Background: The reactivity of AαC, a tobacco smoke carcinogen, was investigated with DNA and albumin of human hepatocytes.
Results: Hepatocytes bioactivate AαC to metabolites, which adduct to DNA and albumin.
Conclusion: Cys34 and Met329 of serum albumin are targets for AαC electrophiles.
Significance: AαC forms macromolecular adducts and induces oxidative stress, which may be contributing factors to liver damage and cancer risk in smokers.
Keywords: Carcinogenesis, Cigarette Smoke, DNA Damage, Oxidative Stress, Protein Chemical Modification
Abstract
2-Amino-9H-pyrido[2,3-b]indole (AαC) is a carcinogenic heterocyclic aromatic amine formed during the combustion of tobacco. AαC undergoes bioactivation to form electrophilic N-oxidized metabolites that react with DNA to form adducts, which can lead to mutations. Many genotoxicants and toxic electrophiles react with human serum albumin (albumin); however, the chemistry of reactivity of AαC with proteins has not been studied. The genotoxic metabolites, 2-hydroxyamino-9H-pyrido[2,3-b]indole (HONH-AαC), 2-nitroso-9H-pyrido[2,3-b]indole (NO-AαC), N-acetyloxy-2-amino-9H-pyrido[2,3-b]indole (N-acetoxy-AαC), and their [13C6]AαC-labeled homologues were reacted with albumin. Sites of adduction of AαC to albumin were identified by data-dependent scanning and targeted bottom-up proteomics approaches employing ion trap and Orbitrap MS. AαC-albumin adducts were formed at Cys34, Tyr140, and Tyr150 residues when albumin was reacted with HONH-AαC or NO-AαC. Sulfenamide, sulfinamide, and sulfonamide adduct formation occurred at Cys34 (AαC-Cys34). N-Acetoxy-AαC also formed an adduct at Tyr332. Albumin-AαC adducts were characterized in human plasma treated with N-oxidized metabolites of AαC and human hepatocytes exposed to AαC. High levels of N-(deoxyguanosin-8-yl)-AαC (dG-C8-AαC) DNA adducts were formed in hepatocytes. The Cys34 was the sole amino acid of albumin to form adducts with AαC. Albumin also served as an antioxidant and scavenged reactive oxygen species generated by metabolites of AαC in hepatocytes; there was a strong decrease in reduced Cys34, whereas the levels of Cys34 sulfinic acid (Cys-SO2H), Cys34-sulfonic acid (Cys-SO3H), and Met329 sulfoxide were greatly increased. Cys34 adduction products and Cys-SO2H, Cys-SO3H, and Met329 sulfoxide may be potential biomarkers to assess exposure and oxidative stress associated with AαC and other arylamine toxicants present in tobacco smoke.
Introduction
Tobacco smoke is a major risk factor not only for lung cancer but also for cancer of the liver, bladder, and gastrointestinal tract (1–4). The combustion of tobacco produces many genotoxicants, including polycyclic aromatic hydrocarbons, nitrosamines, aromatic amines, and heterocyclic aromatic amines (HAAs),2 which are potential human carcinogens (5). AαC was originally discovered as a mutagenic pyrolysis product of protein (6) and subsequently identified in cigarette smoke at levels ranging from 60 to 250 ng/cigarette (7, 8). These quantities are far greater than those of the aromatic amines 4-aminobiphenyl and 2-naphthylamine, which are implicated in the pathogenesis of bladder cancer in smokers (1, 9). Apart from the endocyclic nitrogen atoms, AαC shares the same structure as 2-aminofluorene, one of the most well studied aromatic amine carcinogens (10). Significant levels of AαC were detected in the urine of male smokers of the Shanghai cohort in China, providing evidence that tobacco smoke is a major source of AαC exposure (11). AαC is a liver carcinogen in mice, a transgene colon mutagen, and an inducer of colonic aberrant crypt foci, an early biomarker of colon neoplasia (12–14). Therefore, AαC could play a role in the incidence of liver or digestive tract cancers of smokers.
AαC undergoes metabolic activation by N-oxidation of the exocyclic amine group, by cytochrome P450 (P450) enzymes, to form 2-hydroxyamino-9H-pyrido[2,3-b]indole (HONH-AαC) (Fig. 1) (15, 16). HONH-AαC can undergo conjugation reactions with N-acetyltransferases or sulfotransferases to form unstable esters. These metabolites undergo heterolytic cleavage to form the proposed short lived nitrenium ion of AαC (Fig. 1) (17), which reacts with DNA to form covalent adducts, leading to mutations (18). The genotoxic potential of AαC has been shown in human peripheral blood lymphocytes (19), Chinese hamster ovary cells (18), and human hepatocytes (20), where high levels of AαC-DNA adducts are formed. However, long term stable biomarkers of AαC must be developed for implementation in molecular epidemiology studies that seek to address a role for this chemical in human cancer risk.
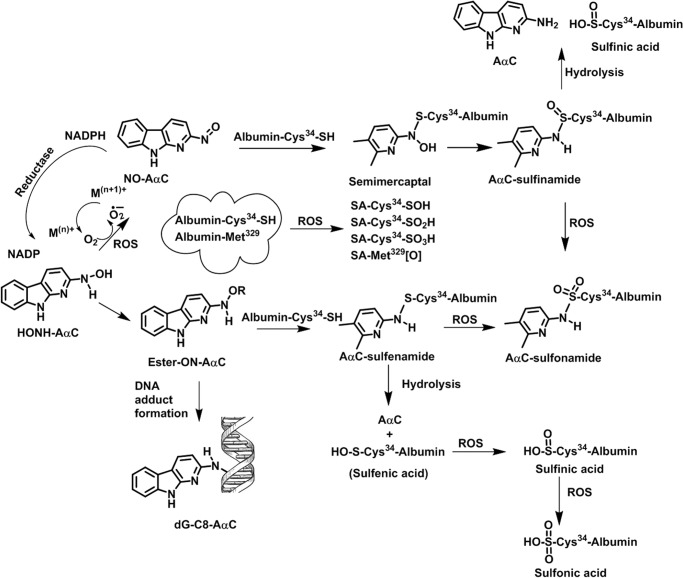
FIGURE 1.
Bioactivation of AαC, albumin adduct formation and induction of ROS in human hepatocytes. AαC undergoes bioactivation by P450 to form HONH-AαC. The HONH-AαC and its N-O-esters react with albumin to form AαC-Cys34 sulfenamide and sulfinamide adducts and also react with DNA to form dG-C8-AαC. HONH-AαC can undergo further oxidation by P450, by transition metals, or by oxygen to form NO-AαC and ROS. A redox cycling mechanism catalyzed by NADPH-P450 reductase can regenerate HONH-AαC (49, 64). The superoxide anion or other ROS can oxidize S-N-linked Cys-AαC to form the sulfonamide linkage. The ROS generated by N-oxidized AαC also directly oxidize albumin to form the sulfinic and sulfonic acids of Cys34 and Met residues of albumin.
DNA adducts of AαC can be measured by sensitive liquid chromatography/mass spectrometry (LC/MS)-based methods (20). However, DNA from biopsy specimens is often unavailable, which restricts the use of this biomarker. The electrophilic N-oxidized metabolites of AαC are also expected to react with proteins (21). The biomonitoring of protein-carcinogen adducts is an alternative approach to assess exposure to hazardous chemicals. Stable carcinogen protein adducts do not undergo repair and are expected to follow the kinetics of the lifetime of the protein (22, 23). The major proteins in blood are hemoglobin (Hb) with a life span of 60 days, and human serum albumin with a half-life of 21 days. The chemistry of reactivity of Hb and albumin with various genotoxicants and toxic electrophiles has been reported (24–26), and several protein-carcinogen adducts have been employed to assess human exposures (23, 27, 28).
Our goal is to develop and implement protein-based biomarkers of AαC and other HAAs (29, 30) in molecular epidemiological studies designed to assess the role of HAAs in human cancers. In this study, we have examined the reactivity of albumin with N-oxidized metabolites of AαC. Cys34 followed by Tyr140 and Tyr150 of albumin were major sites of adduction of AαC electrophiles. The Cys34 and Met329 residues of albumin also served as scavengers of reactive oxygen species (ROS) generated by metabolites of AαC, and the Cys34 sulfinic acid (Cys-SO2H), sulfonic acid (Cys-SO3H), and Met329 sulfoxide were recovered in high yield from human hepatocytes treated with AαC.
Experimental Procedures
Caution
AαC is a potential human carcinogen. AαC and its derivatives must be handled in a well ventilated fume hood with proper use of gloves and protective clothing.
Chemicals and Materials
AαC was purchased from Toronto Research Chemicals (Toronto, Canada). [4b,5,6,7,8,8a-13C6]AαC was a gift from Dr. Daniel Doerge (National Center for Toxicological Research, Jefferson, AR). Albumin, trypsin, chymotrypsin, Pronase E, prolidase, leucine amino peptidase, β-mercaptoethanol, iodoacetamide (IAM), dithiothreitol (DTT), acetic anhydride, and palladium on carbon were obtained from Sigma-Aldrich. LC/MS grade solvents were from Fisher. Tetrahydrofuran was obtained from Alfa Aeser (Ward Hill, MA). Amicon ultracentrifugal filters (10 kDa cut-off) were purchased from Millipore (Billerica, MA). The Pierce albumin depletion kit was purchased from Thermo Scientific (Rockford, IL). Human plasma was purchased from Bioreclamation LLC (Hicksville, NY).
Synthesis of N-Oxidized Metabolites of AαC
2-Nitro-9H-pyrido[2,3-b]indole (NO2-AαC) and NO2-[13C6]AαC were prepared by oxidation of AαC with dimethyldioxirane (17). HONH-AαC and HONH-[13C6]AαC were prepared by reduction of NO2-AαC in tetrahydrofuran with hydrazine, using palladium on carbon as a catalyst (31). HONH-AαC was oxidized to 2-nitroso-9H-pyrido[2,3-b]indole (NO-AαC) with potassium ferricyanide (32). N-(Deoxyguanosin-8-yl)-AαC (dG-C8-AαC) and the isotopically labeled internal standards [13C10]dG-C8-AαC were synthesized as described (33).
Modification of Albumin and Human Plasma with N-Oxidized Metabolites of AαC
Mixed disulfides formed at Cys34 of commercial albumin (34) were reduced by treatment with β-mercaptoethanol (35). The reduced albumin was recovered in 100 mm potassium phosphate buffer (pH 7.4) employing Amicon Ultra centrifugal filters. The reduced albumin contained 0.98 mol of Cys34/mol of albumin upon β-mercaptoethanol treatment. An equimolar solution of HONH-AαC and HNOH-[13C6]AαC or N-acetoxy-AαC and N-acetoxy-[13C6]AαC (15 nmol, in 10 μl of EtOH) or NO-AαC (30 nmol in 10 μl of EtOH) was reacted with albumin (0.6 nmol, 40 μg) in 1 ml of 100 mm potassium phosphate buffer (pH 7.4) at 37 °C for 18 h. N-Acetoxy-AαC and N-acetoxy-[13C6]AαC were prepared in situ by adding equimolar solution of HONH-AαC and HONH-[13C6]AαC (15 nmol) to the solution of albumin, immediately followed by the addition of 450 nmol of acetic anhydride (36), and incubated at 37 °C for 1 h. The unreacted AαC metabolites were removed by ethyl acetate extraction. Human plasma (5 μl, containing ∼200 μg of albumin, 3 nmol) was diluted with 1 ml of PBS and reacted with N-oxidized AαC derivatives as described above. Albumin from plasma was purified by affinity purification by Pierce albumin depletion kit. Other studies on AαC-albumin adduct formation were carried out using lower amounts of N-oxidized AαC (see below).
