Abstract
In this work, we have synthesized 5-thiocyanato-2′-deoxyuridine (SCNdU) along with the C6-deuterated nucleobase 5-thiocyanatouracil (6-D-SCNU) and studied their reactions with radiation-produced electrons. ESR spectra in γ-irradiated nitrogen-saturated frozen homogeneous solutions (7.5 M LiCl in H2O or D2O) of these compounds show that electron-induced S-CN bond cleavage occurs to form a thiyl radical (dU-5-S• or 6-D-U-5-S•) and CN− via the initial π-anion radical (SCNdU•−) intermediate in which the excess electron is on the uracil base. HPLC and LC-MS/MS studies of γ-irradiated N2-saturated aqueous solutions of SCNdU in the presence of sodium formate as a OH-radical scavenger at ambient temperature show the formation of the dU-5S-5S-dU dimer in preference to dU by about 10 to 1 ratio. This shows that both possible routes of electron-induced bond cleavage (dUC5-SCN and S-CN) in SCNdU•− and dU-5-S• formation are preferred for the production of the σ-type uracilyl radical (dU•) by 10 fold. DFT/M06-2x/6-31++G(d,p) calculations employing the polarizable continuum model (PCM) for aqueous solutions show that dU-5-S• and CN− formation was thermodynamically favored by over 15 kcal/mol (ΔG) compared to dU• and SCN− production. The activation barriers for C5-S and S-CN bond cleavage in SCNdU•− amount to 8.7 and 4.0 kcal/mol, respectively, favoring dU-5-S• and CN− formation. These results support the experimental observation of S-CN bond cleavage by electron addition to SCNdU that results in the formation of dU-5-S• and the subsequent dU-5S-5S-dU dimer. This establishes SCNdU as a potential radiosensitizer that could cause intra- and inter-strand crosslinking as well as DNA-protein crosslinking via S-S dimer formation.
Keywords: radiosensitizers, thiocyanatouridine, dimerization, electron spin resonance, DFT calculations
1. Introduction
5-Bromo-2′-deoxyuridine (5-BrdU) and its nucleobase, 5-bromouracil (5-BrU), belong to the class of DNA radiosensitizers that are incorporated into DNA during replication and repair.1–5 Owing to the very similar van der Waals radii of -CH3 and Br, 5-BrdU easily substitutes thymine in DNA without affecting its function.1 Various investigations have demonstrated a correlation between the extent of 5-BrdU incorporation and radiosensitization.4,5 The major mechanism for 5-BrdU-induced radiosensitization has been proposed to be the fast, irreversible attachment of an electron to 5-BrdU incorporated into DNA, leading to the formation of the halouracil π-anion radical; subsequently, the halouracil π-anion radical very rapidly dissociates into the uracil-5-yl π-radical (U•) along with Br− loss via dissociative electron attachment (DEA).1 U•, being a vinyl-type radical, is highly reactive and can undergo rapid addition to double bonds, leading to crosslinking (e.g., addition to the parent C5=C6 double bond of 5-BrU)1,6 and H-atom abstraction1,7 as competitive reactions. U• can abstract a hydrogen atom from an adjacent8–10 sugar residue, leading to a single strand break (SSB). Quite recently, it was demonstrated that U• is sufficiently reactive to trigger the release of a OH-radical from a proximate water molecule.11 We note here that OH-radicals are well-known genotoxic agents which, if produced in sufficient proximity to the double helix, readily lead to DNA strand breaks by H-atom abstraction.1,12 Furthermore, U•- induced H-atom abstraction from sugar residues has been employed as a probe to find various conformations (A, B, Z, and G-quadruplex) of DNA-oligomers.12,13
Under hypoxia, commonly observed in solid tumor cells,14 water radiolysis releases a large number of solvated electrons15 which are, however, not able to damage native DNA16 unless a radiosensitizer such as 5-BrdU is present. Therefore, the employment of some type of radiosensitization seems to be indispensable for efficient ionizing radiation treatment.
Although in vitro experiments showed BrdU to be a potent radiosensitizer,4,5 in vivo tests were much less optimistic. One of the most extended clinical trials on brain cancer patients17 did not show any advantages of radiotherapy with 5-BrdU as an adjuvant agent over irradiation treatment alone. Thus, this situation calls for new sensitizers that would be efficient not only in vitro but also in patients.
Very recently, we investigated the efficiency of electron-induced U• formation on various C5-H substituted uracils.18 Therefore, we carried out DFT modelling of the DEA process in 5-substituted uracils. We assumed two premises: (i) a 5-substituent must increase the electron affinity of the studied derivative compared to that of uracil and (ii) the C5-X (where X stands for a substituent) bond should be weak enough to ensure an efficient dissociation (irreversible reaction) in the π-anion radical formed due to electron attachment. The free energy profiles for the DEA process calculated for only 10 derivatives resulted in two compounds, 5-thiocyanatouracil (SCNU) and 5-oxocyanatouracil (OCNU), which should be comparable or even better radiosensitizers than 5-BrU.18
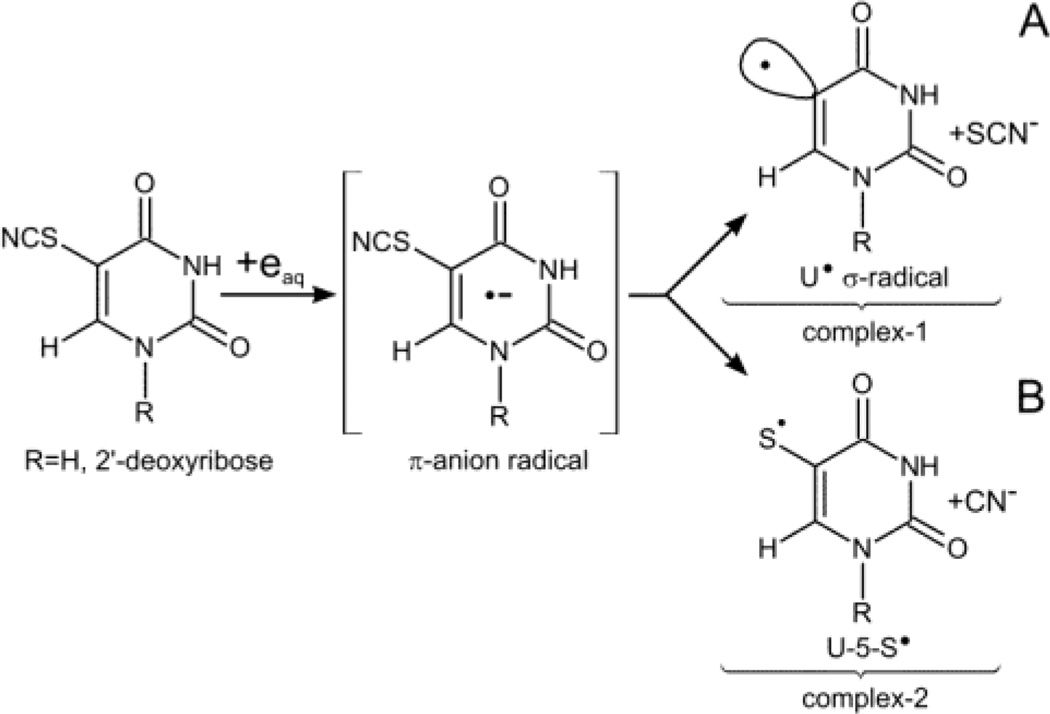
In the current work, the sensitivity of SCNdU (Scheme 1) to electron attachment is investigated through the use of electron spin resonance (ESR) studies at low temperatures, steady-state radiolysis at ambient temperature, and DFT modeling. We demonstrate that π-anion radicals formed due to electron attachment in the nucleoside (SCNdU) or in the C6-deuterated nucleobase 5-thiocyanatouracil (6-D-SCNU) lead to two types of radicals via competitive reactions (Scheme 1). The formation of dU in low abundance as a stable product points to the U• production along with SCN− loss that was postulated in our previous studies.18 This originated from the dissociation of the C5-S bond in the SCNdU π-anion radical, SCNdU•− (Scheme 1). However, in this work, ESR studies at low temperature and product analyses at ambient temperature show that the predominant radical species is the 2′-deoxyuridine-5-thiyl radical (dU-5-S•). dU-5-S• is formed by S-CN bond cleavage of the thiocyanate substituent in SCNdU. The work of Houmam on aryl and benzyl thiocyanates19,20 shows that S-CN bond cleavage via DEA is feasible in the π-anion radical; in the aryl thiocyanate π-anion radical, S-CN bond cleavage is the especially favored process. Thus, our observation of the predominant formation of dU-5-S• from SCNdU•− is in accord with these results and, in fact, is the first report of the base-thiyl radical formation via DEA in a DNA model system. Furthermore, our results show that dU-5-S• dimerizes to form dU-5S-5S-dU. This result allows us to propose that dU-5-S•, when formed in DNA, will lead to both inter and intrastrand crosslinks as well as DNA-protein crosslinks. In addition, the second product of this DEA process, CN−, is highly cytotoxic, as it irreversibly binds to cytochrome oxidase, which effectively halts cellular respiration.21 We thereby propose that SCNdU or SCNU may be an effective radiosensitizer.
