by pulse oximetry during analysis time. The mean SpO2 of the first 3 min of the polygraphic recording was classified as presleep SpO2.
CA Activity Assessment
Venous blood samples (antecubital vein) were collected in EDTA (Ethylenediaminetetraacetic acid) -coated tubes. Whole blood was stored within 30 min at −20°C and subsequently transferred to −70°C. Prior to analysis, samples were slowly thawed, refrozen, and rethawed on ice in order to obtain hemolysis. The assay was adapted from a method previously described by Everaert et al.13 In detail, a buffer consisting of 0.02M HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 0.02M MES (2-(N-morpholino)ethanesulfonic acid), 0.035M potassium chloride (KCl), and 0.015M sodium chloride (NaCl) with a balanced capacity in the pH 6–8 range was prepared. The subsequent analysis was conducted on ice in order to maintain an ambient temperature around zero to 1°C. A separate solution constituting of 200 ml of zero to 1°C distilled water was continuously flushed with 100% CO2 and kept at zero to 1°C for at least 30 min. The blood sample was diluted (1:2000) in saline (0.9%) and 10 mL of the sample was applied into a separate reaction vessel containing 10 ml of the buffer solution. A calibrated pH meter (Docu-pH+, Sartorius, Sweden) was used to continuously monitor pH in the reaction vessel. A baseline pH of 8.00 ± 0.03 was established during approximately 5 min at zero to 1°C. Ten mL of the CO2/distilled water solution was rapidly added into the reaction vessel to yield a final volume of 30 ml. The pH in the reaction vessel was continuously monitored at 1 Hz during 120 sec and graphically displayed (Figure 1). The analysis method was calibrated by duplicate samples and repeated measurements. The intra-assay and interassay variability was 5.0% and 7.6%, respectively.
Figure 1.
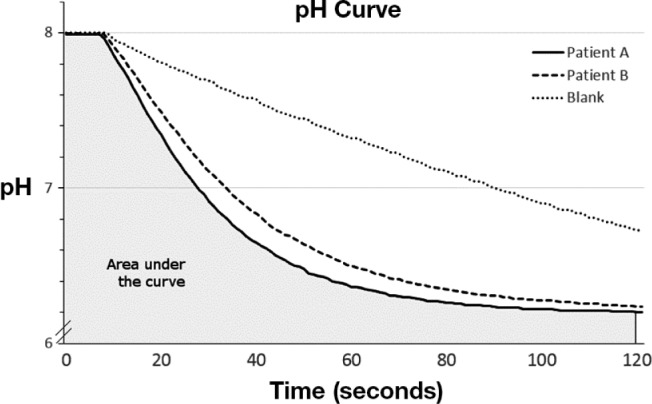
Change of pH in two typical study patients and the control condition using reference blank in the bioassay. The shaded area shows the area under the curve calculated to represent carbonic anhydrase (CA) activity in patient A. In this illustration patient A exhibited a higher CA activity compared with patient B.
pH curves were plotted and analyzed by self-developed software. In order to reflect time of the catalyzed reaction we calculated the area under the curve (AUC, range 802–837) as the sum of all pH assessments during 120 sec. Hence, a higher CA activity corresponds to a lower calculated value (shorter reaction time). For illustrative purposes CA activity was defined in arbitrary units as 850-AUC.
BP and Heart Rate Assessment
Systolic/diastolic BP and heart rate were determined after a minimum of 5 min rest (Omron M6, Omron Healthcare, Kyoto, Japan). An appropriately sized cuff was placed on the right arm at the heart level. Systolic and diastolic BP (nearest 1 mmHg) was determined as the mean of two sequential recordings. Heart rate was expressed as beats per min (bpm).
Statistics
Statistical analysis was performed using IBM SPSS 20 (SPSS Inc, Chicago, IL, USA). The Kolomogorov-Smirnov test was used to determine the distribution of the data. Variables were expressed in median and interquartile range (IQR) or mean ± standard deviation (SD). Spearman correlation was used to study the associations between CA activity, AHI, and ODI4. Pearson correlation was used to study the relationship between CA activity, nocturnal oxygen saturation, and systolic/diastolic BP. Mann-Whitney U test and Kruskal-Wallis test were used for group comparisons. AHI and ODI4 were logarithmized in order to enable parametric statistics. Two multivariate generalized linear models (using logAHI and logODI4 as the dependent variable respectively) controlling for age, sex, body mass index, hypertension status, use of diuretic medication, presleep, and mean nocturnal SpO2 were used to address the independent association between CA activity and OSA severity. Because CA in part is an intraerythrocytic enzyme,5 hemoglobin concentration was controlled in a subgroup of 46 patients with known hemoglobin value. The statistical significance level was set to P < 0.05.
