Abstract
Background:
Congenital long QT syndrome (LQTS) is a familial arrhythmogenic cardiac channelopathy characterized by prolonged ventricular repolarization and increased risk of torsades de pointes-mediated syncope, seizures, and sudden cardiac death (SCD). QT prolongation corrected for heart rate (QTc) is an important diagnostic and prognostic feature in LQTS. Obstructive sleep apnea (OSA) has been increasingly implicated in the pathogenesis of cardiovascular disease, including arrhythmias and SCD. We tested the hypothesis that the presence of concomitant OSA in patients with LQTS is associated with increased QT intervals, both during sleep and while awake.
Methods and Results:
Polysomnography with simultaneous overnight 12-lead electrocardiography (ECG) was recorded in 54 patients with congenital LQTS and 67 control subjects. OSA was diagnosed as apnea-hypopnea index (AHI) ≥ 5 events/h for adults and AHI > 1 event/h for children. RR and QT intervals were measured from the 12-lead surface ECG. QTc was determined by the Bazett formula. Respiratory disturbance index, AHI, and arousal index were significantly increased in patients with LQTS and with OSA compared to those without OSA and control subjects. QTc during different sleep stages and while awake was also significantly increased in patients with LQTS and OSA compared to those without OSA. Severity of OSA in patients with LQTS was directly associated with the degree of QTc.
Conclusions:
The presence and severity of obstructive sleep apnea (OSA) in patients with congenital long QT syndrome (LQTS) is associated with increased QT prolongation corrected for heart rate, which is an important biomarker of sudden cardiac death (SCD). Treatment of OSA in LQTS patients may reduce QT prolongation, thus reducing the risk of LQT-triggered SCD.
Citation:
Shamsuzzaman AS, Somers VK, Knilans TK, Ackerman MJ, Wang Y, Amin RS. Obstructive sleep apnea in patients with congenital long QT syndrome: implications for increased risk of sudden cardiac death. SLEEP 2015;38(7):1113–1119.
Keywords: hypoxia, long-QT syndrome, torsade de pointes, sleep apnea
INTRODUCTION
Congenital long QT syndrome (LQTS) is a familial arrhythmogenic cardiac channelopathy characterized by prolonged ventricular repolarization and increased risk of torsades de pointes-mediated syncope, seizures, and sudden cardiac death (SCD).1,2 Reduced outward potassium current with geno-types LQT1 (KCNQ1) and LQT2 (KCNH2) or excess influx of sodium with genotype LQT3 (SCN5A) in the mutant cardiac ion channel cause lengthened action-potential duration and consequent prolonged QT interval.3 Thus, QT prolongation reflects the functional abnormality in the cardiac ion channel and is a well-established diagnostic and prognostic feature in congenital LQTS.4,5
Obstructive sleep apnea (OSA) recently has been linked to abnormalities in cardiac repolarization.6,7 OSA is characterized by recurrent episodes of cessation of airflow caused by upper-airway inspiratory collapse during sleep, with a consequent decrease in oxygen saturation.8 OSA is a common sleep problem in both adults and children that increasingly has been implicated in the pathogenesis and complications of cardiovascular disease, including arrhythmias9 and SCD.10 Apnea-related acute sympathetic activation during sleep and sustained elevation in sympathetic activity while awake and breathing normally are considered to be important mechanisms of cardiovascular morbidity and mortality in patients with OSA.11,12 Sympathetic activation also plays a critical role for the generation of cardiac arrhythmias in LQTS patients, as evidenced by the effect of beta-adrenergic agonist on spontaneous arrhythmias after experimental QT prolongation with pharmacologic blockade of cardiac ion channels in an experimental animal model.13 In addition, beta-adrenergic blockade is the primary treatment strategy for the prevention of SCD in patients with congenital LQTS, especially patients with sympathetically triggered type 1 LQTS (LQT1).14
A recent study of patients with Brugada syndrome, another cardiac channelopathy caused by loss-of-function sodium channel mutations, who are at increased risk for SCD during sleep, suggested that approximately half of these patients have clinically significant OSA.15 Considering the significant relationship between OSA and cardiovascular mortality in patients with normal hearts, we anticipate that the exaggerated sympathetic activation and abnormality in cardiac repolarization related to OSA has a significant effect on triggering cardiac arrhythmias during sleep or while awake in patients with LQTS who are at increased risk of SCD due to the presence of genetic defects in the heart. Therefore, we assessed whether the presence of OSA in patients with LQTS is associated with increased QT prolongation both during sleep and while awake.
SUBJECTS AND METHODS
Subjects
Patients with congenital LQTS (N = 54; 14 male, 40 female) were recruited from the Heart Center of Cincinnati Children's Hospital Medical Center (CCHMC) for diagnosis of OSA by overnight standard polysomnography (PSG), which was conducted in the CCHMC Sleep Center. Patients with LQTS were divided into two groups: those with and those without OSA, according to the determined apnea-hypopnea index (AHI). OSA was diagnosed as the presence of AHI ≥ 5 events/h for adults (age 18 y or older) and AHI > 1 event/h for children (age younger than 18 y). The patients with OSA included in the study had never been treated for OSA. Control subjects (N = 67; 21 male, 46 female) were recruited for sleep studies from the greater Cincinnati area through local advertisement, and those with occult OSA by overnight PSG (AHI ≥ 5 events/h for adults and AHI > 1 event/h for children) were excluded from the study. The control group was free of any acute or chronic disease and on no medications. The study was approved by the CCHMC Institutional Human Subjects Review Committee.
