Abstract
This study explored the developmental trajectories of postpartum weight from 0.5 to 3 years after childbirth, and aimed to determine the associations between postpartum weight trajectories and pre-pregnancy body mass index and adequacy of gestational weight gain (GWG). Data from the Norwegian Mother and Child Cohort study were used, following 49,528 mothers 0.5, 1.5, and 3 years after childbirth. Analyses were performed using latent growth mixture modeling. Three groups of developmental trajectories of postpartum weight were found, with most women (85.9 %) having a low level of weight retention initially and slight gain over 3 years, whereas 5.6 % of women started at a high postpartum weight retention (on average 7.56 kg) at 0.5 years but followed by a marked weight loss over time (2.63 kg per year on average), and the third trajectory represented women (8.5 %) who had high weight retention high initially (on average 4.67 kg at 0.5 years) and increasing weight over time (1.43 kg per year on average). Pre-pregnancy overweight and obesity and excessive GWG significantly predicted a high postpartum weight trend. Women had substantial variability in postpartum weight development—both initially after birth and in their weight trajectories over time. Early preventive interventions may be designed to assist women with pre-pregnancy overweight and obesity and excessive GWG, which helps to reduce the increasing trend for postpartum weight.
Keywords: Postpartum weight, MoBa, Developmental trajectory, Prepregnancy BMI, Gestational weight gain
Introduction
Obesity among women is increasing globally [1], and excessive postpartum weight retention (PPWR) has been regarded as one of the most important risk factors for this concerning trend [1–4]. Although studies have commonly reported a relatively small average of PPWR, ranging from 0.5 to 1.5 kg during 6–18 months postpartum, substantial variability in weight retention has been documented, ranging from a gain of 26.5 kg to a loss of 12.3 kg 1 year after pregnancy [5, 6]. In general, studies have reported that 13–20 % of mothers retain 5 kg or more above their preconception weight measured 1-year postpartum [7]. However, other studies have documented a decline in mean postpartum weight until 12 months postpartum and then a steady increase [4]. Furthermore, PPWR at 1-year postpartum appears to be an even stronger predictor of overweight 15 years after pregnancy than weight gain during pregnancy itself [8]. Little work has been done to characterize the trajectories of postpartum weight and capture the variability in weight retention or gain over time. Such research is important to understand the natural course of postpartum weight, to identify high-risk groups for overweight and obesity, and help to design targeted prevention programs, which may have important public health impact on obesity and related disorders.
Higher pre-pregnancy body mass index (BMI) has been identified as potential risk factor for PPWR and sub-sequent overweight and obesity but research findings examining the association between pre-pregnancy BMI and postpartum overweight have been inconsistent [7]. A review of studies exploring the relation between pre-pregnancy BMI and PPWR yielded mixed results with some studies reporting a positive association, some no association, and some an inverse association [7]. Non-significant differences in weight retention between women who were normal weight and overweight before pregnancy were reported as well [8]. Limited sample sizes and self-reporting bias have been suggested as the primary reasons for the conflicting findings [7]. However, the patterns of developmental trajectories for postpartum weight across pre-pregnancy BMI groups have rarely been investigated.
Gestational weight gain (GWG) in excess of 2009 Institute of Medicine (IOM) recommendations is strongly and positively associated with high PPWR [7, 9]. Women with excessive GWG are still at a greater risk of being overweight or obese even two decades after the index pregnancy [10]. Nevertheless, it remains unclear how the developmental trajectories of postpartum weight are related to GWG, and, in particular to the adequacy of GWG based on IOM definition [11]. Since women with excessive GWG retained more weight than those who gained within the recommendations [9], focusing on average GWG may not necessarily demonstrate a greater risk for persistent postpartum weight retention or gain. Thus, investigating the impact of adequacy of GWG on the developmental trajectory of postpartum weight may provide results with important implications to the rising trend of overweight and obesity among women of reproductive age.
Generally, many of the prior studies examining pre-pregnancy BMI, GWG and PPWR have been limited by short follow-up periods (~3 years after birth), small sample sizes, and a lack of control for multiple confounding covariates. Thus, little is known about individual differences in the amount of weight retained or gained. In this study, we therefore aim to examine postpartum weight trajectories up to 3 years after birth, and to determine the effect of pre-pregnancy BMI and adequacy of GWG on trajectories of postpartum weight. The current study used data from the population-based longitudinal study—Norwegian Mother and Child Cohort study (MoBa).
