Abstract
Estrogens play a crucial role in breast tumor growth, which is the rationale for the use of antiestrogens, such as tamoxifen, in women with estrogen receptor (ER)-α-positive breast cancer. However hormone resistance is a major clinical problem. Altered growth factor signaling to the ERα pathway has been shown to be associated with the development of clinical resistance. We previously have identified a mutation that replaces arginine for lysine at residue 303 (K303R) of ERα, which confers hypersensitive growth in low levels of estrogen. To determine if the K303R mutation could participate in the evolution of hormone resistance, we generated MCF-7 breast cancer cells stably transfected with either wild-type (WT) or K303R ERα. We found that the mutation confers decreased sensitivity to tamoxifen in the presence of the growth factor heregulin, using anchorage-independent growth assays. K303R ERα-expressing cells were hypersensitive to growth factor signals. Our data suggest that phosphorylation of serine 305 within the hinge domain of ERα might play a key role in increasing ligand-independent activity of the mutant receptor. We hypothesize that the mutation adapts the receptor for enhanced bidirectional cross-talk with the HER2 growth factor receptor pathway, which then impacts on responsiveness to tamoxifen.
Keywords: breast cancer, estrogen receptor, K303R mutant ERα, HER2
Introduction
Estrogens play a crucial role in regulating the growth and differentiation of normal breast epithelium and also of breast cancers, with approximately two-thirds of all breast cancers dependent for their growth on a functional estrogen receptor α (ERα). ERα is a member of the nuclear hormone receptor superfamily that regulates transcription of ER target genes by binding with specific estrogen response elements [1]. However, ERα also regulates the expression of many genes without direct binding to DNA. This occurs via protein-protein interactions with other transcription factors, such as activator protein-1, and with extranuclear signaling complexes that, in turn, modulate downstream gene expression [2, 3]. Therapeutic strategies directed at inhibiting the action of ERα using antiestrogens, such as tamoxifen (Tam), or reducing estrogen levels using aromatase inhibitors, are the standard therapies offered to women with ERα-positive cancer. However, not all patients who have ER positive tumors respond to endocrine therapies (termed de novo resistance), and a large number of patients who do respond will eventually develop disease progression or recurrence while on therapy (acquired resistance).
In previous work we identified an A to G somatic mutation at ERα nucleotide 908 (A908G) in early premalignant breast lesions [4]. This transition introduces a lysine to arginine substitution at residue 303 (K303R ERα) within exon 4, at the border between the hinge and the hormone-binding domain of the receptor; the mutation confers increased sensitivity to subphysiological levels of estrogen [4]. Others have reported that the K303R ERα mutation was not present in invasive tumors [5, 6], but we have demonstrated that the detection method used by these other investigators, standard dye-terminator sequencing, was not sensitive for detection of this specific mutation [7]. In addition, Conway et al have identified this mutation in 6% of breast cancers using a different detection method [8]. Therefore, the exact frequency of this mutation in breast tumors remains to be established. The mutation resides at major post-translational modifications sites (acetylation, ubiquitination, methylation) adjacent to a protein kinase A (PKA) phosphorylation site at serine residue 305 (S305). Cui et al. have demonstrated that this naturally-occurring mutation is a more efficient substrate for phosphorylation by PKA, and is hypoacetylated which subsequently alters estrogen sensitivity [9]. Michalides et al. have suggested that phosphorylation of ERα S305 by PKA induces a switch from antagonistic to agonistic effects of Tam, which induces resistance to this antiestrogen [10]. It has also been shown that the ERα S305 site can be an in vivo substrate for p21-activated kinase 1 (PAK 1)-mediated phosphorylation, and that activation of ERα S305 might confer a conformational change which allows for a better interaction with ligands such as Tam [11, 12].
We know that estrogen regulation of breast cancer cell growth can also be modulated by complex interactions with a variety of peptide growth factors. A large body of evidence supports the idea that rapid membrane effects of ERα may activate various components of growth factor tyrosine kinase signaling, such as that from insulin-like growth factor-IR (IGF-IR), epidermal growth factor receptor (EGFR), and c-erbB2/HER2 [13–15]‥ Furthermore, the kinase cascade signaling initiated by growth factor receptors can activate ERα (termed ligand-independent effects) and its coregulatory proteins, causing an interdependent loop of cross-talk that leads to enhanced tumor cell survival and proliferation [16–19]. Moreover, several preclinical and clinical studies suggest that overexpression of EGFR or HER2, and/or high levels of phosphorylated Akt and extracellular signal-regulated kinases (ERKs) in breast cancers contribute to Tam resistance [20–25]. We therefore hypothesized that the K303R mutation adapts ERα for enhanced reception of intracellular signal transduction, which leads to antiestrogen resistance. To test this hypothesis we used as an experimental model MCF-7 breast cancer cells stably transfected with either wild-type (WT) or the K303R mutant ERα. We found that cells expressing the estrogen hypersensitive K303R ERα mutant showed elevated levels of growth factor signaling, and enhanced cross-talk between the mutant and the HER2 growth factor receptor. These results suggest that the presence of the A908G ERα somatic mutation may be useful as a predictive marker of hormonal response in patients whose tumors exploit ERα and/or growth factor cross-talk to evade treatment.
Materials and methods
Reagents, hormones and antibodies
17β-Estradiol (E2), 4-Hydroxytamoxifen (4-OH), Epidermal growth factor (EGF), Insulin like growth factor −1 (IGF-1), and Heregulin (H) were from Sigma (St. Louis, MO). Herceptin was form Genentech (San Francisco, CA) Antibodies used for immunoblotting were: ERα (6F11) from (Novocastra, Newcastle, United Kingdom), progesterone receptor (PR) from DAKO (Carpinteria, CA), Rho GDIα from Santa Cruz Biotechnology (Santa Cruz, CA), total MAPK, total Akt, total c-Src, phosphorylated p42/44 MAPK (Thr202/Tyr204), Akt (Ser437), c-Src (Tyr416) from Cell Signaling Technology (Beverly, MA), total HER2 from NeoMarker (Fremont, CA), phosphorylated HER2 (Tyr1248), phosphor-ER-S305 from UPSTATE (Temecula, CA), and Living colors™ Full Length polyclonal antibody (Clontech, Mountain View, CA).
Cell culture
MCF-7 breast cancer cells, originally obtained from Dr. Benita Katzenellenbogen (University of Illinois, Urbana, IL), were maintained on plastic in minimal essential medium (MEM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Summit Biotechnology, Fort Collins, CO), 0.1 nmol/L nonessential amino acid, 2 mmol/L L-glutamine, 50 units/ml penicillin/streptomycin, at 37°C with 5% CO2/ 95% air. HeLa cells were obtained form American Type Culture Collection (Manassas, VA), and were maintained in the same media. Generation of the yellow-fluorescent protein (YFP)-tagged expression constructs, YFP-WT ERα and YFP-K303R ERα, has been previously described [9]. MCF-7 cells were stably transfected using Fugene 6 according to the manufacturer’s instructions (Roche, Indianapolis, IN), and individual clones were isolated and expanded with G418 selection. In some experiments we used a pool of stably transfected cells selected for one week with G418 antibiotic. Stably transfected clones were screened for expression of exogenous and endogenous ERα using immunoblot analysis.
Quantitative image analyses by high throughput microscopy (HTM)
PRL-Hela is a cell line specifically engineered for the single cell study of ER function [26]. PRL-Hela cells contain multiple genomic integrations of a replicated prolactin (PRL) promoter/enhancer. The multiple integrations (PRL array) are spatially confined and are visualized by the accumulation of fluorescently tagged ERα. PRL-Hela cells transiently expressing YFP-WT ERα or YFP K303R ERα, pretreated with forskolin (10 µM) for 15 minutes, and then treated with increasing concentration of E2 or Tam for 30 minutes, were fixed and DAPI-stained as previously reported [26, 27]. The cells were imaged using the Cell Lab IC 100 Image Cytometer (Beckman Coulter, Fullerton, CA) with a Nikon 40X Plan S flour 0.90 NA objective. Two channels were imaged: channel 0 (DAPI stain) was used to find the focus and nuclei, when channel 1 was used to image YFP ERα. A proprietary algorithm (GPRC) developed at Beckman Coulter was used to identify and quantify the YFP-ERα-targeted PRL array. The parameters for the GPRC algorithm were: object scale=30 and minimum peak height=10. Foci identified by the GPRC algorithm were masked. The area of the mask in pixels was the measure of PRL array size. Channel 1 was offset 2µm from DAPI focused for cells in all treatment conditions. After image acquisition and application of the GPRC algorithm, the total cell populations for each treatment were progressively filtered (gated) using the same criteria. Nuclei clusters, and mitotic cells were filtered from the total cell population using an intersection of DNA content and DNA clusters gates. In addition, low YFP ERα expression and low aggregate number gates were generated and applied to produce the final cell population to be analyzed. From the final population of cells, the array size was determined using the GPRC mask [28]. The images and masks were visually inspected for accuracy. Unpaired student’s t-test assuming equal variance was performed to determine statistical significance (two-tailed, p<0.05). Standard deviations are shown.
Immunoprecipitation and immunoblot analysis
Cells were starved in phenol red free MEM with 5% charcoal-stripped FBS for 48 h and treated as indicated before lysis [50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 2% NP40, 0,25% deoxycholic acid, 1 mmol/L EDTA, 1 mmol/L Na3VO4, and 1:100 protease inhibitors cocktail; Calbiochem]. For coimmunoprecipitation experiments, we used 1 mg of total cellular protein and a 1:200 dilution of anti-Living colors Full Length polyclonal antisera (Clontech) that recognizes native and denatured forms of recombinant YFP fusion proteins expressed in mammalian cells, and 2 µg of HER2 polyclonal antisera overnight, followed by protein A/G precipitation with rotation at 4°C for 2 h. Immunoprecipitated proteins were washed thrice with lysis buffer. Equal amounts of cell extract and immunoprecipitated proteins were resolved under denaturing conditions by electrophoresis in 8% to 10% polyacrylamide gels containing SDS (SDS-PAGE), and transferred to nitrocellulose membranes (Schleicher & Schuell, Keen, NH) by electroblotting. After blocking the transferred nitrocellulose membranes were incubated with primary antibodies overnight at 4°C, with secondary antibodies goat anti-mouse or goat anti-rabbit antisera (1:3000; Amersham Bioscences; Piscataway, NJ) for 1 h at room temperature and developed with enhanced chemi luminescence reagents (Alpha Innotech, San Leandro, CA).
Anchorage-independent soft agar growth assays
Cells (5000 per well) were plated in 4 ml of 0.35% agarose with 5% charcoal-stripped FBS in phenol red-free MEM, in a 0.7% agarose base in six-well plates. Two days after plating, media containing control vehicle or hormonal treatments (E2 1 nM, heregulin 2 ng/ml, EGF 10ng/ml, IGF-1 10ng/ml with or without Tam 100 nM) was added to the top layer, and the appropriate media was replaced every two days. In some experiments a pool of stably transfected cells were treated with heregulin with or without herceptin (10 µg/ml). After 14 days, 150 µl of MTT was added to each well and allowed to incubate at 37°C for 4 h. Plates were then placed in 4°C overnight and colonies > 50 µm diameter from triplicate assays were counted. Data are the mean colony number of three plates and representative of two independent experiments analyzed for statistical significance (p<0.05) using a two-tailed student’s Test, performed by Graph Pad Prism 5 (GraphPad Software, Inc., San Diego, CA). Standard deviations are shown.