Methods
Polysomnography
PSG was conducted using a computerized system (Grass, Telefactor, Astro Inc., Westwarwick, RI, USA). Recording variables were electroencephalogram (C3-A2, C4-A1, O1-A2, and O2-A1); right and left electrooculogram (EOG); submental, tibial, and intercostal electromyogram (EMG); electrocardiogram (ECG); nasal/oral airflow through nasal pressure sensor; end-tidal CO2 measured at the nose by infrared capnometry using the Nelcor N1000 (Nelcor, Van Nuys, CA, USA); oxygen saturation (SpO2) by finger-pulse oximeter (Nelcor N1000); and rib cage- and abdominal-volume changes with a computer-assisted respiratory inductance plethysmograph (Somnostar, Noninvasive Monitoring System Inc., Miami Beach, FL, USA). Sleep staging was performed according to the rules of Rechtschaffen and Kales.16 All sleep studies were scored, according to the standard criteria set by the American Academy of Sleep Medicine.17 Blinded interpretations of the sleep studies were carried out by the same board-certified sleep specialist. Results obtained from the PSG were sleep duration, percentage of sleep time spent in different stages of sleep, number of arousals from sleep, and AHI. An apnea was defined as complete cessation of airflow for at least 10 sec. Hypopnea was defined as 30% or greater reduction of respiratory signals for at least 10 seconds associated with oxygen desaturation of ≥ 4%. The AHI was calculated as the total number of respiratory events per hour of sleep.
Measurements of the 12-Lead ECG and QT Interval
The 12-lead ECG was recorded continuously and simultaneously with the PSG from surface electrodes using a Ponemah 12-lead ECG Amplifier (Data Sciences International, Valley View, OH, USA). The data were digitized on a computer and stored for subsequent analysis. All signals were sampled at a frequency of 1 kHz. RR and QT intervals were calculated from either the lead II or lead V5 of the 12-lead surface ECG using an ECG analysis module (AD Instruments, Colorado Springs, CO, USA). Lead V5 was selected in some subjects because of noise in lead II ECG, particularly during sleep. However, the same lead II or V5 was used in each subject for calculating QT and RR during awake and sleep stages. The ECG module provides a graphical interface of recorded ECG with automatic identification of beginning and end of each wave form of the ECG waves and manual control on the automatically selected markers of each wave form. RR and QT intervals were measured from segments of recording ranges from 3 to 5 min of inactive wakefulness (eyes closed and lights out, at the beginning of the sleep study) and each stage of sleep without electroencephalogram (EEG) arousals, irregular or abnormal breathing including apnea and hypopnea, abnormal ECG including premature ventricular contractions, and movements including periodic leg movements. In addition, care was taken to select a segment during sleep stages at least 2 min before and 2 min after any of these events. The QT intervals were measured from the onset of the Q- or R-wave to the termination of the T-wave, which was determined by the intersection of a tangent drawn to the steepest slope of the down-sloping portion of the T-wave and the isoelectric baseline. The beginning of Q- and R-wave and ending of T-wave were identified by automated computer program and manually edited after careful observation of the 12-lead ECG. QT prolongation corrected for heart rate (QTc) were calculated by Bazett formula,18 where QTc = QT/RR1/2. The investigators involved in ECG analyses were blinded to the diagnosis of OSA.
Protocols
All studies were carried out in the CCHMC Sleep Laboratory. Simultaneous measurements of overnight PSG and 12-lead ECG were conducted in all study subjects. For studies in the patients with LQTS, the investigators were blinded to the genotype, history of cardiac events, and diagnosis of OSA in these subjects.
Statistical Analysis
Demographic and clinical characteristics including sleep profile were compared between groups. For continuous variables, mean and standard deviation (SD) or median and interquartile range (IQR) are reported. Either one-way analysis of variance (ANOVA) or the nonparametric Kruskal-Wallis test was used to compare the means or medians among three groups (control, LQTS with or without OSA). Further pairwise comparisons were performed, if significant differences were determined from either test. Tukey-Kramer multiplicity adjustment was applied to multiple comparisons involved. For categorical variables, frequencies were reported. Fisher exact test was used to check if there was any difference in the distribution between groups. RR, QT, and QTc intervals were compared between groups and sleep stages by using repeated-measures ANOVA with patient group, sleep stage, and their interaction included in the model. Simulation-based multiplicity adjustment was applied to multiple comparisons of the least square means. Repeated-measures simple and multiple regression analyses were performed to check the association of age, sex, body mass index (BMI), history of beta blocker, and syncope with RR, QT, and QTc intervals in patients with LQTS when awake. The association of AHI, arousal index (AI), and SpO2 with QTc interval in patients with LQTS was investigated using simple linear regression models for the average QTc. Analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA). All tests are two-sided with P < 0.05 used for statistical significance.