Methods and Procedures
Participants and Procedures
MoBa is a prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health [11]. Participants were recruited throughout Norway from 1999 through 2008, and 38.5 % of the invited women consented to participate. The cohort includes 108,000 children and 90,700 mothers. Informed consent was obtained from each MoBa participant upon recruitment. The study was approved by the Regional Committee for Medical Research Ethics in southeastern Norway and the Norwegian National Data Inspectorate. The response rates among women who consented to participate (38.5 %) were 84.5, 72.5, and 58.7 % at 0.5, 1.5, and 3 years postpartum, respectively. A complete description of recruitment and data collection has been published elsewhere [11].
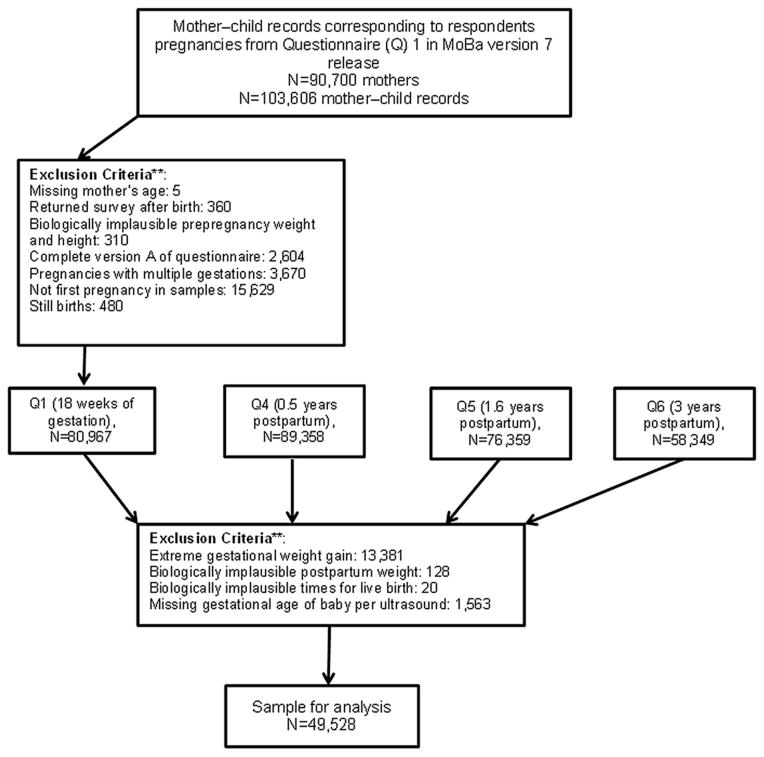
The current study is based on version 7 of the quality assured data files released in 2013. This data version included 103,606 mother–child records. The analysis population included MoBa participants who: (a) had non-missing self-reported age; (b) had a live, singleton birth; (c) had the first pregnancy in the MoBa cohort regardless of parity status, i.e. if a woman enrolled in MoBa more than once (due to additional pregnancies), only the first pregnancy was included; (d) had returned questionnaire 1 before delivery; (e) did not complete an early pilot version of questionnaire 1; (f) had plausible values for pre-pregnancy and postpartum weight (30 kg < weight before and after pregnancy≤300 kg), and height values (>100 cm); (g) did not have extreme GWG values (−10 kg< GWG< 50 kg); (h) did not have extreme postpartum weight values (−10 kg< postpartum weight<16 kg); (i) had biologically viable times for live birth (gestational age of the baby exceeded 140 days but was <309 days); and (j) had a recorded gestational age of baby per ultrasound. Figure 1 demonstrates how we reached the analytic sample of 49,528 mothers who met these criteria. The women who met the selection criteria were more likely to be older and highly educated, and have higher household income and fewer children compared to those women who did not meet the selection criteria. Moreover, research evaluating selection-bias in MoBa found significant differences in the prevalence of pregnancy outcomes and complications, and background characteristics between MoBa participants and the total Norwegian population of mothers; however, no statistically significant differences were found for exposure-outcome associations [12]. This suggests that although selection bias may be a problem with validity in studies of prevalence, it may be less problematic for studies of exposure-outcome associations.
Figure 1.
Flow chart for a sample.**The criteria are not mutually exclusive
Measures
Outcome
Information about postpartum weight was obtained through self-report. Participants reported their weight at 0.5, 1.5, and 3 years after birth. Weight retention or gain (in kg) was calculated using self-reported weight before pregnancy subtracted from postpartum weights at each follow-up time point.