RESULTS
Patient Characteristics
A total of 70 patients were included in the study. Characteristics of participants are shown in Table 1. A history of hyper-tension, other cardiovascular disease (coronary artery disease/ stroke), or diabetes was found in 46%, 19%, and 4% of patients, respectively. The use of beta-blockers, calcium-channel blockers, diuretics, angiotensin-converting enzyme inhibitors, and angiotensin II blockers was 24.3, 11.4, 18.6, 17.1, and 5.7%, respectively, in the patient cohort.
Table 1.
Study participant characteristics.
Association between Sleep Apnea and CA Activity
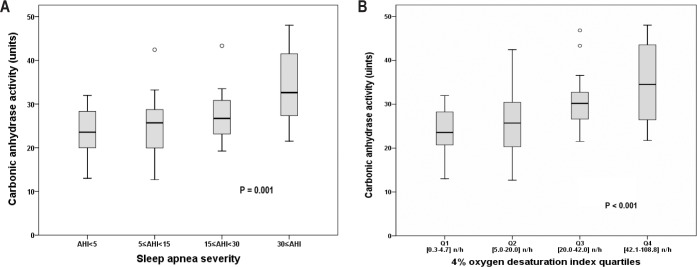
The CA activity in patients without OSA (AHI < 5) and patients with mild (5 ≤ AHI < 15), moderate (15 ≤ AHI < 30) and severe (AHI ≥ 30) OSA was 23.7 ± 5.5, 25.2 ± 8.3, 27.3 ± 6.0, and 33.9 ± 8.5 units, respectively (Kruskal-Wallis test, P = 0.001, Figure 2A). The difference was significant between the severe and the non-OSA groups (Mann-Whitney U test, P < 0.001). CA activity increased in a dose-response manner along with ODI4 quartile (Kruskal-Wallis test, P < 0.001, Figure 2B). CA activity was higher in the two highest quartiles compared with the lowest quartile (Mann-Whitney U test, for Q3 P = 0.001 and for Q4 P < 0.001). There was a positive correlation between CA activity and AHI/ ODI4 (Spearman correlation, r = 0.44 and 0.47, respectively, both P < 0.001, Figure 3A and 3B) as well as LogAHI/LogODI4 (see Figure S1 and Figure S2, supplemental material). Mean nocturnal SpO2 was negatively associated with CA activity (Pearson correlation, R2 = 0.091, P = 0.011, see Figure S3, supplemental material). In two separate generalized linear models controlling for age, sex, body mass index, pre-sleep oxygen saturation, mean nocturnal SpO2, hypertension status, and use of diuretic medication, CA activity was found to be significantly associated with logAHI and logODI4 (P = 0.007 and P = 0.011, respectively, Table 2 and Table 3). This association remained significant after controlling for hemoglobin concentration (see Table S1 and Table S2, supplemental material).
Figure 2.
Box plots of carbonic anhydrase activity in relation to obstructive sleep apnea severity class (Kruskal-Wallis test). (A) Apnea-hypopnea index (AHI) severity category. (B) Quartiles [range] of 4% oxygen desaturation index.
Figure 3.
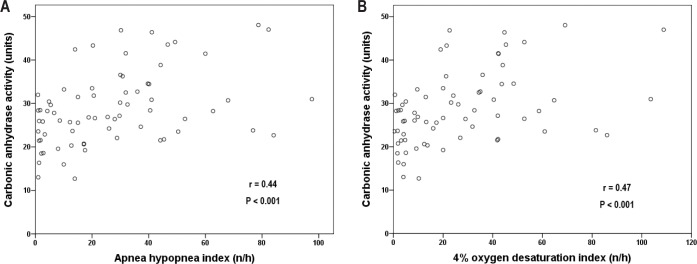
Spearman correlation between carbonic anhydrase activity and obstructive sleep apnea severity: (A) carbonic anhydrase activity and apneahypopnea index. (B) Carbonic anhydrase activity and 4% oxygen desaturation index.
