Abstract
High antibiotic resistance frequencies have become a major public health issue. The decrease in new antibiotics' production, combined with increasing frequencies of multi-drug resistant (MDR) bacteria, cause substantial limitations in treatment options for some bacterial infections. To diminish overall resistance, and especially the occurrence of bacteria that are resistant to all antibiotics, certain drugs are deliberately scarcely used—mainly when other options are exhausted. We use a mathematical model to explore the efficiency of such antibiotic restrictions. We assume two commonly used drugs and one restricted drug. The model is examined for the mixing strategy of antibiotic prescription, in which one of the drugs is randomly assigned to each incoming patient. Data obtained from Rabin medical center, Israel, is used to estimate realistic single and double antibiotic resistance frequencies in incoming patients. We find that broad usage of the hitherto restricted drug can reduce the number of incorrectly treated patients, and reduce the spread of bacteria resistant to both common antibiotics. Such double resistant infections are often eventually treated with the restricted drug, and therefore are prone to become resistant to all three antibiotics. Thus, counterintuitively, a broader usage of a formerly restricted drug can sometimes lead to a decrease in the emergence of bacteria resistant to all drugs. We recommend re-examining restriction of specific drugs, when multiple resistance to the relevant alternative drugs already exists.
Author Summary
Methods for minimizing antibiotic resistance are becoming more important as antibiotic resistance frequencies are rising, coupled with low discovery rates of new antibiotics. In this work we examined the practice of restricting specific drugs to be used only as 'last resort'. The goal of such restrictions is to maintain low resistance levels to certain drugs, and prevent the creation of bacteria resistant to all available treatment options. We used a mathematical model to study the impact of such restrictions, when some resistance to the unrestricted drugs is already present. We estimated the resistance frequencies of common bacteria from hospital data. We find that restricting drugs leads to increased rates of incorrect treatment, and might simultaneously lead to increased emergence of multidrug resistant bacteria. We conclude that restricting specific antibiotics should be done with caution. In some cases lifting restrictions might even delay MDR emergence.
Introduction
Antibiotic resistance management is one of the most pressing public health issues challenging modern medicine [1]. Resistance frequency is continually rising in abundant pathogenic bacteria [2,3], entailing higher risk for patients and increased economic costs [4,5]. Especially perilous are infections of multi-drug resistant (MDR) bacteria, which can lead to higher rates of inadequate treatment and mortality [6–8], and at times are resistant to almost all available antibiotics [9,10]. Even drugs of last resort, with relatively low prescription rate, are revealed to have increasing resistance frequencies [11,12].
The increase in antibiotic resistance frequencies is a consequence of microbial evolution and adaptation, induced by vast anthropogenic antibiotic consumption and dissemination [13]. This arms-race hinges on two main forces, acting at opposite directions: resistance acquisition and spread in bacterial populations, and new antibiotic production [14]. Seeing that rates of new antibiotics' production are steadily decreasing [15] (although efforts are made to motivate the development of new antibiotics [16,17]), we are left to consider measures to impede the emergence of new resistant, and particularly MDR, bacteria. Reducing superfluous antibiotic usage, both in human consumption and veterinary usage, is currently considered the prominent course of action [18,19].
Albeit the benefit of reducing overall superfluous antibiotic usage is easy to justify and seems to have almost no drawbacks; restricting specific antimicrobial agents involves a compromise between increasing resistance to a drug saved for the most acute scenarios, versus increasing the probability of correct treatment.
The rationale of restricting specific drugs is an evolutionary one. The use of a certain antibiotic exerts selective pressure on microbes to acquire resistance to that antibiotic. However, restricting an antibiotic does not amount to complete avoidance of its usage, as it will still be used against infections resistant to all other antibiotics [20]. Thus an increase in the frequency of bacteria resistant to the common antibiotics might result in increased usage of the restricted antibiotic as well.
Recent theoretical studies support these claims: Wu et al. have shown that maintaining variation in stockpiled influenza drugs might substantially reduce resistance [21], while Joyner et al. demonstrated the benefits of developing new antibiotics and expanding the range of possible antimicrobial treatments [22].
In this work we try to assess the conditions under which a restricted antibiotic agent should be considered for broader usage. We use a mathematical model to describe the dynamics of antibiotic resistance within a hospital unit. We model three antibiotics effective against a bacterial pathogen, and bacterial strains with all combinations of resistance to these antibiotics. We concentrate on a scenario where two antibiotics are more frequently used than a third one. Therefore resistance levels to the first two antibiotics are high, while the third antibiotic has thus far been restricted to emergency use only, and the level of resistance against it is lower. This is compatible with 'last resort' antibiotics such as Vancomycin, Linezolid and Daptomycin. We compare the efficiency of antibiotic appliance strategies where one of the antibiotics is restricted, to strategies where all drugs are equally applied. We examine the impact of antibiotics restriction in the context of a commonly used antibiotic prescription strategy: mixing, wherein each drug is prescribed to each patient with equal probability [23–25]. Several works have compared mixing and other strategies [23–27]. Here, we focus on a different question: the potential effect of restricting antibiotics on multi drug resistance within the framework of mixing. We note that similar results were obtained under a 'cycling' strategy (S5 Text).
Data obtained from Rabin medical center, Israel, is used to estimate realistic single and double antibiotic resistance frequencies in incoming patients.
Methods
Ethics statement
The study was approved by the Helsinki Committee of Rabin Medical Center.
