Abstract
Introduction:
The aim of this study is to evaluate the association of vitamin D deficiency and pelvic floor disorders (PFD) including pelvic organ prolapse (POP) and stress urinary incontinence in postmenopausal women.
Materials and Methods:
This is a prospective case control study on 120 women with or without symptoms of PFD. Relevant history and clinical examination were conducted. Serum 25-hydroxy vitamin D levels were measured in all women. Chi square and student t test were used to test significance of association. Logistic regression was used to adjust for age. Odds ratios and 95% confidence intervals were calculated.
Results:
Of the 120 postmenopausal women included, 51 had PFD on clinical examination. Of the 51 cases, 28 women had POP and 14 women had stress incontinence (SUI) while nine women had both POP and SUI. The study showed that vitamin D levels were significantly lower in women with PFD than those without PFD. Menopausal status of more than 5 years was also significantly associated with PFD.
Conclusion:
Findings suggest association of vitamin D deficiency and PFD in postmenopausal women. In addition, postmenopausal women have a high prevalence of vitamin D deficiency indicating a need to evaluate vitamin D levels in these women.
Keywords: Pelvic floor disorders, pelvic organ prolapse, postmenopausal women, stress urinary incontinence, vitamin D deficiency
INTRODUCTION
Pelvic floor disorder (PFD) is a major clinical problem in postmenopausal women.
It is a group of disorders, which includes pelvic organ prolapse (POP), urinary incontinence (UI) and fecal incontinence (FI).[1] These can occur alone or in combination. The associated symptoms are vaginal bulge or protrusion, involuntary leakage of urine, flatus or feces.[2] The overall prevalence of PFD in the non-pregnant women is 24%,[2] which almost doubles as the age of the women increases, with prevalence being 36-49% after 60 years of age.[2,3,4] The various factors contributing to PFD include multiparity, mode of delivery, instrumental delivery, obesity, collagen defects with menopause being well-known risk factors.[1,5,6] It is well known by many epidemiological studies that age is one of the important causes for PFD. This may be due to the age-related changes in the neuromuscular function and connective tissue changes in the elderly women.[2] Recent literature has suggested the role of vitamin D deficiency as contributory to PFD. This prospective case control study intended to evaluate the possible association of vitamin D deficiency and PFD including POP and UI in postmenopausal women attending a tertiary care facility in south India.
MATERIALS AND METHODS
This study was a prospective case control study done in the Department of Obstetrics and Gynecology, in a tertiary care center, South India. The study was approved by the IRB and Ethics committee. Postmenopausal women attending the outpatient clinic were invited to participate in the study. The nature of the study was explained and written informed consent was obtained.
One hundred and twenty postmenopausal women with and without symptoms of POP and stress urinary incontinence were recruited. Women with chronic renal illness, previous vitamin D supplementation, non-ambulant chronically bedridden patients were excluded from the study.
A detailed history included educational status, socioeconomic background, job status, and sunlight exposure was recorded. Obstetric history included parity, age at first delivery, mode of delivery, interval between deliveries, institutional/ home delivery. Other relevant details including associated medical illness, prior abdominal/vaginal surgeries were documented. The calcium intake was calculated from daily milk intake and calcium supplementation history and not from the complete dietary history. The body mass index was calculated. Pelvic examination was done to diagnose POP, which was quantified using the POP quantification system. Urinary leak was demonstrated using the supine stress test and also in standing position.
Venous sample was taken in a clotted tube for serum vitamin D analysis at the time of recruitment. 25-hydroxy vitamin D levels were measured by electro chemiluminescent immunoassay (ECLIA) by Roche. Vitamin D deficiency is defined variably in the literature ranging from 10-30 ng/ml (Endocrine society/vitamin D council, Food and Nutrition Board). We defined vitamin D level of more than 20 ng/ml as normal.
Descriptive statistics (mean, standard deviation) were calculated for continuous variables while frequency distributions were obtained for categorical data. Chi square and Student t test were used to test the statistical significance of associations between groups. Logistic regression was used to adjust for age. Odds ratios and 95% confidence intervals were calculated.
RESULTS
A total of 120 women were included in the study. On clinical examination, 42.5% of women had PFD (N = 51) and 57.5% of women had no PFD (N = 69). Of these 51 women, 54.9% had POP alone (N = 23) and 27.4% had SUI alone (N = 14), while 17.6% had both SUI and POP (N = 9).
