Abstract
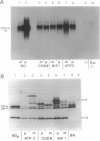
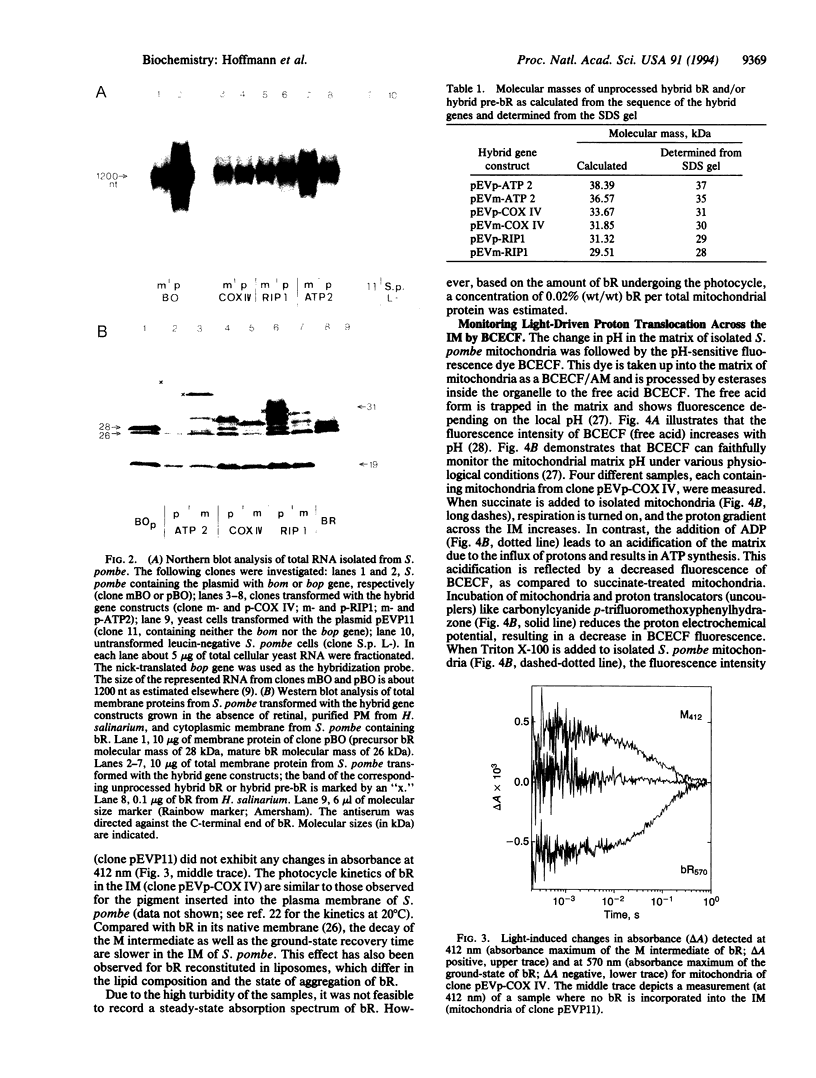
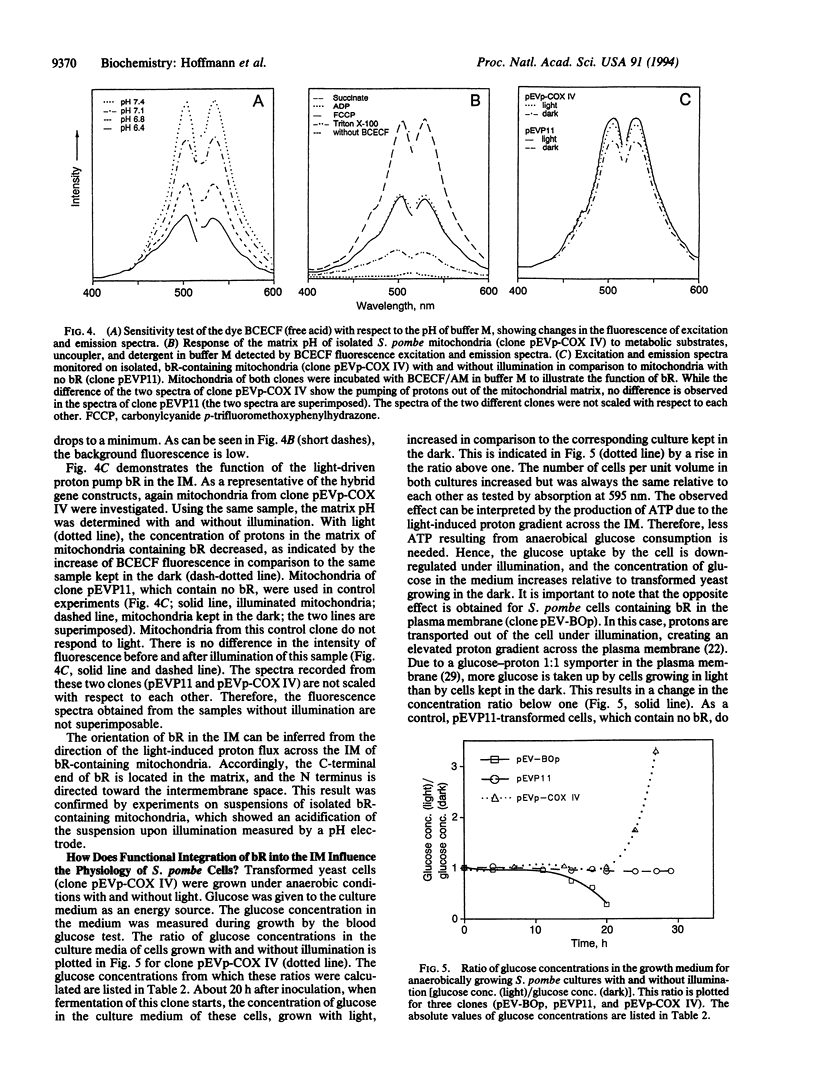
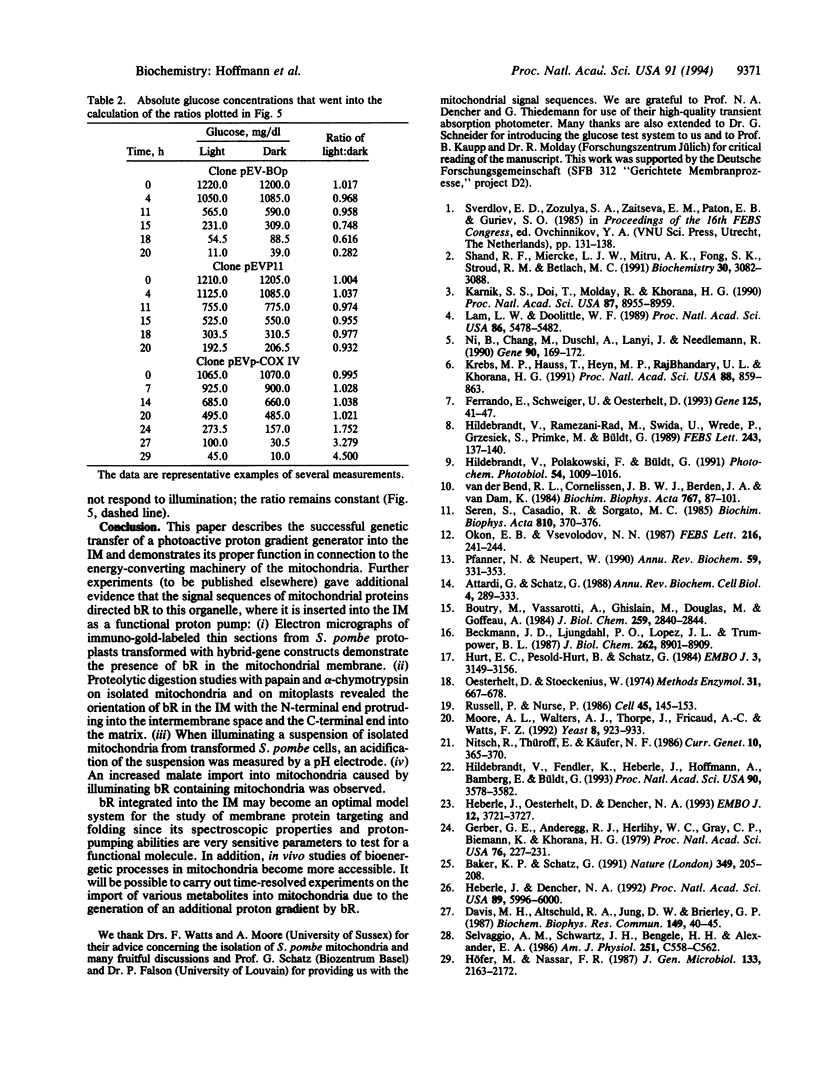
The light-driven proton pump bacteriorhodopsin (bR) from Halobacterium salinarium has been genetically transferred into the inner mitochondrial membrane (IM) of the eukaryotic cell Schizosaccharomyces pombe, where the archaebacterial proton pump replaces or increases the proton gradient usually formed by the respiratory chain. For targeting and integration, as well as for the correct orientation of bR in the IM, the bacterioopsin gene (bop) was fused to signal sequences of IM proteins. Northern and Western blot analysis proved that all hybrid gene constructs containing the bop gene and a mitochondrial signal sequence were expressed and processed to mature bR. Fast transient absorption spectroscopy showed photocycle activity of bR integrated in the IM by formation of the M intermediate. Experiments with the pH-sensitive fluorescence dye 2',7'-bis(2-carboxyethyl)-5 (and -6)-carboxyfluorescein revealed bR-mediated proton pumping from the mitochondrial matrix into the intermembrane space. Glucose uptake measurements under anaerobic conditions showed that yeast cells containing photoactive mitochondria need less sugar under illumination. In summary, our experiments demonstrate the functional genetic transfer of a light energy converter to a naturally nonphotoactive eukaryotic organism.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Baker K. P., Schatz G. Mitochondrial proteins essential for viability mediate protein import into yeast mitochondria. Nature. 1991 Jan 17;349(6306):205–208. doi: 10.1038/349205a0. [DOI] [PubMed] [Google Scholar]
