Abstract
Stroke is a leading cause of morbidity and mortality worldwide. Atrial fibrillation (AF) is an independent risk factor for stroke, increasing the risk five-fold. Strokes in patients with AF are more likely than other embolic strokes to be fatal or cause severe disability and are associated with higher healthcare costs, but they are also preventable. Current guidelines recommend that all patients with AF who are at risk of stroke should receive anticoagulation. However, despite this guidance, registry data indicate that anticoagulation is still widely underused. With a focus on the 2012 update of the European Society of Cardiology (ESC) guidelines for the management of AF, the Action for Stroke Prevention alliance writing group have identified key reasons for the suboptimal implementation of the guidelines at a global, regional, and local level, with an emphasis on access restrictions to guideline-recommended therapies. Following identification of these barriers, the group has developed an expert consensus on strategies to augment the implementation of current guidelines, including practical, educational, and access-related measures. The potential impact of healthcare quality measures for stroke prevention on guideline implementation is also explored. By providing practical guidance on how to improve implementation of the ESC guidelines, or region-specific modifications of these guidelines, the aim is to reduce the potentially devastating impact that stroke can have on patients, their families and their carers.
Keywords: Atrial fibrillation, Guidelines, Oral anticoagulants, Stroke prevention
Introduction
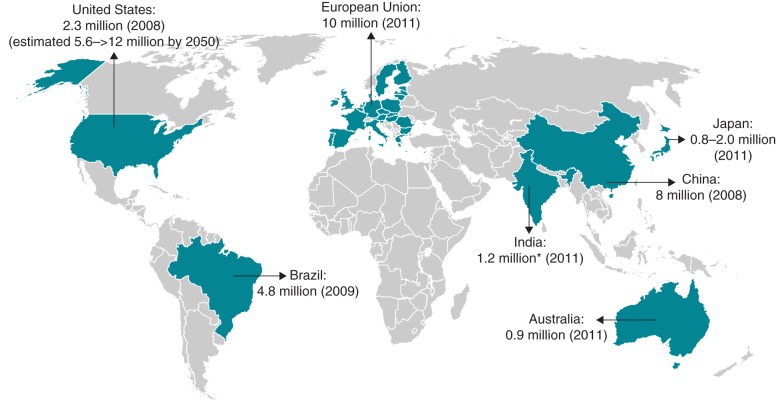
In 2012, stroke was estimated to have contributed to the deaths of ∼6.7 million people worldwide, accounting for nearly 12% of all deaths.1 Stroke causes permanent disability in nearly 5 million people each year.2 Atrial fibrillation (AF) is a significant independent risk factor for stroke, associated with an approximately five-fold excess in risk, but is much less well recognized than, for example, hypertension, for which the excess stroke risk is three-fold.3 In addition, unlike most other cardiovascular risk factors for stroke, the pivotal Framingham Study found an increase in attributable risk due to AF from 1.5% in individuals aged 50–59 years to 23.5% for those aged 80–89 years.3 Atrial fibrillation is the most common sustained cardiac arrhythmia and represents a global problem (Figure 1).4–8 Patients with AF are five times more likely to have a stroke than the general population.3 Atrial fibrillation-related strokes are almost twice as likely to be fatal and, in survivors, cause severe disability, increase the length of hospital stay and decrease the likelihood of patients returning to their own home, compared with non-AF-related strokes.9,10 Atrial fibrillation-related strokes have also been associated with significantly higher mean direct costs per patient than non-AF-related strokes.11 However, AF-related strokes can be prevented and their impact minimized by effective management strategies including increased detection of AF, adherence to stroke prevention guidelines and anticoagulant use in at-risk patients. Left atrial appendage occlusion may also have a role in patients who are unable to receive long-term anticoagulant management, but it is not a recommended alternative to anticoagulation per se.12
Figure 1.
Estimates of the prevalence of AF.4–8 *Based on data from a single community study.
Guidelines have an important role in optimizing evidence-based care, improving health outcomes for individuals and populations and decreasing costs to healthcare systems. Despite the risk of stroke in patients with AF, and the availability of clear global guidelines on the prevention of AF-related stroke since 1999, real-world data suggest that a large proportion of patients [38% of those with a CHADS2 (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke/transient ischaemic attack (doubled)) score of 2 in the Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF) Registry] are still not receiving stroke prophylaxis in line with guideline recommendations.13 With the increased convenience and improved benefit–risk profile of the non-vitamin K antagonist oral anticoagulants (NOACs; rivaroxaban, dabigatran, apixaban, and edoxaban), this situation may improve.