Human Hepatocyte Cell Culture
Human samples were obtained from the Centre de Ressources Biologiques (CRB)-Santé of Rennes. The research protocol was conducted under French legal guidelines and approved by the local institutional ethics committee. Hepatocytes were isolated by a two-step collagenase perfusion, and the parenchymal cells were seeded at a density of ∼3 × 106 viable cells/19.5-cm2 Petri dish in 3 ml of Williams' medium with supplements as reported (20), except fetal calf serum was replaced with human albumin (1 g/liter) pretreated with β-mercaptoethanol. After 2 days, the differentiated cells were incubated with AαC (20, 33).
Albumin and DNA Adduct Formation with AαC in Hepatocytes
Metabolism studies with AαC (0 or 50 μm in DMSO, 0.01% (v/v)) were conducted for 24 h. A solution of 1:1 AαC and [13C6]AαC (50 μm) was employed for characterization of AαC-albumin adducts, whereas the DNA adduct studies employed AαC (50 μm). The culture media containing albumin were removed after 24 h of incubation and immediately stored at −80 °C. The cells were washed with PBS, and cell pellets were collected by centrifugation at 3500 × g for 10 min at 4 °C. Cells were stored at −80 °C until further use. Cell viability was determined by 3-(4,5-dimethyltiazol-2-yl)-2,5-diphenyltetrazolium bromide test, and treatment with AαC did not decrease cell viability (37). The medium was extracted with 3 volumes of ethyl acetate, and then the albumin was recovered with >85% purity by ethanol precipitation. For some analyses, the albumin (100 μg, 1.5 nmol) was alkylated with a 100-fold molar excess of IAM (150 nmol) at 37 °C for 1 h. Excess IAM was removed by Amicon Ultra centrifugal filters.
Trypsin/Chymotrypsin Digestion
The digestion of AαC-modified albumin (10 μg) was carried out using trypsin and chymotrypsin at a protease/protein ratio of 1:50 (w/w) and 1:25 (w/w), respectively, in 100 μl of 50 mm ammonium bicarbonate buffer (pH 8.5) containing CaCl2 (1 mm) at 37 °C for 16–18 h (35).
Pronase E/Leucine Aminopeptidase/Prolidase Digestion
Albumin (10 μg, 150 pmol) was digested with Pronase E, leucine aminopeptidase, and prolidase at protease/protein ratios of 1:2 (w/w), 1:30 (w/w), and 1:8 (w/w), respectively, in 50 mm ammonium bicarbonate buffer (pH 8.5) containing MnCl2 (1 mm) at 37 °C for 20 h (23). The AαC-amino acid adducts were enriched by solid phase extraction (32).
UPLC Mass Spectrometry Parameters for Peptide Analyses
Peptides were resolved with an Atlantis C18 nanoACQUITY column (0.3 mm × 150 mm, 3-μm particle size, 100 Å) (Waters Corp., Milford, MA) using solvent A (5% acetonitrile, 94.99% water, 0.01% formic acid) and solvent B (95% acetonitrile, 4.99% water, 0.01% formic acid) as mobile phases with a Thermo Dionex Ultimate 3000 Nano/Cap LC System connected to an Orbitrap Elite mass spectrometer (Thermo Scientific, San Jose, CA) using an Advance CaptiveSpray source (Auburn, CA) in the positive ionization mode.
A 60-min gradient (99% solvent A to 60% solvent B for 45 min, 60 to 99% solvent B at 45–60 min) and 25-min gradient (99% solvent A to 60% solvent B for 20 min and 60–90% solvent B for 20–25 min) at 5 μl/min flow rate were used, respectively, for data-dependent (DDA) and targeted data acquisition.
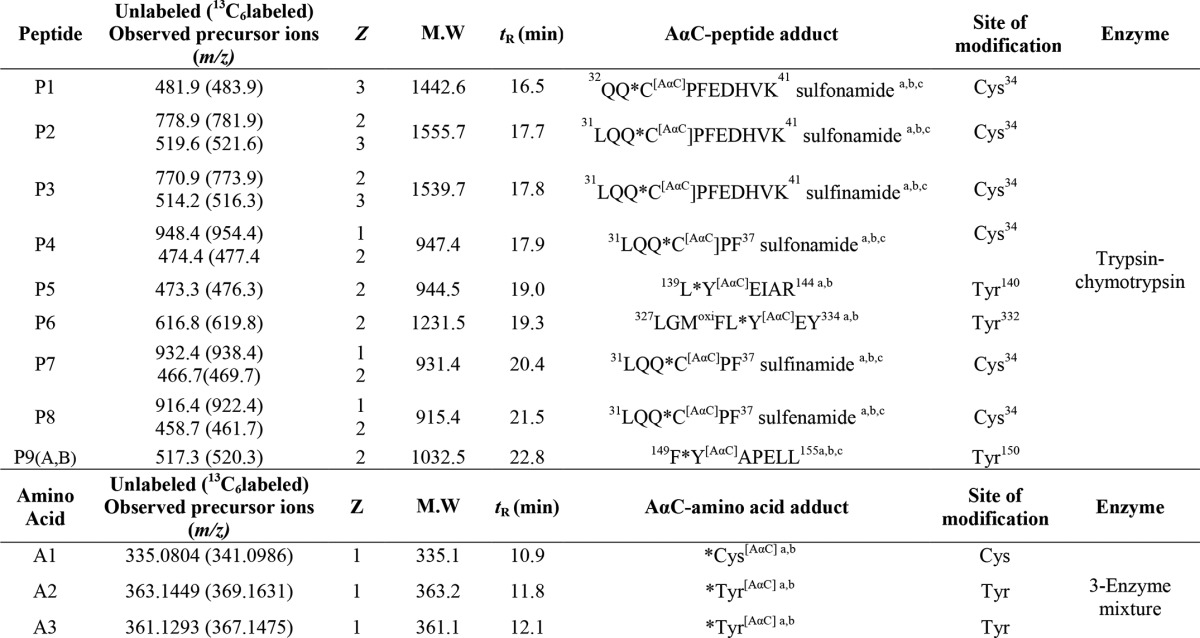
The top five precursor ions for CID-MS/MS analysis with dynamic exclusion for 180 s with three repeats of 60-s repeat duration were selected for DDA. Mass tag DDA (MS tag DDA) was employed to trigger MS/MS on peptides and amino acid adducts displaying the characteristic pattern of a 1:1 isotopic mixture of AαC and [13C6]AαC (38). The partner intensity ratio was 85–100%; m/z difference of 6 (singly charged), 3 (doubly charged), and 2 (triply charged) species. A mass list of precursor ions (Table 1) for AαC-amino acid and AαC-peptide adducts identified from MS tag DDA was used for targeted analysis.
TABLE 1.
AαC-peptide and AαC-amino acid adducts identified using mass tag data-dependent acquisition
Z, Charge state; tR, retention time; M.W, molecular weight; 3-enzyme mixture, Pronase E, leucine aminopeptidase, and prolidase.
a HONH-AαC/HONH-[13C6] AαC-albumin-modified sample.
b N-acetoxy-AαC/N-acetoxy-[13C6] AαC-albumin-modified sample.
c NO-AαC-modified sample.
The tune parameters were as follows: capillary tube temperature, 270 °C; spray voltage, 2.5 kV; S-lens RF level, 68(%); in-source fragmentation, 5 V; collision gas, helium; normalized collision energy, 35 eV; activation Q 0.3; HCD collision gas, argon; HCD collision energy, 20–35. Full scan data were acquired over a mass range of 150–1800 m/z at a resolving power of 120,000 (at 400 m/z), whereas MS/MS data acquisition was carried out with the ion trap or Orbitrap (resolving power 60,000) as mass analyzer with isolation widths 1.5 and 2 m/z. An internal lock mass at m/z 371.1012 (polysiloxane) was employed while using the Orbitrap mass analyzer. The tune parameters were optimized with LQQCPFEHVK and LQQCPF peptides (New England Peptide, Gardner, MA). Mass spectral data were acquired with Xcalibur version 3.0.63 software.
Data Analysis of AαC-Albumin
The BumberDash platform (version 1.4.115) with the Myrimatch search algorithm (version 2.1.138) with a 31-protein subset of the RefSeq human protein database, version 37.3, with their forward and reverse protein sequences were used to search for the AαC-peptide adducts (29, 39). The dynamic modifications used in the Myrimatch configuration file were as follows: oxidation (+15.99 Da) on Met, Cys; deamidation (−17.03 Da) on N-terminal Gln; AαC and [13C6]AαC (+181.06 and 187.11) on Cys, Lys, Tyr, Ser, Thr, Trp, His, Glu, Asp; SO-AαC and SO-[13C6]AαC (+197.06, +203.10) on Cys and SO2-AαC and SO2-[13C6]AαC (+213.05, +219.10); acetylation (+42.01) on Lys, Cys, Tyr, Arg. The search parameters used were as follows: enzyme (trypsin/chymotrypsin), up to two missed cleavages, mass tolerances 1.25 m/z (precursor ions) and 0.5 m/z (product ions). Each identified spectrum was filtered by the IDPicker algorithm (version 3.0.564) with a false discovery rate of 1%. The peptide sequences of identified AαC-peptide and AαC-amino acid adducts were confirmed by manually sequencing.
DNA Isolation and UPLC/MS3 Measurement of AαC-DNA Adducts
DNA was isolated by the phenol/chloroform method (20). DNA (5 μg) was spiked with the isotopically labeled internal standard ([13C10]dG-C8-AαC) at 1 adduct per 106 bases, followed by enzymatic digestion of DNA (40). The UPLC/MS3 measurements of AαC-DNA adducts were performed using a NanoAcquity UPLC system (Waters Corp.) coupled with an LTQ VelosPro ion trap mass spectrometer (Fisher) as described previously (41). The ions were monitored with the MS3 scan mode. For dG-C8-AαC, ions at m/z 449.1 (MS) > 333.1 (MS2) > 209.2, 291.4, 316.4 (MS3) and for the internal standard, [13C10]-dG-C8-AαC, ions at m/z 459.1 (MS) > 338.1 (MS2) > 210.2, 295.4, 321.5 (MS3) were monitored (38).
Results
The metabolic activation of AαC in hepatocytes, the reaction of N-oxidized AαC metabolites with DNA and albumin, and the scavenging of ROS by albumin are summarized in Fig. 1.
The UV spectra of AαC, HONH-AαC, and NO-AαC are displayed in Fig. 2A. NO-AαC displays a red shift in its maximal absorbance compared with AαC and HOHN-AαC. The product ion spectrum of AαC ([M + H]+ at m/z 184.1) displays fragment ions at m/z 167.0 and 157.1, attributed to the loss of NH3 and HCN (Fig. 2B) (9). The product ion spectrum of NO-AαC ([M + H]+ m/z 198.1) displays a sole ion at m/z 168.0 and is assigned to the loss of the NO group (Fig. 2C). The product ion spectrum of HONH-AαC ([M + H]+ at m/z 200.1) displays prominent fragment ions at m/z 182.1 and 183.1, which are attributed to the losses of H2O and OH•, and the product ion at m/z 155.1 is attributed to a loss of H2O and HCN (Fig. 2D).
FIGURE 2.