RESULTS
Characteristics of LQTS and Control Subjects
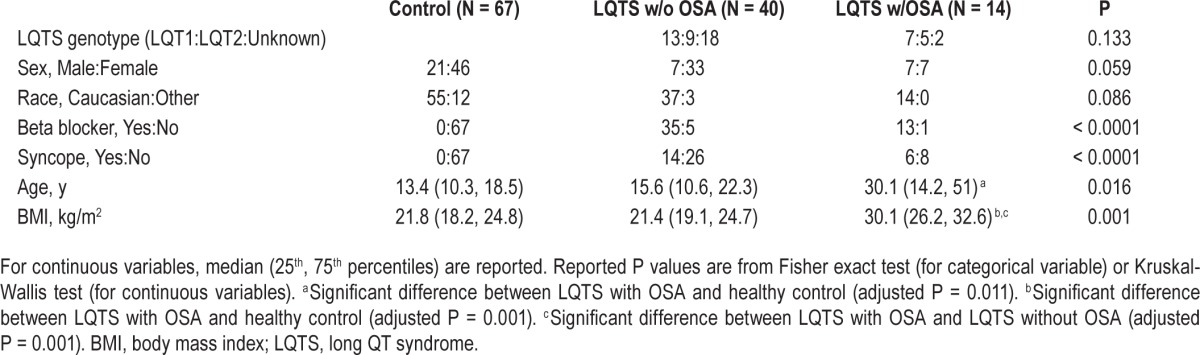
Baseline characteristics of LQTS subjects with or without OSA and control subjects are described in Table 1. All patients with LQTS in the current study received a clinical diagnosis of and were treated for LQTS. OSA was diagnosed in approximately 26% of the patients with LQTS recruited to the study. Patients with LQTS and OSA were significantly older and heavier compared with those with LQTS without OSA and control subjects. Differences in history of beta blocker use (P = 1.0) and syncope (P = 0.75) in patients with LQTS with or without OSA were not significant. The proportion of LQTS genotypes LQT1, LQT2, and unknown genotypes between patients with LQTS with or without OSA were not different, and there were no patients with LQT3 in this study. Unknown LQTS genotypes include those for patients who refused genotyping.
Table 1.
Characteristics of control subjects, patients with LQTS and without OSA, and patients with LQTS and with OSA.
Sleep Profile
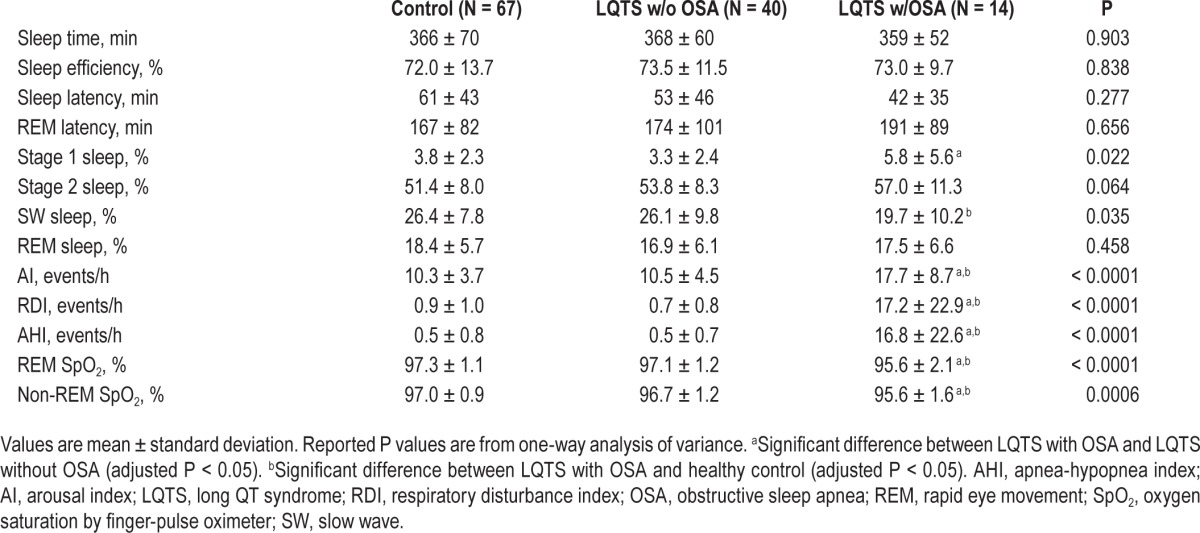
Sleep profiles of LQTS with or without OSA and control subjects are summarized in Table 2. Total sleep time, sleep efficiency, rapid eye movement (REM) sleep latency, and REM sleep percentage were not significantly different between the three groups. The percentage of Stage 1 sleep was increased (adjusted P = 0.016) and there was a tendency of decreased slow wave sleep (adjusted P = 0.057) in patients with LQTS and OSA compared to those with LQTS without OSA. However, the percentages of Stage 2 sleep and REM sleep were not significantly different between the three groups. AI, respiratory disturbance index, and AHI were significantly increased and oxygen saturation during both non-REM and REM sleep stages were significantly reduced in patients with LQTS and OSA compared with either patients with LQTS and without OSA or control subjects.