The model
We have developed a mathematical model consisting of eight differential equations, describing the changes in the frequencies of patients infected with different bacterial strains within a hospital unit. The model is an extension of existing models, used for evaluating and predicting the efficiency of antibiotic treatment strategies [23,24,26,27]. Here we assume there are three antibiotics available for empiric treatment, rather than two, as in most classical models. This extension allows us to consider the contribution of both double and single resistant bacteria to the emergence of triple resistance. Frequencies of resistance can vary significantly between strategies and conditions, so incorporating different combinations of resistant types entails a more realistic depiction of the dynamics.
We denote by S the frequency of patients infected with bacteria susceptible to all three antibiotics; R i is the frequency of patients infected with strains resistant only to antibiotic i, for i ∊ {1, 2, 3}; R i,j is the frequency of patients infected with bacteria resistant to both antibiotics i and j, and only to these antibiotics, for (j, k) ∊ {(1, 2),(1, 3), (2, 3)}; X is the frequency of patients uninfected with any of the bacterial strains. We assume that strains resistant to all three antibiotics are not present yet, and only the rate of their emergence is estimated.
Each infected patient is immediately treated upon admission, with one antibiotic only, and is either cleared from infection or acquires resistance with rate τ. The probability of acquiring resistance to antibiotic i is p i, so that the rate of resistance acquisition upon treatment is τp i, and the rate of clearance is τ(1−p i).
For each strategy, χ i is the fraction of patients initially receiving antibiotic i.
The mixing strategy consists of the application of drugs at constant probabilities throughout time. Using only two drugs, while reserving the third drug to double resistant infections alone, will be termed mix2. Formally:. The application of all three drugs equally will be denoted mix3:.
A patient carrying an infection with a confirmed resistance to antibiotic k has to be treated with either antibiotic i or j. Such a patient will be treated with antibiotic i with probability . E.g. if a patient is found (through microbiological testing) to be resistant only to antibiotic 1, under mix2 he will receive antibiotic 2 only , while under mix3 and he will receive either antibiotic 2 or 3 with equal probability . For simplicity, we do not differentiate between patients acquiring resistance during treatment and those admitted with resistance.
An infection resistant to antibiotic i is assumed to be unaffected by treatment with it. Patients treated incorrectly—i.e., with an antibiotic that their infection is resistant to—are switched to a correct treatment after a delay period [27]. This is modeled by assigning a slower clearance rate to a proportion of the patients carrying resistant infections. The rate of recovery when the delay applies is given by τd, so that for a delay of D days, we define . Thus, for example, a fraction χ 1 of patients of class R 1 will have a recovery rate of τd while a fraction χ 1 + χ 3 of that class will have a recovery rate τ. A fraction will be treated with antibiotic 2, after a delay, and their rate of resistance acquisition will be dτp 2. γ is the rate of spontaneous recovery due to the response of the patient's immune system; β is the rate of bacterial transmissions resulting in infection (for simplicity, super-infection is neglected). Since we assume the bacterial infection considered is not the main reason of hospitalization, patients leave the hospital, or die, at a rate proportional to their frequency, with the proportion constant c. This common assumption allows us to model constant hospital occupancy.
λ V is the rate of patients of class V entering the hospital (e.g., λ S is the rate of patients admitted to the hospital with bacteria susceptible to all three antibiotics). We assume a constant population size, so that the total admission rate is equal to the discharge rate, namely:. For convenience, we also define the auxiliary parameters . f V is the frequency of incoming patients carrying a resistant pathogen of class V out of all patients carrying the pathogen, and will be used throughout this work. The model parameters, interpretations, values used and references (taken from [20,28–35]) are given at Table 1.
Table 1. Parameters, their meaning and values.
| Parameter | Meaning | Value |
|---|---|---|
| β | Rate of bacterial transmission | 0.01–0.5 day-1 [28,29] |
| pi | Probability of developing resistance to antibiotic i during treatment | 0–0.3 [30,31] |
| γ | Rate of clearance due to the patient's immune system | 0.03 day-1 |
| τ(1−pi) | Rate of clearance due to antibiotic i | day-1 [20] |
| c | Rate of patient turnover (length of stay-1) | 0.1 day-1 [28] |
| λV | Entrance rates of patients of class V | Varies (day-1 units) |
| fV | Resistance frequency of incoming patients, of the class V | Varies |
| Resistance frequency of incoming patients, of the restricted antibiotic (antibiotic 3) | 0.001 [32,33] | |
| d | Delay of correct treatment factor | 0.778 [34] |
| π | Hospital size relative to community | [35] |
| Χi | Fraction of patients initially treated with antibiotic i | Determined by strategy |
| Fraction of patients treated with antibiotic i, for which resistance to antibiotic k ≠ i, j was established. | Varies | |
| t1–t0 | Duration of time the dynamics are tested for. | 20–100 years |
The full model is comprised of the following differential equations (for i ∊ {1, 2, 3},(j, k) ∊ {(1, 2),(1, 3), (2, 3)}, i ≠ j ≠ k):
| (1) |
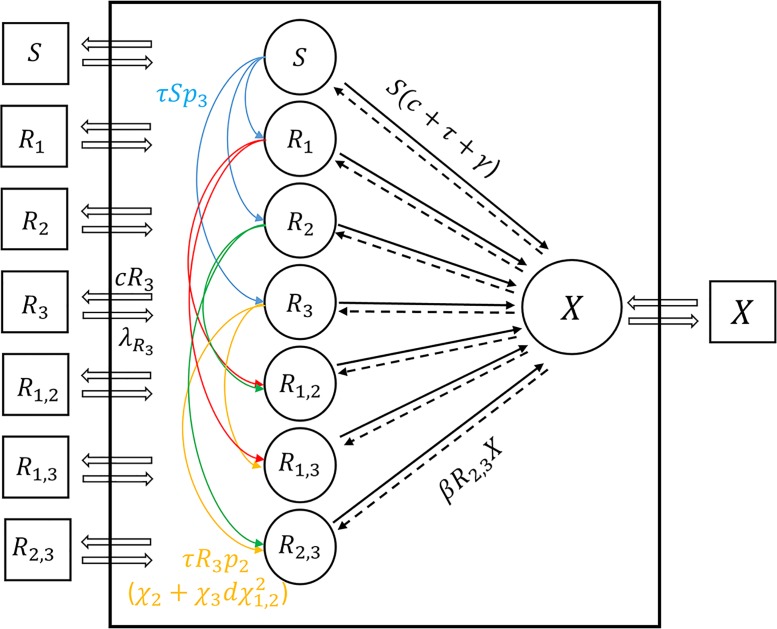
An illustration of the dynamics is given in Fig 1.