Scheme 1.
Formation of U• (ca. 10% yield) and uracil-5-thiyl radical (U-5-S•, ca. 90% yield) by electron addition to 5-thiocyanatouracil (SCNU) or 5-thiocyanato-2′-deoxyuridine (SCNdU) resulting in dissociative electron attachment (DEA).
2. Experimental section
Materials
Cyclohexene (≥98%), acetonitrile and methanol (HPLC grade), 2′-deoxyuridine (≥99%), potassium thiocyanate (≥99%) was purchased from Sigma-Aldrich (Poznań, Poland). Formic acid (≥99%) was purchased from POCH (Gliwice, Poland). 6-Deuterouracil (uracil-6-d1) was purchased from CDN Isotopes Inc. (Quebec, Canada) in a 0.1 vial and stored at room temperature (15–23°C) away from light and moisture. Chlorine gas (Cl2) was purchased from Linde AG (Munich, Germany). Lithium chloride (LiCl) (ultra dry, 99.995% (metals basis)) was obtained from Alfa Aesar (Ward Hill, MA, USA). Potassium ferrocyanide (K4[Fe(CN)6]) and deuterium oxide (D2O) (99.9 atom % D) were purchased from Aldrich Chemical Company Inc. (Milwaukee, WI, USA). Chromasolv® HPLC grade H2O was purchased from Sigma-Aldrich (St Louis, MO, USA). All these compounds were used without further purification.
Synthesis of 5-thiocyanato-2′-deoxyuridine
A 500 mL three-necked, round-bottomed flask equipped with a mechanical stirrer and drying tube was prepared. The flask was filled with an ice–cold solution of Cl2 (1.55 g, 21.80 mmol) in dry acetic acid (300 mL) prepared by passing Cl2 gas through a CaCl2 trap connected to a flask. Dried KSCN (2.34 g, 24.08 mmol) was then added to the mixture for the preparation of ClSCN. The solution was then stirred for 1.5 hours at room temperature. 2′-deoxyuridine (0.5 g, 2.19 mmol) was added in one portion to the solution of ClSCN in acetic acid and stirring was continued for 2.5 hours at room temperature. Half an hour before the end of reaction, 15 mL of cyclohexene was added. After filtration, the solution was evaporated under vacuum. The residue was maintained for 12 hours in 50% methanol. The MeOH was subsequently evaporated and the residue was extracted with hot water. The aqueous phase was purified with preparative HPLC (LC-20AP) with a UV detector (SPD-20A) set at 260 nm. A Gemini (Phenomenex®) reverse-phase C18 column (10 mm × 250 mm, 5 µm in particle size and 110 Å in pore size) with a mobile phase consisting of water, acetonitrile, and 1% formic acid (pH 2.55; 87.7:2:10.3 v/v/v) was used. The flow rate was set at 4 mL/min (see ESI, Figure S8). The resulting product (130 mg) was obtained as a white powder, with a yield of 40%. IR (KBr) 3416 (NH), 2163 (CN), 1726 (CO), 1673 (CO), 1614 (CN), 1297 (CN), 1163 (CH), 1094 (CO), 766 (CH), 665 (CS), 553 (CC); Anal. (C10H11N3O5S): calculated C, 42.10; H, 3.89; N, 14.73; S, 11.23 found C, 41.81; H, 4.00; N, 14.43; S, 11.47; Purity HPLC: 99% tR=21.043 min; ESI-MS [M-H]− = 284.06 (MW = 285.27)
Synthesis of 5-thiocyanato-6-d1-uracil
Following the above-mentioned protocol for SCNdU synthesis, here also a 500 mL three-necked, round-bottomed flask equipped with a mechanical stirrer and drying tube was prepared. Subsequently, the flask was filled with an ice-cold solution of Cl2 (0.53 g, 7.5 mmol) in dry acetic acid (200 mL) prepared by passing Cl2 gas through a CaCl2 trap connected to a flask. For ClSCN preparation, dried KSCN (0.80 g, 8.23 mmol) was added to the mixture. The solution was then stirred for 1.5 hours at room temperature. Uracil-6-d1 (0.1 g, 0.88 mmol) was added in one portion to the solution of ClSCN in acetic acid and stirring was continued for 2.5 hours at room temperature. 10 mL of cyclohexene was added to the reaction mixture half an hour before the completion of the reaction (i.e., substitution of the C5-H atom in the uracil base of 6-D-U by the SCN group). After filtration, the solution was evaporated under vacuum. The residue was extracted with hot water. The aqueous phase was purified with preparative HPLC (LC-20AP) with a UV detector (SPD-20A) set at 260 nm. A Gemini (Phenomenex®) reverse-phase C18 column (10 mm × 250 mm, 5 µm in particle size and 110 Å in pore size) with a mobile phase consisting of water, acetonitrile and 1% formic acid (87.7:2:10.3 v/v/v, pH 2.55) was used (see ESI, Figure S7). The flow rate was set at 4 mL/min. The resulting product was a white powder (25 mg, 35% yield). IR (KBr) 3432 (NH), 2279 (CD), 2160 (CN), 1705 (CO), 1666 (CO), 1188 (CH), 734 (CH), 666 (CS); Anal. (C5H3DN3O2S): calculated C, 35.29; H, 1.78; N, 24.69; S, 18.84 found C, 35.61; H, 1.49; N, 25.01; S, 19.14; Purity HPLC: 99% tR= 5.563 min; ESI-MS [M-H]− = 169.16 (MW = 170.18).
ESR measurements
(A) Preparation of homogeneous solutions
Following methods from our previous studies on various model systems of DNA and RNA,22–27 homogeneous solutions of SCNdU and 6-D-SCNU were prepared by dissolving 2 mg/mL in either 7.5 M LiCl as well as in 7.5 M LiBr in D2O or in H2O in the presence of K4[Fe(CN)6] (6 mg/mL), which acts as a scavenger of radiation produced holes. The purpose of the addition of a hole scavenger is to follow directly and only the formation of the one-electron reduced species and its subsequent reactions via ESR spectroscopy.
(B) pH adjustments
The homogeneous solutions have high ionic strengths (7.5 M LiCl or LiBr); therefore, a pH meter would not provide accurate pH measurements of these solutions. pH values reported in this work were obtained using pH papers and are approximate measurements, as described in our previous efforts.22 The pH of SCNdU in either 7.5 M LiCl as well as in 7.5 M LiBr in D2O or in H2O was adjusted to the range of ca. 5 (native pH of the 7.5 M LiCl or 7.5 M LiBr) to ca. 11 depending on the experiment. These pH adjustments were performed by quickly adding µL amounts of 1 M HCl or 1 M NaOH under ice-cold conditions.
(C) Preparation of glassy samples and their storage
Following our earlier work,22 to remove the dissolved oxygen in these pH-adjusted homogenous solutions, the solutions were bubbled thoroughly with nitrogen. Subsequently, these solutions were immediately drawn into 4 mm Suprasil quartz tubes (Catalog no. 734-PQ-8, WILMAD Glass Co., Inc., Buena, NJ, USA) and were rapidly cooled in liquid nitrogen (77 K). Transparent homogeneous glassy solutions were formed as a result of the rapid cooling of these degassed homogeneous liquid solutions at 77 K. These glassy solutions were later used for γ-irradiation and subsequent progressive annealing experiments. All glassy samples were stored at 77 K in Teflon containers in the dark.