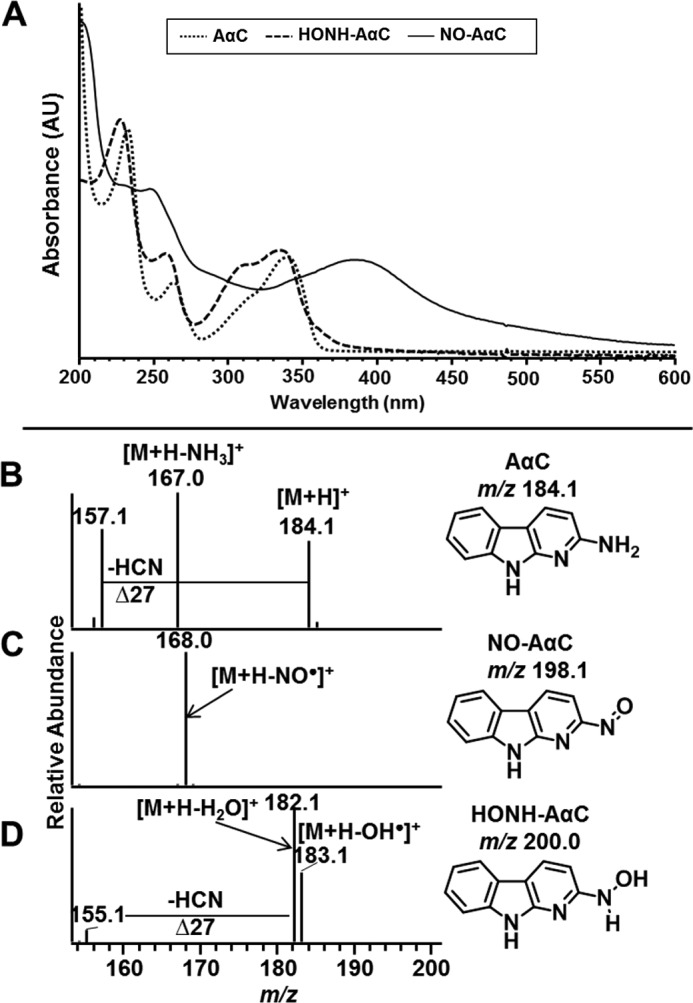
Characterization of AαC and its N-oxidized metabolites. A, UV spectra of AαC, NO-AαC, and HONH-AαC were obtained in methanol. Product ion spectra of AαC (B), NO-AαC (C), and HONH-AαC (D) were acquired by ion trap mass spectrometry.
MS Tag DDA Mapping of AαC-Albumin Peptides and AαC-Amino Acid Adducts
The assignments of AαC-peptide adducts are based on CID and higher-energy collision dissociation (HCD) tandem MS.
The chromatograms of the MS tag DDA experiments of albumin modified with a 50-fold molar excess of N-oxidized AαC and [13C6]AαC (1:1 ratio) were filtered using m/z values of the isotopic pair (i.e. for the radical cation (AαC•+ at m/z 183.1, [13C6]AαC•+ at m/z 189.1) and protonated ions (AαC+ at m/z 184.1 and [13C6]AαC at m/z 190.1)) (Fig. 3). Nine AαC-peptide adducts (P1–P9) were detected in the tryptic/chymotryptic digest. The assignments of the peptide adduct sequences and accurate mass measurements of the amino acid adducts, following digestion with Pronase E, leucine aminopeptidase, and prolidase, are listed in Table 1. Cys34, followed by Tyr140 and Tyr150, formed adducts with HONH-AαC; an additional adduct was formed at Tyr332 with N-acetoxy-AαC. The adduction of NO-AαC primarily occurred at Cys34, followed by much lower levels of adduct formation at Tyr150.
FIGURE 3.
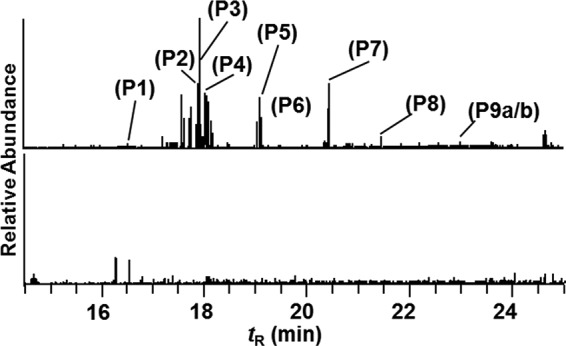
Mass tag data dependent MS2 scanning of trypsin/chymotrypsin digest of albumin modified with a 50-fold molar excess of an equimolar mixture of N-acetoxy-AαC and N-acetoxy-[13C6]AαC. Shown are reconstructed ion chromatograms for AαC at m/z 183.1 and 184.1 from digests of albumin modified with N-acetoxy-AαC/N-acetoxy-[13C6]AαC (top) or digests of non-modified albumin (bottom). The chromatograms were acquired on ions exhibiting mass difference of m/z 6 (for singly charged ions), m/z 3 (for doubly charged ions), and m/z 2 (for triply charged ions). The ion intensities were normalized to the same scale.
MS Characterization of Adducts Formed at Cys34 of Albumin
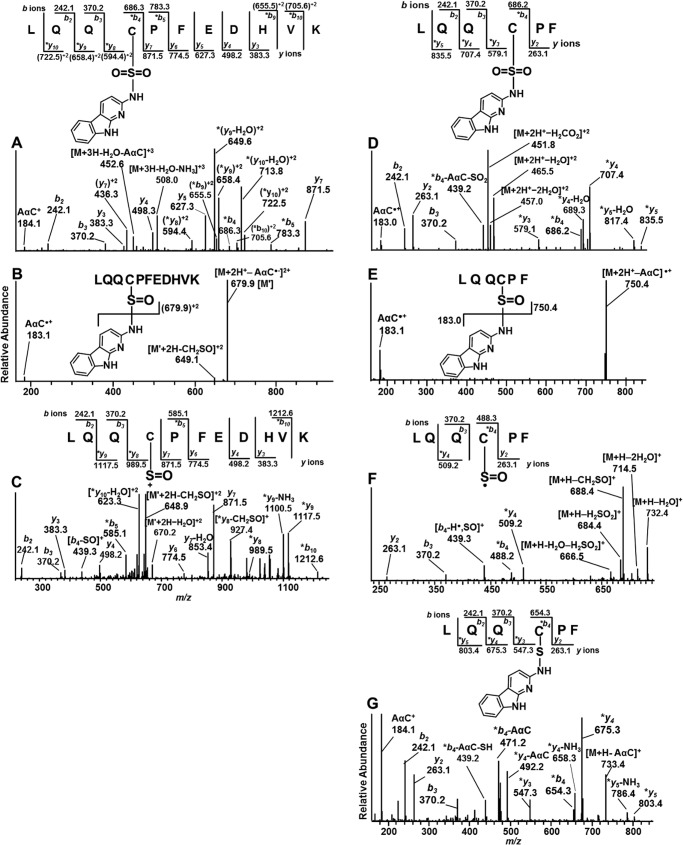
The digestion of AαC-modified albumin with trypsin/chymotrypsin produced peptides containing adducts at 31LQQC*PF36, 32QQCPFEDHVK41 and 31LQQCPFEDHVK41. A total of six adducts with different oxidation states of the sulfur atom were identified. The product ion spectra of several adducts peptides are described in detail below.
LQQ*CPFEDHVK-AαC Adducts
The single missed cleavage peptide containing the proposed sulfonamide adduct LQQ*C[SO2AαC]PF EDHVK (P2) (1555.7 Da), eluted at tR = 17.7 min and occurred as a triply charged species [M + 3H]3+ at m/z 519.6 (Fig. 4A). The 213-Da increase in mass over the non-modified peptide corresponds to the addition of AαC (182 Da) and two oxygen atoms (32 Da) minus one proton (1 Da) on the -SH moiety. The product ion spectrum of m/z 519.6 displayed a series of -b ions and -y ions confirming the sequence assignment. The shift in masses at the *b4 and (*y8)2+ ions identify the site of adduction at the Cys34 residue. The proposed sulfinamide adduct LQQ*C[SOAαC]PFEDHVK (P3) (1539.7 Da) at tR = 17.8 min occurred as a triply charged species [M + 3H]3+ at m/z 514.2, a mass 16 Da less than the sulfonamide adduct. The product ion spectrum of m/z 514.2 contains minor fragment ions at m/z 183.1 (AαC•+) and 184.1 (AαC+), with a base peak at m/z 679.9, corresponding to the sulfonium ion [M + 2H-AαC]2+ (Fig. 4B). The MS3 scan stage product ion spectrum of the proposed LQQ*C[SO]PFEDHVK sulfoxide ion at m/z 679.9 [M′] showed -b and -y ion series and supports the sequence assignment. Doubly charged product ion at m/z 648.9 attributed to the loss of CH2SO from the M′ ion and *b4-SO, *b5,*y8, and *y9 ions supports the proposed structure (Fig. 4C).
FIGURE 4.
A and B, product ion spectra of AαC adducts of LQQC[SO2AαC]PFEDHVK (P2) [M + 3H]3+ at m/z 519.6 (A) and LQQC[SOAαC]PFEDHVK (P3) [M + 3H]3+ at m/z 514.2 (B). C, consecutive reaction monitoring at the MS3 scan stage of LQQC[SOAαC]PFEDHVK (P3) targeting m/z 679.9 product ion from second generation product ion spectrum of m/z 514.2. D, LQQC[SO2AαC]PF (P4) [M + 2H]2+ at m/z 474.7. E, LQQC[SOAαC]PF (P7) [M + 2H]2+ at m/z 466.7. F, third generation product ion spectrum of [M + 2H]2+ at m/z 466.7>750.4>. G, LQQC[AαC]PF (P8) [M + 2H]2+ at m/z 658.7 of the trypsin/chymotrypsin digest of albumin modified with N-acetoxy-AαC and N-acetoxy-[13C6]AαC. *, fragment ions with AαC adduction.
LQQ*CPF-AαC Peptide Adducts
Three additional S-N-linked peptide adducts were identified. The product ion mass spectra of the doubly protonated ions are consistent with the AαC sulfonamide LQQ*C[SO2AαC]PF (P4) ([M + H]+ at m/z 948.4, [M + 2H]+ at m/z 474.7, tR = 17.9 min) (Fig. 4D), AαC sulfinamide LQQ*C[SOAαC]PF (P7) ([M + H]+ at m/z 932.4, [M + 2H]+ at m/z 466.7, tR = 20.4 min) (Fig. 4, E and F), and AαC sulfenamide LQQ*C[AαC]PF (P8) ([M + H]+ at m/z 916.4, [M + 2H]+ at m/z 458.7 tR 21.5 min) (Fig. 4G).
The doubly charged peptide precursor ion [M + 2H]2+ of LQQ*C[AαC]PF (P8) [M + H]+ at m/z 458.7 is a 181-Da increase in mass over the non-modified protonated peptide (m/z 735.3). This increase in mass corresponds to the addition of AαC (182 kDa) minus one proton (1 kDa) from the -SH moiety. The increase in mass of the peptide is consistent with the proposed sulfenamide linkage. The product ion spectrum of [M + 2H]2+ at m/z 458.7 (Fig. 4G) shows the -b ion and -y ion series, where *b4, *y3-*y5 ions provide evidence that adduction of AαC occurred at the sulfhydryl group of Cys34. The product ion at m/z 439.3 (*b4-AαC-SH) occurs via the cleavage of the C–S bond (41, 42). The prominent base peak observed at m/z 184.1 is attributed to protonated AαC (Fig. 4G). It is noteworthy that most arylsulfenamide adducts undergo hydrolysis during proteolytic digestion, which generally makes these adducts difficult to detect (30, 43, 44).