Table 2.
Generalized linear model 1: the association between logAHI and blood carbonic anhydrase activity controlling for sex, age, body mass index, presleep oxygen saturation, mean nocturnal oxygen saturation, hypertension status, and use of diuretic medication.
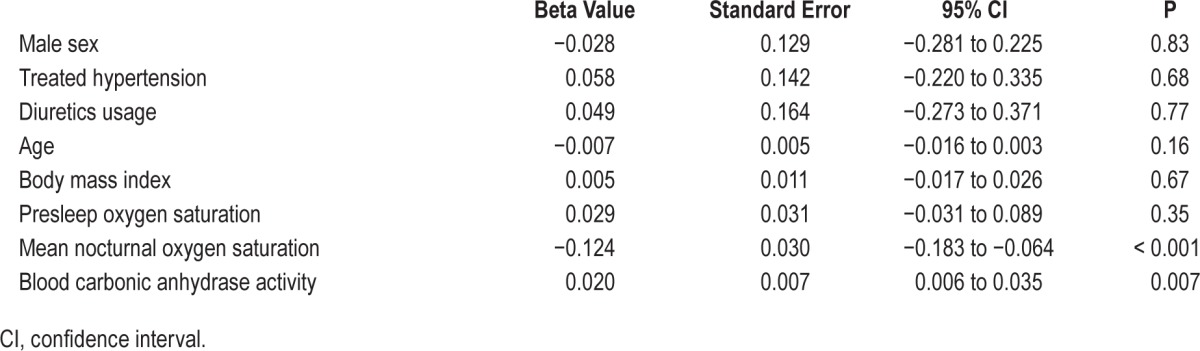
Table 3.
Generalized linear model 2: the association between logODI4 and blood carbonic anhydrase activity controlling for sex, age, body mass index, presleep oxygen saturation, mean nocturnal oxygen saturation, hypertension status, and use of diuretic medication.
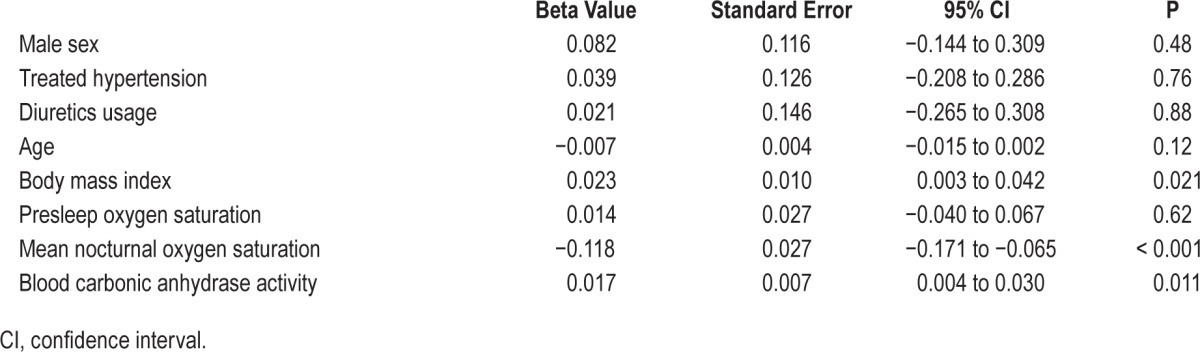
Sitting BP and CA Activity
In a post hoc analysis accounting for all patients irrespective of hypertension status, CA activity was positively associated with resting diastolic BP (Pearson correlation, R2 = 0.142, P = 0.001), but not systolic BP (P = 0.098) (Figure 4A and 4B). In a generalized linear model adjusting for sex, age, body mass index, mean SpO2, and AHI, CA activity was significantly associated with diastolic BP (β = 0.32, P = 0.046). There was no interaction between use of antihypertensive medication and CA activity in relation to diastolic BP (P = 0.096).
Figure 4.