(D) Irradiation and storage of γ-irradiated glassy samples for ESR studies
Following our previous efforts,22 these glassy solutions were γ (60Co)-irradiated using a model 109-GR 9 that utilizes a shielded 60Co irradiator (J.L. Sheppard and Associates, Inc.) (absorbed dose = 400 to 600 Gy) at 77 K. For LC-MS and LC-MS/MS studies (vide infra), N2-bubbled and sealed solutions of SCNdU (0.01 mg/mL in Chromasolv® HPLC grade H2O) were γ-irradiated at varying doses between 50 to 200 Gy at room temperature in the presence of sodium formate (1 mg/mL) as the OH-radical scavenger. During each experiment, one unirradiated sample of SCNdU was left as the control.
(E) Annealing of glassy samples for ESR studies
Following our earlier work,22–27 a variable temperature assembly was employed which passed liquid nitrogen-cooled nitrogen gas past a thermistor and over the sample. The glassy samples were annealed at temperatures in the range of 140 – 170 K for 15 min. Annealing leads to the softening of the glass, and as a result, the π-anion radical of SCNdU or 6-D-SCNU held in homogeneous rigid glass at 77 K becomes mobile and undergoes subsequent reactions. Thus, via progressive annealing, the subsequent reactions of only the π-anion radical of the solute, e.g., SCNdU or 6-D-SCNU, have been followed directly by ESR studies in this work.
(F) Electron Spin Resonance
Following our earlier studies,22–27 immediately after γ-irradiation of the glassy sample at 77 K, the ESR spectrum was recorded at 77 K. Also, immediately after each annealing step, the sample was cooled to 77 K by immersing it immediately in liquid nitrogen (77 K) and the ESR spectrum was recorded at 77 K, which maximizes signal height and allows for comparison of the signal intensities.27 A Varian Century Series X-band (9.3 GHz) ESR spectrometer with an E-4531 dual cavity, 9-inch magnet, and a 200 mW Klystron was used and Fremy’s salt (gcenter = 2.0056, A(N) = 13.09 G) was employed for the field calibration. All ESR spectra have been recorded at 77 K and at 40 dB (20 µW).
Anisotropic simulations of ESR spectra have been performed using the WIN-EPR and SimFonia programs of Bruker as per our previous works.22–27 The simulated spectra thus obtained were compared to the experimental spectra, and the ESR parameters were adjusted for the best fit.22–27
HPLC Conditions
The HPLC separation was performed on a Dionex UltiMate 3000 System with a Diode Array Detector, which was set at 260 nm for monitoring the effluents. The analytes were separated on a Wakopak® Handy ODS column (4.6 mm × 150 mm; 5 µm in particle size and 100 Å in pore size). The mobile phase A consisted of deionized water, acetonitrile (Sigma-Aldrich, Poland) and 1% formic acid (POCH S.A., Poland) (pH 2.55; 87.7:2:10.3, v/v/v) and mobile phase B contained 80% acetonitrile. The gradient initially went from 100% A to 95% A over 20 min, than to 90% A in the next 10 min. The flow rate was set at 1 mL/min.
LC-MS and LC-MS/MS Conditions
LC analyses were performed on an Eksingent Micro LC system (AB SCIEX). The analytes were separated on an Eksingent 5C18-EP-120 column (0.5 × 100 mm, 5 µm, 120 Å). The mobile phase A consisted of deionized water, acetonitrile (Sigma-Aldrich, Poland) and 1% formic acid (POCH S.A., Poland) (pH 2.55; 87.7:2:10.3, v/v/v) and mobile phase B was 80% acetonitrile. The gradient went from 100% A to 90% A in 5 min. The flow rate was 50 µL/min and the injected volume was 2 µL of the sample. The micro LC system was coupled directly to a QTRAP quadrupole time-of-flight (QTOF) mass spectrometer (AB SCIEX) equipped with a duo-electrospray interface operated in negative ionization mode. MS and MS/MS operation parameters were the same for both types of scan and were as follows: the spray voltage was −4.0 kV, the nebulizer gas (N2) pressure was 25 psi, the flow rate was 11 L/min and the source temperature was 200 °C. Each spectrum was obtained by averaging 3 scans, and the time of each scan was 0.2 s.
Computations
All calculations were performed with density functional theory (DFT), using the M06-2x28 hybrid meta-exchange correlation functional and the 6-31++G(d,p) basis set.29 The unconstrained geometry optimizations for stationary points (minima and transition states) on the potential energy surface of SCNdU were carried out in aqueous solution, employing the Polarizable Continuum Model (PCM)30 of water in order to account for the effect of a polar environment.
The Gibbs free energy changes (ΔGs) calculated for particular reaction steps are the differences between the electronic energies of the products and substrates, corrected for zero-point energies, the thermal contributions to the energies, and the pV and entropy terms. These terms were computed in the rigid rotor-harmonic oscillator approximation31 at T = 298 K and p = 1 atm.
Gaussian 0932 code was used for all computations, while the molecule structures were visualized with the GaussView package.33
3. Results
ESR studies
Owing to ionization events in irradiated LiCl aqueous glasses, the electrons formed are predominantly trapped in shallow wells.34 Some traps can be as deep as 2.6 eV; however, generally, the most abundant trapped electron species have been observed at traps ca. 0.5 eV below the continuum.35–37 Prior to their complete solvation, these partially solvated electrons react with the solutes. As a result, the reactions of electrons in the irradiated glasses are primarily due to these partially solvated electrons, which are known as presolvated (prehydrated) electrons (epre−).34–39
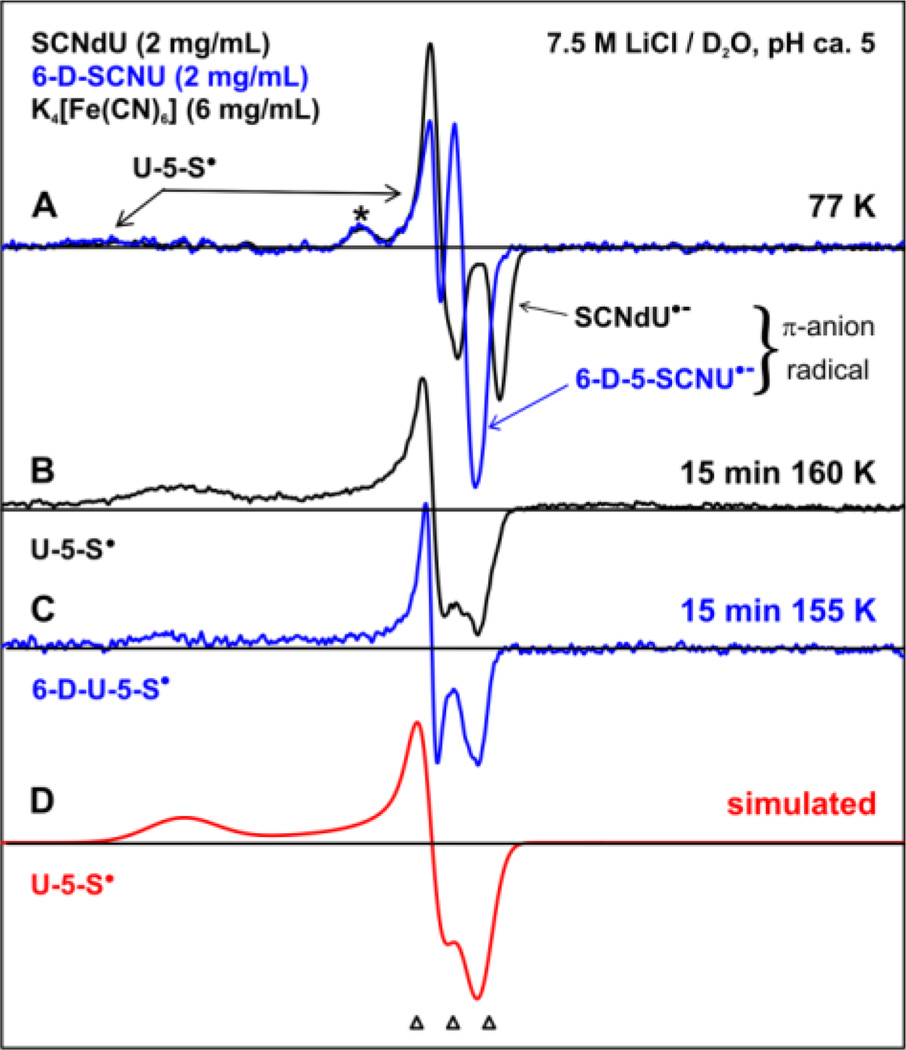
In Figure 1(A), we show the spectrum obtained after subtraction of the Cl2•− ESR spectrum23(b) from the experimentally recorded (77 K) 400 G wide ESR spectrum (black) of one-electron reduced SCNdU by epre− formed by π-irradiation at 77 K at pH (pD) ca. 5 in a homogeneous glassy 7.5 M LiCl/D2O solution in the presence of the hole scavenger K4[Fe(CN)6]. Superimposition of the 77 K spectrum (blue) obtained from a matched sample of 6-D-SCNU at pD ca. 5 on the top of the black spectrum in Figure 1(A) shows that the blue spectrum from one-electron reduction of 6-D-SCNU is identical to the black spectrum, except for the collapse of the central doublet of ca. 16 G in the black spectrum to a singlet in the blue spectrum. This collapse of the central doublet to a singlet on deuteration at C6 in 6-D-SCNU shows that the doublet splitting arises from the C6H proton coupling, which is only in accord with the formation of the π-anion radicals of SCNdU and 6-D-SCNU (SCNdU•− and 6-D-SCNU•−) upon epre− addition to the parent molecules (SCNdU or 6-D-SCNU, Scheme 1). In addition to this central doublet, both spectra in Figure 1(A) show a line component at gzz = 2.085. This g-value is much higher than the observed C-centered and N-centered radicals in DNA;22–27 based on the reported g-values of thiyl radicals,40–43 the line component due to gzz = 2.085 has been assigned to U-5-S•. Another small line component at g = 2.027 (indicated by *) is unassigned. It is not observed in 7.5 M LiBr glasses (see ESI, Figures S2, S4, S5).