MS Characterization of *Cys-AαC Adducts
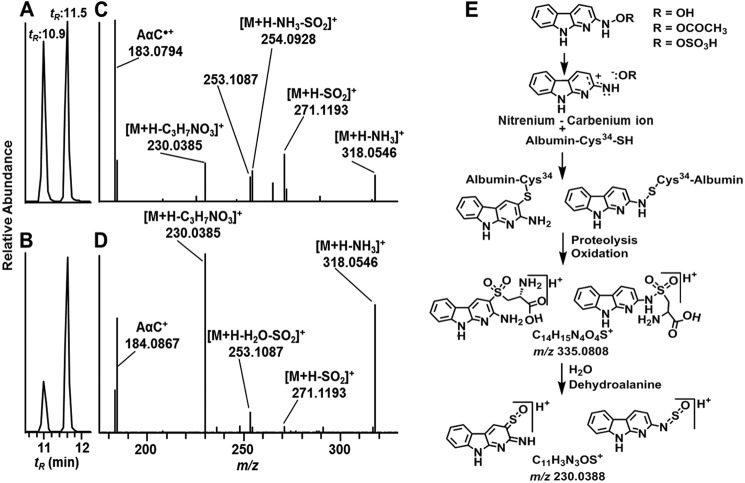
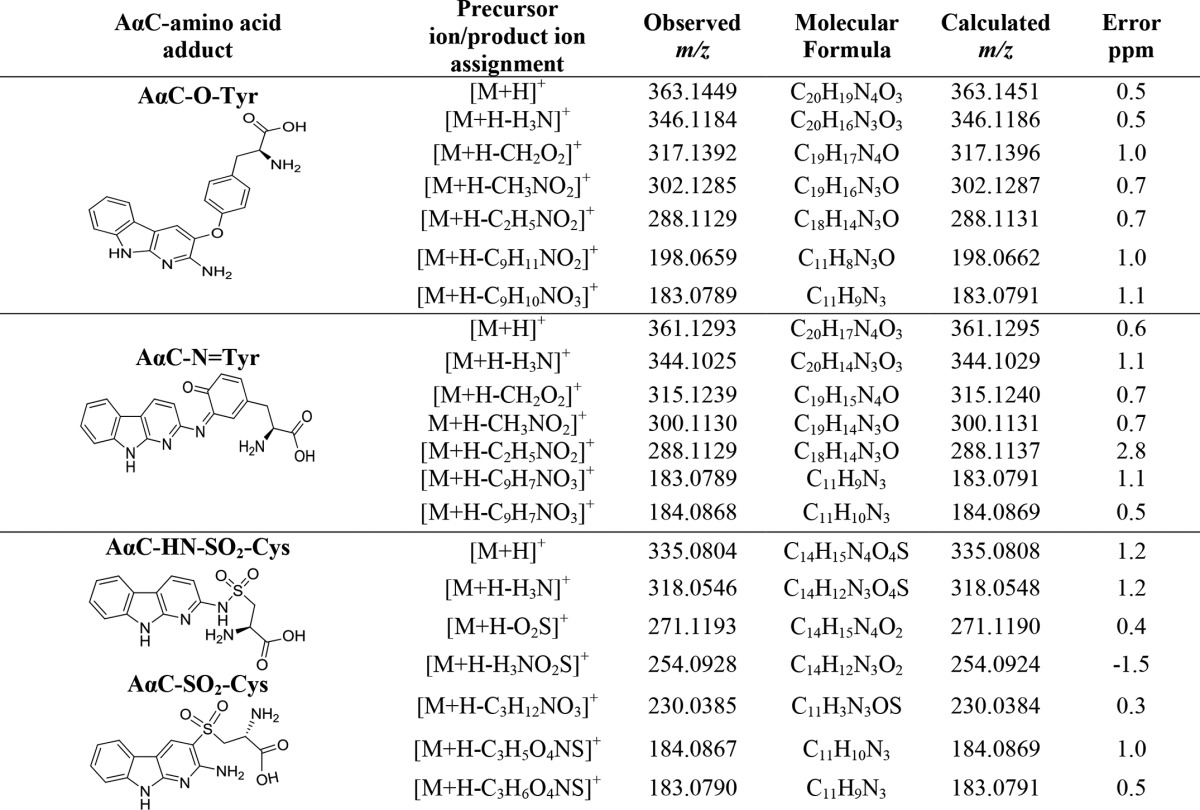
The Cys sulfenamide- and sulfinamide-linked adducts of AαC underwent hydrolysis to produce AαC during proteolysis of albumin with Pronase E, leucine aminopeptidase, and prolidase. Two peaks attributed to Cys-AαC adducts containing the S-dioxide linkage were identified (tR = 10.9 and 11.5 min) (A1) in the UPLC/MS chromatogram (Fig. 5, A and B). The precursor ions [M + H]+ were observed at m/z 335.0806, a value that is within 0.6 ppm of the calculated m/z value of the proposed sulfonamide structure (Table 1). The adducts were present in an approximate ratio of 4:6 in albumin modified with HONH-AαC and in a 2:8 ratio when albumin was modified with N-acetoxy-AαC; Fig. 5, A and B). The adducts were formed at ∼5-fold higher levels in albumin treated with N-acetoxy-AαC than in albumin treated with HONH-AαC. The earlier eluting isomer underwent CID to preferentially form a radical cation at m/z 183.0790 (AαC•+), whereas the second adduct favored the formation of the even electron ion at m/z 184.0867, the m/z of protonated ion of AαC+ (Fig. 5 (C and D) and Table 2). Other notable product ions were detected for both adducts but with different relative abundances: m/z 230.0382 ([AαC + SO]+); m/z 254.0922 ([M + H-NH3-SO2]+); m/z 271.1189 ([M + H-SO2]+); and m/z 318.0547 ([M + H-H2O]) (Fig. 5 (C and D) and Table 2).
FIGURE 5.
AαC-Cys S-dioxide adducts of albumin obtained from Pronase E/prolidase/leucine amino peptidase digest of albumin modified with HONH-AαC and HONH-[13C6]AαC or N-acetoxy-AαC and N-acetoxy-[13C6]AαC. Shown is a total ion chromatogram (TIC) at the MS2 scan stage of [M + H]+ at m/z 335.0806 obtained from albumin modified with HONH-AαC (A) and N-acetoxy-AαC (bottom) (B). The product ion spectra of [M + H]+ at m/z 335.0806 of C[SO2AαC] at 10.9 min (C) and at 11.5 min (D) and proposed structures of isomers are shown. E, proposed formation of cysteine-S-yl-dioxide-AαC isomeric adducts by reaction of Cys with the nitrenium-carbenium ion resonance forms of HONH-AαC and CID fragmentation mechanism of sulfonium ion at m/z 230.0385 in product ion spectra of isomers ([M + H]+ at m/z 335.0806).
TABLE 2.
Accurate mass measurements of AαC amino acid adducts formed with Tyr and Cys
Arylsulfonamides are weak acids, and the nitrogen anion of the sulfonamide linkage can form several tautomeric forms with hindered rotation about the S=N bond (45). However, the prominent differences in the product ion spectra, combined with the inability to interconvert these Cys-AαC adducts at elevated temperature, suggest that the adducts are not conformational isomers.
The nitrenium ion of HONH-AαC can undergo charge delocalization to form the carbenium ion resonance form with electron deficiency centered at the C-3 position of the AαC skeleton (Fig. 5E) (46). We propose that one isomeric Cys-AαC adduct contains an S-N linkage, and the second adduct contains a thioether linkage, formed between the Cys34-SH group and possibly the C-3 atom of AαC (46). The S-N-sulfenamide (or sulfinamide) and thioether adducts undergo oxidation, by ROS generated by aerobic oxidation of N-oxidized AαC metabolites (Fig. 1) or during proteolysis, to form the sulfonamide and sulfone linkages (Fig. 5E) (47). Both adducts undergo CID to form the proposed sulfonium ion at m/z 230.0385 (Fig. 5E). These adducts were not chromatographically resolved in the tryptic/chymotryptic digests of albumin. Large scale syntheses and NMR studies are required to elucidate the respective structures of these AαC-Cys-S-dioxide linked isomers. The Cys34 of albumin was reported to react with N-acetyl-p-benzoquinoneimine, the electrophilic metabolite of acetaminophen, and formed two regioisomeric adducts (42).
MS Characterization of Adducts Formed at Tyr140, Tyr150, and Tyr332 of Albumin
Three adducts were formed between Tyr residues of albumin and AαC (Table 1).
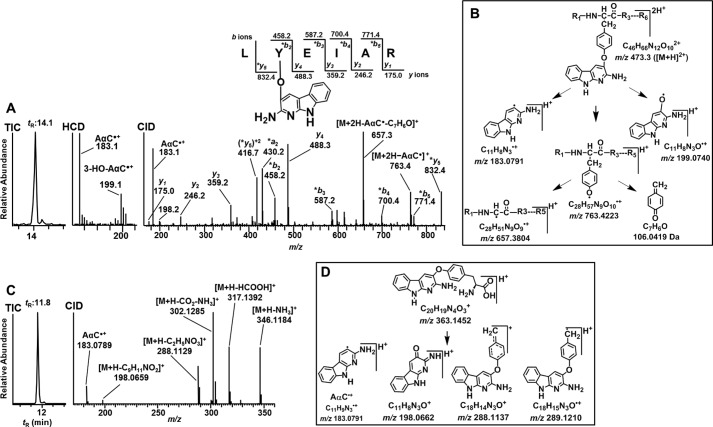
L*YEIAR Peptide Adduct
The product ion spectrum of the L*YEIAR (P5) adduct with a doubly charged peptide precursor ion [M + 2H]2+ at m/z 473.3 (tR = 14.1 min) displayed a series of -b ions and -y ions, which identifies the sequence as 139L*Y[AαC]EIAR144, with the site of adduction at Tyr140 (Fig. 6A). The ion at m/z 763.4 is proposed to arise by the loss of AαC as a radical cation at m/z 183.1 [M + H-AαC•]+, followed by the neutral loss of quinone methide (106.1 Da) [M + H-AαC•-C7H6O]+ to form the ion at m/z 657.4 (Fig. 6B). The mass spectral data support the proposed structure as an O-linked adduct formed between oxygen atom of Tyr and possibly the C-3 atom of the heterocyclic skeleton of AαC (46). Further support for the proposed O-linkage is provided by the lower region of the HCD mass spectrum; ions are observed at m/z 183.0790 (m/z 183.0791, calculated) and m/z 199.0745 (m/z 199.0741, calculated) and attributed to [AαC•]+ and [AαC+O•]+, respectively (Fig. 6, A and B). We recently reported a similar mechanism of fragmentation of an O-linked adduct formed between tyrosine and the HAA 2-amino-1-methyl-6-phenylmidazo[4,5-b]pyridine (PhIP) (32).
FIGURE 6.
Product ion spectrum of AαC-Tyr peptide and amino acid adduct of albumin modified with N-acetoxy-AαC and N-acetoxy-[13C6]AαC. Shown are TIC, HCD, and CID product ion spectra of LY[AαC]EIAR (P5) [M + 2H]2+ at m/z 473.3, tR = 14.1 min (A), and proposed CID fragmentation mechanism of LY[AαC]EIAR (P5) adduct [M + 2H]2+ at m/z 473.3 (B). Shown are TIC (C) and product ion spectrum (D) of amino acid adduct [M + H]+ at m/z 363.1449 (A2) at tR = 11.8 min and proposed CID fragmentation mechanism of AαC-Tyr adducts [M + H]+ at m/z 363.1452 (E).
MS characterization of an O-Linked Tyr-AαC Adduct
The proteolysis of albumin adducts with Pronase E, leucine aminopeptidase, and prolidase produced two Tyr linked adducts of AαC. A first set of Tyr-AαC and Tyr-[13C6]AαC adducts (A2) was observed at m/z 363.1449 (m/z 363.1451, calculated) and m/z 369.1629 (m/z 369.1631, calculated) at tR 11.8 min. The product ion spectrum of the unlabeled Tyr-AαC adduct [M + H]+ at m/z 363.1449 displayed fragment ions at m/z 346.1186, 317.1395, and 302.1285 attributed to the losses of NH3, H2CO2, and NH3 and CO2, respectively (Fig. 6C and Table 2). The product ions at m/z 289.1210 [M + H-C2H5NO2]+ and 288.1137 [M + H-C2H4NO2]+ are proposed to arise by cleavage of the Cα and Cβ bond of tyrosine. The fragment ion at m/z 183.0791 is assigned to AαC•+, and the ion at m/z 198.0659 is tentatively assigned as the protonated ion of the 2-imino-3-oxo derivative of AαC, with the loss of phenylalanine (165.0790 Da) as a neutral fragment. The ion at m/z 198.0659 provides evidence that adduct formation occurred between the 4-HO group of tyrosine and the AαC heterocyclic ring (Fig. 6D).