Predictors
Maternal pre-pregnancy BMI (in kg/m2) was calculated using pre-pregnancy weight and height that were retrospectively reported at 18 weeks of gestation. BMI was categorized according to WHO and the 2009 IOM guideline (i.e. underweight: <18.5, normal weight: 18.5–24.9, overweight: 25.0–29.9 and obese: C30.0).
GWG (in kg) was calculated using weight before pregnancy subtracted from weight at birth that was retrospectively reported at 0.5 years postpartum. The adequacy of GWG was defined as a ratio of observed GWG to expected GWG (the 2009 IOM recommendation) at the gestational age of delivery multiplied by 100, as described elsewhere [13, 14]. The calculated percentage of weight-gain was thereafter categorized as inadequate (less than the lower cutoff of recommendations), adequate (within recommended range), or excessive (greater than the upper cutoff of recommendations) for each pre-pregnancy BMI group. For example, a woman with preconception weight within the normal/healthy weight range (i.e. 18.5–24.9 kg/m2) would be expected to gain 12.28 kg at 37 weeks of gestation. GWG below 85% (i.e., 10.44 kg) of the expected value would be categorized as inadequate, between 85 and 118 % (i.e., 10.44–14.49 kg) as adequate, and above 118 % (i.e., 14.49 kg) as excessive.
Covariates
Covariates included maternal age at delivery, parity (defined as total number of live births), maternal education, total household income, and breastfeeding status at 5 months. Breastfeeding was based on maternal report at 5 months. Predominantly breastfeeding was defined as breastfeeding without any supplements of formula milk or solid food. Although the current WHO recommendation is for exclusive breastfeeding (without any drinks, including water etc.), we applied the less restrictive category of predominant breastfeeding up to 5 months postpartum. If other feeding types had been used (either solid foods or formulas) then they were categorized as “partially breast-feeding”, and a third category was “not breastfeeding” if the child was not receiving any breast milk [15]. All covariates were self-reported with the exception of maternal age and parity, which were captured from the Medical Birth Registry of Norway [16]. Adjusting for these factors allows for the examination of the unique association between postpartum weight and predictors (pre-pregnancy BMI and GWG status).
Statistical Analyses
Latent growth mixture modeling (GMM) was applied to model the developmental trajectories of postpartum weight. GMM is useful to model differences in growth trajectories of individuals over time. This analysis is accomplished by specifying latent trajectory classes (i.e., a categorical latent variable), which allows for differences in growth parameters (intercept and slope) across groups of individuals [17]. GMM also captures heterogeneity in growth parameters across the latent classes, while assuming homogenous growth trajectories within a class [17]. The models were estimated by using the robust maximum likelihood approach; specifically an expectation–maximization algorithm was applied. The time values were coded as 0, 1, and 2.5 representing 0.5, 1.5 and 3 years postpartum respectively. Missing data were modeled by using full-information maximum-likelihood (FIML) method with the assumption that the data are missing at random [18].
Given the study objectives, the framework of analyses for the GMM is presented in Fig. 2. The developmental trajectories of postpartum weight at 0.5, 1.5, and 3 years are estimated by growth parameters (“I”—intercept, initial status of PPWR and “S”—slope, postpartum weight change rate over time). A categorical latent variable “C” represents the unobserved subpopulation membership for each developmental trajectory of postpartum weight, where each subpopulation (latent class) is represented by specific growth parameters (means and variances in “I” and “S”). The GMM is extended by including time-invariant predictors and covariates that predict the membership of subpopulation to each latent class of postpartum weight.
Figure 2.
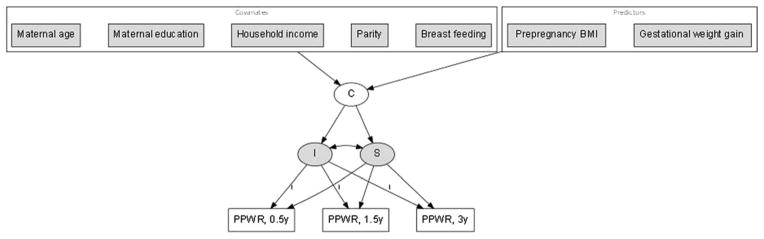
A framework of analyses for a growth mixture model. Note not all variances and covariance for endogenous and exogenous variable, and factor loading for slopes are shown
To perform the above-mentioned framework of analyses, we fitted the conditional GMM that included “C”, predictors, and covariates. Then, the optimal number of latent classes was determined by comparing the Bayesian Information Criteria (BIC), entropy, and the bootstrap likelihood ratio test (BLRT) among growth-mixture models with different numbers of latent classes [17, 19]. The effects of predictors on “C” were estimated by multinomial logit models. Data analyses were performed in Mplus version 6.12. For the analyses, a p value < 0.05 is considered statistically significant.