This consensus document aims to identify barriers to guideline implementation worldwide and to define clear strategies and practice models to help overcome these barriers, focusing on the European Society of Cardiology (ESC) guidelines for the management of AF published in EP Europace in 2012.12 A Medline search was performed to identify guidelines for stroke prevention in patients with AF and barriers to guideline implementation, focusing on registry data but including individual studies where relevant. The Action for Stroke Prevention alliance writing committee also provided their own country-specific experiences and, based on the collated information, identified key barriers to guideline implementation and developed consensus strategies to help overcome these barriers.
Guidelines overview
A focused update of the 2010 ESC guidelines for the management of AF was issued in 2012.12 This was partly in response to positive Phase III clinical trial data with the NOACs dabigatran, rivaroxaban, and apixaban,14–16 and their subsequent approval for stroke prevention in at-risk patients with non-valvular AF. The NOACs have shown equivalent or improved efficacy compared with warfarin in randomized controlled trials, with a reduction in the risk of severe bleeding events, in particular intracranial haemorrhage (ICH).14–17 In addition, they all offer fixed-dose regimens (with some dose reductions mandated in special populations, such as patients with renal impairment) that eliminate the need for the routine coagulation monitoring associated with vitamin K antagonists (VKAs). Edoxaban is currently the only one of these NOACs that is not yet widely approved in this indication. The ESC guidelines now recommend the use of the NOACs in most patients with a CHA2DS2-VASc [Congestive heart failure/left ventricular dysfunction, Hypertension, Age ≥75 years (doubled) Diabetes, Stroke (doubled), Vascular disease, Age 65–74 years, Sex category (female)] score of ≥1 in preference to VKAs (Figure 2), although in certain patients, e.g. those with severe renal impairment or underlying disease on echocardiogram, VKAs remain preferred. Antithrombotic treatment is not recommended in low-risk patients with a CHA2DS2-VASc score of 0.12 However, the latter group represented only 3–7% of patients in two large cohort studies,13,18 indicating that the majority of patients with AF are candidates for oral anticoagulation.
Figure 2.
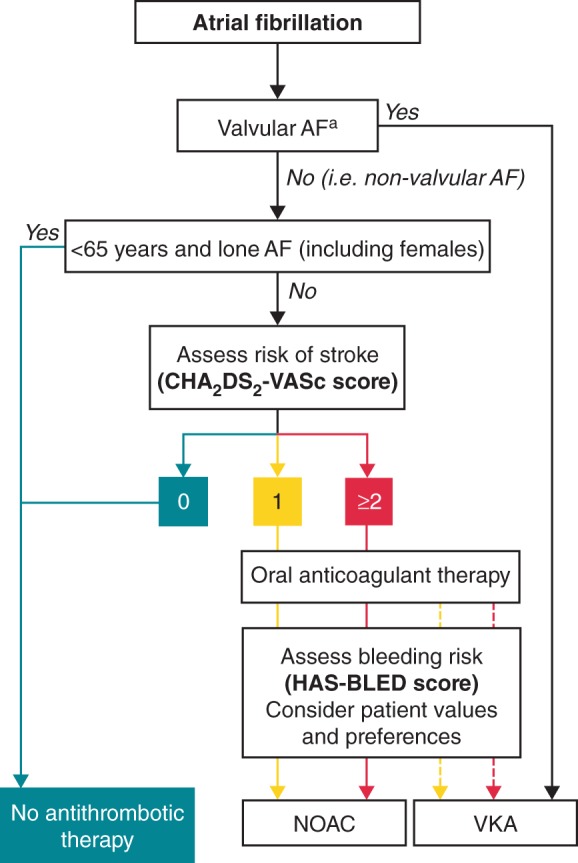
European Society of Cardiology (ESC) guideline recommendations for the prevention of stroke in patients with AF.12 Antiplatelet therapy with ASA plus clopidogrel, or—less effectively—ASA only, should be considered in patients who refuse any oral anticoagulant or cannot tolerate anticoagulants for reasons unrelated to bleeding. If there are contraindications to oral anticoagulation or antiplatelet therapy, left atrial appendage occlusion, closure, or excision may be considered. CHA2DS2-VASc score: turquoise, 0; yellow, 1; red, ≥2. Line: solid, best option; dashed, alternative option. aIncludes rheumatic valvular disease and prosthetic valves. AF, atrial fibrillation; ASA, acetylsalicylic acid; CHA2DS2-VASc, ESC-recommended stroke risk score [Congestive heart failure/left ventricular dysfunction, Hypertension, Age ≥75 years (doubled) Diabetes, Stroke (doubled), Vascular disease, Age 65–74 years, Sex category (female)]; HAS-BLED, [ESC-recommended bleeding risk score, defined as Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly (e.g. age >65 years, frailty, etc.), Drugs/alcohol use]; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist. From Camm et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. European Heart Journal Nov 2012, 33(21) 2719–2747. Reproduced with permission of Oxford University Press (UK) © European Society of Cardiology, www.escardio.org/guidelines