Blocking peptide delivery
A blocking peptide of 13 residues (IKRSKKNSLALSC) from the sequence (residues 298–310) surrounding the S305 residue (in bold) of the human ERα was transferred into cells using a cationic amphiphile molecule, PULSin™ delivery reagent (Polyplus transfection, Illkirch, France), as suggested by manufacturer. Briefly, cells were plated in a 6-well plate with regular growth media, and then starved for 48 h in a phenol red free MEM with 5% charcoal stripped FBS. After starvation cells were washed with PBS to remove all traces of serum, and fresh phenol-red free media without serum was added. The mixture containing the S305 blocking peptide (4 µg/well) diluted in 200 µl of 20 nM Hepes, and 16 µl of PULSin™ was incubated for 15 min at room temperature, and then added to the cells. The media was changed after 4 hours of incubation, cells were treated as indicated, and cellular extracts were prepared. Delivery of R-phycoerythrin was used as a positive control and live cells were observed by fluorescence microscopy after 4 h.
Results
Ligand-independent signaling to the K303R ERα mutant reduces Tam sensitivity
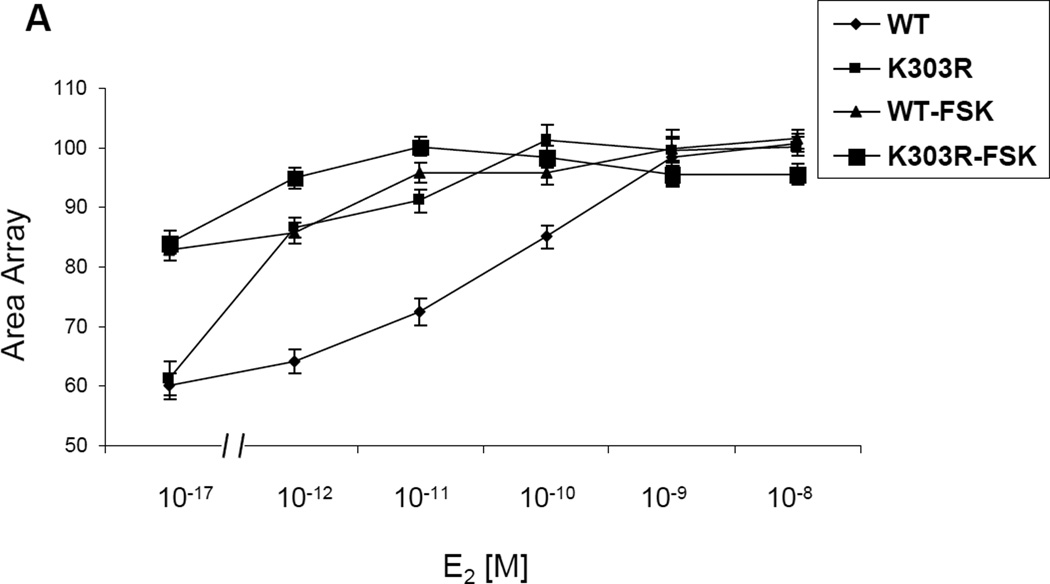
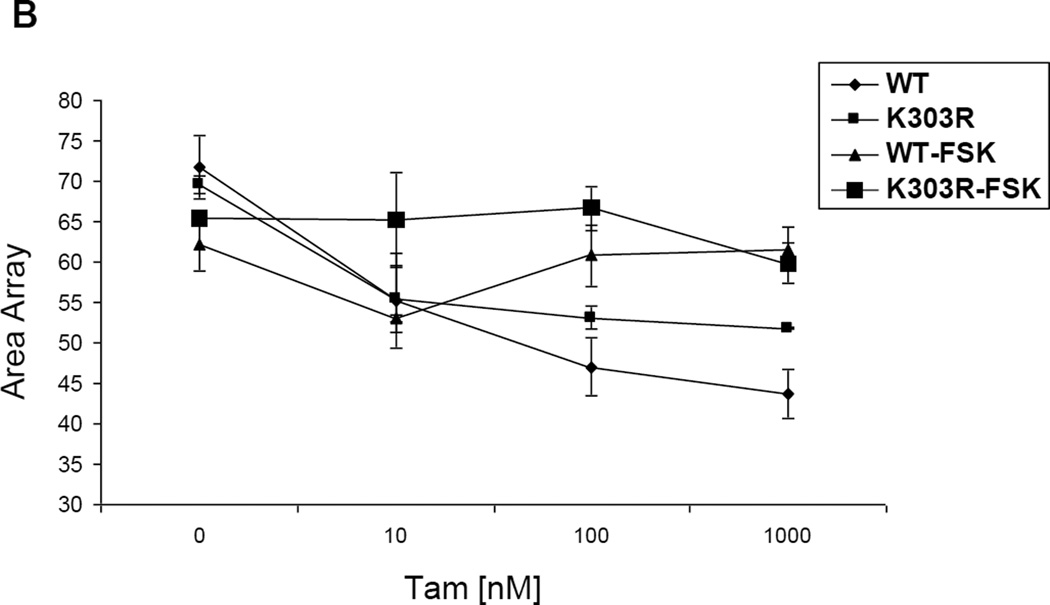
We have previously shown that K303R ERα mutant-overexpressing cells display enhanced ligand-independent activity when stimulated with cyclic AMP, in part because the mutation generates a more efficient substrate for PKA-mediated signaling [9]. It is well known that ligand-independent signaling can influence cellular responsiveness to Tam [29]. For instance, PKA-mediated phosphorylation of ERα at S305 allows the antagonist Tam to behave as an agonist, which then results in ERα-dependent transactivation [10]. Here we have utilized the stable cell line PRL-Hela containing a multi-copy integrated prolactin enhancer/promoter DNA array that is responsive to ER such that when the receptor is expressed as a YFP-fusion protein, the integration site can be easily visualized (PRL-array) [26]. High-throughput microscopy has been used to identify ligand-independent changes in the size of the PRL-array which is an indicator of the chromatin condensation status at the promoter [28]. Arrays rapidly decondense after E2 treatment or condense after anti-estrogen treatment (both within minutes). Thus, in PRL-Hela cells, the array size is an indicator of receptor-mediated transcription function in response to different treatments and allows direct and live observation of ER-dependent chromatin remodelling. Therefore using a mammalian-based, stably transfected prolactin promoter array system, we analyzed live cell dynamics on an estrogen-responsive promoter, to visualize chromatin remodelling induced by E2 and Tam. We transiently transfected PRL-Hela cells with YFP tagged-ERα WT or the YFP-ERα K303R mutant receptor expression vector, and examined E2 (Fig 1a) and Tam’s (Fig 1b) effect on chromatin remodelling in the presence of forskolin, an activator of PKA signaling. Using high through-put microscopy, the amount of chromatin remodelling was quantified [28]. 24h after transfection cells were pretreated with forskolin (FSK), and then treated with E2 or Tam at different doses, as indicated, for 30 minutes. Over 60 nuclei in two separate experiments were analyzed to determine the size of the PRL-reporter array. Vehicle control showed similar values for the array size in the presence of the WT receptor and the mutant (Fig. 1b). In PRL-Hela cells expressing the WT receptor, estrogen treatment increased array size in a dose-dependent manner in estrogen concentrations between 10−12 M and 10−9 M. Mutant-expressing cells showed a linear dose-response in the range of 10−17 M through 10−11 M of estrogen. Indeed, mutant ERα expressing cells exhibited a much lower EC50 (3 × 10−13 M) compared with WT ERα-expressing cells (5.1 × 10−11 M). These data demonstrate that K303R mutant-expressing cells are hypersensitive to estrogen and form larger arrays in the presence of low estrogen concentrations. With forskolin treatment, WT and mutant ERα cells stimulated with estrogen both displayed significantly increased array sizes. WT ERα-expressing cells demonstrated a one-log reduction in the EC50 (4.2 × 10−12 M ± 0.0038 compared to 5.1 × 10−11 M ± 0.0132 in the absence of forskolin) and a significantly increased average array size (Fig. 1a). Mutant-expressing cells also displayed a significantly increased average size, but without a significant change in the EC50 (8 × 10−13 M ± 612.5 compared to 3 × 10−13 M ± 0.0004 in the absence of forskolin). Thus, forskolin increased average array size, and reduced the EC50 of WT ERα cells thus enhancing estrogen sensitivity, whereas forskolin treatment of mutant-expressing cells only increased the array size, suggesting that these cells displayed an inherent hypersensitivity to estrogen. As shown in Fig. 1b, Tam treatment decreased the array size in WT ERα-expressing cells, however the mutant receptor was significantly less responsive to Tam (p<0.0001). Forskolin treatment blocked Tam-induced promoter condensation (reduced size of the array), and the mutant receptor array size was not affected by physiological levels of Tam (100 nM) in the presence of forskolin. All together these results suggest that hormone-independent kinase signaling to the mutant receptor K303R ERα may confer resistance to antiestrogen treatment in breast cancer cells.
Fig. 1.
E2 and Tam effects on large-scale chromatin structure in PRL-Hela cells. Cells transiently expressing YFP-ERα WT (WT) or the YFP-K303R ERα (K303R) mutation were pretreated with forskolin (FSK) for 15’ minutes and then were treated with E2 (a) or Tam (b) at different doses for 30 minutes. After fixing and counter-staining with DAPI, cells were imaged and array size was quantified using HTM as described in Materials and Methods. Results are expressed as array size normalized to control cells obtained from three independent experiments.
MCF-7 K303R-ERα mutant expressing cells exhibit altered growth factor signaling
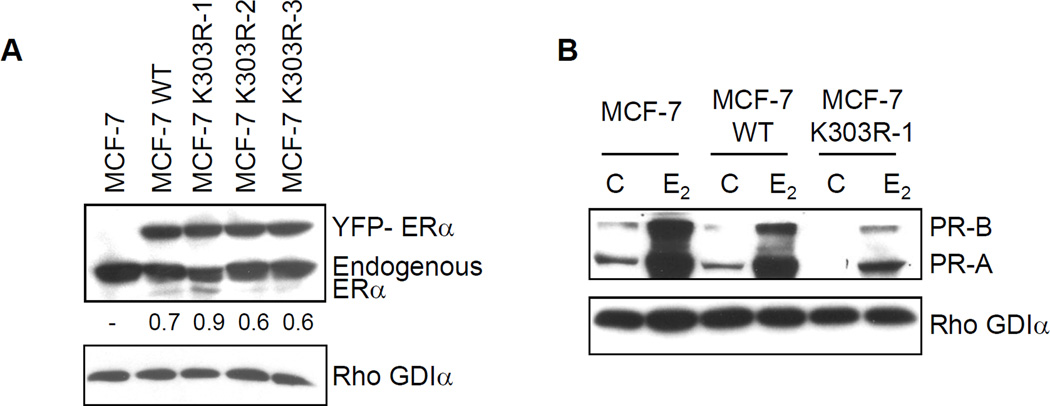
To further explore ligand-independent activation of signaling in mutant-expressing breast cancer cells, we developed MCF-7 ERα-positive human breast cancer cell lines overexpressing either WT or the K303R ERα mutant. We chose to introduce the mutant receptor into parental WT ERα-expressing cells to simulate the situation in invasive human breast tumors, where WT receptor is most frequently co-expressed along with the mutant [7]. To differentiate the exogenously expressed receptor, we tagged the vector with YFP. Stably transfected clones were screened for expression of ERα using immunoblot analysis (Fig. 2a). Parental MCF-7 cells are shown along with one clone stably expressing YFP-WT, and three clones expressing YFP-K303R ERα (MCF-7 K303R-1–3). .