- Beckmann J. D., Ljungdahl P. O., Lopez J. L., Trumpower B. L. Isolation and characterization of the nuclear gene encoding the Rieske iron-sulfur protein (RIP1) from Saccharomyces cerevisiae. J Biol Chem. 1987 Jun 25;262(18):8901–8909. [PubMed] [Google Scholar]
- Boutry M., Vassarotti A., Ghislain M., Douglas M., Goffeau A. Isolation of the structural genes for the alpha and beta subunits of the mitochondrial ATPase from the fission yeast Schizosaccharomyces pombe. J Biol Chem. 1984 Mar 10;259(5):2840–2844. [PubMed] [Google Scholar]
- Davis M. H., Altschuld R. A., Jung D. W., Brierley G. P. Estimation of intramitochondrial pCa and pH by fura-2 and 2,7 biscarboxyethyl-5(6)-carboxyfluorescein (BCECF) fluorescence. Biochem Biophys Res Commun. 1987 Nov 30;149(1):40–45. doi: 10.1016/0006-291x(87)91602-0. [DOI] [PubMed] [Google Scholar]
- Ferrando E., Schweiger U., Oesterhelt D. Homologous bacterio-opsin-encoding gene expression via site-specific vector integration. Gene. 1993 Mar 15;125(1):41–47. doi: 10.1016/0378-1119(93)90743-m. [DOI] [PubMed] [Google Scholar]
- Gerber G. E., Anderegg R. J., Herlihy W. C., Gray C. P., Biemann K., Khorana H. G. Partial primary structure of bacteriorhodopsin: sequencing methods for membrane proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):227–231. doi: 10.1073/pnas.76.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Surface-bound optical probes monitor protein translocation and surface potential changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5996–6000. doi: 10.1073/pnas.89.13.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberle J., Oesterhelt D., Dencher N. A. Decoupling of photo- and proton cycle in the Asp85-->Glu mutant of bacteriorhodopsin. EMBO J. 1993 Oct;12(10):3721–3727. doi: 10.1002/j.1460-2075.1993.tb06049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt V., Fendler K., Heberle J., Hoffmann A., Bamberg E., Büldt G. Bacteriorhodopsin expressed in Schizosaccharomyces pombe pumps protons through the plasma membrane. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3578–3582. doi: 10.1073/pnas.90.8.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt V., Ramezani-Rad M., Swida U., Wrede P., Grzesiek S., Primke M., Büldt G. Genetic transfer of the pigment bacteriorhodopsin into the eukaryote Schizosaccharomyces pombe. FEBS Lett. 1989 Jan 30;243(2):137–140. doi: 10.1016/0014-5793(89)80115-2. [DOI] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Schatz G. The amino-terminal region of an imported mitochondrial precursor polypeptide can direct cytoplasmic dihydrofolate reductase into the mitochondrial matrix. EMBO J. 1984 Dec 20;3(13):3149–3156. doi: 10.1002/j.1460-2075.1984.tb02272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik S., Doi T., Molday R., Khorana H. G. Expression of the archaebacterial bacterio-opsin gene with and without signal sequences in Escherichia coli: the expressed proteins are located in the membrane but bind retinal poorly. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8955–8959. doi: 10.1073/pnas.87.22.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs M. P., Hauss T., Heyn M. P., RajBhandary U. L., Khorana H. G. Expression of the bacterioopsin gene in Halobacterium halobium using a multicopy plasmid. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):859–863. doi: 10.1073/pnas.88.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam W. L., Doolittle W. F. Shuttle vectors for the archaebacterium Halobacterium volcanii. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5478–5482. doi: 10.1073/pnas.86.14.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. L., Walters A. J., Thorpe J., Fricaud A. C., Watts F. Z. Schizosaccharomyces pombe mitochondria: morphological, respiratory and protein import characteristics. Yeast. 1992 Nov;8(11):923–933. doi: 10.1002/yea.320081103. [DOI] [PubMed] [Google Scholar]