In large Phase III studies, the currently approved NOACs showed similar or improved efficacy compared with warfarin for the prevention of primary stroke or systemic embolism.19–21 Several of the NOACs have also demonstrated benefits in the prevention of secondary strokes, with similar efficacy to warfarin, as well as reducing the incidence of ICH.19–21 The risk of a stroke recurrence is 2.5-fold higher in patients with AF who have already had a stroke or transient ischaemic attack.22 These patients may also be at increased risk of falls, have dementia or have limited access to international normalized ratio (INR) monitoring because of decreased mobility, making it problematic to ensure effective VKA therapy.
In addition to incorporation of the NOACs into the recommendations, another significant change to the ESC guidelines was a move away from the use of the CHADS2 score to risk-stratify patients with AF, in favour of the CHA2DS2-VASc score. This was based on evidence that the CHA2DS2-VASc score could be used to more accurately identify truly ‘low-risk’ patients, who would not require antithrombotic therapy.12
The 2012 guidelines also recommend the use of the HAS-BLED [Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly (e.g. age >65 years, frailty, etc.), Drugs/alcohol use] score to assess bleeding risk, but highlight that it should focus efforts on improving the modifiable risk factors for bleeding and should not be used to exclude patients from oral anticoagulant therapy.12
The simultaneous use of old and new guidelines, or of guidelines issued by different organizations, at either a global or regional level, can lead to a degree of confusion or contradictory guidance. In addition, recommendations may differ between guidelines because of variations in the populations for which they are intended. However, recommendations for antithrombotic therapy are, for the most part, consistent between the major guidelines. In 2012, the American College of Chest Physicians (ACCP) issued guidelines on antithrombotic therapy for AF, which provide similar recommendations to the ESC guidelines (based on CHADS2 scoring for risk assessment), including a preference for NOACs over adjusted-dose VKAs in at-risk patients.23 Older guidelines, such as those jointly issued by the American College of Cardiology (ACC), the American Heart Association (AHA), and the ESC in 2006, were developed long before the NOACs were available to clinicians and, in contrast with newer guidelines, recommend acetylsalicylic acid (ASA) as an alternative to warfarin in patients with one moderate risk factor for stroke.24 Updated ACC/AHA guidance, issued in 2014, recommends oral anticoagulation for patients with prior stroke/transient ischaemic attack or CHA2DS2-VASc score ≥2, whereas patients with a score of 1 may be given oral anticoagulation, ASA, or no antithrombotic therapy.25 In 2013, the European Heart Rhythm Association published a practical guide on the use of NOACs in patients with non-valvular AF26 based on the 2012 ESC recommendations.
Country-specific guidelines, such as those from the National Institute for Health and Care Excellence (NICE) in the UK, recommend apixaban, dabigatran, and rivaroxaban as options for the prevention of stroke or systemic embolism in patients with AF.27 A focused update of the Canadian Cardiovascular Society (CCS) Atrial Fibrillation Guidelines, published in 2012, recommends NOACs over other oral anticoagulants in eligible patients.28 In the Asia-Pacific region, several countries have country-specific guidelines for the management of patients with AF but not all yet incorporate recommendations for the use of NOACs. However, the 2014 Japanese guidelines largely support the ESC 2012 guidelines, as does a consensus statement issued in 2013 by the Asia Pacific Heart Rhythm Society on antithrombotic therapy in patients with non-valvular AF.29 Information provided by other organizations, such as the National Stroke Association in the UK or the Canadian Agency for Drugs and Technologies in Health, who issue therapeutic reviews, may also influence clinical practice at a national level.