Fig. 2.
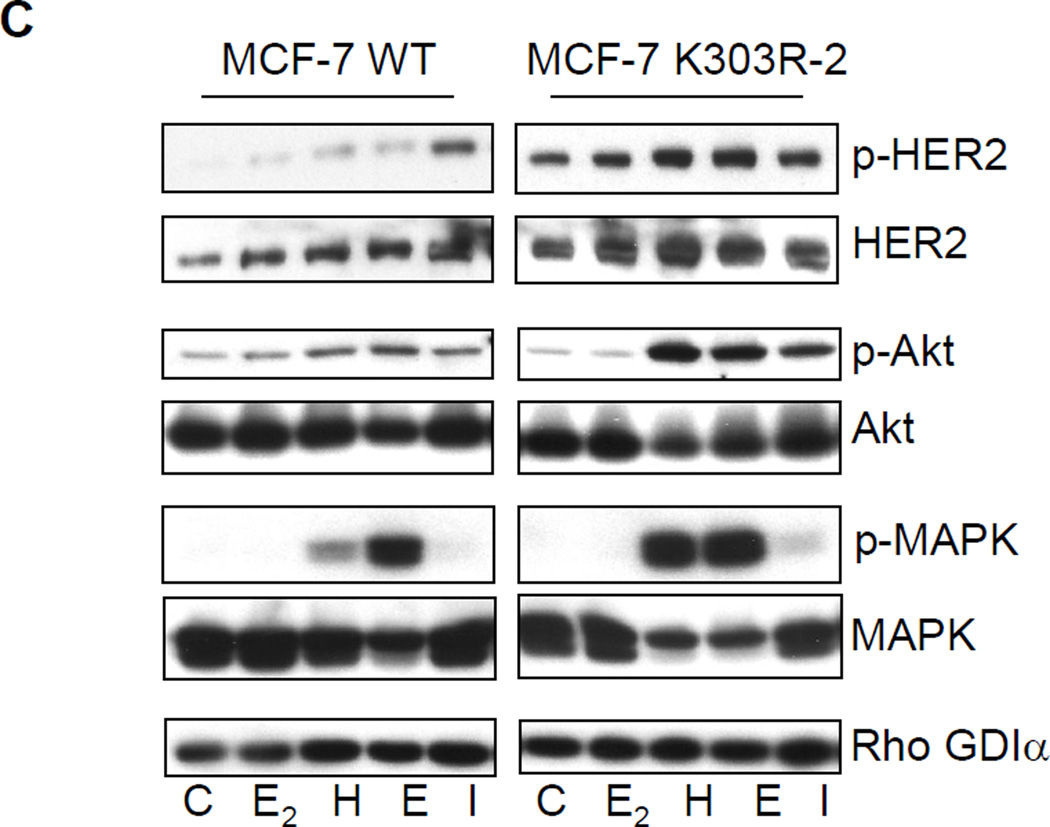
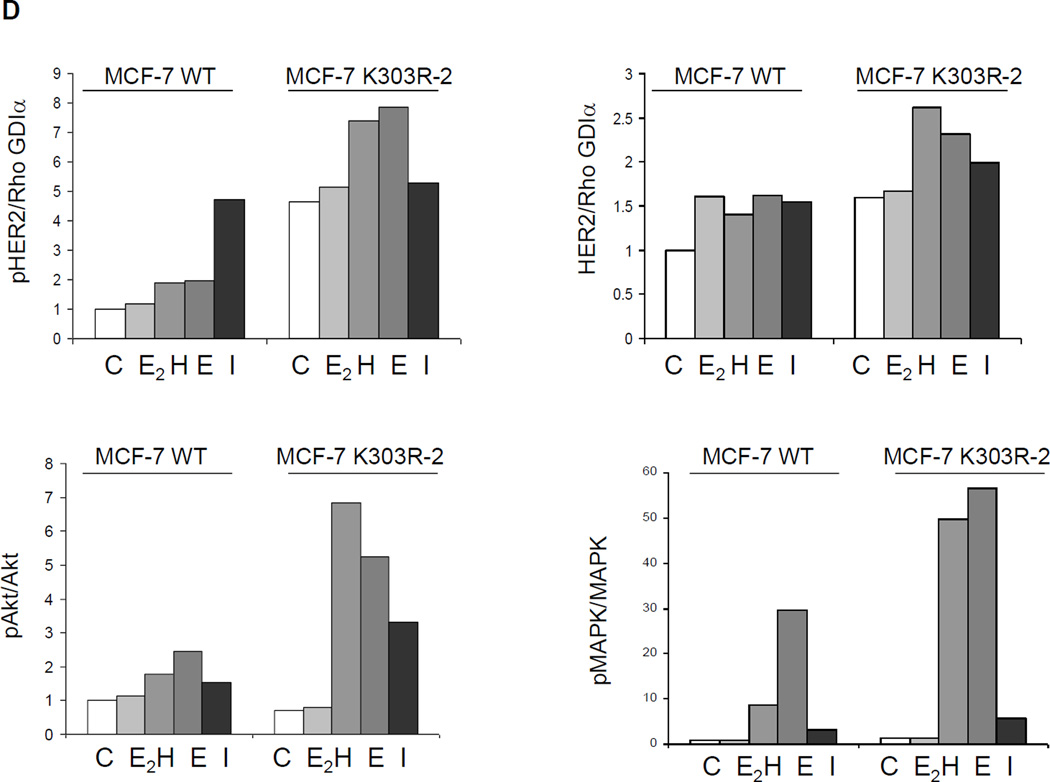
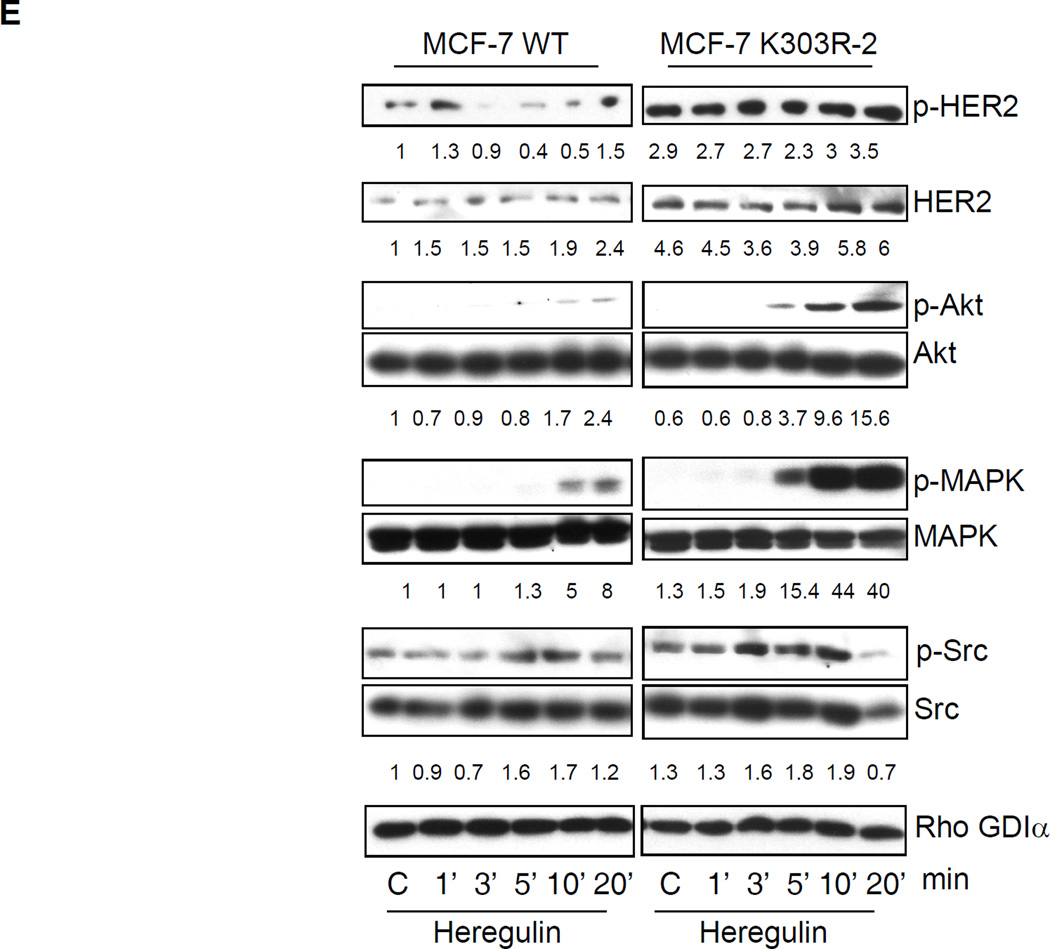
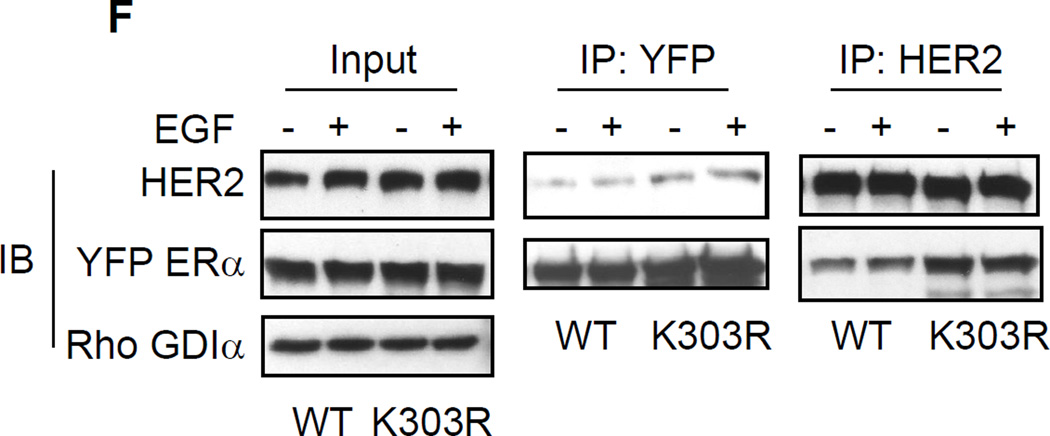
Growth factor signaling in MCF-7 WT-ERα and MCF-7 K303R ERα-expressing cells. (a) MCF-7 cells stably transfected with a yellow-fluorescent protein (YFP)-tagged expression construct YFP-WT ERα (MCF-7 WT) and different clones transfected with the mutant YFP-K303R ERα construct (MCF-7 K303R-1, −2, −3) were screened for expression of exogenous (96 kDa) and endogenous ERα (66 kDa) by immunoblot analysis using an antibody against human ERα. Parental cells (MCF-7) were used as a control for endogenous ERα expression. Rho GDIα was used as a control for equal loading and transfer. Numbers below the blot represent the ratio between YFP-ERα and endogenous ERα protein expression. (b) MCF-7 parental, MCF-7 WT, and MCF-7 K303R-1 cells were serum-starved for 48h, and then treated with or without 1 nM E2 for 24 hours before lysis. Equal amounts of total cellular extract were analyzed for progesterone receptor (PR-A and PR-B) levels by Western blotting. (c) MCF-7 WT and MCF-7 K303R-2 cells were treated with vehicle ethanol (C), 1 nM E2, 2 ng/ml heregulin (H), 10 ng/ml EGF (E) and 10 ng/ml IGF-1 (I) for 10 min before lysis. Levels of phosphorylated (p) HER2 (Tyr1248), Akt (Ser473), and MAPK (Thr202/Tyr204), at the indicated residues, and total non-phosphorylated protein were measured in cellular extracts by immunoblot analysis. (d) Quantitative analysis of the blots shown above. (e) MCF-7 WT and MCF-7 K303R-2 cells were treated with heregulin (H) at 2 ng/ml for different times before lysis. Cellular extracts were analyzed as in panel c, and for p-Src (Tyr416) at the indicated residues. Numbers below the blot represent fold change in protein expression of MCF-7 K303R-2 cells compared to MCF-7 WT cells. (f) MCF-7 WT (WT) and MCF-7 K303R-3 (K303R) cells after 48h of starvation were treated with or without EGF 100 ng/ml for 5 min before lysis. YFP-WT ERα and YFP-K303R ERα proteins were immunoprecipitated using an anti-YFP polyclonal antibody (IP:YFP), or an anti-HER2 polyclonal antibody (IP:HER2), and immunoblotted (IB) with HER2 and anti-ERα antibodies, respectively. Whole-cell lysates (Input) were used as input controls. Rho GDIα was used as a control for equal loading and transfer. Immunoblots are representative of three separate experiments.