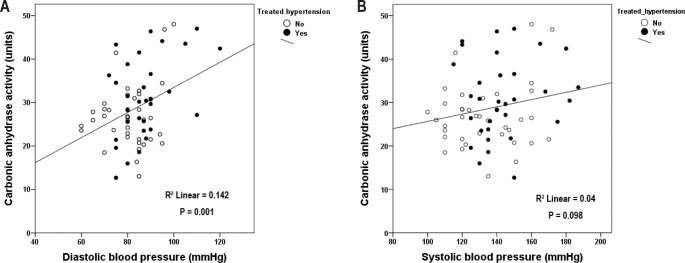
Carbonic anhydrase activity in relation to (A) sitting diastolic blood pressure and (B) sitting systolic blood pressure. Filled circles depict patients on antihypertensive treatment, while open circles represent patients without treatment.
DISCUSSION
In the current study we have shown that whole blood CA activity was related to the severity of sleep apnea and the degree of nocturnal intermittent hypoxia in patients with OSA. Interestingly, we also found that CA activity was associated with office diastolic BP independent of AHI. To the best of our knowledge, this is the first study to quantify CA activity in patients with OSA. Future studies are warranted to further explore the association between CA activity and respiratory control in OSA as well as the possible link between CA activity and hemodynamic control in patients with sleep disordered breathing.
The physiological and pathophysiological role of the CA system in man is complex and multifaceted. Not only are there multiple isoenzymes of CA but the enzymes appear to be involved in specific functions at various locations in the body.14 Previous studies suggest that hypoxia provides an important influence on CA activity in human and chick embryonic development.15–18 Tissue hypoxemia increased CA I and II activity in the human fetus15 and high- altitude hypoxic exposure was associated with increased CA activity in chicken embryo whole blood.18 We found that lower mean nocturnal SpO2, a marker of reduced average oxygenation during the night in OSA, was associated with increased CA activity in whole blood. In addition, there was an increased CA activity in OSA that was most evident in patients with severe disease. Several mechanisms may contribute to the increase of CA activity with hypoxia in OSA. It has been shown that increased CA synthesis may be directly induced by a lower oxygen tension at the molecular level.17 A hypoxia-induced increase of plasma norepinephrine stimulated the synthesis of red cell CA II in chick embryos.19 An alternative possibility is that hypoventilation-induced reduction of tissue oxygen tension following oxidative metabolism results in acidification of tissue, which in turn induces elevated activity of enzymes and transporters involved in cellular pH regulation and erythrocyte acid-base handling.6 Both mechanisms would result in increased CA activity in several compartments of the body.
The effect of altered CA activity on ventilation is multidimensional. Pharmacological inhibition of CA activity may cause respiratory and metabolic acidosis, stimulate ventilation, increase the ventilatory response to hypoxia, and slow the rate of the ventilatory response to CO2 in man.20 Animal experiments have suggested a role of CA in regulating the speed and magnitude of the O2 and CO2 response in the carotid body.21,22 The effect of increased blood CA on CO2 elimination and ventilatory control in patients with OSA remains unclear. Injection of CA has been shown to increase catalysis of HCO3- dehydration and excretion of CO2 in rainbow trout after exercise.23 In a CA II-deficient mouse model, there was a mixed respiratory and metabolic acidosis, probably attributed to CA II deficiency in red blood cells and type II pneumocytes.24 It is likely that sleep apnea events lead to local tissue acidification and CO2 retention. These changes will induce increased tissue and blood CA activity aiming to maintain acid-base balance by rapid catalytic hydration of CO2 to carbonic acid. However, although upper airway collapsibility is an important determinant of OSA,25 it does not determine the severity of the condition. Other factors, including ventilatory instability (high “loop gain”), appear to be important for the frequency of events.26 The ventilatory instability in patients with the most severe OSA may in part be due to increased CA, as observed in the current study. This hypothesis is supported by experimental protocols demonstrating a reduced loop gain and ventilatory response to arousal after CA inhibition by acetazolamide in patients with OSA.27,28 Future studies are needed to further investigate the causal relationship between CA and OSA and whether elevated CA activity in severe OSA can serve as a target for novel sleep apnea treatment.