Guideline implementation and barriers to implementation
Benefits of compliance with guidelines
A criticism of guidelines is that they sometimes rely on evidence that is relatively weak and/or studies that have been conducted with inconsistent methodologies. However, the evidence for the use of NOACs in patients with non-valvular AF comes from very large, multicentre, randomized controlled Phase III studies,14–16 and the primary recommendations made by the ESC in 2012 on anticoagulation in patients with AF generally carried a high level (A or B) of evidence.12 Nevertheless, prospective clinical data for the NOACs are lacking in some specific areas; e.g. a prospective randomized study of rivaroxaban for patients with AF undergoing cardioversion has been published recently,30 but a similar trial for apixaban (NCT02100228) is only just getting underway, and there is no such prospective study planned for dabigatran. Studies to address other data gaps with the NOACs are ongoing.
Published studies support the benefits of compliance with guideline recommendations for anticoagulation in patients with AF. A cross-sectional study of patients admitted to the University of Maastricht Medical Centre between 2003 and 2006 found that 51% of those with known AF who were eligible for oral anticoagulation did not receive it, and that improved adherence to guidelines could potentially have prevented 22% of subsequent ischaemic strokes.31 More recently, a real-world cohort-based study of almost 9000 patients recruited over 10 years found low annual rates of stroke or thromboembolism (0.64%), major bleeding (1.12%), and death (1.08%) among untreated patients classified as low risk according to the ESC 2012 guidelines (i.e. CHA2DS2-VASc score 0).18 These data support the ESC recommendation that these patients should not receive antithrombotic therapy. Furthermore, simulations of patient outcomes from the RE-LY database using ESC-recommended dabigatran treatment protocols found a significant net clinical benefit compared with warfarin, supporting the ESC recommendations.32
Compliance with guidelines in practice
Registry data offer valuable insights into patterns of drug use and can provide a means of tracking uptake of guideline recommendations. For example, global data from the GARFIELD-AF Registry, collected between 2009 and 2011, indicated that ∼27% of all patients with AF were still receiving ASA therapy, compared with ∼60% who received oral anticoagulation (Table 1).13 However, observational data from the European PREFER AF (PREvention oF thromboembolic events—European Registry in Atrial Fibrillation) registry collected between 2012 and 2013 suggest that the introduction of the 2010 ESC guidelines was associated with a move away from the use of ASA to oral anticoagulation.40 Other European registry data show variation in oral anticoagulation use depending on when the data were collected and from which country or countries (Table 1).35–41 Outside of Europe, US registry data indicate a low use of oral anticoagulation across all stroke risk categories.43 Data from the REACH registry (REduction of Atherothrombosis for Continued Health) demonstrated the lower use of oral anticoagulation in patients with AF recruited from Asia (excluding Japan) compared with those recruited from Japan and non-Asian regions.45 These differences highlight that oral anticoagulant use is not yet consistent at a global, country, or regional level and may be influenced by factors such as the specialty of the physicians enrolling patients into the registries and the degree of awareness of updated guidelines.
Table 1.
Atrial fibrillation registries and surveys
| Registry or study | Guidelines | Patients with AF, n | Country/region | Data collection, year | OAC use (%) |
|---|---|---|---|---|---|
| GARFIELD-AF13 | Multiple | 10 614 | Global | 2009–2011 | ∼60 |
| RE-LY AF33 | Multiple | 15 400 | Global | 2008–2011 | 30 |
| GLORIA-AF34 | Multiple | ∼56 000 | Global | 2011 onwards | Awaiting data |
| Euro Heart Survey on AF35 | ACC/AHA/ESC 2001 and ACCP 2004 | 5333 | Europe | 2003–2004 | 64 |
| AFNET36 | ACC/AHA/ESC 2001 | 9582 | Germany | 2004–2006 | 71 |
| ATRIUM37 | ACC/AHA/ESC 2001 and ACCP 2008 | 3667 | Germany | 2009 | 83 |
| Prospective non-interventional study38 | Not specified | 2753 | Germany | 2010 | 64–73a |
| ISAF39 | Not specified | 6036 | Italy | 2011 | 46 |
| PREFER AF42 | ESC 2010 | 7243 | Europe | 2012–2013 | 82 |
| Retrospective, cohort study43 | ACC/AHA/ESC 2006 and ACCP 2008 | 171 393 | USA | 2003–2007 | 43 |
| ORBIT-AF44 | ACC/AHA/ESC 2006 and ACCP 2008 | 10 098 | USA | 2010–2011 | 76 |
| REACH45 | Not specified | ∼300 | Asia (ex. Japan) | 2006–2011 | 36 |
| REACH45 | Not specified | ∼350 | Japan | 2006–2011 | 54 |
| REACH45 | Not specified | ∼6000 | Global (ex. Asia) | 2006–2011 | 55 |