Table 2.
Sleep profile of control subjects, patients with long QT syndrome without obstructive sleep apnea, and patients with long QT syndrome with obstructive sleep apnea.
RR, QT, and QTc Intervals
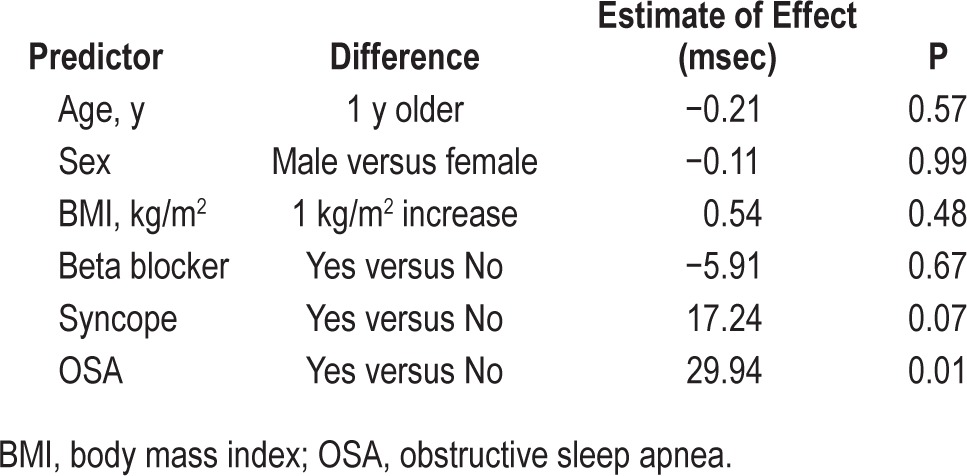
RR intervals, QT intervals, and Bazett-calculated QTc values while awake and during both non-REM and REM sleep stages were increased significantly in patients with LQTS compared to control subjects. Additionally, patients with LQTS and OSA had significantly increased RR, QT, and QTc during awake and sleep compared to patients with LQTS and without OSA (Figure 1). However, sleep stage-related changes in these variables between all three subject groups were not statistically significant. Simple and multiple regression analysis indicated that OSA was significantly associated with QTc intervals while awake, after adjustment of age, sex, BMI, history of beta blocker, and syncope (Table 3).
Figure 1.
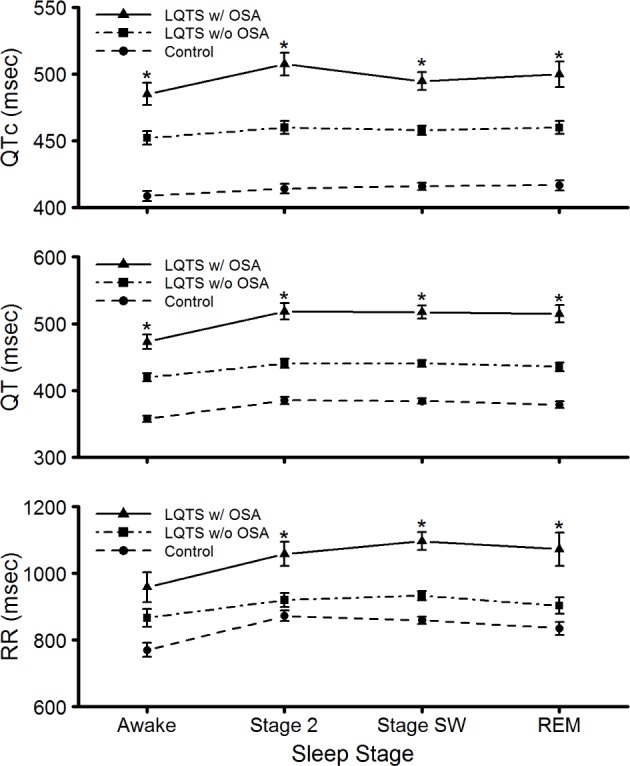
Changes in RR, QT, and QT prolongation corrected for heart rate (QTc) intervals while awake and during non-REM [Stage 2 and slow wave (Stage SW)] and REM sleep in patients with LQTS with or without OSA and control subjects. Data are least square means ± standard error of the mean. *Adjusted P < 0.05 for comparison between patients with LQTS with and without OSA. LQTS, long QT syndrome; OSA, obstructive sleep apnea; REM, rapid eye movement.
Table 3.
Multiple regression analysis of the effects of age, sex, body mass index, history of beta blocker use, syncope and obstructive sleep apnea on QT prolongation corrected for heart rate interval in patients with long QT syndrome while awake.
Effects of Severity of OSA on QTc
Correlation analysis based on simple linear regression indicated that the severity of OSA as described by the level of AHI (r = 0.27 and P = 0.049) and AI (r = 0.40 and P = 0.003) were associated directly with the degree of QTc (Figure 2). However, oxygen desaturation during either non-REM (r = 0.18 and P = 0.201) or REM (r = 0.06 and P = 0.689) sleep was not associated with QTc.