We have also demonstrated an association between whole blood CA activity and diastolic BP independent of sleep apnea, antihypertensive medication, and conventional confounders. Several CA isoenzymes have been intimately associated with physiological regulation of diuresis, fluid shift, vascular resistance, and cardiac contractility.29–32 The proposed mechanisms of action at the molecular level include, but are not limited to, modulation of membranous calcium-activated potassium channels (KCa) and nitric oxide metabolism.33–35 This opens for the possibility that elevated CA activity may be involved in hemodynamic control and particularly in cases associated with hypoxia. It has been shown that reduced CA activity following CA inhibition by acetazolamide included rapid vasodilation in a forearm flow model and reduced pulmonary vascular resistance in an animal model of chronic hypoxia.31,36 Moreover, acetazolamide counteracted BP elevation in normotensive subjects at high altitude, suggesting that CA activity may be involved in blood pressure regulation under hypoxic conditions.37 The isoenzymes CA I and II, both found in erythrocytes and vascular walls, are particularly relevant in the context of hemodynamic regulation.38 It is also worth noting that a wealth of data suggests an elevation of sympathetic activity in OSA.39,40 Interestingly, both in vitro and experimental data suggest that adrenergic agonists induce activation of CA I and II in erythrocytes and vascular smooth muscle.41 The elevated CA activity demonstrated in our study may therefore provide an additive mechanism, on top of sympathetic activation, to modulate hemodynamic control in OSA. Such a potential interaction needs to be addressed in future detailed studies of vascular dynamics in patients with OSA.
Several study limitations need to be addressed. Our study population was a clinical cohort and a normal control group was lacking in the data analysis. Blood gas assessments were not performed in the study. The cross-sectional design did not allow us to draw any conclusions on the causality of the association between apnea severity and CA activity. Future studies including elimination of OSA by continuous positive airway pressure are needed to further address this issue. It cannot be excluded that ongoing medication may have affected CA activity. However, an analysis controlling for use of diuretics did not suggest that this was the case. Our analysis method only permitted assessment of total CA activity in whole blood. It remains unknown whether OSA was associated with alteration of specific CA isoenzymes or a change of CA activity in other compartments of the body. Finally, because of a change of clinical routines, hemoglobin data were only available in a subgroup of patients. However, our subgroup analysis suggested that the association between CA activity and apnea severity was independent of hemoglobin concentration.
It is concluded that CA activity was positively associated with AHI and measures of nocturnal hypoxemia in OSA. Altered CA activity may also be involved in blood pressure control and hypertension development in patients with OSA. Interventional studies are needed to further explore these associations.
DISCLOSURE STATEMENT
This was not an industry supported study. The study was supported by the Swedish Heart and Lung Foundation and regional hospital grant (ALF). The authors have indicated no financial conflicts of interest.
SUPPLEMENTAL MATERIAL
Pearson correlation between carbonic anhydrase activity and logAHI.
Pearson correlation between carbonic anhydrase activity and logODI4.
Pearson correlation between carbonic anhydrase activity and mean nocturnal oxygen saturation.
Generalized linear model addressing the association between logAHI and blood carbonic anhydrase activity controlling for sex, age, body mass index, presleep oxygen saturation, mean nocturnal oxygen saturation, hypertension status, use of diuretic medication, and hemoglobin concentration (n = 46).
Generalized linear model addressing the association between logODI4 and blood carbonic anhydrase activity controlling for sex, age, body mass index, presleep oxygen saturation, mean nocturnal oxygen saturation, hypertension status, use of diuretic medication, and hemoglobin concentration (n = 46).