ACC, American College of Cardiology; ACCP, American College of Chest Physicians; AF, atrial fibrillation; AFNET, Central Registry of the German Competence NETwork on Atrial Fibrillation; AHA, American Heart Association; ATRIUM, Outpatient Registry Upon Morbidity of Atrial Fibrillation; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke/transient ischaemic attack (doubled); ESC, European Society of Cardiology; GARFIELD-AF, Global Anticoagulant Registry in the FIELD; GLORIA-AF, Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation; ISAF, Italian Network of Atrial Fibrillation Management survey; OAC, oral anticoagulant; ORBIT-AF, Outcomes Registry for Better Informed Treatment of Atrial Fibrillation; PREFER AF, PREvention oF thromboembolic events—European Registry in Atrial Fibrillation; REACH, REduction of Atherothrombosis for Continued Health; RE-LY AF, Randomized Evaluation of Long-term anticoagulant TherapY.
aCHADS2 score ≥2 (includes low molecular weight heparin).
Patient and physician-related barriers
Registries can also provide useful information on the reasons for non-adherence to guidelines and identify barriers to their adoption. GARFIELD-AF highlighted that nearly half of patients with a CHADS2 score ≥2 were not receiving VKA therapy because of physician choice, specifically because of concerns over bleeding risk, patient compliance, uncertainty regarding guideline recommendations, fall risk or low risk of stroke.13 Patient factors, such as alcohol misuse, medication refusal, unsuitable co-medication use, or previous bleeding events, were the reasons for not initiating VKA therapy in <16% of cases.13 Similar reasons were given for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF).46 Similarly, in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE) A trial, which enrolled patients who were considered unsuitable for VKA therapy, the primary reason for enrolment in the trial (accounting for ∼50% of patients) was physician judgement that a VKA was inappropriate. Patient preference accounted for ∼26% of patients enrolled, and specific risk of bleeding for ∼23% of patients.47 In the Apixaban vs. Acetylsalicylic Acid to Prevent Strokes in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) study, the most frequent reasons for not prescribing VKAs were physician judgement that INR monitoring could not be achieved at the requisite frequency (43%) and patient preference (37%).48
The Stroke and Atrial Fibrillation Ensemble (SAFE) II study found that having a younger general practitioner and being followed up by a cardiologist were independently associated with the prescription of oral anticoagulants.49 The presence of potential contraindications, lack of an indication, low compliance, and fear of bleeding were reasons given for non-prescription.49 These findings highlight that the lack of physician awareness about oral anticoagulation and how to manage complications is a key barrier to adoption of guideline-recommended therapies. A general survey of Dutch general practitioners also identified environmental factors such as organizational constraints, lack of time, and lack of resources as prominent barriers to guideline adherence.50 Furthermore, uptake of NOACs may be limited by a physician preference for close INR monitoring, particularly in elderly or frail patients.
Another barrier to the adoption of guidelines arises when they are not considered applicable at a country level, possibly because of regional heterogeneity in patient baseline characteristics (e.g. a high proportion of patients not meeting the criteria for recommended treatments or a perception that the studies underpinning recommendations are based on non-representative populations), differences in current standards of care or cultural perceptions of risk vs. benefit of the intervention. For example, in some Asian countries, such as Japan, the recommended target INR in patients with AF ≥70 years of age is lower (1.6–2.6) than the recommended target of 2.0–3.0, which is used more widely.51 Similarly, a lower 15 mg once-daily dose of rivaroxaban (or 10 mg once-daily in patients with creatinine clearance 30–49 mL/min) was specifically tested in Japanese patients.52
Access to guideline-recommended therapies
In some countries, prescribing of the NOACs is restricted at a national, regional, or local level. For example, the National Health Service for Scotland limits the prescription of rivaroxaban to patients who appear compliant with coumarin therapy and yet still have poor INR control, as well as to those who are allergic to or are unable to tolerate coumarins.53 In the UK, the Department of Health follows the NICE guidelines rather than the ESC guidelines. However, even though the NOACs are recommended as a therapeutic option by NICE, many clinical commissioning groups or regional prescribing groups in the UK interpret this ‘option’ as second line, with warfarin compulsory as first-line therapy.54,55 In the UK and Ireland, patients who are closer to the hospital and can attend clinics regularly for coagulation monitoring and dose adjustments are also more likely to receive VKAs than those who live further away. In Eastern European countries (e.g. Hungary), the National Health Service limits the prescription of NOACs to patients who have had a previous stroke or patients with poor INR control on coumarin therapy.56 This restriction is in place because the first-line use of the NOACs is considered financially prohibitive.