Figure 2.
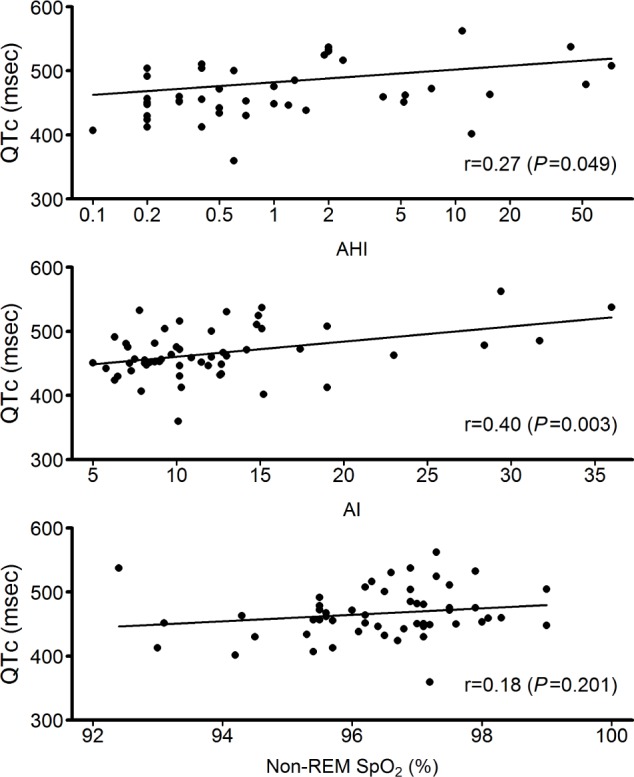
Relationships between QT prolongation corrected for heart rate (QTc) and severity of obstructive sleep apnea. Log scale is used for apnea-hypopnea index (AHI). Both AHI and arousal index (AI) are directly and significantly associated with QTc.
DISCUSSION
Our findings suggest that the presence of OSA in patients with congenital LQTS is associated with increased QTc independent of age, sex, BMI, use of beta blocker, and history of syncope. QTc derived from ECG predicts future cardiac arrhythmias and SCD in patients with LQTS,5 cardiovascular disease,19,20 and even in apparently healthy humans.21 In addition, severity of OSA as expressed by the level of AHI and AI during sleep is directly related to the degree of QT prolongation.
Normal Sleep and QTc
Sleep in healthy individuals is associated with distinct sleep stage-related changes in autonomic and cardiovascular functions.22 The autonomic nervous system contributes importantly to beat-to-beat variation in the QT interval.23 Thus, normal sleep is associated with QT prolongation due to sleep related changes in autonomic function.24 Our previous results indicated that sympathetic activation during REM sleep is associated with increased QTc in normal individuals and that QTc interval is increased during sleep in female patients with LQTS, particularly during REM sleep.25 REM sleep also is associated with arrhythmias, particularly in patients with the LQT2 genotype.26
Acute and Chronic Effects of Sleep Apnea on Autonomic Function
Hypoxemia, hypercapnia, and frequent arousals due to repetitive apneas disrupt the normal physiologic interactions between sleep and the autonomic and cardiovascular systems in patients with OSA.8 The acute hemodynamic consequences of OSA include sympathetic-mediated vasoconstriction and consequent increases in systemic blood pressure and brady-cardia. OSA also is associated with acute sympathetic activation during apneic events that disappears after resumption of breathing during sleep and persistently elevated sympathetic drive during the daytime normoxic awake state while breathing normally.27 Repeated nocturnal apneic events in patients with OSA result in carryover sustained daytime activation of the sympathetic nervous system. Markedly elevated daytime resting sympathetic nerve traffic in patients with OSA may be associated with alteration of QT interval due to direct effects on the ventricular myocardium or an indirect effect on the sinus node, or both. Therefore, OSA may act as a trigger for the development of arrhythmias, particularly in patients with LQTS.
OSA and QTc
Patients with OSA and with normal cardiac structure and function have increased QTc and QT dispersion compared to control subjects closely matched for age, sex, and BMI.28 Increased QT dispersion that was reduced after treating OSA with continuous positive airway pressure has been reported in patients with OSA.29,30 Approximately 26% of the patients with LQTS in our study had clinically significant OSA. In contrast, only approximately 3–5% of the general population in the US have had OSA diagnosed by PSG.31 We determined that the presence of OSA in LQTS was significantly associated with increased QTc (Table 3 and Figure 1), and our results suggest that the severity of OSA in LQTS is directly related to the degree of QTc (Figure 2). OSA in the general population is also associated with increased QT interval variability, which correlates with the severity of OSA.32 Strong association between severity of OSA and QTc and significant improvement of cardiac repolarization after treatment of OSA suggest important dose-dependent effects of OSA on cardiac repolarization in patients with OSA. Thus, successful treatment of OSA also might reduce QT prolongation and therefore the risk of SCD in patients with LQTS.