Results
Characteristics of the Study Population
Descriptive statistics for the characteristics of study population are presented in Table 1. The mean age of women in the study was 30.3 years (SD = 4.64). About 44 % of women were living in households with more than 500,000 NOK annual income (& $84,000). Around two thirds had a college or university education. Close to 50 % of women were first-time mothers, whereas 31.7 % had one child, and 18.9 % of women had two or more children before the current pregnancy. About 51 % of women had predominantly breastfed the current baby during the first 5 months postpartum. As to pre-pregnancy BMI, 67.8 % of women had BMI within the normal range, whereas 3.2 % fell within the underweight range, 21.4 % fell within the overweight range, and 7.6 % fell within the obese range. The mean GWG was 15 kg (SD = 5.72), with about a quarter in the inadequate GWG group, 35.5 % in the recommended GWG group, and 40.2 % of women in the excessive GWG group. The adequacy of GWG was calculated based on WHO and the 2009 IOM guideline for each BMI category.
Table 1.
Descriptive summary of the study population characteristics
| Variables | N | % |
|---|---|---|
| Maternal age in years (M ± S.D.) | 30.28 ± 4.64 | |
| Household income: | ||
| 0–200,000 NOK | 2,824 | 6.18 |
| 201,000–500,000 NOK | 22,535 | 49.34 |
| 501,000–700,000 NOK | 12,528 | 27.43 |
| >700,000 NOK | 7,785 | 17.05 |
| Maternal education: | ||
| <3 years high school | 3,457 | 7.37 |
| Vocational high school | 6,171 | 13.15 |
| 3-year high school | 7,120 | 15.17 |
| Regional technical college/4-year university degree | 19,248 | 41.02 |
| University or technical college, > 4 years | 10,926 | 23.29 |
| Parity: | ||
| 0 | 24,469 | 49.40 |
| 1 | 15,689 | 31.68 |
| 2+ | 9,370 | 18.92 |
| Breast feeding at 5 months: | ||
| Predominant breast feeding | 25,126 | 50.88 |
| Some breast feeding | 16,561 | 33.54 |
| No breast feeding | 7,692 | 15.58 |
| Prepregnancy BMI (kg/m2): | ||
| Normal | 33,242 | 67.80 |
| Underweight | 1,569 | 3.20 |
| Overweight | 10,490 | 21.39 |
| Obese | 3,730 | 7.61 |
| GWG in kg (M ± S.D.) | 15.01 ± 5.72 | |
| GWG adequacy: | ||
| Inadequate | 11,902 | 24.27 |
| Adequate | 17,411 | 35.51 |
| Excessive | 19,718 | 40.22 |
BMI, body mass index; GWG, gestational weight gain
Developmental Trajectories of Postpartum Weight
The mean postpartum weight retained or gained (M ± SD in kg) was 1.36 ± 4.15 at 0.5 years, 1.25 ± 4.18 at 1.5 years, and 1.87 ± 4.39 at 3 years. As an initial exploratory step, we first estimated linear growth curves to describe the trajectory of postpartum weight in all participants by the growth factors (intercept and linear slope). The model indicated that the mean of intercept and slope of postpartum weight were 1.29 (SD = 3.26, p<0.001) and 0.16 (SD = 0.85, p<0.001), showing the average PPWR at 0.5 years to be 1.29 kg, whereas weight increased significantly by 0.16 kg per year on average (BIC = 621,560; root mean square error of approximation = 0.08; Comparative Fit Index = 0.98). The correlation between intercept and slope in this model was negative and significant (−0.76, p<0.001), suggesting that the rate of increase in postpartum weight is dependent on the initial PPWR— those with greater PPWR at 0.5 years tended to have an attenuated increase in weight over time. Also, the significant slope growth factor variance indicated that not all women retained or gained weight at the same rate, but they had significant variability in their rates of postpartum weight change over time.
To examine the heterogeneity of developmental trajectories of postpartum weight from 0.5 to 3 years after birth, GMM was applied. As shown in Table 2, we explored the number of distinct latent trajectory classes and compared them by goodness-of-fit indices—BIC, entropy, and BLRT. The BIC and the BLRT test suggested that the four class model represented a significantly better fit to group the trajectories of postpartum weight than the three class model; however, the three class model had the highest entropy indicating better latent classification [19], on which we based our selection of that model. The model with three trajectories is also in accordance with previous theoretical and empirical work, where weight retention has been categorized as lost weight/no change, moderate, and high retention groups [20].