F*YAPELL-AαC Adducts
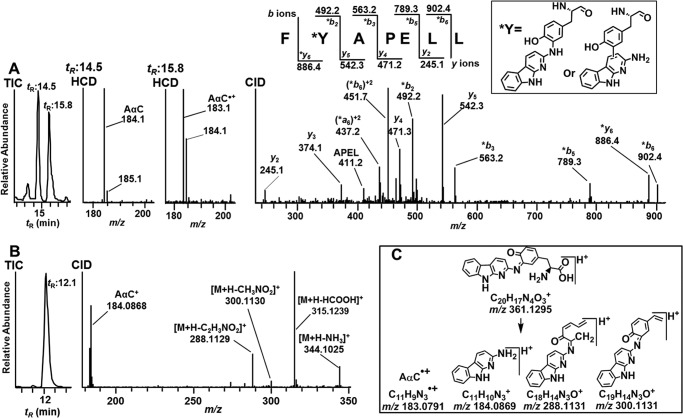
Two minor Tyr adducts of AαC with the peptide sequence 149F*Y[AαC]APELL155 (P9A and P9B; Fig. 3 and Table 1) were identified at tR 14.5 and 15.8 min (Fig. 7A). The full scan spectra of both adducts displayed doubly charged [M + 2H]2+ at m/z 517.3. The CID fragment ions of m/z 517.3 displaced the same characteristic -b ion and -y ion series attributed to the F*Y[AαC]APELL for both peptide adducts (Fig. 7A, CID). The shift in mass between the y5 and *y6 ion series proves that the site of AαC adduction occurred at Tyr150 for both peptides (Fig. 7A). CID did not provide appreciable fragment ions at the low m/z region for either adduct. The doubly protonated ions [M + 2H]2+ at m/z 517.3 were subjected to HCD to examine for potential fragmentation of the bond formed between the Tyr and AαC. The extracted ion chromatograms were generated at m/z 183.0791 and m/z 184.0869 (Fig. 7A, HCD). The earlier eluting adduct (P9A) at tR = 14.5 min displayed a prominent fragment ion at m/z 184.0869, an ion attributed to protonated AαC (Fig. 7A, HCD at tR = 14.5). The linkage of this adduct may have occurred between the C-3 or C-5 atom of the Tyr phenyl ring and the exocyclic amino group of AαC. The HCD product ion spectrum of the second adduct (P9C) at tR = 15.8 min showed a mixture of product ions assigned as the radical cation of AαC•+ and protonated AαC, respectively, at m/z 183.0789 and m/z 184.0868 (Fig. 7A, HCD at tR = 15.8). A Tyr linkage may have formed at the C-3 or C-7 atom of the heterocyclic ring of AαC, based on studies of nucleophilic trapping agents adducting at these sites of the nitrenium ion of AαC (46, 48).
FIGURE 7.
Characterization of isomeric FY[AαC]APELL peptides and an AαC-amine-linked Tyr amino acid adduct of albumin modified with N-acetoxy-AαC and N-acetoxy-[13C6]AαC. A, TIC, HCD, and CID product ion spectra of FY[AαC]APELL (P9) [M + 2H]2+ at m/z 517.3, tR = 14.5 and 15.8 min (CID spectra for both peptides were identical; only the spectrum of peptide at tR 14.5 min is shown). B, TIC and CID product ion spectra of AαC-amine-linked Tyr amino acid adduct [M + H]+ at m/z 361.1299 (A2) at tR 12.1 min. C, proposed CID fragmentation mechanism of AαC-Tyr adduct [M + H]+ at m/z 361.1299. *, fragment ions with AαC adduction.
MS Characterization of an Amine-linked AαC Tyr Adduct
The second set of Tyr-AαC and Tyr-[13C6]AαC adducts (A3) eluted at tR = 12.1 min (Fig. 7B). The protonated ions [M + H]+ were observed at m/z 361.1293 and 367.1475, respectively, a mass 2 Da less than the Tyr-AαC and Tyr-[13C6]-AαC adducts (m/z 363.1449 and 369.1631) described above (Fig. 7B and Table 2). The structure of the adduct is proposed to be a quinoneimine-linked adduct, which occurs by oxidation of P9A (Fig. 7B) during proteolytic digestion with the three-enzyme mixture. The product ion spectrum of [M + H]+ at m/z 361.1293 shows ions at m/z 344.1025, 315.1239, and 300.1130, which are of losses of NH3, H2CO2, and NH3 and CO2, respectively (Fig. 7B and Table 2). The product ion at m/z 288.1129 is proposed to arise following ring opening of the protonated quinoneimine ring (Fig. 7C). The Tyr-[13C6]AαC adduct displays the same product ions as the unlabeled adduct but shifted by 6 m/z.
A third peptide adduct formed between AαC and Tyr was observed only in the albumin modified with N-acetoxy-AαC. The CID-MS/MS spectrum of doubly charged protonated precursor ion at m/z 616.8 at 19.3 min displayed a typical -b and -y ion pattern attributable to LGMFL*Y[AαC]EY (P6) with AαC adduction at Tyr332 (supplemental Fig. S1, top). The [13C6]AαC homologue of this adduct at m/z 619.8 displayed the same pattern of fragmentation (supplemental Fig. S1, bottom).
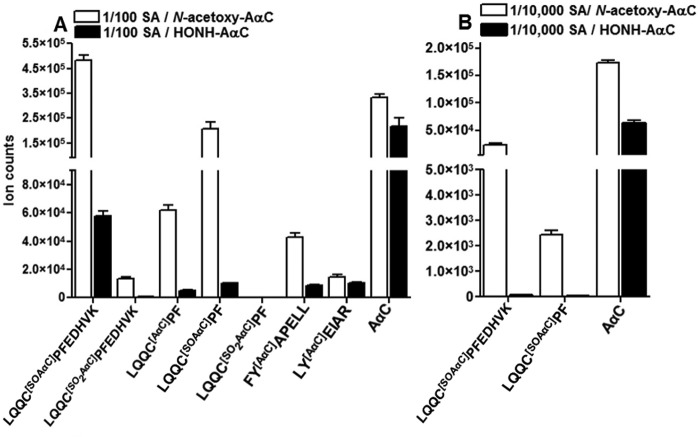
AαC-SA Adduct Formation as a Function of Concentration of N-Oxidized AαC Metabolites
The LQQ*C[SOAαC]PFEDHVK (P3) sulfinamide adduct at Cys34 accounted for 73–80% of the total ion counts of AαC-peptide adducts, when commercial albumin was reacted with a 50-fold molar excess of N-oxidized AαC metabolites. In vivo, the exposure to AαC occurs at much lower levels than the physiological concentration of albumin, and relative abundances of adducts formed may be different from those adducts formed at elevated exposures to AαC in vitro. Therefore, albumin was treated with HONH-AαC or N-acetoxy-AαC over a million-fold range of carcinogen/mol of albumin (1–10−6) (Fig. 8, A and B). We also examined the effect of plasma matrix components on the reactivity of albumin with N-oxidized AαC metabolites. Representative UPLC/MS chromatograms of the peptide adducts recovered from commercial albumin or albumin in plasma modified with 1 molar eq of N-acetoxy-AαC are shown in supplemental Fig. S2, A and B.
FIGURE 8.
Ion counts of AαC-peptide adducts targeting Cys34 and Tyr140 Tyr150 and free AαC recovered from trypsin/chymotrypsin digests of albumin modified with 1/10−2 molar eq of HONH-AαC or N-acetoxy-AαC, 0.3 μg digest/injection (A) and albumin modified with 1/10−4 molar eq of HONH-AαC or N-acetoxy-AαC, 1.0 μg of digest/injection (B). Data are plotted as mean and S.D. (error bars) of ion counts (n = 3).
The Tyr-peptide adducts of AαC at Tyr140, Tyr150, and Tyr332 residues were only detected when albumin was reacted with ≥ 0.01 mol N-acetoxy-AαC per mol albumin. In contrast, adducts were still formed at Cys34 with a 1 × 10−5 molar ratio of N-acetoxy-AαC/albumin (Fig. 8, A and B), and LQQ*C[SOAαC]PFEDHVK sulfinamide was the major adduct. The amounts of AαC-peptide adducts formed were ∼5–40 times higher in reactions of albumin conducted with N-acetoxy-AαC than those amounts of albumin adducts formed with HONH-AαC (Fig. 8, A and B). The level of AαC adduct formation with albumin in plasma was severalfold lower than adduct levels formed with commercial albumin (data not shown). Plasma albumin adducts were not detected at Tyr140, Tyr150, or Tyr332 residues at any concentration of N-oxidized AαC, and the sulfinamide LQQ*C[SOAαC]PFEDHVK was the predominant adduct.
AαC-DNA and AαC-Albumin Adduct Formation in Human Hepatocytes
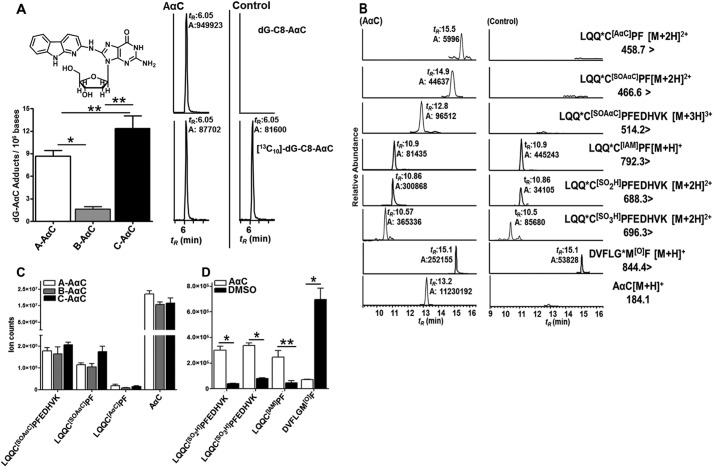
Reactive N-oxidized intermediates of AαC are formed and adduct to DNA in human hepatocytes and albumin in human hepatocytes (Fig. 1) (20, 33). The basal activities of P450 1A1 and 1A2, two major isoforms involved in N-oxidation of AαC (16, 17), were measured in human hepatocytes from three donors using ethoxyresorufin and methoxyresorufin as substrates (33). The level of dG-C8-AαC adduct formation from the two donors (A and C) with the highest P450 1A1 and 1A2 activities produced higher levels of dG-C8-AαC than donor B with low enzyme activity (Fig. 9A).
FIGURE 9.
A, estimates of dG-C8-AαC adduct formation in hepatocytes of donors A, B, and C treated with 50 μm AαC. Shown are UPLC-/MS3 chromatograms of dG-C8-AαC from hepatocytes treated with 50 μm AαC and with DMSO (control). For dG-C8-AαC, ions at m/z 449.1 (MS) > 333.1 (MS2) > 209.2, 291.4, 316.4 (MS3) (top) and for the internal standard, [13C10]dG-C8-AαC, ions at m/z 459.1 (MS) > 338.1 (MS2) > 210.2, 295.4, 321.5 (MS3) (bottom) were monitored. B, UPLC-ESI/MS2 chromatograms of AαC-peptide adducts and Cys and Met oxidation products recovered from trypsin/chymotrypsin digests of albumin from hepatocytes of donor A treated with AαC and [13C6] AαC (50 μm) and donor A treated with DMSO (control). Shown are ion counts of AαC-peptide adducts and AαC (C) and Cys34 sulfinic acid, Cys34 sulfonic acid, Met329 sulfoxide, and LQQC34PF alkylated with IAM obtained from trypsin/chymotrypsin digests of albumin of hepatocytes of donor A, B and C treated with a 50 μm equimolar mixture of AαC and [13C6]AαC for 24 h or treated with DMSO (control) (D). Values are reported as the mean and S.D. (error bars) (n = 3). *, p < 0.01 using one-way ANOVA for donors A, B, and C treated with 50 μm AαC; **, p < 0.05 for comparison between donors A and B and donors B and C using Tukey's multiple-comparison test for DNA adducts. *, p < 0.01; **, p < 0.05, AαC-treated versus control (DMSO-treated) (two-tailed Student's t test) for peptide adducts.