It is well accepted that the response to endocrine therapies in human breast cancer patients correlates with ERα and progesterone receptor (PR) levels. Several studies have shown that patients with ERα/PR-positive breast cancers derive greater benefit from adjuvant hormonal therapy than those patients whose tumors lack PR [30–32] however it must be noted that other studies have not always found this result [33]. It has been hypothesized that high growth factor signaling activity in breast cancers may be associated with decreased PR levels (for a review see [34]), and elegant studies have shown that up-regulation of pMAPK Erk1/2 leads to a loss of PR via degradation by the 26S proteasome [35]. In clinical samples we have previously reported a borderline significant inverse correlation between the presence of the K303R ERα mutation and the PR-B isoform [7], and we have shown that a reduction in PR-B levels was associated with a poorer response to endocrine therapy [36]. Therefore, we first evaluated the levels of PR-A and B in our parental MCF-7 cells, and two stably transfected clones (Fig. 2b). Parental MCF-7 cells expressed both PR isoforms, with PR-A as the predominant form in these cells. PR is a known estrogen-induced gene, and as expected we saw higher levels of both PR-A and B in MCF-7 cells after estrogen (E2) treatment. In comparison, MCF-7 WT-overexpressing cells exhibited slightly less PR induction with E2. In contrast, under control, basal conditions, K303R ERα mutant-overexpressing cells demonstrated much lower levels of both PR isoforms, and less were induced with E2 treatment. Lower levels of PR mRNA levels were also detected in estrogen-stimulated mutant cells using Real-Time polymerase chain reaction (qPCR), (p = 0.0008, data not shown). These results suggest that growth factor signals might be altered in the mutant-overexpressing cells.
To test for altered intracellular signaling, we next examined the effects of short-term treatments with E2, heregulin (H), EGF (E), and IGF-1 (I) on phosphorylation levels of downstream growth factors signaling components, such as HER2, Akt, and MAPK using immunoblot analysis (Fig. 2c) which is quantitatively represented in Fig. 2d. Cells were maintained under estrogen-depleted conditions for 2 days, and then treated for 10 minutes with E2 or different growth factors as indicated, and cellular extracts were prepared. MCF-7 K303R mutant cells showed constitutively higher levels of phosphorylated HER2 as well as total HER2, compared to MCF-7 WT ERα-overexpressing cells (Fig. 2c). To begin to understand the mechanism associated with higher levels of total HER2 in the mutant cells we analyzed the expression level of HER2 mRNA in different clones of mutant ERα expressing cells, by qPCR; but we did not found differences in HER2 mRNA levels between WT and K303R ERα expressing cells (data not shown). This result suggests that post-translational modification and increase in protein stability could be involved in the HER2 up-regulation that we found in mutant ERα-expressing cells, and we are currently exploring this possibility. Treatment with E2, heregulin, EGF, and IGF-1 induced higher levels of pHER2 in mutant-overexpressing cells, but only a small induction was seen in MCF-7 WT cells which contain low endogenous levels of HER2. Treatment with heregulin or EGF led to increased phosphorylation of the downstream signaling molecules Akt and MAPK in mutant-overexpressing cells compared with MCF-7 WT cells. IGF-1 treatment also induced enhanced phosphorylation of Akt, but increased levels of phosphorylated MAPK were only detected in K303R ERα mutant-overexpressing cells. In both cell lines, no enhancement of Akt and MAPK phosphorylation was seen with estrogen treatment.
To investigate if the kinetics of growth factor signaling might be altered in mutant-expressing cells, we performed a time-course study with heregulin treatment in WT ERα and K303R ERα mutant cells (Fig. 2e). After two days of starvation cells were treated for 1, 3, 5, 10, or 20 minutes with heregulin, and then lysed as described in Materials and Methods. As shown in Fig. 2e a rapid response to heregulin was seen within 1’ in MCF-7 WT cells with activation of phospho-HER2, and phospho-AKT/phosphoMAPK within 10–20’ (numerical quantitation relative to control WT is shown beneath each immunoblot). In contrast, mutant-expressing cells exhibited enhanced activation of pHER2, pAKT, and pMAPK. Compared with WT-expressing cells, these molecules were all stimulated at earlier time points in mutant cells; heregulin induced phosphorylation of MAPK and Akt by 5 minutes, but levels of these phosphorylated kinases were not detectable at this time-point in WT ERα cells. We saw that the non receptor tyrosine kinase c-Src also exhibited an earlier time-point of activation (by 3 min), and higher phosphorylation levels at the activating c-Src tyrosine residue 416 (Tyr416) was seen in K303R ERα cells. These collective data demonstrate that the mutant-overexpressing cells are hypersensitive to growth factor signal transduction, and suggest that the mutant receptor could impact on ligand-independent signaling pathways commonly known to be employed to evade anti-hormonal therapeutic strategies in breast cancer.
Previous reports have shown that ERα and growth factor pathways can interact at different levels, through a direct association or complex formation of ERα with key signaling molecules such as c-Src, Shc, and the p85α regulatory subunit of PI3K [37–40]. C-Src family tyrosine kinases are involved in signaling of a number of different growth factor receptors, including EGFR/HER2, in breast cancer cells. In previous work we have reported that MCF-7 cells stably expressing the K303R ERα mutant receptor exhibited increased c-Src kinase activity and c-Src tyrosine phosphorylation, when compared with WT ERα-expressing cells [41]. Therefore we next addressed whether the mutation might alter the ability of the receptor to bind with the HER2 tyrosine kinase receptor, which is expressed at higher levels in mutant cells (Fig. 2c). To evaluate potential protein-protein interactions between WT and mutant receptors with HER2 in our model system, we treated MCF-7 cells overexpressing WT or the mutant for 5’ with EGF 100 ng/ml and then lysates were prepared (Fig. 2f). Equal amounts of protein were immunoprecipitated with either anti-YFP antisera or anti-HER2 antisera followed by immunoblot for HER2 and YFP-ERa. As shown in Fig. 2f, similar amounts of input from the whole-cell lysates were used. In the absence of treatment, both WT ERa and HER2 resided in a protein complex; and EGF treatment slightly increased the amount of the protein in the complex. The levels of mutant receptor bound to HER2 under basal conditions were higher, and EGF treatment did not further increase the amount of receptor in the complex. The bottom panel shows that equal amounts of YFP-ERa and HER2 were immunoprecipitated under all conditions tested. These results suggest that the K303R ERα mutant may be constitutively associated with HER2, which could be involved in the enhanced bidirectional crosstalk that we report between ERα and growth factor receptor signaling pathways.
Tamoxifen fails to inhibit anchorage-independent growth induced by heregulin in MCF-7 K303R ERα-overexpressing cells
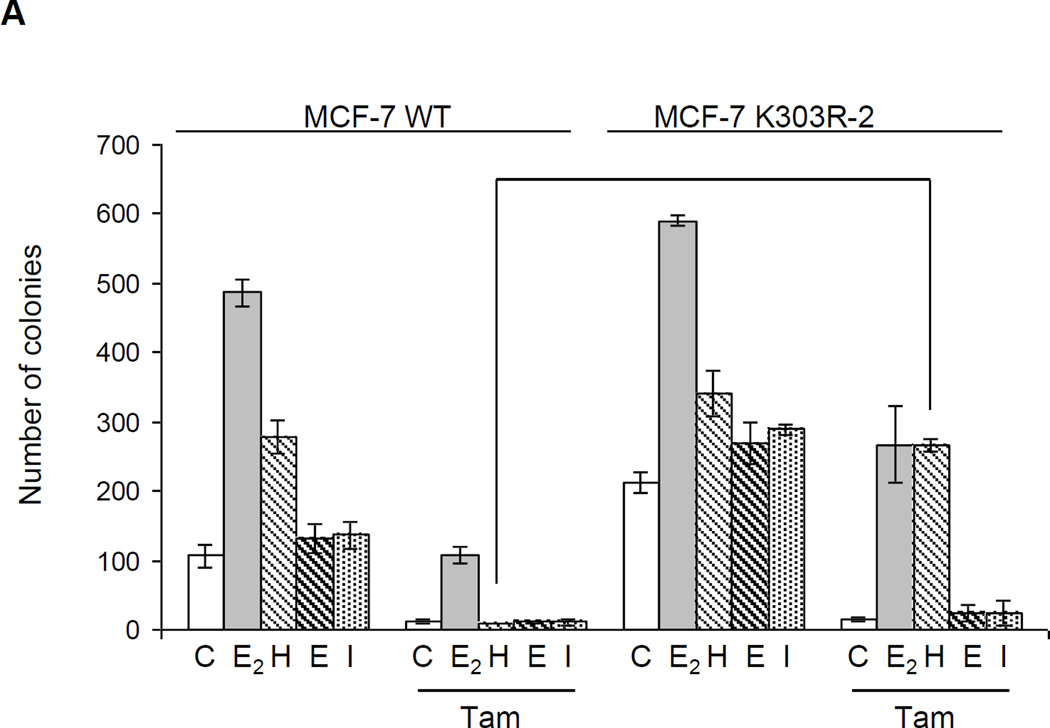
It is already appreciated that altered crosstalk between several receptor tyrosine kinases and ERα may contribute to endocrine resistance [18], and it has been shown that elevated levels of HER2 contribute to resistance in a tamoxifen-resistant subline of MCF-7 cells compared to unselected parental cells [42]. Since the K303R ERα mutation conferred enhanced heregulin-mediated signaling, we next addressed whether this alteration might confer a decrease in response to antiestrogen therapy. To test this question we next performed anchorage-independent growth assays using our two ERα-overexpressing models. Cells were plated in soft agar and then treated with estrogen (E2, 1 nM) or heregulin (H, 2 ng/ml), EGF (10ng/ml, E), IGF-1 (10ng/ml, I) in the presence or absence of tamoxifen (Tam, 100 nM). After 14 days of growth colonies >50 µM in diameter were counted (Fig. 3a). Control (C) basal growth of mutant-expressing cells was higher compared to WT-expressing cells (p=0.001); as expected treatment with estrogen as well as heregulin increased the number of colonies in both cell lines, while EGF and IGF-1 slightly increased proliferation but only in MCF-7 K303R ERα-overexpressing cells. Tam treatment induced a significant reduction in the number of colonies in MCF-7 WT cells under control conditions, and was able to inhibit growth of cells stimulated with estrogen or heregulin (reduction in colonies 78% and 96%, respectively). In contrast Tam treatment was less efficient at inhibiting estrogen or heregulin-stimulated proliferation in mutant-overexpressing cells (reductions were only 54% and 21%, respectively), but effectively reduced EGF and IGF-1 stimulated growth in these cells Thus, response to Tam was greatly affected by the enhanced growth factor signaling by heregulin in mutant cells; this enhanced sensitivity prevented the major antagonistic activity of tamoxifen on the proliferation of mutant-expressing cells.