Table 2.
Indices of goodness-of-fit of GMM
| No. of latent classes | BIC | Entropy | BLRT
|
|
|---|---|---|---|---|
| Value (df) | p | |||
| 1 | 534,594 | - | - | |
| 2 | 531,010 | 0.76 | 3936.5(33) | <0.001 |
| 3 | 529,483 | 0.78 | 2065.7(33) | <0.001 |
| 4 | 528,743 | 0.50 | 986.6(33) | <0.001 |
GMM, growth mixture model; BIC, Bayesian Information Criteria, BLRT, the bootstrap likelihood ratio test
Figure 3 illustrates the estimated and sample means of postpartum weight for each trajectory over time. In accordance with the GMM results (estimated means for I and S in parentheses), the first trajectory represented women with a high PPWR (I = 4.67, p< 0.001) and who displayed an increasing trend over time (S = 1.43, p<0.001). Mothers in the second trajectory experienced initial high PPWR (I=7.56, p<0.001) with a sharp decline over time (S = −2.63, p<0.001). Women in the third trajectory started with low PPWR (I = 0.35, p<0.001) and increased at a low level over time (S=0.19, p<0.001). Henceforward, these trajectories are referred as initial high retention—continued gain (IHR-CG), initial high retention—sharp decline (IHR-SD), and initial low retention—continued low gain (ILR-CLG), respectively.
Fig. 3.
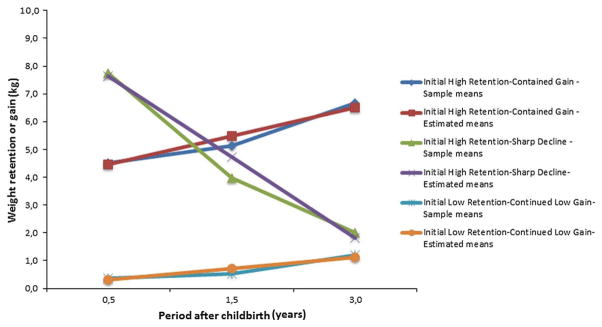
Sample and estimated means of PPWR among the latent developmental trajectories
Based on the most likely latent class membership, the proportion of participants was 8.5 % (N = 3,679) for IHR- CG class; 5.6 % (N = 2,407) for IHR-SD class; and 85.9 % (N = 37,019) for ILR-CLG class. The variances of all growth factors in each class were significant (p<0.001), indicating that there were substantial inter-individual differences in the initial status of PPWR and rate of changes in weight after birth.
Developmental Trajectories of Postpartum Weight, Pre-pregnancy BMI and Adequacy of GWG
Table 3 presents the multinomial logistic model results for the effect of pre-pregnancy BMI and adequacy of GWG on the latent class membership of postpartum weight, while controlling for multiple covariates, such as maternal age, maternal education, household income, parity, and breast feeding. To obtain odds ratios (ORs), the ILR-CLG was set as the reference class. Also, normal pre-pregnancy BMI and the adequate GWG group were set as reference categories for pre-pregnancy BMI and GWG, respectively. Under- weight before pregnancy and inadequate GWG were significantly and negatively associated with IHR-CG (ORs 0.56 and 0.63, respectively) and IHR-SD (ORs 0.43 and 0.39, respectively) classes versus the ILR-CLG class, suggesting lower odds of being in the IHR-CG and IHR-SD classes if underweight before pregnancy or having low GWG. Overweight and obesity before pregnancy and excessive GWG were significantly and positively associated with IHR-CG (ORs 3.16, 3.45, and 2.90, respectively) and IHR-SD (ORs 2.52, 3.26, and 6.06, respectively) classes versus the ILR-CLG class, suggesting higher odds of IHR-CG and IHR-SD class membership for these groups.
Table 3.
Multinomial logistic regression results for effects of prepregnancy BMI and adequacy of GWG on the latent class membership of postpartum weight
| Predictor | Latent classes of postpartum weight | |
|---|---|---|
| Initial High Retention- Continued Gain (N= 3,679) | Initial High Retention- Sharp Decline (N=2,407) | |
| OR (95% CI)1 | OR (95% CI)1 | |
| Prepregnancy BMI: | ||
| Normal | (reference group) | (reference group) |
| Underweight | 0.56(0.35–0.89)* | 0.43(0.19–0.97)* |
| Overweight | 3.16(2.67–3.74)*** | 2.52(1.83–3.47)*** |
| Obese | 3.45(2.69–4.43)*** | 3.26(2.40–4.41)*** |
| GWG adequacy: | ||
| Adequate | (reference group) | (reference group) |
| Inadequate | 0.63(0.50–0.80)** | 0.39(0.23–0.65)*** |
| Excessive | 2.90(2.418–3.49)*** | 6.06(4.21–8.73)*** |
BMI, body mass index; GWG, gestational weight gain
p< 0.05,
p< 0.01,
p< 0.001
adjusted for maternal age, maternal education, household income, parity, and breast feeding at 5 months
Initial Low Retention -Continued Low Gain (N= 37,019) is the referent class in this multinomial model.