Fig 1. An illustration of the dynamic system presented in Eq 1.
The black frame represents the hospital, with hollowed arrows signifying patients moving in and out between the hospital and the community; circles representing patient frequencies within the hospital, and squares representing the frequencies of patients infected with bacterial strains in the community, with respect to variable names within the shapes (see methods). Colored arrows show the direction of resistance acquisition due to treatment; solid black arrows are recovery from infected to cleared states, while dashed lines are infections. Several arrows are marked with the corresponding rates of flow between variables.
We derived an analytical approximation of the equilibrium state of (Eq 1) (see S1 Text for the analysis and agreement between the approximation and the numerical solution of the equilibrium).
We note that our model can be reduced to a two-drug model, if all the parameters and variables pertaining to double resistance and to resistance to the third drug are set to zero. We can model a strategy where only the first drug is used, except for treatment of resistant infections, and two alternative strategies of mixing and cycling both drugs (see supplementary material III).
Adding community feedback
We also include in our model the impact of different strategies on resistance rates in the community. Given that the mean patient length of stay is days, then on average a fraction c of the patients leave (and enter) each day. We will denote the ratio of the hospital size to the size of the community it serves as π. The number of hospital beds per capita can serve as an estimate for π, and the estimate is usually within the range , with for developed countries [35].
We assume that the total incidence of infection by the modeled pathogen is constant and model only the change in the distribution of different types of resistant infections within the community.
This is modeled by changing the frequencies of different incoming patients (which are proportional to the entrance rates) from constant in (Eq 1) to dynamic. We add to (Eq 1) the following ODEs:
For V ∊ {S, R 1, R 2, R 3, R 1,2, R 1,3, R 2,3}.
Thus the entrance rates of all infected patients () become dynamic and are affected by the efflux of the various strains to the community. The chosen method of modeling assumes that the discharged patients are replacing patients in the community (which are admitted into the hospital) at rate c, with the weight of each discharged patient, relative to the community, being π. Although models explicitly computing the dynamics in the community exist [36], we chose a simplified model that captures the main component, of varying strength of influence of the hospital on the community (π).
Measuring the strategies' efficiency
We employ two criteria to measure the efficiency of each treatment strategy:
First, we measure the mean frequency of incorrectly treated patients. This measure was chosen since inadequate treatment leads to higher mortality, in single-resistant and MDR bacteria [6,37,38]. The mean frequency of initially incorrectly treated patients under a strategy σ ∊ {mix2, mix3}, for i ∊ {1, 2, 3},(j, k) ∊ {(1, 2),(1, 3), (2, 3)}, i ≠ j ≠ k), is:
Where t 0, t 1 are the times of beginning and end of measuring, respectively. Second, we measure the emergence rate of triple resistant bacteria. We estimate triple resistance emergence as proportional to the number of double resistant infections treated with the only antibiotic they are susceptible to. Therefore, the mean emergence rate of triple resistance under a treatment strategy σ is proportional (with the proportion constant being the clearance rate) to:
We used several methods to decide which parameters are most influential in our system. First, we used the analytical approximation of the equilibrium state of the ODE system without the community feedback to see the influence of various parameters on the system (S1 Text). In addition, we performed a parameter sensitivity analysis (PSA) of the system (S4 Text). The result of the PSA suggested that the resistance frequencies of incoming patients are the most influential parameters on the dynamics. We thus focus on these parameters and use estimates from clinical data for their values. We also performed pairwise comparisons of parameters to determine the effect of their interaction on the emergence of triple resistance (S2 Text).
To further investigate the implications of our model, we estimated realistic resistance frequencies of incoming patients, using data gathered from patients in Rabin medical center, Israel, dating 2009–2012, that had positive blood cultures for bacteremia. We used antibiotic resistance profiles of blood cultures obtained up to 48 hours after patient admission, to avoid estimating resistant infections acquired within the hospital. Antibiotics co-occurring in resistance tests were divided to all possible pairs, and frequencies of single and double resistance were estimated (corresponding to f 1, f 2 and f 1,2). Only observations with ≥50 instances of testing for resistance of both antibiotics were considered, resulting in 73 pairs of antibiotics (see S1 Table).
Results
We concentrate on a scenario in which two antibiotics (in our model, antibiotics 1 and 2) are frequently used, and resistance levels to them are not low. In contrast, antibiotics 3 is assumed to have thus far been restricted for emergency use only, and the frequency of resistance to it is still low. We examine the change prompted by enabling the broad usage of antibiotic 3 under the mixing strategy.
The mixing strategy can be thought of as the default strategy applied in hospitals, when physicians are prescribing antibiotics from a predetermined set, with no specific time-dependent preference. We use our mathematical model to examine the effect of mixing the three antibiotics available versus mixing only two and restricting the third (mix3 and mix2, respectively).