Table 1 documents the baseline demographic and clinical characteristics comparing the cases with PFD against controls without such disorders.
Table 1.
Description of the sample
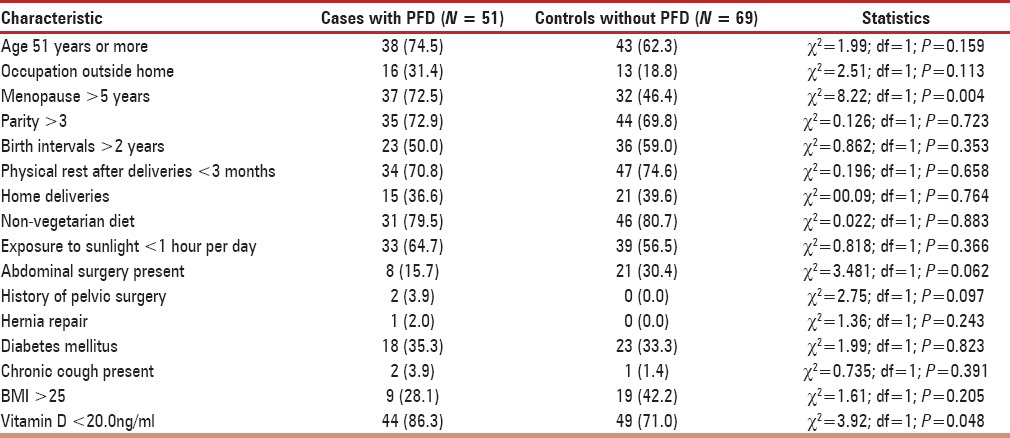
Out of the 120 women, 77.5% were vitamin D deficient (N = 93). Out of which, vitamin D levels were significantly lower (t = –2.16; df = 98.22; P = 0.034) among cases with PFD (mean 13.15 ng/ml; SD 1.01) than among controls without PFD (17.98 ng/ml; SD 16.61). Menopausal status of more than 5 years showed statistically significant association with PFD (χ2= 8.22; df = 1; P = 0.004).
The relationships between vitamin D levels (OR 2.88; 95% CI 1.08, 7.68; P = 0.034) and menopause of more than 5 years (OR = 3.06; 95% CI 1.41, 6.64; P = 0.005) remained statistically significant when age was adjusted using backwards conditional logistic regression. Based on the milk intake and calcium supplementation history, calcium intake was less than 500 mg/day in 61.7% (N = 37) of those without PFD and 81.7% (N = 49%) of those with PFD. The prescribed calcium tablets in calcium supplements contained 250 IU of vitamin D3.
POP seem to be associated with age over 51 years (χ2= 6.47; df = 1; P = 0.011), menopause more than 5 years (χ2= 15.12; df = 1; P = 0.000), history of abdominal surgery (χ2= 5.21; df = 1; P = 0.022), and history of pelvic surgery (χ2= 4.56; df = 1; P = 0.033). Vitamin D levels were not significantly related to POP. Menopause > 5 years (OR = 6.12; 95% CI 2.31, 16.22; P = 0.000) remained statistically significant when age was adjusted using backwards conditional logistic regression. The other variables were not statistically significantly related to POP.
SUI was statistically significantly associated with obesity (χ2= 4.24; df = 1; P = 0.039), limited exposure to sunlight (less than 1 hour per day) (χ2= 4.20; df = 1; P = 0.040) with a trend toward significance in women with serum vitamin D levels below 20.0 ng/ml (χ2= 3.47; df = 1; P = 0.062). Other variables were not significantly related to SUI after adjusting for age using logistic regression.
DISCUSSION
Vitamin D has been extensively studied mainly because of its high prevalence and its association with bone absorption.[7] Recently, the clinical importance of vitamin D is noted in physiological impact on various systems.