Restricted access can also be a result of administrative barriers. For example, in Italy and Hungary, a limited number of specialists are allowed to prescribe the NOACs; to finalize the prescription, these specialists are required to fill out an electronic case report form, contributing to a compulsory national survey (www.agenziafarmaco.gov.it). This is a time-consuming process and, as such, the national Regulatory Authorities have indirectly discouraged routine implementation of the ESC guidelines for antithrombotic treatment. The bureaucratic situation in Italy is mirrored in other countries: in Ireland and some parts of England, justification forms must be completed to allow physicians to prescribe the NOACs and, in Spain, patients must have an INR that is recorded to be out of range three times in a row before a patient can be prescribed any of these drugs, and this may take weeks.
Access issues can also arise when updates to guidelines are delayed. For example, the European Stroke Organisation (ESO) has not updated its guidelines on secondary stroke prevention since 2008,57 and so the NOACs are not included. This influences the daily practice of stroke specialists: in some Eastern European countries, for instance, physicians face financial penalties or even imprisonment if they are not compliant with the guidelines recommended for their specialty, even if the guidelines are outdated and do not reflect the latest clinical advances in the field. In cases such as these, pre-existing guidelines, though relevant when they were published, are themselves a barrier to adopting new approaches or therapies. An update to the ESO guidelines is anticipated.
Even if guidelines are up to date, it does not necessarily guarantee access to recommended therapies. For example, a group of Spanish medical experts developed a consensus document in line with the ESC guidelines that was approved by the Spanish Ministry of Health for national use.58 However, access to NOACs is still often restricted by local authorities because warfarin is considered to be as effective as the NOACs and is a low-cost drug, albeit with high monitoring costs. Therefore, the newer drugs are often limited to patients who are unstable on warfarin or who have had an ischaemic stroke and are at high risk of ICH.
Financial barriers to guideline-recommended therapies
One of the major reasons for restricted access to new guideline-recommended therapies is perceived cost. Restrictions tend to be made based on a consideration of short-term budget impact, such as the lower acquisition costs of VKAs compared with the NOACs, rather than the potential longer term economic impact of events that might have been prevented. Non-vitamin K antagonist oral anticoagulants do not require routine coagulation monitoring, which, in one US study, was shown to cost between $291 and $943 annually per patient.59 The National Institute for Health and Care Excellence has estimated the annual cost of INR monitoring, including transport costs, at £656 in the first year and £540 thereafter.60 Longer term cost savings relate to the direct costs of managing the consequences of anticoagulation, which are summarized in Table 2. For example, considering that NOACs reduce the risk of ICH by at least half compared with warfarin, their use could contribute substantially to long-term cost savings (Table 3). A study in Denmark published in 1999 estimated that, after ICH, the mean total cost of healthcare and social services during the first year was 123 200 DKK or US$22 000.63 More recently, a study in the USA analysing medicine and pharmacy claims for patients with AF estimated the mean unadjusted all-cause health costs in the year after a warfarin claim to be $41 903 for patients with at least one ICH.64 Despite this, it is often difficult in practical terms to implement a scheme in which a more expensive therapy is paid for from one budget (in this case, drug costs) to provide cost savings that relate to a separate budget (blood tests and monitoring).
Table 2.
Overview of event costs. Adapted from Kleintjens et al.61
| Eventa | Acute (per event) (€) | Rehabilitation (per event)b (€) | Long-term follow-up (per 3 months) (€) |
|---|---|---|---|
| Minor stroke | 5946 | 3204 | 244 |
| Major stroke | 12 247 | 17 734 | 2216 |
| Systemic embolism | 5124 | Not reported | Not reported |
| Clinically relevant non-major extracranial bleeding event | 23 | Not reported | Not reported |
| Major extracranial bleeding event | 3510 | Not reported | Not reported |
| Intracranial bleeding eventc | 7699 | 17 734 | 2216 |
| Myocardial infarction | 7891 | Not reported | Not reported |
aThe range of event costs tested in sensitivity analyses was ±25% of the mean.
bBased on unpublished results (K. Putman, personal communication).
cBased on market share and prices of locally available brands.