Discussion
The current study reveals that for most women, the trajectory of postpartum weight represented fairly moderate weight change—2 kg in the 3 year after birth. However, 8.5 % of women reported an increasing trend in their weight, retaining around 5 kg at 0.5 years and increasing by 1.43 kg per year on average during the observation interval. Another 5.6 % of women reported initial high weight retention—about 8 kg at 0.5 years, followed by considerable loss of weight—2.63 kg per year on average. Previous investigations have also reported quite marked average variations with weight retention ranging from a gain of 26.5 kg to a loss of 12.3 kg 1 year after pregnancy [5, 6], but none of these studies examined the individual variability in the weight trajectories in more detail. Importantly, our study implies the 8.5 % of women who continued to gain weight over the 3 years after childbirth were more likely to be overweight or obese before pregnancy and have experienced excessive GWG. Such associations are particularly concerning, given that the prevalence of obesity among Norwegian women has increased over the last 30 years, from 9–10 % in 1985 to 18 % in 2013 [21, 22]. If pre-pregnancy obesity is a causal risk factor for PPWR, then increasing obesity trends may negatively influence postpartum weight trajectories for a significant number of mothers. Although little is known about risk factors that influence a pattern of high postpartum weight (IHR-CG women), a few longitudinal studies have suggested socioeconomic, psychological, and lifestyle factors may contribute [20, 23–25]. For example, Siega-Riz et al. [20] identified a number of predictors for a high weight retention at 3 and 12 months postpartum including: young maternal age; unemployment; low educational attainment; short sleep duration (B5 h); having an infant hospitalized after going home; disordered eating behaviors, and high total energy intake. The results in this paper indicate that postpartum weight retention or gain can still be a significant issue for women with relatively high income and education.
Additionally, a Danish population-based study revealed that feeling distress, depression, anxiety, and low socioeconomic status were associated with high PPWR at 6 and 18 months postpartum [24]. Some biological factors have been also implicated, such as high serum insulin concentration during pregnancy, insulin resistance, and the G protein b3 subunit 825T allele [1, 26]. However, it is still unclear whether these predictors are causally associated with postpartum weight or whether they differentiate IHR-CG women from IHR-SD or ILR-CLG women. Each of these aspects of postpartum weight predictors will be followed in our future research.
Interestingly, 5.6 % of women in this study experienced a noticeable declining trend in their weight development, even though they started with a high PPWR at 0.5 years postpartum and were more likely to have a high pre-pregnancy BMI (C25) and excessive GWG. Although studies identified some women with average weight loss about 12.6 kg in the 1 year postpartum [5, 6] or 13.6 kg in 2.5 years after birth [27], factors related to such marked weight loss have not been adequately studied. It is relevant to determine whether this declining trend is transitory or has permanent effects on long-term weight status. It is perhaps not surprising that women with pre-pregnancy overweight and obesity and excessive GWG were more likely to be classified as having IHR-CG or IHR-SD trajectories than an ILR-CLG trajectory, even while controlling for some confounding factors. Despite inconsistent findings regarding the effects of pre-pregnancy BMI on postpartum weight and limited evidence about the association between adequacy of GWG and postpartum weight, there has been a well-documented positive association between postpartum weight, pre-pregnancy BMI, and GWG [7, 9, 20].