Effects of Hypoxia on QT Intervals During Sleep
Apneic events during sleep are associated with sympathetic activation11 and bradyarrhythmias.33,34 Acute hypoxia in healthy volunteers has been associated with significant prolongation of QT intervals, which returned to normal following withdrawal of the hypoxic stimuli.35 In patients with LQTS, vagal brady-arrhythmias during an apneic event will further prolong the QT interval. Therefore, extreme QT prolongation in the setting of severe sympathetic activation and consequent increases in plasma catecholamine might trigger life-threatening arrhythmias during sleep in patients with LQTS who have OSA. QT-interval prolongation also has been reported during nocturnal hypoxemia in patients with chronic obstructive airway disease36 or coronary artery disease with intermittent decreases in oxygen saturation of blood.37 Ventricular arrhythmias that have been observed frequently in patients with severe OSA38 correlate with the degree of oxygen desaturation and effective therapy and are associated with suppression of arrhythmias.39 In our study, patients with LQTS and OSA had significantly reduced SpO2 during both non-REM and REM sleep compared to LQTS without OSA and control subjects. Therefore, exposure to chronic hypoxia resulting in markedly elevated sympathetic activity during sleep may be a critical mechanism for increased QT prolongation during both wakefulness and sleep in patients with LQTS and OSA.
Repeated Arousals During Sleep and QTc
EEG arousal is common at the end of apneic episodes during sleep. Our results demonstrate that the subjects with LQTS with OSA had significantly elevated AI compared to those with LQTS without OSA and control subjects. Arousal from sleep activates upper-airway dilator muscles and prevents prolonged apnea, while also markedly increasing sympathetic drive, heart rate, and blood pressure. Arousal from sleep, which is typical for patients with OSA, also may be an important trigger for cardiac arrhythmias during sleep in patients with LQTS.40 Sleep deprivation occurs in patients with OSA as a result of repetitive nocturnal arousals and also has been associated with autonomic and circulatory dysregulation.41 A recent study determined that only 1 night of sleep deprivation in healthy individuals was associated with significant increases in maximal QT interval (QTmax), QT interval dispersion (QTd) and QTc dispersion (QTcd) compared to regular sleep.42 Therefore, sleep fragmentation accompanying OSA in patients with LQTS may be associated with increased QT prolongation.
Sympathetic Activation and Cardiac Arrhythmias in LQTS
Historically, cardiac events in patients with LQTS are thought to be associated with sympathetic activation and imbalance in cardiac sympathetic drive.43 Thus, future cardiac arrhythmias and SCD in patients with congenital LQTS can be prevented with adrenoreceptor antagonist and left-cardiac sympathectomy in high-risk patients with LQTS.44 Animal studies have suggested that QT prolongation after pharmacologic blockade of cardiac ion channels did not spontaneously produce arrhythmias until sympathetic stimulation with beta adrenergic agonist was introduced.13 Therefore, acute sympathetic surge during apneic events while asleep and chronically elevated sympathetic drive in patients with LQTS and OSA may play a critical role for triggering cardiac events in patients with preexisting cardiac ion channelopathy.
LQTS Genotype and Risk of SCD
History of syncope, age, sex, and LQTS genotype play a critical role in the risk of SCD in patients with congenital LQTS.45–48 Individuals with genotype LQT1 have arrhythmogenic triggers related to adrenergic stimulation, such as exertion and particularly swimming.49 In contrast, a majority of individuals with genotype LQT2 and most of the patients with LQT3 are especially at risk for sleep related cardiovascular events.46,48 We found that the presence and severity of OSA between LQT1 and LQT2 patients were not significantly different. Unfortunately, there were no patients with LQT3 in our study.
Limitations
The patients with LQTS with OSA in our study were older on average than the patients with LQTS without OSA. The LQTS with OSA subjects also were heavier compared to those without OSA, and obesity is a well-established risk factor for OSA. Therefore, multivariate analyses were conducted for adjustment of age and BMI (Table 3), and the differences in QTc reported in the current study were not due to differences in age or BMI between the two groups. In addition, we found that history of use of beta blockers and syncope were not associated with increased QTc in the LQTS subjects with OSA.
CONCLUSIONS
Both congenital LQTS and OSA are independently associated with increased risk for cardiac arrhythmias and SCD. OSA with QT-interval prolongation has been considered as a lethal combination, particularly in infants, often causing sudden and unexpected death.50 We have determined a previously unknown pathophysiologic mechanism of increased risk for SCD in patients with LQTS. Thus, diagnosis of OSA in patients with congenital LQTS could be used as a novel bio-marker for assessment of risk for future cardiac events. The positive directional relationship between AHI and QTc that we determined suggests dose-dependent effects of OSA severity on the degree of QT prolongation. Importantly, OSA is a treatable risk factor for cardiovascular diseases, and treatment of OSA may improve autonomic dysfunction and reduce QT prolongation and therefore also reduce the risk of SCD in patients with LQTS. Future study will determine the effects of treatment of OSA on autonomic functions and QTc in patients with congenital LQTS.