Fig. 3.
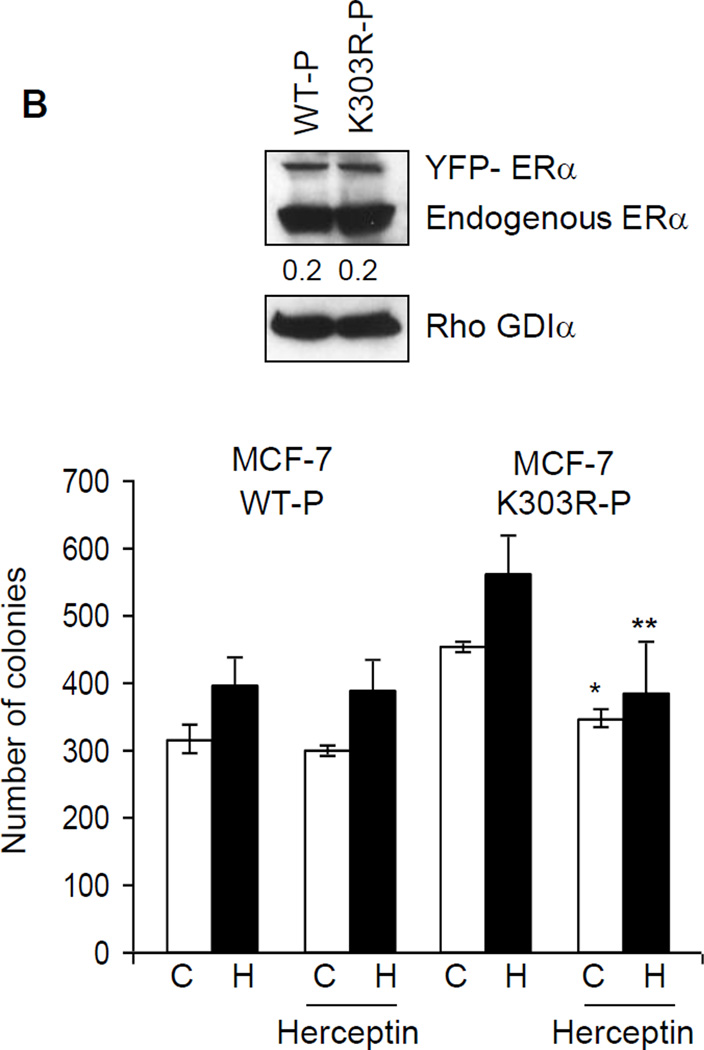
Heregulin treatment reduced the ability of tamoxifen to inhibit anchorage-independent growth of MCF-7 K303R cells. (a) MCF-7 WT and MCF-7 K303R-2 stably transfected cells were seeded (5000/well) in 0.35% agarose and then treated with vehicle (C), E2 (1 nM), heregulin (2 ng/ml, H), EGF (10ng/ml, E), IGF-1 (10ng/ml, I) with or without Tam (100 nM). Cells were allowed to grow for 14 days and the number of colonies >50 µm were quantified and the results were graphed. *p=0.0001 vs control (C) of MCF-7 WT cells. (b) Immunoblot analysis showing YFP-ERα and endogenous ERα protein expression (upper panel) in the pool of stable transfectants. Numbers below the blot represent fold change in protein expression of MCF-7 K303R-2 cells compared to MCF-7 WT cells. MCF-7 WT-P and MCF-7 K303R–P pool of transfected and overexpressing cells were plated in soft agar and then untreated or treated with heregulin 2 ng/ml (H) in the presence or absence of Herceptin (10 µg/ml). *p=0.0002 vs control (C); **p=0.03 vs heregulin (H) treated cells (bottom panel); standard deviations are shown.
Next, we examined whether the HER2 pathway found to be up-regulated in the mutant-expressing cells, was responsible for the higher growth of mutant cells compared to WT ERα cells. We therefore evaluated anchorage-independent growth of either mutant or WT ERα cells after herceptin treatment. Herceptin is a humanized monoclonal antibody directed against the extracellular domain of HER2, and was developed as an agent to inhibit the growth of HER2-overexpressing tumor cells [43, 44]. Cells were plated in soft agar and then treated with heregulin in the presence or absence of herceptin (10 µg/ml). After 14 days of growth, colonies were counted (Fig 3b). As expected since HER2 levels are low in WT cells, herceptin had no effect on their growth either under basal conditions or with heregulin treatment. In contrast, herceptin significantly inhibited anchorage-independent growth of K303R mutant cells in control untreated conditions, and with heregulin treatment (*p=0.0002 vs control; **p=0.03 vs heregulin treated cells). These data confirm that the HER2 pathway may be responsible for the higher constitutive growth of mutant-overexpressing cells rendering these cells more sensitive to the inhibitory effect of this selective HER2-targeted agent.
Phosphorylation at serine residue 305 (S305) of ERα K303R mutant is involved in growth factor signaling up-regulation
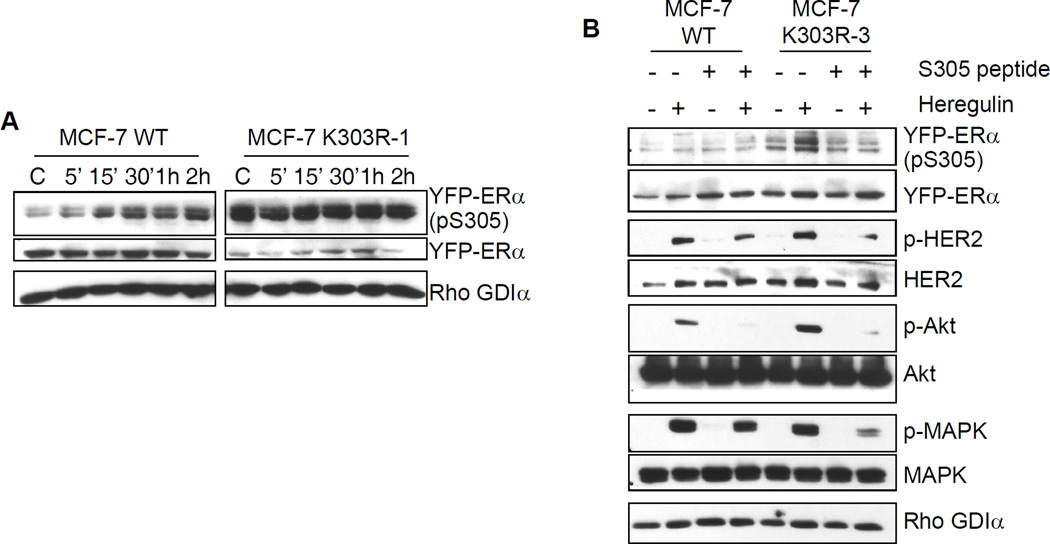
ERα is a known target of several post-translational modifications, such as phosphorylation, sumoylation, and acetylation [9, 45–47]. For instance, receptor phosphorylation, which regulates receptor affinity, coregulator protein binding, and transcriptional activity, can be induced in the absence of ligand via cross-talk with various signal transduction pathways [48]. We previously have reported that the K303R ERα mutant is a more efficient substrate for phosphorylation by PKA at S305 which enhanced hormone sensitivity and stimulated cellular growth [9]. We hypothesize that the phosphorylation status of S305 in the mutant receptor may control receptor activity, and be a conduit for enhanced downstream cross-talk with growth factor signaling networks. To explore this possibility, we first evaluated the phosphorylation status of the S305 residue in either WT ERα or K303R mutant cells after estrogen treatments between 5 minutes to 2 hours. Cellular extracts were subjected to immunoblot assay using a specific anti-phospho-S305 ERα antibody (Fig. 4a). Estrogen treatment enhanced the phosphorylated levels of S305 within 15’ in YFP-WT-expressing cells, and these levels remained elevated at 2 hours. In contrast, K303R ERα mutant-expressing cells exhibited elevated levels of pS305 under basal control conditions, and this elevated phosphorylation remained constant with longer estrogen treatments. Since, it is well established that ERα can be activated in a ligand-independent manner by MAPK [49] at serine 118 (S118), we also evaluated the levels of phospho-S118 in WT ERα or K303R ERα-overexpressing cells under the same conditions described above. We did not detect significant changes in S118 phosphorylation patterns between the two cell lines (data not show). These results suggest that constitutively higher phosphorylation of S305 in the mutant receptor might play a role in the ligand-independent activation of the receptor itself. It is possible that this enhanced S305 phosphorylation within the mutant might play a key role in the observed up-regulation of growth factor signaling cascades seen in these cells as well.
Fig. 4.