REFERENCES
- 1.McNicholas WT, Bonsigore MR. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–78. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 2.Hedner J, Grote L, Bonsignore M, et al. The European Sleep Apnoea Database (ESADA): report from 22 European sleep laboratories. Eur Respir J. 2011;38:635–42. doi: 10.1183/09031936.00046710. [DOI] [PubMed] [Google Scholar]
- 3.Maren TH. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967;47:595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- 4.Chegwidden WR, Carter ND. Introduction to the carbonic anhydrases. In: Chegwidden WR, Carter ND, Edward YH, editors. The carbonic anhydrases-new horizons. Birkhäuser Basel: 2000. pp. 13–28. [Google Scholar]
- 5.Esbaugh AJ, Tufts BL. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir Physiol Neurobiol. 2006;154:185–98. doi: 10.1016/j.resp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Juel C, Lundby C, Sander M, Calbet JA, Hall G. Human skeletal muscle and erythrocyte proteins involved in acid-base homeostasis: adaptations to chronic hypoxia. J Physiol. 2003;548:639–48. doi: 10.1113/jphysiol.2002.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messonnier L, Kristensen M, Juel C, Denis C. Importance of pH regulation and lactate/H+ transport capacity for work production during supramaximal exercise in humans. J Appl Physiol. 2007;102:1936–44. doi: 10.1152/japplphysiol.00691.2006. [DOI] [PubMed] [Google Scholar]
- 8.Teng YH, Tsai HT, Hsieh YS, et al. Elevated erythrocyte carbonic anhydrase activity is a novel clinical marker in hyperventilation syndrome. Clin Chem Lab Med. 2009;47:441–5. doi: 10.1515/CCLM.2009.102. [DOI] [PubMed] [Google Scholar]
- 9.Tojima H, Kunitomo F, Kimura H, Tatsumi K, Kuriyama T, Honda Y. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax. 1988;43:113–9. doi: 10.1136/thx.43.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskandari D, Zou D, Karimi M, Stenlof K, Grote L, Hedner J. Zonisamide reduces obstructive sleep apnoea: a randomised placebo-controlled study. Eur Respir J. 2014;44:140–9. doi: 10.1183/09031936.00158413. [DOI] [PubMed] [Google Scholar]
- 11.Nussbaumer-Ochsner Y, Latshang TD, Ulrich S, Kohler M, Thurnheer R, Bloch KE. Patients with obstructive sleep apnea syndrome benefit from acetazolamide during an altitude sojourn: a randomized, placebo-controlled, double-blind trial. Chest. 2012;141:131–8. doi: 10.1378/chest.11-0375. [DOI] [PubMed] [Google Scholar]
- 12.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events - Rules: Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 13.Everaert N, Willemsen H, Hulikova a, et al. The importance of carbonic anhydrase II in red blood cells during exposure of chicken embryos to CO2. Respir Physiol Neurobiol. 2010;172:154–61. doi: 10.1016/j.resp.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Supuran CT. Carbonic anhydrases--an overview. Curr Pharm Des. 2008;14:603–14. doi: 10.2174/138161208783877884. [DOI] [PubMed] [Google Scholar]
- 15.Akbar SA, Brown PR. Human erythrocyte CAI and CAII isoenzymes in hypoxemic and anemic fetuses. Clin Biochem. 1996;29:57–62. doi: 10.1016/0009-9120(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 16.Liao SY, Lerman MI, Stanbridge EJ. Expression of transmembrane carbonic anhydrases, CAIX and CAXII, in human development. BMC Dev Biol. 2009;9:22. doi: 10.1186/1471-213X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Million D, Zillner P, Baumann R. Oxygen pressure-dependent control of carbonic anhydrase synthesis in chick embryonic erythrocytes. Am J Physiol. 1991;261:R1188–96. doi: 10.1152/ajpregu.1991.261.5.R1188. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Zhang LF, Song ML, Bao HG, Zhao CJ, Li N. Highly efficient dissociation of oxygen from hemoglobin in Tibetan chicken embryos compared with lowland chicken embryos incubated in hypoxia. Poult Sci. 2009;88:2689–94. doi: 10.3382/ps.2009-00311. [DOI] [PubMed] [Google Scholar]
- 19.Dragon S, Glombitza S, Gotz R, Baumann R. Norepinephrine-mediated hypoxic stimulation of embryonic red cell carbonic anhydrase and 2,3-DPG synthesis. Am J Physiol. 1996;271:R982–9. doi: 10.1152/ajpregu.1996.271.4.R982. [DOI] [PubMed] [Google Scholar]
- 20.Swenson ER, Hughes JM. Effects of acute and chronic acetazolamide on resting ventilation and ventilatory responses in men. J Appl Physiol. 1993;74:230–7. doi: 10.1152/jappl.1993.74.1.230. [DOI] [PubMed] [Google Scholar]
- 21.Travis DM. Molecular CO2 is inert on carotid chemoreceptor: demonstration by inhibition of carbonic anhydrase. J Pharmacol Exp Ther. 1971;178:529–40. [PubMed] [Google Scholar]