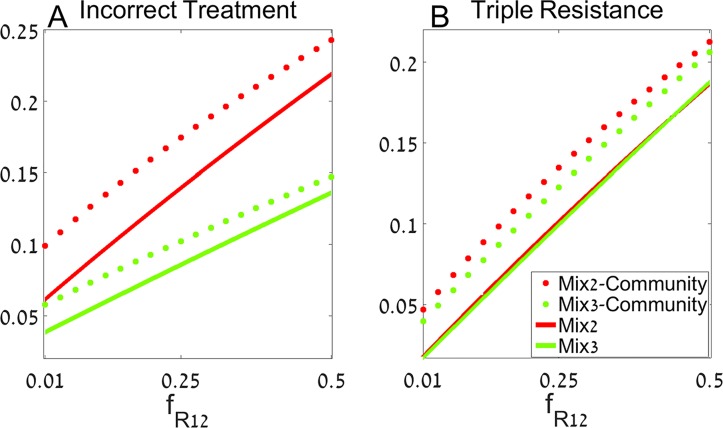
We start by examining the frequency of double and single resistance of incoming patients' infections, with respect to the commonly used antibiotics ( and , respectively). Whereas resistances to different antibiotics might be independent (), selection for resistance could drive the double resistance frequencies to higher values than expected by random. In Fig 2 we set the values of single resistance frequency to , while varying on the horizontal axis.
Fig 2. The mixing strategy for varying double resistance frequencies.
We measure the fraction of incorrect treatment (A), and the rate of triple resistance emergence (B) for varying levels of double resistance to the commonly used antibiotics (). Red curves represent the results under mixing 2 antibiotics and restricting antibiotic 3 (mix2), while green curves are the results under mixing 3 antibiotics (mix3). Dotted and solid lines are the results of the model with and without community feedback, respectively. Parameters are , and the rest are given at Table 1. The system is simulated for 20 years and other parameter values are given in the text.
The vertical axis presents the mean fraction of incorrectly treated patients (Fig 2A), and mean triple resistance emergence rate (Fig 2B) over 20 years. We plot the results of the model assuming no effect of hospital resistance frequencies on the community (solid lines) and of the model with community feedback (dotted lines). We set the double resistance frequencies for the third restricted antibiotic to , as a conservative estimation. Similar results are obtained when resistance to antibiotic 3 is assumed to be independent of resistance to antibiotics 1 and 2. Other parameters are . References for the parameter values used are given in Table 1.
We can see that introducing the previously restricted drug to common usage decreases the prevalence of incorrect treatment in our model, whether regarding community feedback or not (Fig 2A). This is intuitive, as a relatively small fraction of the incoming patients is resistant to antibiotic 3, and thus using it leads to a decreased probability of incorrect treatment.
When considering the emergence of triple resistance, the picture is more complex. On the one hand, the unrestricted usage of the antibiotic 3 limits the spread of double resistance, most common of which is resistance to antibiotics 1 and 2. On the other hand, more frequent usage of antibiotic 3 causes more patients to acquire resistance to it during treatment. This can be seen when examining the triple resistance emergence (Triple(σ)). If we assume that resistance to both common drugs is significantly higher than resistance to the third drug, then Triple(σ) is dominated by the term:
The homogeneity in time of the mixing strategies allows for a simple analysis. E(σ) under mix2 becomes
while for mix 3 it becomes
Thus the condition for reducing triple resistance emergence under mix3 is approximately E(mix2) > E(mix3), which implies that triple resistance is lower under mix3 at a time point t if
| (2) |
Thus mix3 improves as switching to a correct treatment is done faster. Had patients been immediately treated with an effective drug (d = 1), the strategy best minimizing R 1,2 would have reduced triple resistance emergence. When a delay occurs, mix3 will have to reduce R 1,2 by a factor of , relative to mix2. This is a lower bound (i.e. mix3 will have to reduce double resistance more than the mentioned factor) since the neglected terms (R 1,3, R 2,3) are higher under mix3 than under mix2. Furthermore, under mix3 R 1,3, R 2,3 might increase with time due to selective pressure, leading to a substantial underestimation of the bound in (Eq 2).
We should note that if one strategy is able to decrease both incorrect treatment and triple resistance emergence more efficiently than another, then this strategy should be preferred. However, when one strategy is only better at reducing incorrect treatment, while producing higher triple resistance, the costs and benefits of the two effects should be considered.
Furthermore, we examined the effects of various parameters on the emergence of triple resistance under mix3 relative to mix2 (S4 Text). We note that triple resistance emergence can be decomposed to two main components: the resistance emanating from the common double resistant strain (R 1,2), mainly affected by infection; and from the rare double resistant infections (R 1,3 + R 2,3), which are usually a result of resistance acquisition. Typically, mix3 decreases R 1,2 and increases R 1,3 + R 2,3, in comparison to mix2.
The parameters can act in opposing ways on these two terms.
High transmission rates (β) increase the effectiveness of mix3, since the main advantage of using the restricted drug is inhibiting the spread of R 1,2 (Fig A in S4 Text). The opposite is true for the patient turnover rate (c): high turnover limits the potential time for transmission, and requires higher transmission rates for R 1,2 to be substantial and for mix3 to be effective (Fig C in S4 Text). High antibiotic clearance rates (τ) increase the advantage of mix3, under which R 1,2 patients have a higher probability of being correctly treated (Fig A and B in S4 Text. Similar results are obtained when the rate of clearance τ varies while the rate of acquisition τp i is kept constant: Fig E in S4 Text). These parameters act similarly on R 1,3 + R 2,3, but the relative weight of R 1,2 is usually higher than that of R 1,3 + R 2,3. Thus high transmission rates, low turnover rates, and high clearance rates are conditions where restriction might be inefficient for reducing triple resistance emergence. However, antibiotic clearance rates also have a strong interaction with the delay of correct treatment (1/d). When the delay of treatment is short but realistic—about two days ()—mix3 is more efficient than mix2 even for low clearance rates (Fig B in S4 Text). This corresponds to the result obtained above in (Eq 2).