Vitamin D is a fat soluble vitamin. It plays an important role in the bone growth and maintenance of bone mineral density. It influences the intestinal absorption of calcium, which has an influence on bone strength.[7] The prevalence of vitamin D deficiency in the postmenopausal women in studies done globally shows a wide range from 1.6% to 86% depending on the regional location and seasonal variation.[7] In a study done in South India, the prevalence of vitamin D deficiency in postmenopausal women ranges from 50% to 80%.[8,9]
The role of vitamin D in muscle strength, neuromuscular function and postural stability has been evaluated in the recent studies.[10] Since pelvic floor muscle is also composed of skeletal muscles, it is likely to be affected by the vitamin D levels. A few retrospective studies have shown an association between vitamin D deficiency and PFD.[11,12] Elderly women are more likely to be vitamin D deficient due to the reduced activity of the skin to convert 25 hydroxycholecalciferol to active form,[10] which indicates that vitamin D deficiency could be a precipitating factor for PFD in postmenopausal women. Vitamin D has been implicated in the proper functioning of the musculoskeletal system. The mechanism proposed includes role of vitamin D in calcium absorption, protecting muscle cell from insulin resistance and inflammation.[10,11,13] Vitamin D receptors have been identified in the skeletal and smooth muscles.[14,15] Though randomized studies provide inconclusive evidence, many observational studies support the role of vitamin D in muscle function and efficacy.[11,13,16] Various studies have proven the role of vitamin D in the proper functioning of the skeletal muscle, though evidence of vitamin D role in smooth muscle is yet to be proven.[17,18] It has been shown that vitamin D increases the skeletal muscle proliferation and helps in proper functioning by binding to the vitamin D receptors (VDR) in the muscle.[12] Since pelvic floor muscles are composed of both smooth and skeletal muscles, it is plausible that the physiological action of pelvic floor be modulated by vitamin D levels.
The sources of vitamin D includes fish oil, egg yolk and liver and also from the action of sunlight on the skin. Hydroxylation of vitamin D to 25-hydroxylate vitamin D3 occurs in the liver followed by formation of the active form of vitamin D, the 1,25 hydroxylate vitamin D3 in the kidney.[16] Vitamin D increases the intestinal absorption of calcium and in combination with calcium; vitamin D plays a major role in maintenance of bone and muscle strength.[16] In the present study, the data on duration of sunlight exposure was obtained through history and was grouped as those with exposure less than one hour, one to two hours and more than two hours. The extent of exposure and exact duration of exposure was not available in minutes for those with exposure of less than one hour per day. This has been a limitation of this study.
The pelvic floor function is a complex mechanism comprising of muscles, connective tissue with neurological factor to support the pelvic viscera.[19] Even though the PFD are caused by various factors including fascial detachment, muscular weakness and neurological dysfunction, the levator ani plays a major role in the maintenance of urinary continence and prevention of POP.[13] It is plausible to suggest the effect of vitamin D in maintenance of pelvic floor muscle function and thereby its role in prevention of PFD.
In a prospective study by Dallosso et al., on association between dietary nutrients and overactive bladder symptoms in 5,816 women showed that women with higher intake of vitamin D had lower risk of overactive bladder syndrome.[20] Badalian et al., in a survey of 1881 women under National Health and Nutrition Examination Survey (NHANES) showed that vitamin D deficiency was a risk factor for developing PFD.[11] With increasing vitamin D levels, the risk of PFD reduced irrespective of the age. This study showed significant difference in vitamin D levels in those with PFD as compared to those without PFD. Our finding that vitamin D deficiency was associated with PFD is similar to that of Badalian et al., and Dallosso et al. The overall prevalence of vitamin D deficiency in the postmenopausal women in this study was 77.5%, which is similar to the previous studies done in South India.[8,9]
This study attempted to study the risk factors for clinical PFD, POP and SUI. It employed a case control study design. While the study recruited patients with and without symptoms of PFD, the statistical associations were only significant when a clinical diagnosis of PFD, POP and SUI were made on clinical examination and cases were compared with the controls on the different variables collected.
The strengths of this study include a relatively large sample, a systematic evaluation of risk factors and outcomes. The fact that risk factors were assessed prior to the evaluation of outcome also meant a reduction in interviewer bias. Vitamin D levels were assessed using a standard test. The use of multivariate logistic regression to adjust for the effects of age also means an exclusion of this common confounding variable. Its limitations include its cross-sectional design.
These findings are of sufficient strength to suggest the association of PFD and vitamin D deficiency in postmenopausal women. In addition, the high prevalence of vitamin D deficiency in postmenopausal women indicates a need for evaluation of vitamin D levels in these women.
Financial support and sponsorship
Nil.
Conflict of interest
There are no conflicts of interest.