- 22.Buckler KJ, Vaughan-Jones RD, Peers C, Nye PC. Intracellular pH and its regulation in isolated type I carotid body cells of the neonatal rat. J Physiol. 1991;436:107–29. doi: 10.1113/jphysiol.1991.sp018542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood C, Munger R. Carbonic anhydrase injection provides evidence for the role of blood acid-base status in stimulating ventilation after exhaustive exercise in rainbow trout. J Exp Biol. 1994 doi: 10.1242/jeb.194.1.225. 194225–53. [DOI] [PubMed] [Google Scholar]
- 24.Lien YH, Lai LW. Respiratory acidosis in carbonic anhydrase II-deficient mice. Am J Physiol. 1998;274:L301–4. doi: 10.1152/ajplung.1998.274.2.L301. [DOI] [PubMed] [Google Scholar]
- 25.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Younes M, Ostrowski M, Thompson W, Leslie C, Shewchuk W. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–90. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 27.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590:1199–211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards BA, Connolly JG, Campana LM, et al. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep. 2013;36:281–5. doi: 10.5665/sleep.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miracle CM, Rieg T, Blantz RC, Vallon V, Thomson SC. Combined effects of carbonic anhydrase inhibitor and adenosine A1 receptor antagonist on hemodynamic and tubular function in the kidney. Kidney Blood Press Res. 2007;30:388–99. doi: 10.1159/000108625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan P, Leppilampi M, Pastorekova S, et al. Carbonic anhydrase gene expression in CA II-deficient (Car2-/-) and CA IX-deficient (Car9-/-) mice. J Physiol. 2006;571:319–27. doi: 10.1113/jphysiol.2005.102590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. In vivo evidence for K(Ca) channel opening properties of acetazolamide in the human vasculature. Br J Pharmacol. 2001;132:443–50. doi: 10.1038/sj.bjp.0703825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaughan-Jones RD, Villafuerte FC, Swietach P, Yamamoto T, Rossini A, Spitzer KW. pH-Regulated Na(+) influx into the mammalian ventricular myocyte: the relative role of Na(+)-H(+) exchange and Na(+)-HCO Co-transport. J Cardiovasc Electrophysiol. 2006;17:S134–S40. doi: 10.1111/j.1540-8167.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 33.Pickkers P, Garcha RS, Schachter M, Smits P, Hughes AD. Inhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazide. Hypertension. 1999;33:1043–8. doi: 10.1161/01.hyp.33.4.1043. [DOI] [PubMed] [Google Scholar]
- 34.Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol. 2009;297:H2068–74. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 35.Chobanyan-Jurgens K, Schwarz A, Bohmer A, et al. Renal carbonic anhydrases are involved in the reabsorption of endogenous nitrite. Nitric Oxide. 2012;26:126–31. doi: 10.1016/j.niox.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Pichon A, Connes P, Quidu P, et al. Acetazolamide and chronic hypoxia: effects on haemorheology and pulmonary haemodynamics. Eur Respir J. 2012;40:1401–9. doi: 10.1183/09031936.00216011. [DOI] [PubMed] [Google Scholar]
- 37.Parati G, Revera M, Giuliano A, et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J. 2013;34:759–66. doi: 10.1093/eurheartj/ehs140. [DOI] [PubMed] [Google Scholar]
- 38.Puscas I, Gilau L, Coltau M, et al. Hypotensive effect of calcium channel blockers is parallel with carbonic anhydrase I inhibition. Clin Pharmacol Ther. 2000;68:443–9. doi: 10.1067/mcp.2000.110559. [DOI] [PubMed] [Google Scholar]
- 39.Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. 1988;6:S529–31. doi: 10.1097/00004872-198812040-00166. [DOI] [PubMed] [Google Scholar]
- 40.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–8. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 41.Puscas I, Coltau M, Gilau L, et al. Catecholamine-induced vasoconstriction is sensitive to carbonic anhydrase I activation. Braz J Med Biol Res. 2001;34:339–45. doi: 10.1590/s0100-879x2001000300007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pearson correlation between carbonic anhydrase activity and logAHI.
Pearson correlation between carbonic anhydrase activity and logODI4.
Pearson correlation between carbonic anhydrase activity and mean nocturnal oxygen saturation.
Generalized linear model addressing the association between logAHI and blood carbonic anhydrase activity controlling for sex, age, body mass index, presleep oxygen saturation, mean nocturnal oxygen saturation, hypertension status, use of diuretic medication, and hemoglobin concentration (n = 46).
Generalized linear model addressing the association between logODI4 and blood carbonic anhydrase activity controlling for sex, age, body mass index, presleep oxygen saturation, mean nocturnal oxygen saturation, hypertension status, use of diuretic medication, and hemoglobin concentration (n = 46).