The mass chromatograms of AαC-albumin peptide adducts, following tryptic/chymotryptic digestion of albumin recovered from hepatocytes, are shown in Fig. 9B. The relative ion abundances of the peptide adducts are summarized in Fig. 9C, and product ion spectra are presented in supplemental Fig. S3. The major adduct, based on ion counts, is LQQ*C[SOAαC]PFEDHVK sulfinamide, followed by LQQ*C[SOAαC]PF and LQQ*C[AαC]PF. The occurrence of the LQQ*C[SOAαC]PFEDHVK as the major adduct in hepatocytes is similar to the findings of commercial albumin and albumin in plasma treated with N-oxidized AαC metabolites (Figs. 7A, 8A, and 9 (B and C)). The LQQ*C[AαC]PF sulfenamide and LQQ*C[SOAαC]PF sulfinamide were also identified but occurred at lower ion abundances (Fig. 9 (B and C) and supplemental Fig. S3 (A–C)). LQQ*C[SO2AαC]PF and LQQ*C[SO2AαC]PFEDHVK were not detected.
In the absence of stable, isotopically labeled internal standards, quantitative peptide adduct measurements and correlations to DNA adduct levels of AαC cannot be determined. However, the ion counts of the major albumin peptide adduct, LQQ*C[SOAαC]PFEDHVK sulfinamide, were greatest in donor C, who also harbored the highest level of dG-C8-AαC (Fig. 9, A–C). Very high levels of AαC also were recovered in all of the hepatocytes (Fig. 9, B and C).The occurrence of AαC is attributed to hydrolysis of S-N-linked albumin-AαC adducts during proteolysis and not to unmetabolized AαC bound to albumin, because the isolation procedure effectively removed all unbound AαC from albumin in the cell culture media.3
Oxidative Status of Albumin-Cys34 and Albumin-Met329 in Human Hepatocytes Exposed to AαC
N-Oxidized metabolites of arylamines generate ROS (49). We sought to determine if AαC had induced oxidative stress in hepatocytes by identification of oxidation products of Cys34 and Met residues of albumin (50–52). The oxidized sulfinic LQQ*C[SO2H]PF and sulfonic LQQ*C[SO3H]PFEDHVK acids of Cys34 of albumin were monitored (29, 32), and LQQCPF was measured following derivatization of albumin with IAM (29). Elevated levels of the protonated [M + H]2+ peptides for LQQ*C[SO2H]PFEDHVK at m/z 688.3 and LQQ*C[SO3H]PFEDHVK at m/z 696.3 (29) were detected in all three sets of hepatocytes treated with AαC (Fig. 9D). The product ion spectra at m/z 688.3 and 696.3 displayed typical -b and -y ion series type fragment ions (supplemental Fig. S4, A and B) and permitted the assignments as the peptide sequences of the Cys sulfinic and sulfonic acids (29). The levels of LQQ*C[SO2H]PFEDHVK and LQQ*C[SO3H]PFEDHVK in AαC/[13C6]AαC-treated hepatocytes were 6–8 and 4–6 times higher, respectively, than the levels in untreated controls, and the levels of IAM-derivatized LQQ*CPF were decreased by more than 10-fold in AαC-treated hepatocytes (Fig. 9, B and D). A Myrimatch search for oxidation sites in albumin also identified oxidation at the Met329 residue of albumin. The product ion spectrum of trypsin/chymotryptic peptide DVFLGM[O]F at [M + H]+ at m/z 844.4 representing Met329 oxidation resulted in typical -b and -y types of fragment ions, where -*y2-*y6 and -*b6 product ions further confirmed oxidation at Met329 (supplemental Fig. S4C). From targeted analysis, the level of DVFLGM[O]F was ∼6 times higher in AαC/[13C6]AαC-treated hepatocytes than in untreated hepatocytes (Fig. 9, B and D).
Discussion
Primary human hepatocytes are an ideal ex vivo model system for studying metabolism, bioactivation, and mechanisms of toxicity of carcinogens, because cofactors are present at physiological concentrations, and biotransformation pathways may closely simulate those that occur in vivo (53). In this study, we investigated the metabolic activation of AαC, a rodent liver carcinogen, in human hepatocytes and examined the reactivity of its genotoxic N-oxidized metabolites of AαC with DNA and albumin. Our goal is to develop and implement albumin-based biomarkers of AαC and other HAAs in molecular epidemiological studies designed to assess the role of HAAs in human cancers (54). The Cys34 residue was the major nucleophilic site of albumin to form adducts with AαC, followed by Tyr140 and Tyr150, which formed adducts at minor levels. Another HAA, PhIP (55), also primarily formed adducts at the Cys34 of albumin with considerably lower levels of adducts occurring at Tyr140 and Tyr150 (56). In contrast, the Tyr140 and Tyr150 residues of albumin are the preferred site for adduct formation of neurotoxic organophosphate compounds (57–59).
The preliminary characterization AαC-albumin adducts was performed in vitro. The formation of AαC-albumin adducts was greatly enhanced by the in situ generation of N-acetoxy-AαC, a reactive intermediate of HONH-AαC, which undergoes heterolytic cleavage to produce the nitrenium ion (48). N-Acetoxy-AαC is a penultimate metabolite of AαC that adducts to DNA and proteins (17). The N-acetoxy intermediates of HON-AαC and other N-hydroxylated HAAs, such as 2-hydroxyamino-3-methylimidazo[4,5-f]quinoline and 2-hydroxyamino-3,8-dimethylimidazo[4,5-f]quinoxaline, are unstable and cannot be isolated (60). However, the in situ formation of these N-acetoxy intermediates in the presence of DNA, by reaction of the N-hydroxylated HAAs with acetic anhydride, increased the levels of DNA adducts by 10–30-fold (36, 61, 62). Using a similar reaction scheme, we showed that adduct formation of HON-AαC with albumin was also greatly enhanced by the in situ generation of N-acetoxy-AαC (Fig. 8, A and B). The Lys, Arg, Cys, and Tyr residues of albumin can potentially compete with HONH-AαC and undergo acetylation with acetic anhydride and influence the formation of different AαC-albumin adducts. However, the same AαC-albumin adducts were formed by reaction of albumin with a 50-fold molar excess of HONH-AαC, NO-AαC, or N-acetoxy-AαC generated in situ (Table 1). Moreover, the Myrimatch search engine did not detect acetylation at Lys, Arg, Cys, or Tyr residues of albumin under these reaction conditions (100 mm potassium phosphate buffer (pH 7.4, 37 °C). Under low reaction conditions with HONH-AαC (10,000:1, albumin/HONH-AαC), the levels of Cys34 adducts were 50-fold greater when albumin was reacted with HONH-AαC in the presence of acetic anhydride than by reaction of albumin with HONH-AαC alone, thereby demonstrating that N-acetoxy-AαC is efficiently formed in situ and readily reacts with nucleophilic sites of albumin to form covalent adducts (Fig. 8, A and B).
Human hepatocytes efficiently bioactivated AαC to electrophilic N-oxidized metabolites, which formed covalent adducts with DNA and albumin (Fig. 9). The DNA adduct, dG-C8-AαC, was formed at relatively high levels, ranging from 2 to 12 adducts/106 DNA bases, consistent with our previous data (20, 33). dG-C8-AαC was also previously identified in salivary DNA of smokers (63). The Cys34 residue was the sole site of albumin found to form adducts with AαC metabolites in hepatocytes; both sulfenamide and sulfinamide adducts were identified. However, the ion counts of AαC recovered from the albumin digest were 100-fold or greater than the ion counts of any of the AαC-Cys adducts. These findings signify that a large proportion of the S-N-linked albumin-AαC adducts underwent hydrolysis during proteolysis. In the absence of stable peptide adducts or isotopically labeled LQQ*C[SOAαC]PFEDHVK peptides for internal standards, it is difficult to determine the relative reactivity of N-oxidized AαC metabolites with DNA and albumin in hepatocytes.
HONH-AαC and NO-AαC undergo redox cycling in hepatocytes and produce ROS (Fig. 1) (49, 64). Previous studies reported that structurally related aromatic amines, some of which are present in tobacco smoke (1, 66), deplete glutathione levels in the liver or ex vivo in hepatocytes of rodents and induce oxidative DNA damage (67–69); however, the oxidation of albumin was not reported in those studies. In our study, we show that albumin scavenges ROS produced by metabolites of AαC in human hepatocytes by formation of the oxidized Cys34-containing peptides LQQ*C[SO2H]PFEDHVK, LQQ*C[SO3H]PFEDHVK, and the methionine oxidation peptide DVFLGM[O]F. The level of Cys34 of albumin alkylated with IAM prior to proteolytic digestion decreased by more than 90% in hepatocytes treated with AαC and provides strong evidence that Cys34 of albumin is scavenging ROS (Fig. 9C). Together with Cys34, six Met residues of albumin display antioxidant activity toward O2⨪, H2O2, and HOCl (51, 52). Met329 was the primary site of Met oxidation in albumin from human hepatocytes treated with AαC. The oxidation of Met residues, particularly Met329, has been observed in albumin of hemodialysis patients (52).
The major adducts of AαC formed with albumin were determined in human hepatocytes by employing data-dependent scanning and bottom-up proteomics approaches, and the adduction products were the same as those adducts formed in vitro with commercial albumin reacted with N-oxidized AαC intermediates. The dose of AαC (50 μm) employed in human hepatocytes is greater than daily human exposure to AαC but comparable with the doses employed in studies investigating the genotoxicity of AαC (18, 19). The amount of AαC arising in mainstream smoke is 60–250 ng/cigarette (7, 8). Thus, the intracellular or plasma levels of AαC found in humans exposed to this tobacco carcinogen are considerably lower than the amount of AαC employed in our hepatocyte study. However, dG-C8-AαC formation occurs in a concentration-dependent manner in human hepatocytes treated with AαC over a 10,000-fold concentration range (1 nm to 10 μm), signifying that the reactive N-oxidation metabolites of AαC are formed at physiological exposure levels (20). More sensitive mass spectrometry-based methods are required for measuring AαC-albumin adducts and albumin oxidation products in hepatocytes at these lower exposure conditions to AαC.
The Cys34 of albumin accounts for 80% of total free thiol content in plasma and is considered a major antioxidant and scavenger of electrophiles in plasma (34). A number of genotoxicants and toxic electrophiles form adducts with the Cys34 of rodent or human albumin (23). Many of these adducts have been characterized primarily in vitro, and several adducts have been detected in humans. Albumin adducts have been identified with acrylamide (70), nitrogen mustard (71), α,β-unsaturated aldehydes (26), the neurotoxin brevetoxin B (72), acetaminophen (42), benzene (23), and several N-oxidized HAAs of diverse structures (32, 35, 56, 73). In addition, aldehydes produced in tobacco smoke are scavenged by Cys34 of albumin in vitro (74). Immunochemical techniques have shown carbonyl residues are formed with albumin exposed to cigarette smoke extract, and the carbonylation of albumin, by tobacco smoke, has been detected in lung tissue biopsy samples of smokers (75). Spectrophotometric assays have shown a decrease in the free Cys34 content of albumin following exposure to cigarette smoke extracts in vitro (74, 76). The diminution of free Cys34 content may be attributed to adducts formed with aldehydes or by oxidation with ROS (26, 74, 77); however, a correlation between the level of adduct formation at Cys34 of albumin and cigarette smoking constituents remains to established in vivo. Recently, elevated levels of Cys34-SO2H of albumin were detected in plasma of smokers in a small pilot study (65). AαC, other HAAs, and structurally related aromatic amines present in tobacco smoke represent a class of chemicals in tobacco smoke that may contribute to the chemical modification or oxidation of Cys34 and the oxidation of Met residues of albumin.