Figure 1.
ESR spectra recorded at 77 K of γ-irradiated (77 K) N2-saturated 7.5 M LiCl/D2O solutions of SCNdU and 6-D-SCNU with a hole scavenger, K4[Fe(CN)6]. (A) The π-anion radical of SCNdU and 6-D-SCNU upon epre− addition. Both spectra also show the line components from U-5-S• (indicated by arrows). (B) U-5-S• spectrum after annealing at 160 K for 15 min. (C) 6-D-U-5-S• spectrum from the 6-D-5-SCNU sample obtained after annealing at 155 K for 15 min. (D) An anisotropic simulation of the spectra (B) and (C) of U-5-S• using g-tensor principal values. The three reference markers (open triangles) show the position of Fremy’s salt resonance with the central marker at g = 2.0056 and 13.09 G marker spacing.
Upon annealing from 155 to 160 K for 15 min, SCNdU•− and 6-D-SCNU•− resulted in the spectra shown in Figures 1(B) and 1(C), respectively. Employing an anisotropic simulation by using g-tensor principal values (2.0110, 2.0000, 2.0700), anisotropic linewidth (8, 10, 25) G, and mixed Lorentzian/Gaussian lineshape = 1, a simulated spectrum (red, Figure 1(D)) was obtained; this spectrum matches the spectra in Figures 1 (B) and 1(C) quite well. Based on the g-tensor principal values of S-centered radicals in the literature,40–43 spectra (B) and (C) are both assigned to U-5-S•. Comparison of spectrum 1 (B or C) with the spectra in Figure 1(A) clearly shows that the central doublet from SCNdU•− and 6-D-SCNU•− in Figure 1(A) is lost on annealing, with increase in the signal intensity of U-5-S•. The small line component at gzz = 2.027 (indicated by *) is also lost. Moreover, the apparent gzz values = 2.085 due to U-5-S• decrease to 2.070 upon annealing from 77 K to 160 K. In the frozen matrix, increased interaction of U-5-S• with the surrounding solvent on annealing to 160 K somewhat sharpens the low field signal and decreases the apparent gzz value to 2.070. This behavior is consistent for the near orbitally degenerate radicals, such as thiyl radicals.40–43
Thus, in our ESR studies, we have unequivocally identified the π-anion radical of SCNdU and its deuterated analog 6-D-SCNU, which undergoes cleavage of the S-CN bond forming U-5-S• (see Scheme 1). It is apparent from Figure 1 that U-5-S• is the only radical species found at 160 K. These results clearly establish that the formation of U-5-S• via S-CN bond cleavage in SCNdU•− and in 6-D-SCNU•− is a lower energy path than U• production by C5-S bond breakage along with SCN− loss (Scheme 1).
Our results show that the formation of U-5-S• is not affected by the solvent (D2O vs. H2O), the glassy solution (7.5 M LiCl vs. 7.5 M LiBr), the ionic strength (7.5 M vs. 15 M), the pH range of ca. 3 to 11, or the SCNdU concentration; these results support the U-5-S• assignment (see ESI, Figures S1 to S4, S9, and S10). The lack of SCNdU concentration dependence for U-5-S• formation from SCNdU•− supports the unimolecular mechanism presented in Scheme 1. Moreover, unlike the aliphatic thiyl radicals,40–43 U-5-S• does not react with molecular oxygen at low temperature (see ESI, Figure S5).
Radiolysis at Ambient Temperature
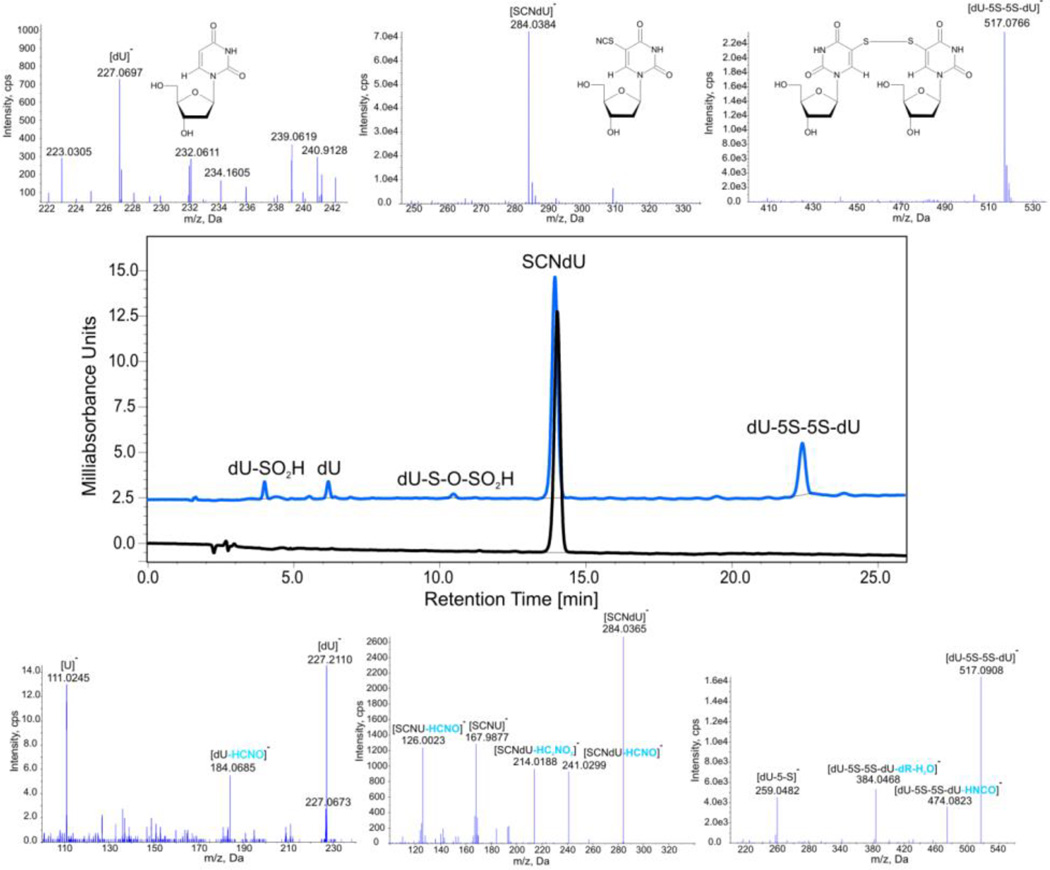
In order to verify the stability of products due to the rise in U-5-S• at ambient temperature, where water radiolysis leads to solvated electrons, we carried out a room temperature steady-state radiolysis of SCNdU solution saturated with N2 and containing sodium formate as a hydroxyl radical scavenger. HPLC traces before and after radiolysis are depicted in the middle panel of Figure 2. The energy delivered via ionizing radiation leads to the substantial decay of the substrate. Comparing the chromatograms before and after radiolysis, one can observe two products: a major product with the retention time of 22.41 min and a minor one with the retention time of 6.17 min (Figure 2). The comparison of the radiolyte chromatogram, shown in Figure 2, with the HPLC traces of 2′-deoxyuridine (dU) measured under the same conditions (not shown) proves that the