- Ni B. F., Chang M., Duschl A., Lanyi J., Needleman R. An efficient system for the synthesis of bacteriorhodopsin in Halobacterium halobium. Gene. 1990 May 31;90(1):169–172. doi: 10.1016/0378-1119(90)90456-2. [DOI] [PubMed] [Google Scholar]
- Nischt R., Thüroff E., Küfer N. F. Molecular cloning of a ribosomal protein gene from the fission yeast Schizosaccharomyces pombe. Curr Genet. 1986;10(5):365–370. doi: 10.1007/BF00418408. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Okon E. B., Vsevolodov N. N. Does bacteriorhodopsin energize the membranes of animal mitochondria under light? FEBS Lett. 1987 Jun 1;216(2):241–244. doi: 10.1016/0014-5793(87)80697-x. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. The mitochondrial protein import apparatus. Annu Rev Biochem. 1990;59:331–353. doi: 10.1146/annurev.bi.59.070190.001555. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986 Apr 11;45(1):145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- Selvaggio A. M., Schwartz J. H., Bengele H. H., Alexander E. A. Kinetics of the Na+-H+ antiporter as assessed by the change in intracellular pH in MDCK cells. Am J Physiol. 1986 Oct;251(4 Pt 1):C558–C562. doi: 10.1152/ajpcell.1986.251.4.C558. [DOI] [PubMed] [Google Scholar]
- Shand R. F., Miercke L. J., Mitra A. K., Fong S. K., Stroud R. M., Betlach M. C. Wild-type and mutant bacterioopsins D85N, D96N, and R82Q: high-level expression in Escherichia coli. Biochemistry. 1991 Mar 26;30(12):3082–3088. doi: 10.1021/bi00226a015. [DOI] [PubMed] [Google Scholar]