In summary, AαC, a rodent liver carcinogen (12), undergoes bioactivation and forms adducts with DNA and albumin and induces oxidative stress in human hepatocytes. Albumin is a potent scavenger of ROS species generated by AαC metabolites. AαC, other HAAs, and many aromatic amines that arise in mainstream tobacco smoke (1, 5, 66) undergo N-oxidation in humans (54). Some of these metabolites form adducts with DNA and protein and also induce oxidative stress, which may be contributing factors to liver damage and cancer risk in smokers. The Cys34 adducts of N-oxidized HAAs and arylamines or their hydrolysis products and elevated levels of Cys34-SO2H and Cys34-SO3H of albumin may be potential biomarkers to assess exposure to these hazardous chemicals in tobacco smokers.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 CA134700 and R01CA134700-03S1 of the Family Smoking Prevention and Tobacco Control Act. This work was also supported by PNREST Anses, Cancer TMOI AVIESAN (2013/1/166). The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. S1–S4.
K. Phatak, unpublished observations.
- HAA
- heterocyclic aromatic amines
- AαC
- 2-amino-9H-pyrido[2,3-b]indole
- N-acetoxy-AαC
- N-acetyloxy-2-amino-9H-pyrido[2,3-b]indole
- HONH-AαC
- 2-hydroxyamino-9H-pyrido[2,3-b]indole
- NO-AαC
- 2-nitroso-9H-pyrido[2,3-b]indole
- NO2-AαC
- 2-nitro-9H-pyrido[2,3-b]indole
- P450
- cytochrome P450
- PhIP
- 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- IAM
- iodoacetamide
- DDA
- data-dependent acquisition
- CID
- collision-induced dissociation
- HCD
- high-energy collision dissociation
- UPLC
- ultraperformance liquid chromatography
- ROS
- reactive oxygen species
- dG-C8-AαC
- N-(deoxyguanosin-8-yl)-AαC
- IAM
- iodoacetamide
- TIC
- total ion chromatogram.
References
- 1. International Agency for Research on Cancer (1986) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoking, pp. 127–293, International Agency for Research on Cancer, Lyon, France [Google Scholar]
- 2. Lüchtenborg M., White K. K., Wilkens L., Kolonel L. N., Le Marchand L. (2007) Smoking and colorectal cancer: different effects by type of cigarettes? Cancer Epidemiol. Biomarkers Prev. 16, 1341–1347 [DOI] [PubMed] [Google Scholar]
- 3. Giovannucci E. (2001) An updated review of the epidemiological evidence that cigarette smoking increases risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev. 10, 725–731 [PubMed] [Google Scholar]
- 4. Vineis P., Alavanja M., Buffler P., Fontham E., Franceschi S., Gao Y. T., Gupta P. C., Hackshaw A., Matos E., Samet J., Sitas F., Smith J., Stayner L., Straif K., Thun M. J., Wichmann H. E., Wu A. H., Zaridze D., Peto R., Doll R. (2004) Tobacco and cancer: recent epidemiological evidence. J. Natl. Cancer Inst. 96, 99–106 [DOI] [PubMed] [Google Scholar]
- 5. Hoffmann D., Hoffmann I., El-Bayoumy K. (2001) The less harmful cigarette: a controversial issue: a tribute to Ernst L. Wynder. Chem. Res. Toxicol. 14, 767–790 [DOI] [PubMed] [Google Scholar]
- 6. Yoshida D., Matsumoto T., Yoshimura R., Matsuzaki T. (1978) Mutagenicity of amino-α-carbolines in pyrolysis products of soybean globulin. Biochem. Biophys. Res. Commun. 83, 915–920 [DOI] [PubMed] [Google Scholar]
- 7. Yoshida D., Matsumoto T. (1980) Amino-α-carbolines as mutagenic agents in cigarette smoke condensate. Cancer Lett. 10, 141–149 [DOI] [PubMed] [Google Scholar]
- 8. Zhang L., Ashley D. L., Watson C. H. (2011) Quantitative analysis of six heterocyclic aromatic amines in mainstream cigarette smoke condensate using isotope dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Nicotine Tob. Res. 13, 120–126 [DOI] [PubMed] [Google Scholar]
- 9. International Agency for Research on Cancer (2002) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Tobacco Smoke and Involuntary Smoking, pp. 1005–1403, International Agency for Research on Cancer, Lyon, France [Google Scholar]
- 10. Kriek E. (1992) Fifty years of research on N-acetyl-2-aminofluorene, one of the most versatile compounds in experimental cancer research. J. Cancer Res. Clin. Oncol. 118, 481–489 [DOI] [PubMed] [Google Scholar]
- 11. Turesky R. J., Yuan J. M., Wang R., Peterson S., Yu M. C. (2007) Tobacco smoking and urinary levels of 2-amino-9H-pyrido[2,3-b]indole in men of Shanghai, China. Cancer Epidemiol. Biomarkers Prev. 16, 1554–1560 [DOI] [PubMed] [Google Scholar]
- 12. Sugimura T., Wakabayashi K., Nakagama H., Nagao M. (2004) Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 95, 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okonogi H., Ushijima T., Shimizu H., Sugimura T., Nagao M. (1997) Induction of aberrant crypt foci in C57BL/6N mice by 2-amino-9H-pyrido[2,3-b]indole (AαC) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx). Cancer Lett. 111, 105–109 [DOI] [PubMed] [Google Scholar]
- 14. Zhang X. B., Felton J. S., Tucker J. D., Urlando C., Heddle J. A. (1996) Intestinal mutagenicity of two carcinogenic food mutagens in transgenic mice: 2-amino-l-methyl-6-phenylimidazo [4,5-b]pyridine and amino(α)carboline. Carcinogenesis 17, 2259–2265 [DOI] [PubMed] [Google Scholar]
- 15. Niwa T., Yamazoe Y., Kato R. (1982) Metabolic activation of 2-amino-9H-pyrido[2,3-b]indole by rat-liver microsomes. Mutat. Res. 95, 159–170 [DOI] [PubMed] [Google Scholar]
- 16. Raza H., King R. S., Squires R. B., Guengerich F. P., Miller D. W., Freeman J. P., Lang N. P., Kadlubar F. F. (1996) Metabolism of 2-amino-α-carboline: a food-borne heterocyclic amine mutagen and carcinogen by human and rodent liver microsomes and by human cytochrome P4501A2. Drug Metab. Dispos. 24, 395–400 [PubMed] [Google Scholar]
- 17. King R. S., Teitel C. H., Kadlubar F. F. (2000) In vitro bioactivation of N-hydroxy-2-amino-α-carboline. Carcinogenesis 21, 1347–1354 [PubMed] [Google Scholar]
- 18. Turesky R. J., Bendaly J., Yasa I., Doll M. A., Hein D. W. (2009) The impact of NAT2 acetylator genotype on mutagenesis and DNA adducts from 2-amino-9H-pyrido[2,3-b]indole. Chem. Res. Toxicol. 22, 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Majer B. J., Kassie F., Sasaki Y., Pfau W., Glatt H., Meinl W., Darroudi F., Knasmüller S. (2004) Investigation of the genotoxic effects of 2-amino-9H-pyrido[2,3-b]indole in different organs of rodents and in human derived cells. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 802, 167–173 [DOI] [PubMed] [Google Scholar]
- 20. Nauwelaërs G., Bellamri M., Fessard V., Turesky R. J., Langouët S. (2013) DNA adducts of the tobacco carcinogens 2-amino-9H-pyrido[2,3-b]indole and 4-aminobiphenyl are formed at environmental exposure levels and persist in human hepatocytes. Chem. Res. Toxicol. 26, 1367–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller J. A. (1970) Carcinogenesis by chemicals: an overview: G. H. A. Clowes memorial lecture. Cancer Res. 30, 559–576 [PubMed] [Google Scholar]
- 22. Törnqvist M., Fred C., Haglund J., Helleberg H., Paulsson B., Rydberg P. (2002) Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 778, 279–308 [DOI] [PubMed] [Google Scholar]
- 23. Rappaport S. M., Li H., Grigoryan H., Funk W. E., Williams E. R. (2012) Adductomics: characterizing exposures to reactive electrophiles. Toxicol. Lett. 213, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liebler D. C. (2002) Proteomic approaches to characterize protein modifications: new tools to study the effects of environmental exposures. Environ. Health Perspect. 110, 3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rubino F. M., Pitton M., Di Fabio D., Colombi A. (2009) Toward an “omic” physiopathology of reactive chemicals: thirty years of mass spectrometric study of the protein adducts with endogenous and xenobiotic compounds. Mass Spectrom. Rev. 28, 725–784 [DOI] [PubMed] [Google Scholar]
- 26. Aldini G., Regazzoni L., Orioli M., Rimoldi I., Facino R. M., Carini M. (2008) A tandem MS precursor-ion scan approach to identify variable covalent modification of albumin Cys34: a new tool for studying vascular carbonylation. J. Mass Spectrom. 43, 1470–1481 [DOI] [PubMed] [Google Scholar]
- 27. Skipper P. L., Tannenbaum S. R. (1990) Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis 11, 507–518 [DOI] [PubMed] [Google Scholar]
- 28. Yu M. C., Skipper P. L., Tannenbaum S. R., Chan K. K., Ross R. K. (2002) Arylamine exposures and bladder cancer risk. Mutat. Res. 506, 21–28 [DOI] [PubMed] [Google Scholar]
- 29. Peng L., Turesky R. J. (2014) Optimizing proteolytic digestion conditions for the analysis of serum albumin adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, a potential human carcinogen formed in cooked meat. J. Proteomics 103, 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peng L., Turesky R. J. (2013) Capturing labile sulfenamide and sulfinamide serum albumin adducts of carcinogenic arylamines by chemical oxidation. Anal. Chem. 85, 1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Westra J. G. (1981) A rapid and simple synthesis of reactive metabolites of carcinogenic aromatic amines in high yield. Carcinogenesis 2, 355–357 [DOI] [PubMed] [Google Scholar]
- 32. Peng L., Turesky R. J. (2011) Mass spectrometric characterization of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine N-oxidized metabolites bound at Cys34 of human serum albumin. Chem. Res. Toxicol. 24, 2004–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nauwelaers G., Bessette E. E., Gu D., Tang Y., Rageul J., Fessard V., Yuan J. M., Yu M. C., Langouët S., Turesky R. J. (2011) DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes. Chem. Res. Toxicol. 24, 913–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carballal S., Radi R., Kirk M. C., Barnes S., Freeman B. A., Alvarez B. (2003) Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry 42, 9906–9914 [DOI] [PubMed] [Google Scholar]
- 35. Turesky R. J., Skipper P. L., Tannenbaum S. R. (1987) Binding of 2-amino-3-methylimidazo[4,5-f]quinoline to hemoglobin and albumin in vivo in the rat: identification of an adduct suitable for dosimetry. Carcinogenesis 8, 1537–1542 [DOI] [PubMed] [Google Scholar]
- 36. Turesky R. J., Markovic J. (1994) DNA adduct formation of the food carcinogen 2-amino-3-methylimidazo[4,5-f]quinoline at the C-8 and N2 atoms of guanine. Chem. Res. Toxicol. 7, 752–761 [DOI] [PubMed] [Google Scholar]