signal at 6.17 min is from dU. The identity of this product is also confirmed by the LC-MS and LC-MS/MS analyses (Figure 2). The parent anion of dU with a m/z ratio equal to 227.0697 (see Table 1 for comparison between the actual and theoretical mass) corresponds to the most intense MS signal (see the upper part of Figure 2). The fragmentation of this anion (see the bottom part of Figure 2) shows the loss of water, HCNO or a sugar fragment. A similar analysis allows the signal at 22.41 min (Figure 2) to be identified. The parent anion with the m/z ratio of 517.0766 (see Table 1) corresponds to the dimer, dU-5S-5S-dU, that arises due to the dimerization of U-5-S•; its structure is shown in Figure 2. The appropriate fragmentation spectrum (see Figure 2, the lower panel) confirms this proposal, since signals corresponding to the loss of HNCO, deoxyribose and water, as well as cleavage of the S-S bond (formation of dU-S(-H) anion), are observed. The presence of two additional small HPLC peaks at 3.99 min, dU-SO2H, and 10.46 min, dU-S-O-SO2H, (see Figure 2) confirms the involvement of U-5-S• and its dimerization product in the studied process. The formation of small amounts of dU-SO2H and dU-S-O-SO2H points to the fact that the residual oxygen produced during radiolysis may react with U-5-S•, which accounts for the dU-SO2− observed in the LC-MS analysis (see ESI, Figure S6). Comparison of these results obtained at ambient temperature with those obtained via ESR spectra at low temperatures suggest that the reaction of molecular oxygen with U-5-S• has a thermal activation barrier, because U-5-S• reacts with molecular oxygen at ambient temperatures but not at temperatures below 170 K (compare Figure 2 with Figure S5 in the ESI). The small amounts of these reactants remains in line with the fact that only residual oxygen is present in the studied system.
Figure 2.
HPLC and MS analysis of a solution of SCNdU. Middle panel – the HPLC traces of SCNdU before (black) and after (blue) γ-irradiation with a dose of 50 Gy; upper part –the MS spectra (in the negative ionization mode) of the selected HPLC signals; lower panel – the MS/MS spectra of the selected HPLC signals along with ion identities.
Table 1.
Comparison of the theoretical (calculated) and actual (measured) m/z values for the [M−H]− Ions identified in the MS analyses.
| Ion | Calculated mass [Da] | Measured mass [Da] |
|---|---|---|
| [dU-5S-5S-dU(−H)]− | 517.0705 | 517.0766 |
| [SCNdU(−H)]− | 284.0347 | 284.0384 |
| [dU-S-O-SO2]− | 338.9962 | 339.0005 |
| [dU-SO2]− | 291.0292 | 291.0294 |
| [dU(−H)]− | 227.0673 | 227.0697 |
The data presented above unequivocally show that the SCNdU π-anion radical leads to two parallel reactions which produce quite different products. In one path, the C5-S bond is broken in SCNdU•− and the secondary U• reacts with the formate anion, resulting in dU as a stable product. On the other path, the S-CN bond cleavage in the thiocyanate substituent produces U-5-S•, which ultimately forms a stable dimer (dU-5S-5S-dU). Moreover, neither the ESR spectral results nor the product analyses studies present any observable evidence of perthyl, i.e., R-S-S• intermediates.44
Computational Results
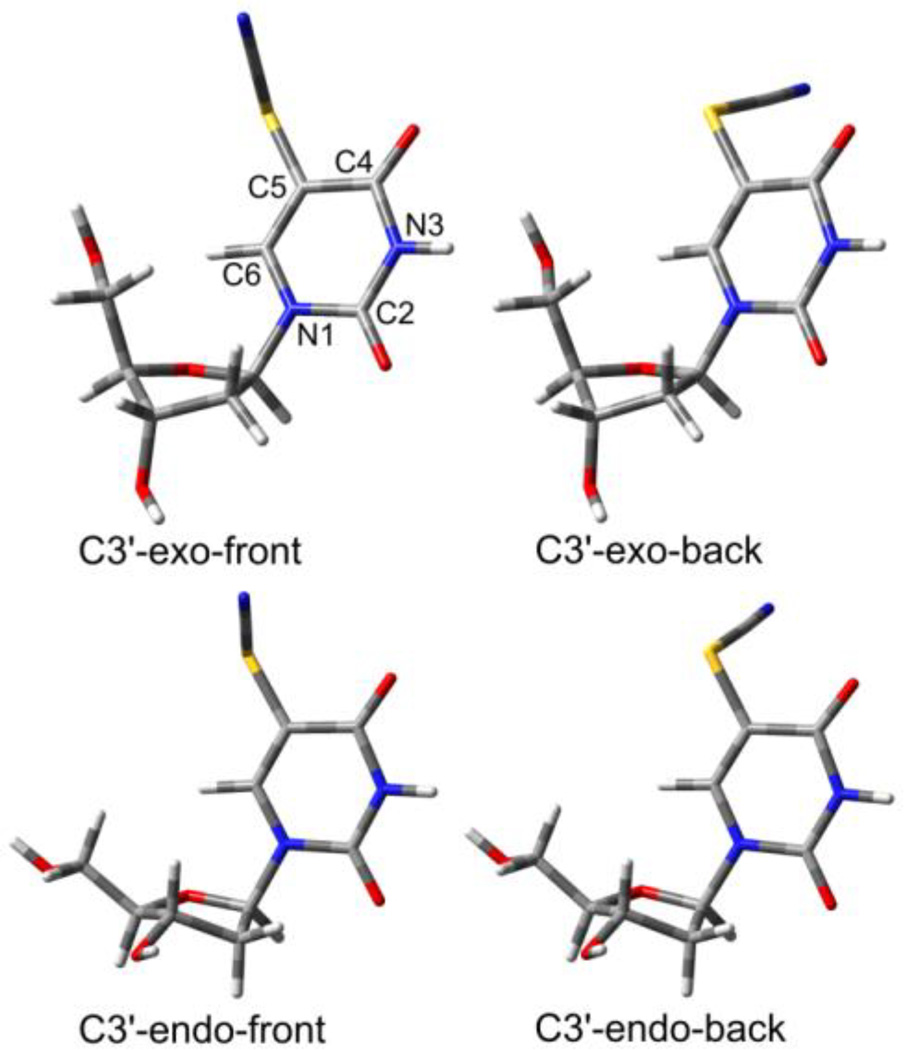
The mechanisms already suggested by the above-described ESR results and steady-state radiolysis (Scheme 1) can be confirmed using computational chemistry. In order to accomplish this, one of the most accurate and effective DFT methods has been used, namely the M06-2x functional, which estimates reaction thermodynamics and kinetics with high accuracy.28 In Figure 3, the optimized geometries of the substrate are displayed. Two types of conformational freedom were taken into consideration: sugar ring puckering (C3′-exo/C3′-endo) and rotation of the -SCN substituent around the C5-S bond (front/back). These resulted in the four conformers of SCNdU, with the C3′-exo-front conformer being the most thermodynamically stable (see Figure 3).