The probabilities of resistance acquisition (p 1, p 2, p 3) also play a role in the dynamics' outcome. As expected, mix3 will be less effective if resistance to antibiotic 3 is easily acquired—high p 3 relative to p 1, p 2 (Fig D in S4 Text).
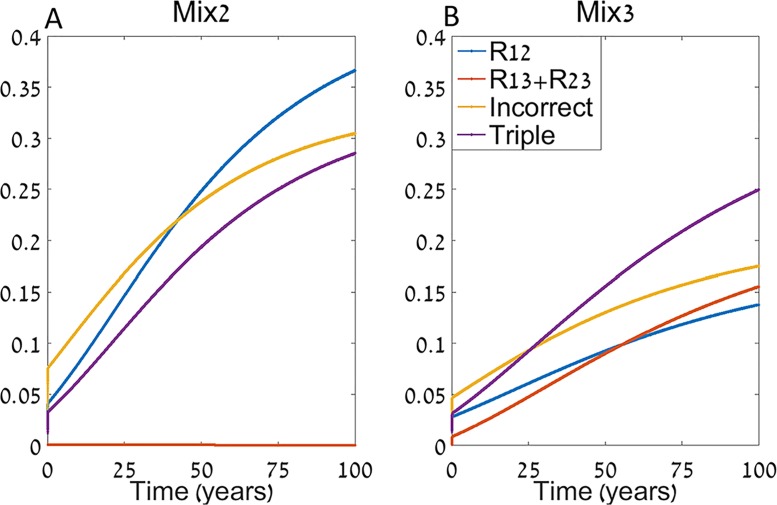
Another important factor that can affect the hospital dynamics is the feedback from the community. In Fig 3 we again decompose the frequency of resistant strains to R 1,2 and R 1,3 + R 2,3, and compare the levels of incorrect treatment and triple resistance emergence for mix2 (Fig 3A) and mix3 (Fig 3B).
Fig 3. Time series of mix2 (A) and mix3 (B).
We plot the frequencies of double resistant infections resistant to the third antibiotic (R 1,3 + R 2,3), the double resistant infections resistant to the two commonly used antibiotics (R 1,2), the measured incorrectly treated patients, and the emergence of triple resistance. The model is simulated for an extended period of time (100 years) to capture long term effects and the rest of the parameters are as in Fig 2.
The parameters used are as in Fig 2, with f 1,2 = 0.5 f 1. One might presume that mix3 will be inefficient in the long term, as it results in increased R 1,3 + R 2,3 over time. In contrast, the dynamics show that the rapid increase of R 1,2 under mix2 might be a more serious pitfall under these parameters, and the sum of all double resistant infections is lower under mix3 than under mix2. As a result mix3 stays more efficient than mix2 for extended periods of time. Decreasing the number of hospital beds per capita relative to the community (π) reduces this effect, with π = 0 equivalent to the model without community.
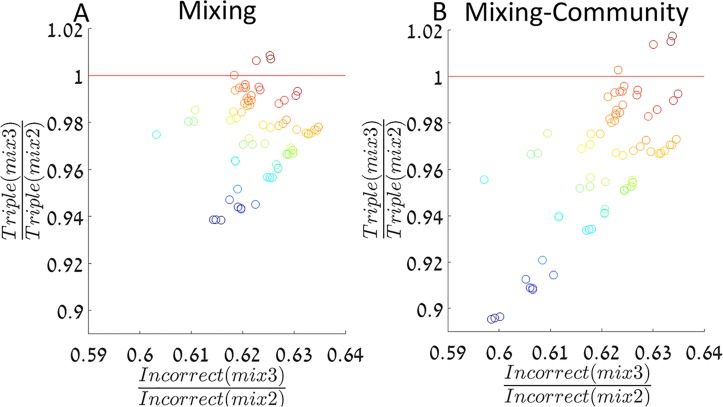
To further investigate the implications of our model, we estimated single and double resistance frequencies of the common drugs (f 1, f 2 and f 1,2) from patients in Rabin medical center, Israel, dating 2009–2012 (see Methods). We simulated our model using the estimated resistance frequencies among incoming patients, with the rest of the parameters as in Fig 2. Fig 4 presents the ratios of incorrectly treated patients and triple resistance emergence, in mix3 relative to mix2, for both the model without community feedback (Fig 4A) and with community feedback (Fig 4B).
Fig 4. The mixing strategy for estimated resistance frequencies.
For each data point, its location on the X axis represents the predicted ratio of incorrect treatment under mix3 relative to mix2 according to our model, and its location on the Y axis represents the predicted ratio of triple resistance emergence. A red line is drawn where the strategies inhibit triple resistance equally well, so below the line mix3 reduces both incorrect treatment and triple resistance emergence more efficiently than mix2. Panels A and B present the results of the models without community feedback and with it, respectively. Antibiotic resistance frequencies among incoming patients are estimated from data (see Methods and S1 Table). The color indicates the estimated resistance frequencies to the common antibiotics (). The system is simulated for 20 years and the rest of the parameters are as in Fig 2.