To the best of our knowledge, this study represents the first longitudinal investigation of the developmental trajectories of postpartum weight using a large, population- based sample (N & 50,000). Nonetheless, the study also has limitations. First, the present study had a low response rate. Of invited women, only 38.5 % agreed to participate in MoBa. However, this rate is characteristic of large epidemiologic studies and does not necessarily imply biased estimates of associations [28]. Second, the sample is rather homogeneous, as participants are predominantly white and more educated with a high average household income. Participants may therefore represent a healthier segment of population, which may limit the generalizability of study findings to the general population of childbearing women in Norway. The inclusion of more women with higher education and income and fewer births could underestimate postpartum weight retention or gain. On the other hand, studying predominantly older women may overestimate the development of postpartum weight, as maternal age over 40 years may be associated with greater postpartum weight retention or gain [23]. Third, excluding a larger proportion of women (i.e., those who did not meet the selection criteria) could be a source for selection bias. Fourth, while self-reported weights have been shown to be reliable [29], there is a tendency for overweight or obese women to underestimate their weight, and for underweight women to overestimate their weight [7]. Moreover, another concern is that weight at birth is reported retrospectively 0.5 year postpartum. This might be inaccurate, as many of the participants may not remember or might not even know their weight at birth. Future research could extract weight at birth from the Medical Birth Registry of Norway, whereas such data are currently incomplete. Fifth, although the current study controlled for some confounders, the associations between postpartum weight and prepregnancy BMI and GWG could be confounded by multiple bio-psychosocial and lifestyle factors. Finally, since the latent trajectories analysis is based on the data driven procedure, it requires replication to confirm the results. In summary, the results suggest three distinct developmental trends of postpartum weight, with significant individual variability in both initial weight retention and rates of weight gain or loss over the 3 year after birth. Women with prepregnancy overweight and obesity and excessive GWG were more likely to retain weight initially and gain over time. Although most women retained or gained weight at a low level, those with an increasing trend in postpartum weight should be assisted through prevention program. Specifically, these findings emphasize the importance of developing acceptable and nonjudgmental approaches to assist women at high risk for postpartum weight, and to maintain weight gain within IOM recommendations during pregnancy. Additional studies are necessary to explore multiple bio-psychosocial and lifestyle risk factors and pathways associated with high postpartum weight. In our future research, we will investigate how biological, psychological, social and behavioral risk factors associate with the developmental trajectories of postpartum weight, and how this weight development relates to the risk of maternal and child overweight and obesity.
Acknowledgments
We are grateful to all the participating families in Norway who take part in this ongoing cohort study. This research analysis was funded by the Research Council of Norway (Grant No. 196226V50). Dr. Zerwas is supported by the CTSA (UL1RR025747), the NIH Building Interdisciplinary Careers in Women’s Health Award (K12-HD01441), and K01MH100435. MoBa was funded by Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (Contract No. N01-ES-75558) and NIH/NINDS (Grant No. 1 UO1 NS 047537-01 and Grant No. 2 UO1 NS 047537-06A1).
Footnotes
Conflict of Interest
Dr. Bulik is a consultant for Shire Pharmaceuticals and an author for Walker Books and Pearson.
Contributor Information
Dawit S. Abebe, Email: shawelus@yahoo.com, dawit.abebe@nova.no, Norwegian Social Research (NOVA), P.O. Box 3223, Elisenberg, 0208 Oslo, Norway. Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Tilmann Von Soest, Norwegian Social Research (NOVA), P.O. Box 3223, Elisenberg, 0208 Oslo, Norway. Division of Mental Health, Norwegian Institute of Public Health, Oslo, Norway. Department of Psychology, University of Oslo, Oslo, Norway.
Ann Von Holle, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Stephanie C. Zerwas, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Leila Torgersen, Division of Mental Health, Norwegian Institute of Public Health, Oslo, Norway.
Cynthia M. Bulik, Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA. Department of Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA. Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
References
- 1.Davis EM, Stange KC, Horwitz RI. Child- bearing, stress and obesity disparities in women: A public health perspective. Maternal and Child Health Journal. 2012;16(1):109–118. doi: 10.1007/s10995-010-0712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siega-Riz AM, Evenson KR, Dole N. Pregnancy- related weight gain—A link to obesity? Nutrition Reviews. 2004;62(7 Pt 2):S105–S111. doi: 10.1111/j.1753-4887.2004.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 3.Linne Y, Dye L, Barkeling B, et al. Weight development over time in parous women—The SPAWN study—15 years follow-up. International Journal of Obesity and Related Metabolic Disorders. 2003;27(12):1516–1522. doi: 10.1038/sj.ijo.0802441. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt NM, Nicholson WK, Schmitt J. The association of pregnancy and the development of obesity— Results of a systematic review and meta-analysis on the natural history of postpartum weight retention. International Journal of Obesity. 2007;31(11):1642–1651. doi: 10.1038/sj.ijo.0803655. [DOI] [PubMed] [Google Scholar]