DISCLOSURE STATEMENT
This was not an industry supported study. These studies were supported by a Scientist Development Award from the American Heart Association (0730129N) and Clinical Translational Research Center, Cincinnati Children's Hospital Medical Center. This work also was supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program (MJA), and National Institutes of Health grants HL070302 and HL065176 (VKS). Dr. Somers has served as a consultant for Cardiac Concepts, Sepracor, Boston Scientific, and ResMed and is an investigator on studies funded with grants from the Respironics Sleep and Breathing Foundation, Sorin, Inc., and Select Research. Dr. Ackerman is a consultant for Boston Scientific, Gilead Sciences, Medtronic, and St. Jude Medical and receives royalties from Transgenomic for FAMILION-LQTS and FAMILION-CPVT genetic tests. None of these companies were involved in this study. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Rhonda D. Szczesniak, PhD and Matthew C. Fenchel, MS for statistical review, Jennifer Jef-fries, RN, BS, for contributing to the regulatory management of the study, and J. Denise Wetzel, CCHMC Medical Writer, for critical review of the manuscript.
Footnotes
A commentary on this article appears in this issue on page 1005.
REFERENCES
- 1.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–80. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz PJ. The congenital long QT syndromes from genotype to phenotype: clinical implications. J Intern Med. 2006;259:39–47. doi: 10.1111/j.1365-2796.2005.01583.x. [DOI] [PubMed] [Google Scholar]
- 3.Roden DM, Lazzara R, Rosen M, Schwartz PJ, Towbin J, Vincent GM. Multiple mechanisms in the long-QT syndrome. Current knowledge, gaps, and future directions. The SADS Foundation Task Force on LQTS. Circulation. 1996;94:1996–2012. doi: 10.1161/01.cir.94.8.1996. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome. An update. Circulation. 1993;88:782–4. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- 5.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–74. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 6.Barta K, Szabo Z, Kun C, et al. The effect of sleep apnea on QT interval, QT dispersion, and arrhythmias. Clin Cardiol. 2010;33:E35–9. doi: 10.1002/clc.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicek D, Lakadamyali H, Gokay S, Sapmaz I, Muderrisoglu H. Effect of obstructive sleep apnea on heart rate, heart rate recovery and QTc and P-wave dispersion in newly diagnosed untreated patients. Am J Med Sci. 2012;344:180–5. doi: 10.1097/MAJ.0b013e318239a67f. [DOI] [PubMed] [Google Scholar]
- 8.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 9.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol. 1983;52:490–4. doi: 10.1016/0002-9149(83)90013-9. [DOI] [PubMed] [Google Scholar]
- 10.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–14. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 11.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand. 2003;177:385–90. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 13.Antzelevitch C. Sympathetic modulation of the long QT syndrome. Eur Heart J. 2002;23:1246. doi: 10.1053/euhj.2002.3287. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz PJ, Crotti L, Insolia R. Long-QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–77. doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macedo PG, Brugada J, Leinveber P, et al. Sleep-disordered breathing in patients with the Brugada syndrome. Am J Cardiol. 2011;107:709–13. doi: 10.1016/j.amjcard.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales A. A manual of standardized terminology. Techniques and scoring systems for sleep stages of human subjects. Los Angeles, CA: UCLA Brain Information Service, Brain Research Institute; 1968. [Google Scholar]
- 17.Iber C, Ancoli-Israel S, Chesson AJ, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Bazett H. An analysis of the time-relations of electrocardiograms. Heart. 1918;7:353–70. [Google Scholar]
- 19.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–94. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 20.Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88:315–25. doi: 10.1016/j.mayocp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Montanez A, Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med. 2004;164:943–8. doi: 10.1001/archinte.164.9.943. [DOI] [PubMed] [Google Scholar]
- 22.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–7. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 23.Murakawa Y, Inoue H, Nozaki A, Sugimoto T. Role of sympathovagal interaction in diurnal variation of QT interval. Am J Cardiol. 1992;69:339–43. doi: 10.1016/0002-9149(92)90230-v. [DOI] [PubMed] [Google Scholar]
- 24.Browne KF, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q-T interval in man during sleep. Am J Cardiol. 1983;52:55–9. doi: 10.1016/0002-9149(83)90068-1. [DOI] [PubMed] [Google Scholar]
- 25.Lanfranchi PA, Shamsuzzaman AS, Ackerman MJ, et al. Sex-selective QT prolongation during rapid eye movement sleep. Circulation. 2002;106:1488–92. doi: 10.1161/01.cir.0000030183.10934.95. [DOI] [PubMed] [Google Scholar]