Figure 3.
3D visualization of the optimized geometries of four neutral SCNdU conformers.
The excess electron localizes on the pyrimidine ring of SCNdU, which leads to its folding (the N1-C2-N3-C4 dihedral angle (for atom numbering see Figure 3) amounts to 13 degrees, while it is equal to 0 in the neutral molecule). The driving force of the ring folding is related to a decrease in the antibonding interactions in the π* orbital to which the excess electron is attached. On the other hand, Mulliken population analyses indicate that the electron localizes mainly on the C6 atom, since it amounts to −0.862 in the anion radical.
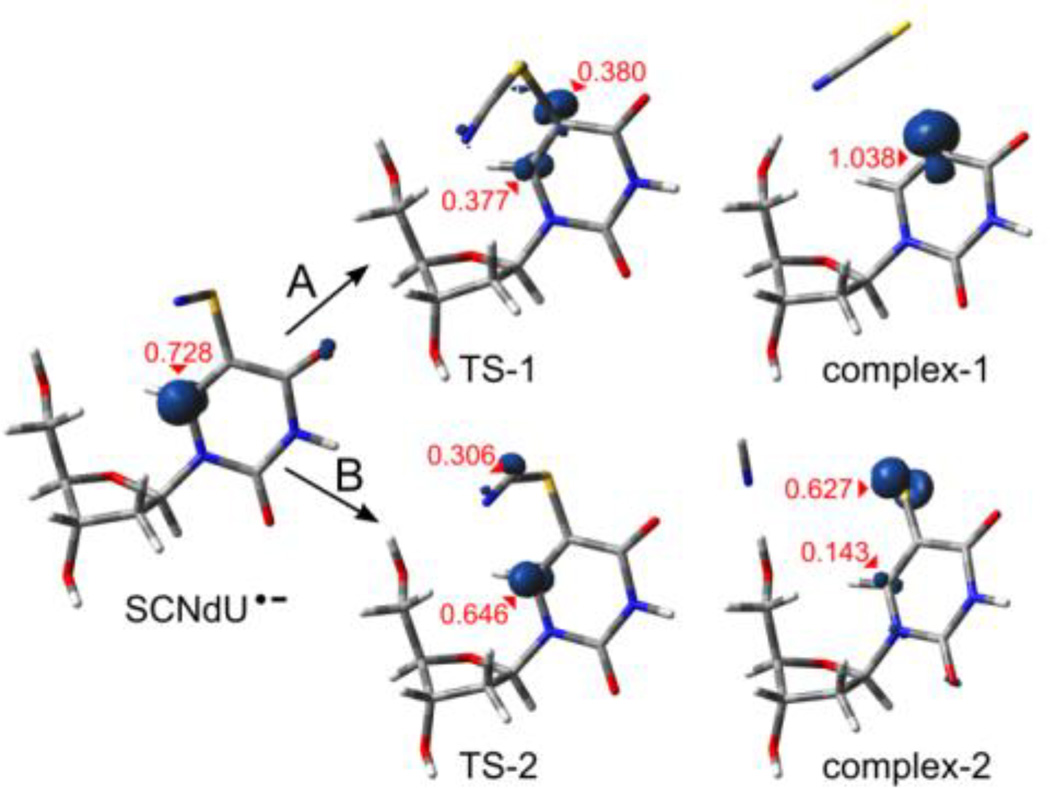
The adiabatic electron affinities (AEAG), calculated as the differences in free enthalpy of the neutral and corresponding anion radicals in their optimal geometries, were found to be significantly positive. These results and the spin density analysis indicate that all the considered structures form electronically stable π-anion radicals (see SCNdU•− in Figure 4 and the AEAG values in Table 2). These radical anions may basically transform via two competitive degradation paths: one leading to the detachment of the anionic SCN− substituent via complex-1 (see path A, Scheme 1 and Figure 4) and another one resulting in the detachment of CN− via complex-2 (path B, Scheme 1 and Figure 4).
Figure 4.
SCNdU•− degradation path A – leading via transition state TS-1 to a C5-SCN bond break (complex-1) and B – via TS-2 to an S-CN bond break (complex-2), shown via 3D geometry visualization along with the spin density surfaces (for the density value of 0.02 e/A3). The highest Mulliken spin values are marked with red.
Table 2.
Adiabatic electron affinities (AEAG) and thermodynamic (∆G) and activation (∆G*) barriers, calculated for the electron-induced degradation of SCNdU. All values are given in kcal/mol, free enthalpy scale.
| Sugar ring conformation |
SCNdU → SCNdU•− |
SCNdU•− → complex-1 |
SCNdU•− → dU•+ SCN− |
SCNdU•− → complex-2 |
SCNdU•− → dU-5-S•+ CN− |
||
|---|---|---|---|---|---|---|---|
| AEAG | ∆G* | ∆G | ∆G | ∆G* | ∆G | ∆G | |
| C3'-exo-front | 60.8 | 8.69 | −1.60 | 0.88 | 4.07 | −16.21 | −16.57 |
| C3'-exo-back | 61.2 | 10.69 | 4.32 | 1.08 | 4.66 | −12.12 | −16.37 |
| C3'-endo-front | 62.2 | 8.85 | 2.33 | 1.65 | 4.04 | −15.70 | −15.44 |
| C3'-endo-back | 62.2 | 9.46 | 4.94 | 1.72 | 5.72 | −11.16 | −15.36 |
The first of the considered paths was found to be thermodynamically allowed only in the case of C3′-exo-front conformation. Indeed, the C5-S bond in the C3′-exo-front radical is likely to be broken, as the kinetic barrier of this process is relatively low (see ΔG* = 8.69 kcal/mol in Table 2), and the thermodynamic stimulus is also favorable (ΔG = −1.60 kcal/mol). The degradation of the remaining radical conformers is unfavorable due to the positive thermodynamic stimuli (from 2.3 to 4.9 kcal/mol, see Table 2). A complete separation of monomers forming complex-1 is slightly unfavorable for each conformer considered (ΔG = 0.88 to 1.72 kcal/mol, see Table 2).
On the other hand, breaking the S-C bond inside the SCN substituent occurs quite efficiently. The activation free energy for this cleavage is about 4–6 kcal/mol (see Table 2), while the thermodynamic stimuli are highly negative (ΔG lower than −11 kcal/mol). The complete separation of the reaction products is thermodynamically favorable (see Table 2).
These results show that the excess electron readily attaches to SCNdU, owing to its large electron affinity (above 60 kcal/mol, see Table 2), which is comparable to the AEA of 5-BrU (57 kcal/mol at B3LYP/6-31++G(d,p)) and the PCM model of water45. However, the later fate of the SCNdU π-anion radical differs substantially from that of 5-BrdU•−. Indeed, the path which leads to the release of SCN− and the U• formation, which is fully analogous to the release of Br− from 5-BrdU•− and has been confirmed by the combined DFT and photoelectron spectroscopy studies,18 is shown to be a minor decomposition channel of SCNdU•− both in glassy solution at low temperature as well as in aqueous solution at ambient temperature.
4. Disscussion
The dominant reaction path found in both the low-temperature ESR experiments and steady-state radiolysis at ambient temperature is the dissociation of the S-CN bond in the π-anion radical of SCNdU. This reaction leads to two products of significant cytotoxicity: the cyanide anion, CN−, and the uracil-5-thiyl radical, U-5-S•. The former species is a well-known poison whose cytotoxicity is attributed to the cessation of aerobic metabolism in the cell;21 CN− binds irreversibly to cytochrome oxidase in mitochondria.21 The radical product of this DEA process, U-5-S•, has potential to be quite cytotoxic. U-5-S• should show comparable chemistry to aryl thiyl radicals, which are not good H atom abstracting agents.19,20,44 The formation of stable dimer products, i.e., dU-5S-5S-dU, suggests that U-5-S• should be able to form intra- and interstrand cross-links as well as DNA-protein crosslinks. These crosslinks are especially cytotoxic, as they are difficult to repair, and clusters of several inter-strand crosslinks are sufficient to terminate the cell.46 Finally, since DNA interacts permanently with proteins such as histones, replication and repair enzymes in the cellular environment,47,48 there is a high probability that U-5-S• formed in the double-helix would lead to DNA-protein cross-links via the formation of disulfide bonds from reactions with cysteine side chains.