The colors represent the sum of the estimated resistances in each sample (). We can see that mix3 reduces incorrect treatment by ~40% relative to mix2 (Fig 4, horizontal axis), and has contrasting effects on triple resistance emergence: Albeit mix3 increases triple resistance emergence rate by about 1% for some samples, it usually reduces it by up to 10%, relative to mix2 (Fig 4, vertical axis). We see that under low levels of resistance among the infections of incoming patients, mix3 is strictly preferred over mix2—it reduces both triple resistance emergence and incorrect treatment. When higher levels of resistance are present, the dynamics may change so that refraining from treating with antibiotic 3, except when the other two antibiotics fail, decreases triple emergence. This is once again the trade-off between effective short term treatment and the risk of creating new MDR bacteria. We can also see that both ratios of incorrect treatment and triple resistance emergence rates tend to be smaller, indicating a stronger preference for mix3, when the hospital population has an effect on the community (Fig 4, compare panel A to panel B). This occurs due to the quick rise of R 1,2 under mix2, and its influence on the community resistance, which is moderated under the more diverse treatment of mix3.
Discussion
In this work we examined whether restricting the usage of certain antibiotic agents, i.e. keeping them as 'last resorts', is effective in inhibiting the spread of MDR bacteria. We developed a model describing hospital dynamics of a bacterial pathogen potentially treated by three available antibiotics. We assumed that the pathogen is treated by two commonly used drugs, and a third drug is only used when an infection is resistant to both common drugs. We examined the scenario where the common drugs have been used for a long enough period, so that resistance to each of the drugs, and double resistance to both of them, is not very uncommon. We tested the efficiency of restricting versus using the third drug extensively, for a common antibiotic usage strategy: mixing. The criteria for efficiency were defined as the mean number of incorrectly treated patients, and the rate of triple resistance emergence. We considered dynamic resistance frequencies in the community, affected by patients discharged from the hospital, as well as constant frequencies.
We conclude that introducing the previously restricted antibiotic to broad usage always decreases incorrect treatment, and can even simultaneously reduce the emergence of triple resistance sometimes.
A similar question can be asked considering only two antibiotics; one commonly used and one restricted. In such a simple model, the usage of two antibiotics will decrease the level of incorrect treatment, and under some conditions also the resistance emergence (see S3 Text). However, the simple model does not apply when double resistant strains are already present, as is the case for many antibiotics (see S1 Table). One major difference between the models is the rate of conversion from the sensitive type to the multiply resistant type. More steps of resistance acquisition are required for multiple resistance in the 3-antibiotics model, and treatment strategies using all drugs or restricting some of them may affect the intermediate resistant types differently.
However, an important insight to be gained from the simple model is that infections can be crudely separated to resistant and sensitive to the restricted drug. This decomposition was found useful in analyzing the influence of the model parameters on the emergence of triple resistance.
We found that when bacterial transmission is high, restricting antibiotics is usually not the preferred strategy. Long hospitalization times have a similar effect, and if bacterial transmission is indeed substantial, restricting antibiotics may not be advisable if the patients' mean length of stay is above several days.
Additionally, if antibiotic clearance times are intermediate (less than weeks), or even low but the switch to correct treatment is rapid (less than two days), restricting antibiotics also performs poorly.
We extended our model and considered the interaction between the hospital and the community: where resistance frequencies in the community are affected by the efflux of resistant infections discharged from the hospital, and resistance frequencies among incoming patients are affected by resistance in the community. The weight of the community in the emergence of MDR is hard to determine. The cost of resistance, for instance, could cause a reversion of resistance in bacterial populations that were not recently exposed to a certain antibiotic. As a consequence, the community resistance frequencies would be less influenced by the patient efflux, which is equivalent to low effect of the hospital on the community in our model. However, the significance of the cost of resistance is debatable and perhaps reversion to susceptibility is not often feasible in relevant time scales [39,40].
Therefore, we examined the dynamics with both a relatively high and a negligible influence of the hospital on the community. The community feedback tended to decrease the efficiency of restricting antibiotics. The high usage of only two drugs gave rise to a steady increase in double resistant strains, in the hospital as well as in the community, inevitably treated with the restricted drug and increasing the emergence of triple resistance. Finally, we simulated our model using incoming single and double resistance frequencies estimated from data. Again, restricting antibiotics was usually the less efficient strategy, both with and without the community feedback.
Interestingly, it was recently shown that hospital size affects resistance frequencies [41]. Small hospitals may facilitate extinction of rare resistant strains, and can have substantial effect on the rare infections resistant to the third drug. However, this phenomenon may be mitigated by the resistant strains in the community, and entails further attention.
We focus on mixing a prominent antibiotic prescription strategy, but other treatment strategies may also be considered. Similar results were obtained when running our analyses on the cycling strategy, where drugs are periodically switched every 100 days (S5 Text). Another example is combination therapy, in which patients are treated with several drugs simultaneously. Our model is applicable for this strategy if each of the drugs in the restricted combination have not been abundantly used in the formerly prescribed combinations. To some extent, single drugs in our model (i.e. antibiotics 1, 2, and 3) can be used to describe combinations of drugs, with the addition of a term for partial resistance, due to the overlap of drugs between different combinations. However, the efficiency and toxicity of combination therapy are still a subject of controversy [42]. Moreover, we have previously shown that combination therapy might increase MDR significantly if genetic variation rates are stress-induced [26], suggesting that its use should be carefully considered in such cases.
One could claim that last resort drugs should be reserved for severe infections, to minimize resistance in high risk patients. In contrast, we show that although the usage of the restricted drug can increase its marginal resistance levels, the probability of getting infected by bacteria resistant to all drugs is also decreased. Thus, severe infections with limited treatment options are also minimized.