Serine residue 305 (S305) in the K303R ERα mutant is involved in growth factor signal up-regulation. (a) MCF-7 WT and MCF-7 K303R-1 cells were treated for different times with E2 (1nM) before lysis. Cellular extracts were analyzed for phosphorylation levels of S305 YFP ERα (pS305) and total non-phosphorylated YFP-ERα. (b) Cells were incubated with the S305 peptide (4 µg/well) for 4 hours in serum-free media and then treated with or without heregulin (2 ng/ml) for 10 min before lysis. Levels of phosphorylated (p) S305 YFP-ERα, HER2 (Tyr1248), Akt (Ser473), and MAPK (Thr202/Tyr204), and total non-phosphorylated proteins were measured in cellular extracts by immunoblot analysis. Blots are representative of three separate experiments. Rho GDIα was used as a control for equal loading and transfer
To test this hypothesis we performed immunoblot analysis to evaluate the phosphorylation levels of a number of growth factor signaling components after incubation with an S305 blocking peptide. To block phosphorylation at S305 we delivered a peptide (S305 peptide, residues 298 to 310) to the cells. After peptide delivery, the cells were subjected to short term treatments (10 min) with heregulin and then growth factor signaling molecules were analyzed by immunoblot analysis (Fig. 4b). Heregulin enhanced S305 phosphorylation in K303R mutant-overexpressing cells, but had no effect WT receptor. Phosphorylation of the mutant receptor was abrogated by the S305 peptide. Addition of the S305 blocking peptide also inhibited heregulin-induced phosphorylation of HER2, Akt, and MAPK in both cell lines. Interestingly, reduction in phospho-HER2 and MAPK levels were more pronounced in mutant-expressing cells compared with WT, suggesting that the mutant cells were more sensitive to the inhibitory effect of S305 blocking peptide. These data indicate that phosphorylation of the S305 residue may be crucial in mediating enhanced cross-talk between HER2 and mutant ERα, and suggest that phosphorylation blockade might be a potential therapeutic strategy to block mutant function.
Discussion
Despite the improvements in the efficacy of hormonal therapies for the treatment of breast cancer patients with ER-positive tumors, de novo and acquired resistance remain major clinical problems that limit the efficacy of these therapies. In most cases, ERα remains essential to the problem of resistance due to its intimate cross-talk with growth factor signaling pathways [50, 51]. In this study, we show that expression of K303R ERα mutant in ERα-positive MCF-7 breast cancer cells confers a decreased sensitivity to tamoxifen treatment in the presence of growth factor stimulation. Furthermore, this naturally-occurring mutant is constitutively phosphorylated at S305, and shows an enhanced bidirectional cross-talk with the HER2 signaling pathway.
The A to G somatic mutation of ERα at nucleotide 908 (A908G) was previously identified in about 30% of premalignant breast lesions, but at a higher frequency (50%) in invasive breast tumors [4, 7]. The mutation was found to be associated with biologic measures of poor outcome, including elevated HER2 protein, larger tumor size and axillary lymph node positivity. To date no other somatic ERα mutation has been identified in more than a few invasive breast cancers [52], making this mutation novel. Recently, in a study using a population-based, case-control study design, the A908G mutation was detected, but at a low frequency (7%), in invasive breast tumors [53], confirming our identification of the mutant in cancer. We previously demonstrated that the exogenous expression of the K303R ERα mutant in MCF-7 breast cancer cells conferred a hypersensitive growth in very low physiological levels of estrogen (10−12 to 10−11 M) [4]. In the present study we focused on the K303R ERα mutation and its potential role in modulating hormonal response in breast cancer cells, and on the molecular pathways that could be involved in its hormone action.
Our studies showed that growth factor signaling pathways were up-regulated in K303R ERα-expressing cells. In particular, MCF-7 mutant ERα expressing cells showed constitutively higher levels of total and phosphorylated HER2, the tyrosine kinase receptor that belongs to the epidermal growth factor receptors family. Preliminary quantitative RT-PCR analysis demonstrated that HER2 mRNA levels were not increased in mutant expressing cells compared to WT ERα-expressing cells. Thus, transcriptional regulation may not be the major mechanism for the observed increased levels of HER2 in mutant cells. In the present study we did not investigate the possible post-transcriptional mechanisms associated with higher levels of HER2, but instead focused on the effects that HER2 overexpression induced on downstream cell signaling and hormone responsiveness in mutant-expressing cells. It is well known that HER2 catalytic activity can amplify the signal of other c-erbB family receptors by the formation of HER2-containing heterodimers, which increases ligand binding affinity and receptor stability [54, 55]. Moreover, it has been shown that c-neu, the mouse homolog of HER2, is able to multimerize, be phosphorylated, and thus activated when present at high density on the cell surfaces [56]. Both mechanisms result in the amplified activation of downstream signaling cascade, such as Akt and MAPK, which are involved in cell survival and proliferation.
We found that the peptide growth factors heregulin and EGF strongly enhanced phosphorylation of the two major downstream signaling cascades Akt and MAPK, in mutant-expressing cells compared to WT ERα-expressing cells. Furthermore, analysis of rapid kinetics showed that many of these downstream molecules, as well as the c-Src non receptor tyrosine kinase, were stimulated at earlier time points in mutant-expressing cells. The rapid responses of these downstream kinase cascades to heregulin suggest that the presence of K303R ERα mutation could modify the responsiveness of the cells to the growth factor signaling possibly through enhanced non-genomic activity of the mutant receptor.
Recent research into the mechanisms associated with Tam resistance suggest that some of the same growth factor receptor pathways implicated in adaptive hypersensitivity, such as Akt and MAPK, or specific oncogenes involved in intracellular signal transduction, become activated and are used to bypass normal hormonal responsiveness. Several reports indicate that the up-regulation of HER2 tyrosine kinase signaling in breast cancer plays an important role in the development of endocrine resistance [57]. Preclinical studies have demonstrated that HER2 overexpression in ERα positive MCF-7 human breast cancer xenografts rendered them resistant to Tam [58], and markedly increased levels of EGFR and HER2 were found in some sublines of MCF-7 cells with acquired Tam resistance [59, 60]. Tam resistance in these cells was reversed by EGFR/HER2 tyrosine kinase inhibitors, and combined treatment with these inhibitors and Tam was effective in reversing resistance in xenograft models [61], thereby strongly implicating this signaling network in resistance. Although HER2 overexpression occurs only in a minority of ERα-positive patients [62], clinical studies confirm that HER2-overexpressing tumors are less responsive to Tam treatment [63, 64]. In a previous retrospective study we found an association between the K303R ERα mutation and elevated HER2 levels in invasive breast cancer [7]. Here we observed increased HER2 levels in K303R mutant-expressing cells that was concomitant with an altered response to Tam with growth factor stimulation.
We also demonstrated that mutant-expressing cells exhibited a higher level of growth under all conditions tested. Importantly, Tam sensitivity was significantly affected with estrogen and heregulin treatments. Our data indicate that the Tam-resistant phenotype associated with the mutant was most pronounced in the presence of growth factor activation; in the presence of heregulin Tam inhibited soft agar growth only 21% compared with a 96% inhibition in WT ERα-expressing cells. We speculate that bidirectional cross-talk between the HER2 and mutant receptors could play a role in conferring a selective advantage in terms of growth to those patients that express the K303R ERα mutant that are treated with Tam. We are currently examining for the presence of the mutant in a retrospective cohort of patients treated with Tam who have long-term clinical follow-up.
Different therapeutic agents targeting the activity of the c-erbB family of receptors have been recently developed and tested in patients. For instance, herceptin (Trastuzumab™), a monoclonal antibody against HER2, was approved for therapeutic use in patients with HER2-overexpressing breast cancer [65, 66]. By binding to the juxtamembrane domain of HER2 [67], this agent blocks HER2 homo- and heterodimerization with the other members of the c-erbB family, and thereby interrupts the activation of downstream proliferative signaling. Here we show that herceptin elicited its antiproliferative effects either under basal conditions or with hergulin treatment in K303R ERα mutant cells, but did not affect growth of the WT cells. These findings confirm our hypothesis that the HER2 pathway, which appears to be elevated in mutant cells, could be involved in the regulation of cell growth in breast tumor cells bearing the mutation, probably through increased crosstalk between HER2 and ERα pathways.
The existence of bidirectional cross-talk between ERα and growth factor receptor pathways, and its involvement in the development of endocrine resistance has been well documented [16, 19]. Several studies have demonstrated direct or indirect activation of growth factor signaling via ERα. For example, ligand-independent activation of serine 118 ERα by EGFR/MAPK-mediated phosphorylation regulates growth of tamoxifen-resistant MCF-7 breast cancer cells [68]. Chung et al. have also demonstrated that HER2 and ERα can directly interact at the cell membrane [69], and this interaction protected breast cancer cells from Tam-induced apoptosis. Moreover, membrane or cytoplasmic ERα can induce phosphorylation of EGFR through activation of G-proteins, c-Src, and matrix metalloproteinases [70], and can directly interact with adaptor proteins such as c-Src, Shc and the p85α regulatory subunit of PI3K [37–40]. These processes activate downstream kinases that in turn activate ERα and its coregulatory proteins, thus also enhancing genomic activities of the receptor [71, 72]. All together these effects amplify the bidirectional crosstalk which multiplies signals between and downstream of the growth factor receptors and ERα, thus sustaining survival and proliferative signals in breast cancer cells.
The present findings suggest an enhanced hormone-independent physical association/complex between the mutant receptor and HER2 compared to WT receptor. This suggests that the mutation, present in the hinge region of the receptor, may increases the ability of ERα to interact with HER2 or other components of the complex. As yet, the interaction surface between HER and ERα has not been defined. Altered interactions as we describe may imply that Tam may not antagonize the mutant receptor because the HER2 pathway may be dominant and non-genomic action predominates. This is consistent because we have already demonstrated that the mutation can alter coregulator protein binding. The mutation demonstrates enhanced binding ability to bind to the TIF-2 coactivator [4], and the AIB coactivator at very low and physiological levels of estradiol, but decreased binding to the corepressor NCoR1. Mutant receptor binding to BRCA-1 has also been shown to be enhanced. These collective data suggest that altered affinity for ER coregulators, and possibly signaling molecules such as HER2, could be one mechanisms by which the K303R mutation confers hypersensitivity to low levels of estrogen, and reduced sensitivity to Tam. The mutant receptor appears to exhibit increased ligand-independent activity which bypasses antiestrogen treatment.
To explore why the K303R ERα mutation has increased cross-talk with the HER2 pathway, we focused on the differences in post-translational modifications between WT and mutant receptor. It is known that ERα activity can be modulated by several post-translational modifications, such as protein phosphorylation [45], acetylation [46], ubiquitination [73], and sumoylation [47]. The majority of studies in this field have focused their attention on the phosphorylation status of the receptor, and its effect on receptor activity. For instance, receptor phosphorylation by different kinases such as c-Src, PKA, MAPK, and Akt can all regulate receptor affinity, coregulator protein binding, and transcriptional activity [45]. We have previously shown that the K303R ERα mutation renders the receptor a more efficient substrate for PKA-induced phosphorylation at residue S305 which has distinct biological results—enhanced hormone sensitivity for growth [9]. Phosphorylation at the S305 residue can also be mediated by both protein kinase A (PKA) and p21-activated kinase-1 (PAK-1) signaling network [9, 12].