In addition to the electron-induced dominant reaction path by loss of CN− resulting in U-5-S• formation (Scheme 1 path B), a minor path by loss of SCN− (Scheme 1 path A) from SCNdU•− leads, at ambient temperature, to the formation of a highly cytotoxic species, U•. Several recent radiation-chemical studies by Hunting on synthetic DNA with 5-BrdU incorporated in the place of thymidine have demonstrated that 5-BrdU sensitizes DNA to the aqueous electrons formed in water radiolysis.9 The electron-induced radiosensitization observed in 5-BrdU incorporated DNA results from the production of U• that is formed via the attachment of an electron to 5-BrdU via the irreversible release of bromide anion by DEA. Depending on the DNA conformation9,45,46 and its local sequence,47 U• abstracts a hydrogen from the adjacent sugar residue (B-DNA, single stranded DNA), ultimately forming a strand break or leading, in a sequence of secondary processes, to interstrand cross-links (mismatched B-DNA) or to alkali labile sites (A-DNA). Thus, our radiation-chemical study predicts that electron addition to SCNdU is likely to be an effective radiosensitizer, since electron addition to SCNdU leads to the formation of three highly cytotoxic species – CN−, U-5-S•, and U•.
5. Conclusions
In the current work, the degradation of 5-thiocyanato-2'-deoxyuridine (SCNdU) induced by excess electron attachment has been studied using low-temperature ESR, steady-state radiolysis at ambient temperature, and molecular modeling at the DFT level. The title compound was synthesized via the reaction of chlorothiocyanate with 2'-deoxyuridine in dry acetic acid. Then, the γ-irradiated N2-saturated frozen homogeneous solutions (7.5 M LiCl in H2O or in D2O) containing SCNdU resulted in ESR spectra having contributions from two radical species. Our ESR studies unambiguously establish the pre-hydrated electron-induced cleavage of the S-CN bond to form the uracil base-thiyl radical (U-5-S•) intermediate along with CN− by dissociative electron attachment (DEA). Moreover, comparison of the ESR spectrum for totally protiated SCNdU with that obtained from a matched sample of the nucleobase SCNU having deuteration at the C6 position of the uracil base unequivocally demonstrates that the primary anion radical formed in the studied system is a typical π*-anion radical in which the unpaired spin is mainly localized in the uracil ring at C6.
The HPLC and LC-MS/MS analyses carried out for γ-irradiated and N2-saturated aqueous solutions of SCNdU, containing sodium formate as the OH-radical scavenger, show that hydrated electrons produced the dU-5S-5S-dU dimer by recombination of U-5-S• in preference to the anticipated formation of the σ-type uracilyl radical via the removal of a thiocyanate anion to form the dU by about 10 to 1.
Applying the M06-2x/6-31++G(d,p) method and the polarizable continuum model (PCM) of water, the complete separation of U-5-S• and CN− anion was found to be thermodynamically more favorable by ca. −15.4 kcal/mol (ΔG) than breaking the C5-SCN bond. Similarly, the activation barrier for the cleavage of the C5-S bond in the 5-thiocyanate-2′-deoxyuridine radical anion is more than 2-fold larger than that related to the breakage of the S-CN bond in the thiocyanate substituent.
Thus, this radiation chemical study of electron addition to SCNdU establishes SCNdU as a potential radiosensitizer. SCNdU•− leads to U• formation, similar to 5-BrdU•−, in low yield (10%). However, SCNdU•− results in a predominant (90%) formation of U-5-S•. The latter species will likely lead to both intra- and inter-strand DNA crosslinking as well as DNA-protein crosslinking via the formation of S-S dimers. Since cross-links are among the most cytotoxic forms of damage,1,9 such dimers would significantly impede DNA damage repair activity, which might enhance, in turn, the extent of DNA damage in cancer cells.
Supplementary Material
Acknowledgments
This work was supported by the Polish National Science Centre (NCN) under the Grant No. 2012/07/N/ST5/01877 (MZ) and by Foundation for Polish Science (LC). The financial support of Polish Ministry of Science and Higher Education (MNiSW) via Funds for Science and Polish Technology Programme that enabled the LC-MS system for the Nucleic Acid Laboratory at the Department of Chemistry, University of Gdansk (Grant No. 775/FNiTP/127/2013 (J.R.) to be purchased is greatly acknowledged. TJW, CH, AA, and MDS thank the National Cancer Institute of the National Institutes of Health (Grant RO1CA045424) for support. Calculations have been carried out in Wroclaw Center for Networking and Supercomputing (http://www.wcss.wroc.pl), grant No. 209 (LC). The authors thank Prof. Anil Kumar for performing the initial calculations showing S-CN bond cleavage in the SCNdU anion radical.
Footnotes
Electronic Supplementary Information:
Electronic Supplementary Information (ESI) available: (i) Figure S1 - ESR spectra recorded at 77 K of matched γ-irradiated (77 K) N2-saturated 7.5 M LiCl/D2O and 7.5 M LiCl/H2O solutions of SCNdU with a hole scavenger, K4[Fe(CN)6]. (ii) Figure S2 - ESR spectra recorded at 77 K of matched γ-irradiated (77 K) N2-saturated 7.5 M LiCl/H2O and 7.5 M LiBr/H2O solutions of SCNdU with a hole scavenger, K4[Fe(CN)6]. (iii) Figure S3 - ESR spectra recorded at 77 K of matched γ-irradiated (77 K) N2-saturated 7.5 M LiCl/H2O and 15 M LiCl/H2O solutions solutions of SCNdU with a hole scavenger, K4[Fe(CN)6]. (iv) Figure S4 - ESR spectra recorded at 77 K of matched γ-irradiated (77 K) N2-saturated 7.5 M LiBr/H2O solutions with pHs ca. 5 and ca. 11 of SCNdU with a hole scavenger, K4[Fe(CN)6]. (v) Figure S5 - ESR spectra recorded at 77 K of matched γ-irradiated (77 K) N2-saturated 7.5 M LiBr/H2O solutions with pH ca. 11 and in the presence and absence of oxygen of SCNdU with a hole scavenger, K4[Fe(CN)6]. (vi) Figure S6- MS and MS/MS spectra (in negative ionization mode) of the additional products of the γ-radiolysed solution of SCNdU (dose: 50 Gy) and ion identities. (vii) Figures S7 and S8 - HPLC chromatogram and MS spectra (in negative ionization mode) of synthesized and purified 6-deutero-5-thiocyanatouracil (6-D-5-SCNU) and SCNdU respectively. (viii) Figure S9- ESR spectra recorded at 77 K of matched γ-irradiated (77 K) N2-saturated 7.5 M LiCl/D2O solutions with two different concentrations of SCNdU. (ix) ESR spectra recorded at 77 K of matched γ-irradiated (77 K) N2-saturated 7.5 M LiCl/D2O solutions with pHs ca. 3, 5, and ca. 9 of SCNdU. (x) Stationary points geometries – Cartesian coordinates and absolute values of electronic energy and free enthalpy.