Our results suggest that the current frequency of resistance to alternative drugs is an important component to be considered when deciding upon the restriction or usage of an antibiotic. Since resistance frequencies can substantially vary between communities [43,44], a sound local estimate of them might be need. This could be obtained by analyzing the records of resistance screens performed on incoming patients, often already available at no additional cost, and perhaps also by sampling commensal bacteria, whose resistance pattern are correlated with the resistance pattern of the infecting bacteria [45]. Analysis of local resistance frequencies could contribute to properly deciding whether to restrict or broadly use certain antibiotics, as well as to monitor and estimate the efficiency of this decision. Moreover, such data has also been proposed for 'informed' strategies: dynamically changing the proportions of drugs prescribed as a function of recent resistance instances within the hospital [46]. Incorporating restricted drug usage with these strategies might lead to even better results at minimizing resistance emergence.
In conclusion, we show that restricted usage of certain drugs, when resistance to the alternative drugs is already present, may sometimes hinder the fight against MDR bacteria. We would like to emphasize that we do not encourage superfluous or increased usage of antibiotics, but rather suggest that changing the proportions of currently used drugs may assist the fight against antibiotic resistance. We hope that our work will promote clinical studies and empirical assessment of the benefits and pitfalls of such a change in treatment policies.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We wish to thank Ranit Aharonov for many helpful comments and suggestions.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research has been supported in part by the Israeli Science Foundation 1568/13 (LH), and by a fellowship from the Manna Program in Food Safety and Security (UO). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, et al. (2013) Antibiotic resistance—the need for global solutions. The Lancet infectious diseases 13: 1057–1098. 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 2. Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, et al. (2012) Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clinical Microbiology and Infection 18: 413–431. 10.1111/j.1469-0691.2012.03821.x [DOI] [PubMed] [Google Scholar]
- 3. Kraker M, Jarlier V, Monen J, Heuer O, Sande N, et al. (2013) The changing epidemiology of bacteraemias in Europe: trends from the European Antimicrobial Resistance Surveillance System. Clinical Microbiology and Infection 19: 860–868. 10.1111/1469-0691.12028 [DOI] [PubMed] [Google Scholar]
- 4. Maragakis LL, Perencevich EN, Cosgrove SE (2008) Clinical and economic burden of antimicrobial resistance. 10.1586/14787210.6.5.751 [DOI] [PubMed] [Google Scholar]
- 5. De Kraker ME, Davey PG, Grundmann H, Group BS (2011) Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: estimating the burden of antibiotic resistance in Europe. PLoS medicine 8: e1001104 10.1371/journal.pmed.1001104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peralta G, Sánchez MB, Garrido JC, De Benito I, Cano ME, et al. (2007) Impact of antibiotic resistance and of adequate empirical antibiotic treatment in the prognosis of patients with Escherichia coli bacteraemia. Journal of antimicrobial chemotherapy 60: 855–863. [DOI] [PubMed] [Google Scholar]
- 7. Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y (2006) Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrobial agents and chemotherapy 50: 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nseir S, Di Pompeo C, Cavestri B, Jozefowicz E, Nyunga M, et al. (2006) Multiple-drug-resistant bacteria in patients with severe acute exacerbation of chronic obstructive pulmonary disease: Prevalence, risk factors, and outcome*. Critical care medicine 34: 2959–2966. [DOI] [PubMed] [Google Scholar]
- 9. McGowan JE Jr (2006) Resistance in nonfermenting gram-negative bacteria: multidrug resistance to the maximum. The American journal of medicine 119: S29–S36. [DOI] [PubMed] [Google Scholar]
- 10. Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, et al. (2010) Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. The Lancet infectious diseases 10: 597–602. 10.1016/S1473-3099(10)70143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nature medicine 10: S122–S129. [DOI] [PubMed] [Google Scholar]
- 12. Leeb M (2004) Antibiotics: a shot in the arm. Nature 431: 892–893. [DOI] [PubMed] [Google Scholar]
- 13. Rodríguez-Rojas A, Rodríguez-Beltrán J, Couce A, Blázquez J (2013) Antibiotics and antibiotic resistance: a bitter fight against evolution. International Journal of Medical Microbiology 303: 293–297. 10.1016/j.ijmm.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 14.McClure NS, Day T (2014) A theoretical examination of the relative importance of evolution management and drug development for managing resistance. Proceedings of the Royal Society B: Biological Sciences 281: 20141861. [DOI] [PMC free article] [PubMed]
- 15. Shlaes DM, Sahm D, Opiela C, Spellberg B (2013) The FDA Reboot of Antibiotic Development. Antimicrobial agents and chemotherapy 57: 4605–4607. 10.1128/AAC.01277-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boucher HW, Talbot GH, Benjamin DK, Bradley J, Guidos RJ, et al. (2013) 10×'20 Progress—development of new drugs active against gram-negative bacilli: an update from the infectious diseases society of america. Clinical infectious diseases 56: 1685–1694. 10.1093/cid/cit152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. POLICY IP (2010) The 10 ‘20 initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clinical Infectious Diseases 50: 1081–1083. 10.1086/652237 [DOI] [PubMed] [Google Scholar]
- 18. Singer RS, Finch R, Wegener HC, Bywater R, Walters J, et al. (2003) Antibiotic resistance—the interplay between antibiotic use in animals and human beings. The Lancet infectious diseases 3: 47–51. [DOI] [PubMed] [Google Scholar]
- 19. Wellington EM, Boxall A, Cross P, Feil EJ, Gaze WH, et al. (2013) The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria. The Lancet infectious diseases 13: 155–165. 10.1016/S1473-3099(12)70317-1 [DOI] [PubMed] [Google Scholar]
- 20.Medicine JH (2013) Antibiotic Guidelines 2013–2014. http://www.hopkinsmedicine.org/amp/guidelines/Antibiotic_guidelines.pdf.