Several reports have identified the serine residue at 305 as a physiologically important site that modifies response to Tam. In particular it has been demonstrated, using fluorescence resonance energy transfer (FRET) analysis, that PKA signaling to ERα S305 causes a conformational arrest in the ERα and switches Tam from an antagonist to an agonist [10]. Michalides et al. have also demonstrated that PKA phosphorylation at S305 ERα induces Tam resistance through an altered orientation of ERα towards the co-activator SRC-1 [74]. In addition, S305 ERα phosphorylation by PAK-1 up-regulates cyclin D1 expression in breast cancer cells [75]. Here we show that K303R mutant cells have elevated phosphorylation levels of S305 compared with WT-expressing cells suggesting that the mutant have constitutive ligand-independent activity. The contribution of the S305 site in enhanced cross-talk with HER2 was established using a blocking Peptide. We show that heregulin-stimulation enhanced S305 phosphorylation in mutant expressing cells, but it did not significantly influence the phosphorylation status of WT receptor. Interestingly, we found that the S305 peptide affected heregulin-induced ERα phosphorylation, and prevented downstream phosphorylation events, such as activation of MAPK and Akt. These effects were even more prominent in K303R mutant cells possibly due to prominent role of the S305 site in ligand-independent activity of the mutant receptor. A similar experimental approach was used by Varricchio et al. [76]. They demonstrated that a six aminoacid peptide surrounding the phosphotyrosine residue 537 was able to block ER/c-Src interactions, cyclin D1 expression, and growth of MCF-7 and LNCaP cells.
We have previously shown that transcriptional activity of the mutant receptor was induced at very low concentrations of estradiol (10−12 M), and only the specific lysine to arginine substitution at 303 residue resulted in a receptor with enhanced sensitivity to estrogen. We have also demonstrated that cAMP-dependent signaling can enhance the receptor’s intrinsic sensitivity to hormone, and that blocking PKA activity reversed the hypersensitive proliferative phenotype in mutant-expressing cells [9]. The data obtained herein using live cell dynamics agrees with these earlier results. The live cell high through-put analyses allowed us to study mutant receptor/promoter interaction and chromatin remodelling [26, 28]. Our results confirm that the K303R mutant is inherently hypersensitivity to estrogen, and only WT receptor showed increased sensitivity to estrogen after forskolin treatment. We used the same experimental approach to test for altered Tam activity in the presence of forskolin. Tam treatment inhibited transcriptional responses in WT, but at statistically significant lower levels in mutant cells. Forskolin treatment blocked Tam-induced promoter condensation, suggesting that ligand-independent kinase signaling to the mutant receptor decreased tamoxifen sensitivity.
Clinical studies have reported that breast tumors with HER2 amplification show reduced levels of PR [77], and the absence of PR is a marker of a more aggressive phenotype [78]. Patients whose tumors lack PR derive less benefit from adjuvant hormonal therapy [30–32]. In vitro studies suggest that amplified growth factor signaling may underlie the reduction of PR levels in breast cancer cells. [79]. In this report we found that PR protein expression was almost undetectable in K303R cells under basal conditions, and estrogen induced only a small increase in PR content, compared to the induction elicited in WT-expressing cells. The lack of PR expression observed in K303R mutant-expressing cells may be the consequence of altered growth factor signaling that contributed to the Tam-resistant phenotype observed in our model system.
We conclude that the K303R ERα hypersensitive phenotype involves an integration of post-translational modification events, such as phosphorylation at S305, with enhanced bidirectional cross-talk between the mutant and growth factor receptors such as HER2, and that genomic and nongenomic mechanisms probably contribute to Tam resistance. Because our molecular and biological data demonstrate that the ERα mutation may be resistant to Tam, we suggest that the mutation is a potential novel predictive marker of hormonal response in breast cancer tumors. In addition, our molecular studies suggest that use of a specific blocking peptide to prevent S305 phosphorylation of the mutant may reduce ligand-independent activity, and be a new therapeutic approach to treat patients with mutation-positive tumors which are resistant to Tam therapy.
Acknowledgements
The authors would like to thank A. Beyer for expert technical, and R. Brown for administrative assistance. This work was supported by NIH/NCI CA72038 to SAWF, and by an AIRC grant 2007 to CG.
References
- 1.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Malley BW. Molecular biology. Little molecules with big goals. Science. 2006;313(5794):1749–1750. doi: 10.1126/science.1132509. [DOI] [PubMed] [Google Scholar]
- 3.Bjornstrom L, Sjoberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 4.Fuqua SAW, Wiltschke C, Zhang QX, Borg A, Castles CG, Friedrichs WE, Hopp T, Hilsenbeck S, Mohsin S, O’Connell P, Allred DC. A hypersensitive estrogen receptor-α mutation in premalignant breast lesions. Cancer Research. 2000;60(15):4026–4029. [PubMed] [Google Scholar]
- 5.Tebbit CL, Bentley RC, Olson JA, Jr, Marks JR. Estrogen receptor alpha (ESR1) mutant A908G is not a common feature in benign and malignant proliferations of the breast. Genes Chromosomes Cancer. 2004;40(1):51–54. doi: 10.1002/gcc.20017. [DOI] [PubMed] [Google Scholar]
- 6.Davies MP, O’Neill PA, Innes H, Sibson DR. Hypersensitive K303R oestrogen receptor-alpha variant not found in invasive carcinomas. Breast Cancer Res. 2005;7(1):R113–R118. doi: 10.1186/bcr965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herynk MH, Parra I, Cui Y, Beyer A, Wu MF, Hilsenbeck SG, Fuqua SA. Association between the estrogen receptor alpha A908G mutation and outcomes in invasive breast cancer. Clin Cancer Res. 2007;13(11):3235–3243. doi: 10.1158/1078-0432.CCR-06-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conway K, Parrish E, Edmiston SN, Tolbert D, Tse CK, Geradts J, Livasy CA, Singh H, Newman B, Millikan RC. The estrogen receptor-alpha A908G (K303R) mutation occurs at a low frequency in invasive breast tumors: results from a population-based study. Breast Cancer Res. 2005;7(6):R871–R880. doi: 10.1186/bcr1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Y, Zhang M, Pestell R, Curran EM, Welshons WV, Fuqua SA. Phosphorylation of estrogen receptor alpha blocks its acetylation and regulates estrogen sensitivity. Cancer Res. 2004;64(24):9199–9208. doi: 10.1158/0008-5472.CAN-04-2126. [DOI] [PubMed] [Google Scholar]
- 10.Michalides R, Griekspoor A, Balkenende A, Verwoerd D, Janssen L, Jalink K, Floore A, Velds A, van’t Veer L, Neefjes J. Tamoxifen resistance by a conformational arrest of the estrogen receptor alpha after PKA activation in breast cancer. Cancer Cell. 2004;5(6):597–605. doi: 10.1016/j.ccr.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Wang RA, Mazumdar A, Vadlamudi RK, Kumar R. P21-activated kinase-1 phosphorylates and transactivates estrogen receptor-alpha and promotes hyperplasia in mammary epithelium. EMBO J. 2002;21(20):5437–5447. doi: 10.1093/emboj/cdf543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rayala SK, Talukder AH, Balasenthil S, Tharakan R, Barnes CJ, Wang RA, Aldaz M, Khan S, Kumar R. P21-activated kinase 1 regulation of estrogen receptor-alpha activation involves serine 305 activation linked with serine 118 phosphorylation. Cancer Res. 2006;66(3):1694–1701. doi: 10.1158/0008-5472.CAN-05-2922. [DOI] [PubMed] [Google Scholar]
- 13.Fan P, Wang J, Santen RJ, Yue W. Long-term treatment with tamoxifen facilitates translocation of estrogen receptor alpha out of the nucleus and enhances its interaction with EGFR in MCF-7 breast cancer cells. Cancer Res. 2007;67(3):1352–1360. doi: 10.1158/0008-5472.CAN-06-1020. [DOI] [PubMed] [Google Scholar]
- 14.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17(3):309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 15.Song RX, Santen RJ. Membrane initiated estrogen signaling in breast cancer. Biol Reprod. 2006;75(1):9–16. doi: 10.1095/biolreprod.105.050070. [DOI] [PubMed] [Google Scholar]
- 16.Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res. 2001;7(12 Suppl):4429s–4435s. discussion 4411s-4412s. [PubMed] [Google Scholar]
- 17.Nicholson RI, McClelland RA, Robertson JF, Gee JM. Involvement of steroid hormone and growth factor cross-talk in endocrine response in breast cancer. Endocr Relat Cancer. 1999;6(3):373–387. doi: 10.1677/erc.0.0060373. [DOI] [PubMed] [Google Scholar]
- 18.Schiff R, Massarweh S, Shou J, Osborne CK. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9(1 Pt 2):447S–454S. [PubMed] [Google Scholar]
- 19.Schiff R, Massarweh SA, Shou J, Bharwani L, Mohsin SK, Osborne CK. Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res. 2004;10(1 Pt 2):331S–336S. doi: 10.1158/1078-0432.ccr-031212. [DOI] [PubMed] [Google Scholar]
- 20.Arpino G, Green SJ, Allred DC, Lew D, Martino S, Osborne CK, Elledge RM. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: a southwest oncology group study. Clin Cancer Res. 2004;10(17):5670–5676. doi: 10.1158/1078-0432.CCR-04-0110. [DOI] [PubMed] [Google Scholar]
- 21.Berry DA, Muss HB, Thor AD, Dressler L, Liu ET, Broadwater G, Budman DR, Henderson IC, Barcos M, Hayes D, Norton L. HER-2/neu and p53 expression versus tamoxifen resistance in estrogen receptor-positive, node-positive breast cancer. J Clin Oncol. 2000;18(20):3471–3479. doi: 10.1200/JCO.2000.18.20.3471. [DOI] [PubMed] [Google Scholar]
- 22.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Tenorio G, Stal O. Activation of AKT/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br J Cancer. 2002;86(4):540–545. doi: 10.1038/sj.bjc.6600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gee JM, Robertson JF, Ellis IO, Nicholson RI. Phosphorylation of ERK1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int J Cancer. 2001;95(4):247–254. doi: 10.1002/1097-0215(20010720)95:4<247::aid-ijc1042>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 25.Nicholson S, Halcrow P, Sainsbury JR, Angus B, Chambers P, Farndon JR, Harris AL. Epidermal growth factor receptor (EGFr) status associated with failure of primary endocrine therapy in elderly postmenopausal patients with breast cancer. Br J Cancer. 1988;58(6):810–814. doi: 10.1038/bjc.1988.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharp ZD, Mancini MG, Hinojos CA, Dai F, Berno V, Szafran AT, Smith KP, Lele TP, Ingber DE, Mancini MA. Estrogen-receptor-alpha exchange and chromatin dynamics are ligand- and domain-dependent. J Cell Sci. 2006;119(Pt 19):4101–4116. doi: 10.1242/jcs.03161. [DOI] [PubMed] [Google Scholar]