- 26.Lanfranchi PA, Ackerman MJ, Kara T, et al. Gene-specific paradoxical QT responses during rapid eye movement sleep in women with congenital long QT syndrome. Heart Rhythm. 2010;7:1067–74. doi: 10.1016/j.hrthm.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–6. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- 28.Voigt L, Haq SA, Mitre CA, Lombardo G, Kassotis J. Effect of obstructive sleep apnea on QT dispersion: a potential mechanism of sudden cardiac death. Cardiology. 2011;118:68–73. doi: 10.1159/000324796. [DOI] [PubMed] [Google Scholar]
- 29.Dursunoglu D, Dursunoglu N. Effect of CPAP on QT interval dispersion in obstructive sleep apnea patients without hypertension. Sleep Med. 2007;8:478–83. doi: 10.1016/j.sleep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Roche F, Barthelemy JC, Garet M, Duverney D, Pichot V, Sforza E. Continuous positive airway pressure treatment improves the QT rate dependence adaptation of obstructive sleep apnea patients. Pacing Clin Electrophysiol. 2005;28:819–25. doi: 10.1111/j.1540-8159.2005.00188.x. [DOI] [PubMed] [Google Scholar]
- 31.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 32.Baumert M, Smith J, Catcheside P, et al. Variability of QT interval duration in obstructive sleep apnea: an indicator of disease severity. Sleep. 2008;31:959–66. [PMC free article] [PubMed] [Google Scholar]
- 33.Brown LK, Miller A, Stacy C. Sleep apnea syndrome and nocturnal bradyarrhythmias. Arch Intern Med. 1986;146:608. [PubMed] [Google Scholar]
- 34.Zwillich C, Devlin T, White D, Douglas N, Weil J, Martin R. Bradycardia during sleep apnea. Characteristics and mechanism. J Clin Invest. 1982;69:1286–92. doi: 10.1172/JCI110568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roche F, Reynaud C, Pichot V, et al. Effect of acute hypoxia on QT rate dependence and corrected QT interval in healthy subjects. Am J Cardiol. 2003;91:916–9. doi: 10.1016/s0002-9149(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 36.Tirlapur VG, Mir MA. Nocturnal hypoxemia and associated electrocardiographic changes in patients with chronic obstructive airways disease. N Engl J Med. 1982;306:125–30. doi: 10.1056/NEJM198201213060301. [DOI] [PubMed] [Google Scholar]
- 37.De Olazabal JR, Miller MJ, Cook WR, Mithoefer JC. Disordered breathing and hypoxia during sleep in coronary artery disease. Chest. 1982;82:548–52. doi: 10.1378/chest.82.5.548. [DOI] [PubMed] [Google Scholar]
- 38.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910–6. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javaheri S. Effects of continuous positive airway pressure on sleep apnea and ventricular irritability in patients with heart failure. Circulation. 2000;101:392–7. doi: 10.1161/01.cir.101.4.392. [DOI] [PubMed] [Google Scholar]
- 40.Ali RH, Zareba W, Moss AJ, et al. Clinical and genetic variables associated with acute arousal and nonarousal-related cardiac events among subjects with long QT syndrome. Am J Cardiol. 2000;85:457–61. doi: 10.1016/s0002-9149(99)90772-5. [DOI] [PubMed] [Google Scholar]
- 41.Kato M, Phillips BG, Sigurdsson G, Narkiewicz K, Pesek CA, Somers VK. Effects of sleep deprivation on neural circulatory control. Hypertension. 2000;35:1173–5. doi: 10.1161/01.hyp.35.5.1173. [DOI] [PubMed] [Google Scholar]
- 42.Ozer O, Ozbala B, Sari I, et al. Acute sleep deprivation is associated with increased QT dispersion in healthy young adults. Pacing Clin Electrophysiol. 2008;31:979–84. doi: 10.1111/j.1540-8159.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz PJ. Another role for the sympathetic nervous system in the long QT syndrome? J Cardiovasc Electrophysiol. 2001;12:500–2. doi: 10.1046/j.1540-8167.2001.00500.x. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz PJ, Locati EH, Moss AJ, Crampton RS, Trazzi R, Ruberti U. Left cardiac sympathetic denervation in the therapy of congenital long QT syndrome. A worldwide report. Circulation. 1991;84:503–11. doi: 10.1161/01.cir.84.2.503. [DOI] [PubMed] [Google Scholar]
- 45.Liu JF, Jons C, Moss AJ, et al. Risk factors for recurrent syncope and subsequent fatal or near-fatal events in children and adolescents with long QT syndrome. J Am Coll Cardiol. 2011;57:941–50. doi: 10.1016/j.jacc.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zareba W, Moss AJ, Schwartz PJ, et al. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–5. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 47.Vincent GM, Timothy KW, Leppert M, Keating M. The spectrum of symptoms and QT intervals in carriers of the gene for the long-QT syndrome. N Engl J Med. 1992;327:846–52. doi: 10.1056/NEJM199209173271204. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 49.Ackerman MJ, Tester DJ, Porter CJ, Edwards WD. Molecular diagnosis of the inherited long-QT syndrome in a woman who died after near-drowning. N Engl J Med. 1999;341:1121–5. doi: 10.1056/NEJM199910073411504. [DOI] [PubMed] [Google Scholar]
- 50.Smith TA, Mason JM, Bell JS, Francisco JT. Sleep apnea and Q-T interval prolongation--a particularly lethal combination. Am Heart J. 1979;97:505–8. doi: 10.1016/0002-8703(79)90399-5. [DOI] [PubMed] [Google Scholar]