- 21. Wu JT, Leung GM, Lipsitch M, Cooper BS, Riley S (2009) Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy. PLoS medicine 6: e1000085 10.1371/journal.pmed.1000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joyner ML, Manning CC, Canter BN (2012) Modeling the effects of introducing a new antibiotic in a hospital setting: A case study. Mathematical biosciences and engineering: MBE 9: 601–625. 10.3934/mbe.2012.9.601 [DOI] [PubMed] [Google Scholar]
- 23. Bonhoeffer S, Lipsitch M, Levin BR (1997) Evaluating treatment protocols to prevent antibiotic resistance. Proceedings of the National Academy of Sciences 94: 12106–12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lipsitch M, Bergstrom CT, Levin BR (2000) The epidemiology of antibiotic resistance in hospitals: paradoxes and prescriptions. Proceedings of the National Academy of Sciences 97: 1938–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergstrom CT, Lo M, Lipsitch M (2004) Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proceedings of the National Academy of Sciences of the United States of America 101: 13285–13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Obolski U, Hadany L (2012) Implications of stress-induced genetic variation for minimizing multidrug resistance in bacteria. BMC medicine 10: 89 10.1186/1741-7015-10-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. zur Wiesch PA, Kouyos R, Abel S, Viechtbauer W, Bonhoeffer S (2014) Cycling empirical antibiotic therapy in hospitals: meta-analysis and models. PLoS pathogens 10: e1004225 10.1371/journal.ppat.1004225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Austin DJ, Bonten MJ, Weinstein RA, Slaughter S, Anderson RM (1999) Vancomycin-resistant enterococci in intensive-care hospital settings: transmission dynamics, persistence, and the impact of infection control programs. Proceedings of the National Academy of Sciences 96: 6908–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooper B, Lipsitch M (2004) The analysis of hospital infection data using hidden Markov models. Biostatistics 5: 223–237. [DOI] [PubMed] [Google Scholar]
- 30. Fish DN, Piscitelli SC, Danziger LH (1995) Development of resistance during antimicrobial therapy: a review of antibiotic classes and patient characteristics in 173 studies. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 15: 279–291. [PubMed] [Google Scholar]
- 31. Milatovic D, Braveny I (1987) Development of resistance during antibiotic therapy. European journal of clinical microbiology 6: 234–244. [DOI] [PubMed] [Google Scholar]
- 32. Ross JE, Anderegg TR, Sader HS, Fritsche TR, Jones RN (2005) Trends in linezolid susceptibility patterns in 2002: report from the worldwide Zyvox Annual Appraisal of Potency and Spectrum Program. Diagnostic microbiology and infectious disease 52: 53–58. [DOI] [PubMed] [Google Scholar]
- 33. Tamma PD, Robinson GL, Gerber JS, Newland JG, DeLisle CM, et al. (2013) Pediatric Antimicrobial Susceptibility Trends across the United States. Infection Control and Hospital Epidemiology 34: 1244–1251. 10.1086/673974 [DOI] [PubMed] [Google Scholar]
- 34. Andrews JM, Testing BWPoS (2001) BSAC standardized disc susceptibility testing method. Journal of Antimicrobial Chemotherapy 48: 43–57. [DOI] [PubMed] [Google Scholar]
- 35. http://data.worldbank.org/indicator/SH.MED.BEDS.ZS. Last Accessed 10.05.2014.
- 36. Cooper B, Medley G, Stone S, Kibbler C, Cookson B, et al. (2004) Methicillin-resistant Staphylococcus aureus in hospitals and the community: stealth dynamics and control catastrophes. Proceedings of the National Academy of Sciences of the United States of America 101: 10223–10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, et al. (2003) Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. The American journal of medicine 115: 529–535. [DOI] [PubMed] [Google Scholar]
- 38. Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH (2000) The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest Journal 118: 146–155. [DOI] [PubMed] [Google Scholar]
- 39. Andersson DI, Hughes D (2010) Antibiotic resistance and its cost: is it possible to reverse resistance? Nature Reviews Microbiology 8: 260–271. 10.1038/nrmicro2319 [DOI] [PubMed] [Google Scholar]
- 40. Björkman J, Nagaev I, Berg O, Hughes D, Andersson DI (2000) Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287: 1479–1482. [DOI] [PubMed] [Google Scholar]
- 41. Kouyos RD, zur Wiesch PA, Bonhoeffer S (2011) On being the right size: the impact of population size and stochastic effects on the evolution of drug resistance in hospitals and the community. PLoS pathogens 7: e1001334 10.1371/journal.ppat.1001334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Paul M, Silbiger I, Grozinsky S, Soares-Weiser K, Leibovici L (2014) Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev 1. [DOI] [PubMed] [Google Scholar]
- 43. García-Rey C, Aguilar L, Baquero F, Casal J, Dal-Ré R (2002) Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. Journal of clinical microbiology 40: 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Alpuche C, Garau J, Lim V (2007) Global and local variations in antimicrobial susceptibilities and resistance development in the major respiratory pathogens. International journal of antimicrobial agents 30: 135–138. [DOI] [PubMed] [Google Scholar]
- 45. Obolski U, Alon D, Hadany L, Stein GY (2014) Resistance profiles of coagulase-negative staphylococci contaminating blood cultures predict pathogen resistance and patient mortality. Journal of Antimicrobial Chemotherapy: dku156. [DOI] [PubMed] [Google Scholar]
- 46. Kouyos RD, zur Wiesch PA, Bonhoeffer S (2011) Informed switching strongly decreases the prevalence of antibiotic resistance in hospital wards. PLoS computational biology 7: e1001094 10.1371/journal.pcbi.1001094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.