REFERENCES
- 1.Greer WJ, Richter HE, Bartolucci AA, Burgio KL. Obesity and pelvic floor disorders: A systematic review. Obstet Gynecol. 2008;112:341–9. doi: 10.1097/AOG.0b013e31817cfdde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, et al. Pelvic Floor Disorders Network. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300:1311–6. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunskaar S, Lose G, Sykes D, Voss S. The prevalence of urinary incontinence in women in four European countries. BJU Int. 2004;93:324–30. doi: 10.1111/j.1464-410x.2003.04609.x. [DOI] [PubMed] [Google Scholar]
- 4.Abed H, Rogers RG. Urinary incontinence and pelvic organ prolapse: Diagnosis and treatment for the primary care physician. Med Clin North Am. 2008;92:1273–93. doi: 10.1016/j.mcna.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Lukacz ES, Lawrence JM, Contreras R, Nager CW, Luber KM. Parity, mode of delivery, and pelvic floor disorders. Obstet Gynecol. 2006;107:1253–60. doi: 10.1097/01.AOG.0000218096.54169.34. [DOI] [PubMed] [Google Scholar]
- 6.MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG. 2000;107:1460–70. doi: 10.1111/j.1471-0528.2000.tb11669.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaugris S, Heaney RP, Boonen S, Kurth H, Bentkover JD, Sen SS. Vitamin D inadequacy among post-menopausal women: A systematic review. QJM. 2005;98:667–76. doi: 10.1093/qjmed/hci096. [DOI] [PubMed] [Google Scholar]
- 8.Paul TV, Thomas N, Seshadri MS, Oommen R, Jose A, Mahendri NV. Prevalence of osteoporosis in ambulatory postmenopausal women from a semiurban region in Southern India: Relationship to calcium nutrition and vitamin D status. Endocr Pract. 2008;14:665–71. doi: 10.4158/EP.14.6.665. [DOI] [PubMed] [Google Scholar]
- 9.Harinarayan CV. Prevalence of vitamin D insufficiency in postmenopausal south Indian women. Osteoporos Int. 2005;16:397–402. doi: 10.1007/s00198-004-1703-5. [DOI] [PubMed] [Google Scholar]
- 10.Venning G. Recent developments in vitamin D deficiency and muscle weakness among elderly people. BMJ. 2005;330:524–6. doi: 10.1136/bmj.330.7490.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badalian SS, Rosenbaum PF. Vitamin D and pelvic floor disorders in women: Results from the National Health and Nutrition Examination Survey. Obstet Gynecol. 2010;115:795–803. doi: 10.1097/AOG.0b013e3181d34806. [DOI] [PubMed] [Google Scholar]
- 12.Parker-Autry CY, Markland AD, Ballard AC, Downs-Gunn D, Richter HE. Vitamin D status in women with pelvic floor disorder symptoms. Int Urogynecol J. 2012;23:1699–705. doi: 10.1007/s00192-012-1700-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker-Autry CY, Burgio KL, Richter HE. Vitamin D status: A review with implications for the pelvic floor. [Last cited on 2012 Jul 23];Int Urogynecol J. 2012 23:1517–26. doi: 10.1007/s00192-012-1710-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22415704 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–9. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 15.Crescioli C, Morelli A, Adorini L, Ferruzzi P, Luconi M, Vannelli GB, et al. Human bladder as a novel target for vitamin D receptor ligands. J Clin Endocrinol Metab. 2005;90:962–72. doi: 10.1210/jc.2004-1496. [DOI] [PubMed] [Google Scholar]
- 16.Nair R, Maseeh A. Vitamin D: The “sunshine” vitamin. J Pharmacol Pharmacother. 2012;3:118–26. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu K, Austin N, Devine A, Bruce D, Prince RL. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J Am Geriatr Soc. 2010;58:2063–8. doi: 10.1111/j.1532-5415.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- 18.Janssen HC, Samson MM, Verhaar HJ. Muscle strength and mobility in vitamin D-insufficient female geriatric patients: A randomized controlled trial on vitamin D and calcium supplementation. Aging Clin Exp Res. 2010;22:78–84. doi: 10.1007/BF03324819. [DOI] [PubMed] [Google Scholar]
- 19.Raizada V, Mittal RK. Pelvic floor anatomy and applied physiology. Gastroenterol Clin North Am. 2008;37:493–509. doi: 10.1016/j.gtc.2008.06.003. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallosso HM, McGrother CW, Matthews RJ, Donaldson MM Leicestershire MRC Incontinence Study Group. Nutrient composition of the diet and the development of overactive bladder: A longitudinal study in women. Neurourol Urodyn. 2004;23:204–10. doi: 10.1002/nau.20028. [DOI] [PubMed] [Google Scholar]