- 5.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: A review of the evidence. Annals of Behavioral Medicine. 2003;26(2):149–159. doi: 10.1207/S15324796ABM2602_07. [DOI] [PubMed] [Google Scholar]
- 6.Linne Y, Barkeling B, Rossner S. Long-term weight development after pregnancy. Obesity Reviews. 2002;3(2):75–83. doi: 10.1046/j.1467-789x.2002.00061.x. [DOI] [PubMed] [Google Scholar]
- 7.Gunderson EP. Childbearing and obesity in women: Weight before, during, and after pregnancy. Obstetrics and Gynecology Clinics of North America. 2009;36(2):317–332. doi: 10.1016/j.ogc.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linne Y, Dye L, Barkeling B, et al. Long-term weight development in women: A 15-year follow-up of the effects of pregnancy. Obesity Research. 2004;12(7):1166–1178. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- 9.Nehring I, Schmoll S, Beyerlein A, et al. Gestational weight gain and long-term postpartum weight retention: A meta-analysis. American Journal of Clinical Nutrition. 2011;94(5):1225–1231. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 10.Mamun AA, Kinarivala M, O’Callaghan MJ, et al. Associations of excess weight gain during pregnancy with long- term maternal overweight and obesity: Evidence from 21 y postpartum follow-up. American Journal of Clinical Nutrition. 2010;91(5):1336–1341. doi: 10.3945/ajcn.2009.28950. [DOI] [PubMed] [Google Scholar]
- 11.Magnus P, Irgens LM, Haug K, et al. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa) International Journal of Epidemiology. 2006;35(5):1146–1150. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 12.Nilsen RM, Vollset SE, Gjessing HK, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatric and Perinatal Epidemiology. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 13.Webb JB, Siega-Riz AM, Dole N. Psychosocial determinants of adequacy of gestational weight gain. Obesity (Silver Spring) 2009;17(2):300–309. doi: 10.1038/oby.2008.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodnar LM, Siega-Riz AM, Simhan HN, et al. Severe obesity, gestational weight gain, and adverse birth outcomes. American Journal of Clinical Nutrition. 2010;91(6):1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perrin EM, Von Holle A, Zerwas S, et al. Weight- for-length trajectories in the first year of life in children of mothers with eating disorders in a large Norwegian cohort. International Journal of Eating Disorders. 2014 doi: 10.1002/eat.22290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irgens L, Bergsjø P, Lie R. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstetricia et Gynecologica Scandinavica. 2000;79:435–439. [PubMed] [Google Scholar]
- 17.Muthén B. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. Handbook of quantitative methodology for the social sciences. Thousand Oaks, CA: Sage; 2004. pp. 345–368. [Google Scholar]
- 18.Muthén LK, Muthén BO. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 2010. Mixture modeling with longitudinal data; pp. 197–232. [Google Scholar]
- 19.Nylund KL, Asparouhov T, Muthén B. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling. 2007;14(4):535–569. [Google Scholar]
- 20.Siega-Riz AM, Herring AH, Carrier K, et al. Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity. 2010;18(10):1996–2003. doi: 10.1038/oby.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer HE, Tverdal A. Development of body weight in the Norwegian population. Prostaglandins Leukotrienes and Essential Fatty Acids. 2005;73(1):3–7. doi: 10.1016/j.plefa.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 doi: 10.1016/s0140-6736(14),60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olson CM, Strawderman MS, Hinton PS, et al. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. International Journal of Obesity and Related Metabolic Disorders. 2003;27(1):117–127. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen P, Baker JL, Henriksen TB, et al. Influence of psychosocial factors on postpartum weight retention. Obesity. 2011;19(3):639–646. doi: 10.1038/oby.2010.175. [DOI] [PubMed] [Google Scholar]
- 25.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstetrics and Gynecology. 2005;106(6):1349–1356. doi: 10.1097/01.AOG.0000185480.09068.4a. [DOI] [PubMed] [Google Scholar]
- 26.Krause KM, Lovelady CA, Peterson BL, et al. Effect of breast-feeding on weight retention at 3 and 6 months postpartum: Data from the North Carolina WIC Programme. Public Health Nutrition. 2010;13(12):2019–2026. doi: 10.1017/S1368980010001503. [DOI] [PubMed] [Google Scholar]
- 27.Ostbye T, Krause KM, Swamy GK, et al. Effect of breastfeeding on weight retention from one pregnancy to the next: Results from the North Carolina WIC program. Preventive Medicine. 2010;51(5):368–372. doi: 10.1016/j.ypmed.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 28.IOM, NRC. Weight gain during pregnancy: Reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 29.Spencer EA, Appleby PN, Davey GK, et al. Validity of self-reported height and weight in 4808 EPIC. Oxford; 2002. [DOI] [PubMed] [Google Scholar]