- 37. Langouët S., Coles B., Morel F., Becquemont L., Beaune P., Guengerich F. P., Ketterer B., Guillouzo A. (1995) Inhibition of CYP1A2 and CYP3A4 by oltipraz results in reduction of aflatoxin B1 metabolism in human hepatocytes in primary culture. Cancer Res. 55, 5574–5579 [PubMed] [Google Scholar]
- 38. Tang Y., Kassie F., Qian X., Ansha B., Turesky R. J. (2013) DNA adduct formation of 2-amino-9H-pyrido[2,3-b]indole and 2-amino-3,4-dimethylimidazo[4,5-f]quinoline in mouse liver and extrahepatic tissues during a subchronic feeding study. Toxicol. Sci. 133, 248–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tabb D. L., Fernando C. G., Chambers M. C. (2007) MyriMatch: highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J. Proteome Res. 6, 654–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goodenough A. K., Schut H. A., Turesky R. J. (2007) Novel LC-ESI/MS/MS n method for the characterization and quantification of 2′-deoxyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by 2-D linear quadrupole ion trap mass spectrometry. Chem. Res. Toxicol. 20, 263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kang P., Dalvie D., Smith E., Zhou S., Deese A. (2007) Identification of a novel glutathione conjugate of flutamide in incubations with human liver microsomes. Drug Metab. Dispos. 35, 1081–1088 [DOI] [PubMed] [Google Scholar]
- 42. Damsten M. C., Commandeur J. N., Fidder A., Hulst A. G., Touw D., Noort D., Vermeulen N. P. (2007) Liquid chromatography/tandem mass spectrometry detection of covalent binding of acetaminophen to human serum albumin. Drug Metab. Dispos. 35, 1408–1417 [DOI] [PubMed] [Google Scholar]
- 43. Saito K., Kato R. (1984) Glutathione conjugation of arylnitroso compound: detection and monitoring labile intermediates in situ inside a fast atom bombardment mass spectrometer. Biochem. Biophys. Res. Commun. 124, 1–5 [DOI] [PubMed] [Google Scholar]
- 44. Eyer P., Galleman D. (1996) in The Chemistry of Amine, Nitroso, Nitro and Related Groups, Part 1 (Patai S., ed) pp. 999–1040, John Wiley & Sons, Chichester, UK [Google Scholar]
- 45. Lemke T. L., Roche V. F., Zito S. W. (2012) Review of Organic Functional Groups: Introduction to Medicinal Organic Chemistry, 5th Ed., pp. 85–87, Lippincott Williams & Wilkins, Baltimore, MD [Google Scholar]
- 46. Novak M., Kazerani S. (2000) Characterization of the 2-(α-carbolinyl)nitrenium ion and its conjugate base produced during the decomposition of the model carcinogen 2-N-(pivaloyloxy)-2-amino-α-carboline in aqueous solution. J. Am. Chem. Soc. 122, 3606–3616 [Google Scholar]
- 47. Steen H., Mann M. (2001) Similarity between condensed phase and gas phase chemistry: fragmentation of peptides containing oxidized cysteine residues and its implications for proteomics. J. Am. Soc. Mass Spectrom. 12, 228–232 [DOI] [PubMed] [Google Scholar]
- 48. Novak M., Nguyen T. M. (2003) Unusual reactions of the model carcinogen N-acetoxy-N-acetyl-2-amino-α-carboline. J. Org. Chem. 68, 9875–9881 [DOI] [PubMed] [Google Scholar]
- 49. Murata M., Kawanishi S. (2011) Mechanisms of oxidative DNA damage induced by carcinogenic arylamines. Front. Biosci. (Landmark Ed.) 16, 1132–1143 [DOI] [PubMed] [Google Scholar]
- 50. Colombo G., Clerici M., Giustarini D., Rossi R., Milzani A., Dalle-Donne I. (2012) Redox albuminomics: oxidized albumin in human diseases. Antioxid. Redox Signal. 17, 1515–1527 [DOI] [PubMed] [Google Scholar]
- 51. Finch J. W., Crouch R. K., Knapp D. R., Schey K. L. (1993) Mass spectrometric identification of modifications to human serum albumin treated with hydrogen peroxide. Arch. Biochem. Biophys. 305, 595–599 [DOI] [PubMed] [Google Scholar]
- 52. Bruschi M., Petretto A., Candiano G., Musante L., Movilli E., Santucci L., Urbani A., Gusmano R., Verrina E., Cancarini G., Scolari F., Ghiggeri G. M. (2008) Determination of the oxido-redox status of plasma albumin in hemodialysis patients. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 864, 29–37 [DOI] [PubMed] [Google Scholar]
- 53. Guillouzo A. (1998) Liver cell models in in vitro toxicology. Environ. Health Perspect. 106, 511–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Turesky R. J., Le Marchand L. (2011) Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem. Res. Toxicol. 24, 1169–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Felton J. S., Jagerstad M., Knize M. G., Skog K., Wakabayashi K. (2000) in Food Borne Carcinogens Heterocyclic Amines (Nagao M., Sugimura T., eds) pp. 31–71, John Wiley & Sons Ltd., Chichester, UK [Google Scholar]
- 56. Peng L., Dasari S., Tabb D. L., Turesky R. J. (2012) Mapping serum albumin adducts of the food-borne carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by data-dependent tandem mass spectrometry. Chem. Res. Toxicol. 25, 2179–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. John H., Breyer F., Thumfart J. O., Höchstetter H., Thiermann H. (2010) Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) for detection and identification of albumin phosphylation by organophosphorus pesticides and G- and V-type nerve agents. Anal. Bioanal. Chem. 398, 2677–2691 [DOI] [PubMed] [Google Scholar]
- 58. Noort D., Hulst A. G., van Zuylen A., van Rijssel E., van der Schans M. J. (2009) Covalent binding of organophosphorothioates to albumin: a new perspective for OP-pesticide biomonitoring? Arch. Toxicol. 83, 1031–1036 [DOI] [PubMed] [Google Scholar]
- 59. Li B., Schopfer L. M., Hinrichs S. H., Masson P., Lockridge O. (2007) Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry assay for organophosphorus toxicants bound to human albumin at Tyr411. Anal. Biochem. 361, 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Turesky R. J., Rossi S. C., Welti D. H., Lay J. O., Jr., Kadlubar F. F. (1992) Characterization of DNA adducts formed in vitro by reaction of N -hydroxy-2-amino-3-methylimidazo[4,5-f]quinoline and N -hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline at the C-8 and N2 atoms of guanine. Chem. Res. Toxicol. 5, 479–490 [DOI] [PubMed] [Google Scholar]
- 61. Snyderwine E. G., Roller P. P., Adamson R. H., Sato S., Thorgeirsson S. S. (1988) Reaction of the N-hydroxylamine and N-acetoxy derivatives of 2-amino-3-methylimidazo[4,5-f]quinoline with DNA. Synthesis and identification of N-(deoxyguanosin-8-yl)-IQ. Carcinogenesis 9, 1061–1065 [DOI] [PubMed] [Google Scholar]
- 62. Frederiksen H., Frandsen H., Pfau W. (2004) Syntheses of DNA-adducts of two heterocyclic amines, 2-amino-3-methyl-9H-pyrido[2,3-b]indole (MeAaC) and 2-amino-9H-pyrido[2,3-b]indole (AaC) and identification of DNA-adducts in organs from rats dosed with MeAaC. Carcinogenesis 25, 1525–1533 [DOI] [PubMed] [Google Scholar]
- 63. Bessette E. E., Spivack S. D., Goodenough A. K., Wang T., Pinto S., Kadlubar F. F., Turesky R. J. (2010) Identification of carcinogen DNA adducts in human saliva by linear quadrupole ion trap/multistage tandem mass spectrometry. Chem. Res. Toxicol. 23, 1234–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim D., Kadlubar F. F., Teitel C. H., Guengerich F. P. (2004) Formation and reduction of aryl and heterocyclic nitroso compounds and significance in the flux of hydroxylamines. Chem. Res. Toxicol. 17, 529–536 [DOI] [PubMed] [Google Scholar]
- 65. Grigoryan H., Li H., Iavarone A. T., Williams E. R., Rappaport S. M. (2012) Cys34 adducts of reactive oxygen species in human serum albumin. Chem. Res. Toxicol. 25, 1633–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hecht S. S. (2003) Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 3, 733–744 [DOI] [PubMed] [Google Scholar]
- 67. Siraki A. G., Chan T. S., Galati G., Teng S., O'Brien P. J. (2002) N-Oxidation of aromatic amines by intracellular oxidases. Drug Metab. Rev. 34, 549–564 [DOI] [PubMed] [Google Scholar]
- 68. Neumann H. G. (2007) Aromatic amines in experimental cancer research: tissue-specific effects, an old problem and new solutions. Crit. Rev. Toxicol. 37, 211–236 [DOI] [PubMed] [Google Scholar]
- 69. Tsuneoka Y., Dalton T. P., Miller M. L., Clay C. D., Shertzer H. G., Talaska G., Medvedovic M., Nebert D. W. (2003) 4-aminobiphenyl-induced liver and urinary bladder DNA adduct formation in Cyp1a2(−/−) and Cyp1a2(+/+) mice. J. Natl. Cancer Inst. 95, 1227–1237 [DOI] [PubMed] [Google Scholar]
- 70. Noort D., Fidder A., Hulst A. G. (2003) Modification of human serum albumin by acrylamide at cysteine-34: a basis for a rapid biomonitoring procedure. Arch. Toxicol. 77, 543–545 [DOI] [PubMed] [Google Scholar]
- 71. Noort D., Fidder A., Hulst A. G., Woolfitt A. R., Ash D., Barr J. R. (2004) Retrospective detection of exposure to sulfur mustard: improvements on an assay for liquid chromatography-tandem mass spectrometry analysis of albumin-sulfur mustard adducts. J. Anal. Toxicol. 28, 333–338 [DOI] [PubMed] [Google Scholar]
- 72. Wang Z., Ramsdell J. S. (2011) Analysis of interactions of brevetoxin-B and human serum albumin by liquid chromatography/mass spectrometry. Chem. Res. Toxicol. 24, 54–64 [DOI] [PubMed] [Google Scholar]
- 73. Reistad R., Frandsen H., Grivas S., Alexander J. (1994) In vitro formation and degradation of 2-amino-1-methyl-6- phenylimidazo[4,5-b]pyridine (PhIP) protein adducts. Carcinogenesis 15, 2547–2552 [DOI] [PubMed] [Google Scholar]
- 74. Colombo G., Aldini G., Orioli M., Giustarini D., Gornati R., Rossi R., Colombo R., Carini M., Milzani A., Dalle-Donne I. (2010) Water-Soluble α,β-unsaturated aldehydes of cigarette smoke induce carbonylation of human serum albumin. Antioxid. Redox. Signal. 12, 349–364 [DOI] [PubMed] [Google Scholar]
- 75. Hackett T. L., Scarci M., Zheng L., Tan W., Treasure T., Warner J. A. (2010) Oxidative modification of albumin in the parenchymal lung tissue of current smokers with chronic obstructive pulmonary disease. Respir. Res. 11, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Colombo G., Rossi R., Gagliano N., Portinaro N., Clerici M., Annibal A., Giustarini D., Colombo R., Milzani A., Dalle-Donne I. (2012) Red blood cells protect albumin from cigarette smoke-induced oxidation. PLoS One 7, e29930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Colzani M., Aldini G., Carini M. (2013) Mass spectrometric approaches for the identification and quantification of reactive carbonyl species protein adducts. J. Proteomics 92, 28–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.