References
- 1.von Sonntag C. The Chemical Basis of Radiation Biology. London U.K.: Taylor and Francis; 1987. [Google Scholar]
- 2.Lawrence TS, Davis MA, Maybaum J, Stetson PL, Ensminger WD. Radiat. Res. 1990;123:192. [PubMed] [Google Scholar]
- 3.Watanabe R, Nikjoo H. Int. J. Radiat. Biol. 2002;78:953. doi: 10.1080/0955300021000024270. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella TJ, Dobson PA, Mitchell JB, Fornance AJ. Int. J. Radiat. Oncol., Biol., Phys. 1987;13:733. doi: 10.1016/0360-3016(87)90292-6. [DOI] [PubMed] [Google Scholar]
- 5.Iliakis G, Kurtzman S, Pantelias G, Okayasu R. Radiat. Res. 1989;119:286. [PubMed] [Google Scholar]
- 6.Rivera E, Schuler RH. J. Phys Chem. 1983;87:3966. [Google Scholar]
- 7.Mertens R, von Sonntag C. Angew. Chem. Int. Ed. 1994;33:1262. [Google Scholar]
- 8.Zimbrick JD, Ward JF, Myers LS., Jr Int. J. Radiat. Biol. 1969;16:525. doi: 10.1080/09553006914551581. [DOI] [PubMed] [Google Scholar]
- 9.Cecchini S, Girouard S, Huels MA, Sanche L, Hunting DJ. Radiat. Res. 2004;162:604. doi: 10.1667/rr3267. [DOI] [PubMed] [Google Scholar]
- 10.Park Y, Polska K, Rak J, Wagner JR, Sanche L. J. Phys Chem. B. 2012;116:9676. doi: 10.1021/jp304964r. [DOI] [PubMed] [Google Scholar]
- 11.Chomicz L, Petrovici A, Archbold I, Adhikary A, Kumar A, Sevilla MD, Rak L. Chem. Commun. 2014;50:14605. doi: 10.1039/c4cc07089e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Tashiro R, Sugiyama H. Nature Prot. 2007;2:78. doi: 10.1038/nprot.2006.467. [DOI] [PubMed] [Google Scholar]
- 13.Sugiyama H. Bull. Chem. Soc. Jpn. 2007;80:823. [Google Scholar]
- 14.Oronsky BT, Knox SJ, Scicinski J. Trans. Oncol. 2011;4:256. doi: 10.1593/tlo.11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.von Sonntag C. Free-radical-induced DNA damage and its repair. Heidelberg, Germany: Springer-Verlag; 2006. Formation of Reactive Free Radicals in an Aqueous Environment. [Google Scholar]
- 16.Rezaee M, Sanche L, Hunting D. Radiat. Res. 2013;179:323. doi: 10.1667/RR3185.1. [DOI] [PubMed] [Google Scholar]
- 17.Prados MD, Scott C, Sandler H, Buckner JC, Phillips T, Schultz C, Urtasun R, Davis R, Gutin P, Cascino TL, Greenberg HS, Curran WJ., Jr Int. J. Radiat. Oncol. Biol. Phys. 1999;45:1109. doi: 10.1016/s0360-3016(99)00265-5. [DOI] [PubMed] [Google Scholar]
- 18.Chomicz L, Zdrowowicz M, Kasprzykowski F, Rak J, Buonaugurio A, Wang Y, Bowen KH. J. Phys Chem. Lett. 2013;4:2853. [Google Scholar]
- 19.Houmam A, Ahmed EM, Still IWJ. J. Am. Chem. Soc. 2003;125:7258. doi: 10.1021/ja028542z. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed EM, Doai H, McLaughlin CK, Houmam A. J. Am. Chem. Soc. 2006;128:6595. doi: 10.1021/ja056730u. [DOI] [PubMed] [Google Scholar]
- 21.Hall AH, Dart R, Bogdan G. Ann. Emerg. Med. 2007;49:806. doi: 10.1016/j.annemergmed.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 22.(a) Adhikary A, Khanduri D, Sevilla MD. J. Am. Chem. Soc. 2009;131:8614. doi: 10.1021/ja9014869. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Adhikary A, Sevilla MD. J. Phys Chem. B. 2011;115:8947. doi: 10.1021/jp202664j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Adhikary A, Kumar A, Heizer AN, Palmer BJ, Pottiboyina V, Liang Y, Wnuk SF, Sevilla MD. J. Am. Chem. Soc. 2013;135:3121. doi: 10.1021/ja310650n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Khanduri D, Adhikary A, Sevilla MD. J. Am. Chem. Soc. 2011;133:4527. doi: 10.1021/ja110499a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adhikary A, Kumar A, Palmer BJ, Todd AD, Sevilla MD. J. Am. Chem. Soc. 2013;135:12827. doi: 10.1021/ja406121x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adhikary A, Kumar A, Palmer BJ, Todd AD, Heizer AN, Sevilla MD. Int. J. Radiat. Biol. 2014;90:433. doi: 10.3109/09553002.2014.884293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adhikary A, Khanduri D, Pottiboyina V, Rice CT, Sevilla MD. J. Phys Chem. B. 2010;114:9289. doi: 10.1021/jp103403p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adhikary A, Kumar A, Rayala R, Hindi RM, Wnuk SF, Sevilla MD. J. Am. Chem. Soc. 2014;136:15646. doi: 10.1021/ja5083156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Truhlar DG. Chem. Theory Comput. 2011;7:669. [Google Scholar]
- 29.(a) Ditchfield R, Hehre WJ, Pople JA. J. Chem Phys. 1971;54:724. [Google Scholar]; (b) Hehre WJ, Ditchfield R, Pople JA. J. Chem Phys. 1972;56:2257. [Google Scholar]
- 30.(a) Miertuš S, Scrocco E, Tomasi J. Chem. Phys. 1981;55:117. [Google Scholar]; (b) Miertuš S, Tomasi J. Chem. Phys. 1982;65:239. [Google Scholar]; (c) Cossi M, Barone V, Cammi R, Tomasi J. Chem. Phys. Lett. 1996;255:327. [Google Scholar]
- 31.McQuarrie DA, Simon JD. Molecular Thermodynamics. Sausalito, CA, USA: University Science Books; 1999. [Google Scholar]
- 32.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery J, Peralta JAJE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Rev. C.01. Wallingford, CT: 2009. [Google Scholar]
- 33.Dennington R, Keith T, Millam J. GaussView, Version 5. Shawnee Mission KS: Semichem Inc.; 2009. [Google Scholar]
- 34.Gillis HA, Teather GG, Buxton GV. Can. J. Chem. 1978;56:1889. [Google Scholar]
- 35.Sevilla MD, Swarts S, Bearden R, Morehouse KM, Vartanian T. J. Phys Chem. 1981;85:923. [Google Scholar]
- 36.Kheir JF, Chomicz L, Engle AM, Rak J, Sevilla MD. J. Phys Chem. B. 2013;117:2872. doi: 10.1021/jp400176c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrovici A, Adhikary A, Kumar A, Sevilla MD. Molecules. 2014;19:13486. doi: 10.3390/molecules190913486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolff RK, Aldrich JE, Penner TL, Hunt JW. J. Phys Chem. 1975;79:210. [Google Scholar]
- 39.Bartels DM, Gosztola D, Jonah CD. J. Phys Chem. A. 2001;105:8069. [Google Scholar]
- 40.Lu QB, Baskin JS, Zewail AH. J. Phys Chem. B. 2004;108:10509. [Google Scholar]
- 41.Becker D, Swarts SG, Champagne M, Sevilla MD. Int. J. Radiat. Biol. 1988;53:767. doi: 10.1080/09553008814551121. [DOI] [PubMed] [Google Scholar]
- 42.Sevilla MD, Yan M, Becker D. Biochem. Biophys. Res. Commun. 1988;155:405. doi: 10.1016/s0006-291x(88)81100-8. [DOI] [PubMed] [Google Scholar]
- 43.Covès J, Le Hir de Fallois L, Le Pape L, Décout JL, Fontecave M. Biochemistry. 1996;35:8595. doi: 10.1021/bi960355o. [DOI] [PubMed] [Google Scholar]
- 44.Alfassi ZB, editor. S-Centered Radicals. New York: John Wiley & Sons, Inc.; 1999. [Google Scholar]
- 45.Chomicz L, Rak J, Storoniak P. J. Phys Chem. B. 2012;116:5612. doi: 10.1021/jp3008738. [DOI] [PubMed] [Google Scholar]
- 46.Magana-Schwencke N, Henriques JA, Chanet R, Moustacchi E. Proc. Natl. Acad. Sci. U.S.A. 1982;79:1722. doi: 10.1073/pnas.79.6.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mandel-Gutfreund Y, Schueler O, Margalit H. J. Mol Biol. 1995;253:370. doi: 10.1006/jmbi.1995.0559. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman MM, Khrapov MA, Cox JC, Yao J, Tong L, Ellington AD. Nucleic Acids Res. 2004;32:D174. doi: 10.1093/nar/gkh128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.