- 27.Berno V, Amazit L, Hinojos C, Zhong J, Mancini MG, Sharp ZD, Mancini MA. Activation of estrogen receptor-alpha by E2 or EGF induces temporally distinct patterns of large-scale chromatin modification and mRNA transcription. PLoS ONE. 2008;3(5):e2286. doi: 10.1371/journal.pone.0002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berno V, Hinojos CA, Amazit L, Szafran AT, Mancini MA. High-resolution, high-throughput microscopy analyses of nuclear receptor and coregulator function. Methods Enzymol. 2006;414:188–210. doi: 10.1016/S0076-6879(06)14011-2. [DOI] [PubMed] [Google Scholar]
- 29.Smith CL, Conneely OM, O’Malley BW. Modulation of the ligand-independent activation of the human estrogen receptor by hormone and antihormone. Proc Natl Acad Sci U S A. 1993;90(13):6120–6124. doi: 10.1073/pnas.90.13.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21(10):1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 31.Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, Carter RD, Rivkin SE, Borst JR, Belt RJ, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992;10(8):1284–1291. doi: 10.1200/JCO.1992.10.8.1284. [DOI] [PubMed] [Google Scholar]
- 32.Elledge RM, Green S, Pugh R, Allred DC, Clark GM, Hill J, Ravdin P, Martino S, Osborne CK. Estrogen receptor (ER) and progesterone receptor (PgR), by ligand-binding assay compared with ER, PgR and pS2, by immuno-histochemistry in predicting response to tamoxifen in metastatic breast cancer: a Southwest Oncology Group Study. Int J Cancer. 2000;89(2):111–117. [PubMed] [Google Scholar]
- 33.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL. Efficacy of letrozole extended adjuvant therapy according to estrogen receptor and progesterone receptor status of the primary tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J Clin Oncol. 2007;25(15):2006–2011. doi: 10.1200/JCO.2006.09.4482. [DOI] [PubMed] [Google Scholar]
- 34.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–7735. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci U S A. 2000;97(3):1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hopp TA, Weiss HL, Hilsenbeck SG, Cui Y, Allred DC, Horwitz KB, Fuqua SA. Breast cancer patients with progesterone receptor PR-A-rich tumors have poorer disease-free survival rates. Clin Cancer Res. 2004;10(8):2751–2760. doi: 10.1158/1078-0432.ccr-03-0141. [DOI] [PubMed] [Google Scholar]
- 37.Migliaccio A, Di Domenico M, Castoria G, Nanayakkara M, Lombardi M, de Falco A, Bilancio A, Varricchio L, Ciociola A, Auricchio F. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res. 2005;65(22):10585–10593. doi: 10.1158/0008-5472.CAN-05-0912. [DOI] [PubMed] [Google Scholar]
- 38.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci U S A. 2002;99(23):14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ. Linkage of rapid estrogen action to MAPK activation by ERalpha-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16(1):116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 40.Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61(16):5985–5991. [PubMed] [Google Scholar]
- 41.Herynk MH, Beyer AR, Cui Y, Weiss H, Anderson E, Green TP, Fuqua SA. Cooperative action of tamoxifen and c-Src inhibition in preventing the growth of estrogen receptor-positive human breast cancer cells. Mol Cancer Ther. 2006;5(12):3023–3031. doi: 10.1158/1535-7163.MCT-06-0394. [DOI] [PubMed] [Google Scholar]
- 42.Nicholson RI, Hutcheson IR, Harper ME, Knowlden JM, Barrow D, McClelland RA, Jones HE, Wakeling AE, Gee JM. Modulation of epidermal growth factor receptor in endocrine-resistant, oestrogen receptor-positive breast cancer. Endocr Relat Cancer. 2001;8(3):175–182. doi: 10.1677/erc.0.0080175. [DOI] [PubMed] [Google Scholar]
- 43.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 44.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 45.Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Mol Endocrinol. 2006;20(12):3120–3132. doi: 10.1210/me.2006-0068. [DOI] [PubMed] [Google Scholar]
- 46.Kim MY, Woo EM, Chong YT, Homenko DR, Kraus WL. Acetylation of estrogen receptor alpha by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol Endocrinol. 2006;20(7):1479–1493. doi: 10.1210/me.2005-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L. Sumoylation of the estrogen receptor alpha hinge region regulates its transcriptional activity. Mol Endocrinol. 2005;19(11):2671–2684. doi: 10.1210/me.2005-0042. [DOI] [PubMed] [Google Scholar]
- 48.Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68(1):1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 49.Karas RH, Gauer EA, Bieber HE, Baur WE, Mendelsohn ME. Growth factor activation of the estrogen receptor in vascular cells occurs via a mitogen-activated protein kinase-independent pathway. J Clin Invest. 1998;101(12):2851–2861. doi: 10.1172/JCI1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Encarnacion CA, Ciocca DR, McGuire WL, Clark GM, Fuqua SA, Osborne CK. Measurement of steroid hormone receptors in breast cancer patients on tamoxifen. Breast Cancer Res Treat. 1993;26(3):237–246. doi: 10.1007/BF00665801. [DOI] [PubMed] [Google Scholar]
- 51.Brunner N, Frandsen TL, Holst-Hansen C, Bei M, Thompson EW, Wakeling AE, Lippman ME, Clarke R. MCF7/LCC2: a 4-hydroxytamoxifen resistant human breast cancer variant that retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Res. 1993;53(14):3229–3232. [PubMed] [Google Scholar]
- 52.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr Rev. 2004;25(6):869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 53.Conway K, Parrish E, Edmiston SN, Tolbert D, Tse CK, Moorman P, Newman B, Millikan RC. Risk factors for breast cancer characterized by the estrogen receptor alpha A908G (K303R) mutation. Breast Cancer Res. 2007;9(3):R36. doi: 10.1186/bcr1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274(13):8865–8874. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
- 55.Wang LM, Kuo A, Alimandi M, Veri MC, Lee CC, Kapoor V, Ellmore N, Chen XH, Pierce JH. ErbB2 expression increases the spectrum and potency of ligand-mediated signal transduction through ErbB4. Proc Natl Acad Sci U S A. 1998;95(12):6809–6814. doi: 10.1073/pnas.95.12.6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samanta A, LeVea CM, Dougall WC, Qian X, Greene MI. Ligand and p185c–neu density govern receptor interactions and tyrosine kinase activation. Proc Natl Acad Sci U S A. 1994;91(5):1711–1715. doi: 10.1073/pnas.91.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev. 2008;29(2):217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24(2):85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 59.Nicholson RI, Gee JM, Knowlden J, McClelland R, Madden TA, Barrow D, Hutcheson I. The biology of antihormone failure in breast cancer. Breast Cancer Res Treat 80 Suppl. 2003;1:S29–S34. doi: 10.1023/a:1025467500433. discussion S35. [DOI] [PubMed] [Google Scholar]
- 60.Hutcheson IR, Knowlden JM, Madden TA, Barrow D, Gee JM, Wakeling AE, Nicholson RI. Oestrogen receptor-mediated modulation of the EGFR/MAPK pathway in tamoxifen-resistant MCF-7 cells. Breast Cancer Res Treat. 2003;81(1):81–93. doi: 10.1023/A:1025484908380. [DOI] [PubMed] [Google Scholar]
- 61.Kurokawa H, Lenferink AE, Simpson JF, Pisacane PI, Sliwkowski MX, Forbes JT, Arteaga CL. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000;60(20):5887–5894. [PubMed] [Google Scholar]
- 62.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 63.De Laurentiis M, Arpino G, Massarelli E, Ruggiero A, Carlomagno C, Ciardiello F, Tortora G, D’Agostino D, Caputo F, Cancello G, Montagna E, Malorni L, Zinno L, Lauria R, Bianco AR, De Placido S. A meta-analysis on the interaction between HER-2 expression and response to endocrine treatment in advanced breast cancer. Clin Cancer Res. 2005;11(13):4741–4748. doi: 10.1158/1078-0432.CCR-04-2569. [DOI] [PubMed] [Google Scholar]
- 64.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95(5):353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 65.Yeon CH, Pegram MD. Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. Invest New Drugs. 2005;23(5):391–409. doi: 10.1007/s10637-005-2899-8. [DOI] [PubMed] [Google Scholar]
- 66.Tokunaga E, Oki E, Nishida K, Koga T, Egashira A, Morita M, Kakeji Y, Maehara Y. Trastuzumab and breast cancer: developments and current status. Int J Clin Oncol. 2006;11(3):199–208. doi: 10.1007/s10147-006-0575-4. [DOI] [PubMed] [Google Scholar]
- 67.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89(10):4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Britton DJ, Hutcheson IR, Knowlden JM, Barrow D, Giles M, McClelland RA, Gee JM, Nicholson RI. Bidirectional cross talk between ERalpha and EGFR signaling pathways regulates tamoxifen-resistant growth. Breast Cancer Res Treat. 2006;96(2):131–146. doi: 10.1007/s10549-005-9070-2. [DOI] [PubMed] [Google Scholar]
- 69.Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between Her2/neu and cell membrane estrogen receptor in breast cancer. Int J Cancer. 2002;97(3):306–312. doi: 10.1002/ijc.1614. [DOI] [PubMed] [Google Scholar]
- 70.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23(5):1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270(5241):1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 72.Martin MB, Franke TF, Stoica GE, Chambon P, Katzenellenbogen BS, Stoica BA, McLemore MS, Olivo SE, Stoica A. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology. 2000;141(12):4503–4511. doi: 10.1210/endo.141.12.7836. [DOI] [PubMed] [Google Scholar]
- 73.Nawaz Z, Lonard DM, Dennis AP, Smith CL, O’Malley BW. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci U S A. 1999;96(5):1858–1862. doi: 10.1073/pnas.96.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zwart W, Griekspoor A, Berno V, Lakeman K, Jalink K, Mancini M, Neefjes J, Michalides R. PKA-induced resistance to tamoxifen is associated with an altered orientation of ERalpha towards co-activator SRC-1. EMBO J. 2007;26(15):3534–3544. doi: 10.1038/sj.emboj.7601791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balasenthil S, Barnes CJ, Rayala SK, Kumar R. Estrogen receptor activation at serine 305 is sufficient to upregulate cyclin D1 in breast cancer cells. FEBS Lett. 2004;567(2–3):243–247. doi: 10.1016/j.febslet.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 76.Varricchio L, Migliaccio A, Castoria G, Yamaguchi H, de Falco A, Di Domenico M, Giovannelli P, Farrar W, Appella E, Auricchio F. Inhibition of estradiol receptor/Src association and cell growth by an estradiol receptor alpha tyrosine-phosphorylated peptide. Mol Cancer Res. 2007;5(11):1213–1221. doi: 10.1158/1541-7786.MCR-07-0150. [DOI] [PubMed] [Google Scholar]
- 77.Dowsett M, Harper-Wynne C, Boeddinghaus I, Salter J, Hills M, Dixon M, Ebbs S, Gui G, Sacks N, Smith I. HER-2 amplification impedes the antiproliferative effects of hormone therapy in estrogen receptor-positive primary breast cancer. Cancer Res. 2001;61(23):8452–8458. [PubMed] [Google Scholar]
- 78.Balleine RL, Earl MJ, Greenberg ML, Clarke CL. Absence of progesterone receptor associated with secondary breast cancer in postmenopausal women. Br J Cancer. 1999;79(9–10):1564–1571. doi: 10.1038/sj.bjc.6690249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cui X, Zhang P, Deng W, Oesterreich S, Lu Y, Mills GB, Lee AV. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003;17(4):575–588. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]