Table 3.
Absolute percentage annual risk of ICH stratified by stroke risk in patients with non-valvular AF receiving oral anticoagulation therapy for stroke prevention. Adapted from Rognoni et al.62
| Intracranial bleeding, absolute annual risk (%) |
|||
|---|---|---|---|
| CHADS2 ≤ 1 | CHADS2 = 2 | CHADS2 ≥ 3 | |
| Warfarin | 0.48 | 0.65 | 1.01 |
| Rivaroxaban (20 mg od) | Not investigated | 0.44 | 0.68 |
| Apixaban (5 mg bid) | 0.2 | 0.27 | 0.42 |
| Dabigatran (150 mg bid) | 0.2 | 0.26 | 0.52 |
bid, twice daily; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke/transient ischaemic attack (doubled); od, once daily; ICH, intracranial haemorrhage.
The lack of monitoring of the NOACs may also represent a financial barrier to their implementation. In some countries, primary care practices and anticoagulation clinics receive a financial incentive for providing VKA monitoring services, which could be considered under threat with the introduction of the NOACs. However, with appropriate training of personnel, these facilities could be repurposed to take on a role in the initiation and management of the NOACs and management of co-morbidities and to provide valuable guidance and reassurance to patients about their anticoagulation care. Indeed, overall risk factor control, not limited to the use of NOACs, is the most important therapeutic intervention for patients with AF.
Financial barriers to appropriate VKA management specific to individual countries or regions also exist. In many Eastern and Central European countries, both INR monitoring and travel costs (for blood sampling) are covered by the healthcare system. However, blood sampling takes place in large centralized laboratories that are often a long distance from where the patient lives, meaning that substantial work time is lost through travelling to and from appointments; this can also have a negative impact on patient compliance.
In Brazil and many South East Asian countries, only 30% of patients have private health insurance and, therefore, have access to the newer therapies for stroke prevention in patients with AF. In contrast, patients treated in the public sector typically continue to receive warfarin or another VKA because these drug costs are supported by the healthcare system. In Brazil and Mexico, the local authorities can subsidize more expensive medications, but it takes time to get new therapies included on these lists and, because it is not a national responsibility, differences in access exist between local authorities.
In cost-effectiveness analyses, the NOACs have been shown to be cost-effective compared with warfarin.61,65–74 Robust and well-designed cost-effectiveness analyses can be important when arguing the case for consideration of new therapeutic options with policy makers. However, these analyses are not without limitations. Their general applicability can be limited because of differences in healthcare systems between countries. Furthermore, cost-effectiveness analyses may be inadequate because they do not take into consideration all factors, particularly indirect costs including loss of work for patients or carers due to INR clinic visits and associated travel costs.75 Additionally, even demonstrating the cost-effectiveness of a drug within the parameters defined in the analysis may not provide a comparison of cost-effectiveness relative to other established treatments.
In addition to their cost-effectiveness, the relative effectiveness and safety of the NOACs compared with the VKAs is an important benefit. The convenience to patients in terms of lack of monitoring and dietary restrictions, which is likely to improve persistence and adherence and therefore clinical outcomes, should also not be underestimated.
Best practice in European Society of Cardiology guideline implementation
Registry data highlight that it can take several years for guideline recommendations to be implemented in clinical practice, and even then, recommendations may not be applied properly or consistently. It is also clear that there is wide variation between countries regarding which recommendations are implemented and how this is achieved. When the writing committee rated the importance of certain factors for guideline implementation, variation between countries did exist, but factors such as AF screening, diagnosis, and stroke risk assessment were rated of high importance across the group.
The barriers to guideline implementation that the group identified (summarized in Table 4) fall into three main categories: practical, educational, and access related. Practical issues relate primarily not only to optimal diagnosis and risk stratification of patients with AF but also to the applicability of the ESC guidelines to non-European populations. Lack of awareness of the ESC guidelines and delays in updates to local or national guidelines are the key educational barriers that exist, despite the efforts of the ESC to promote its guidelines through various methods, including ‘train the teacher’ programmes, ESC guideline implementation toolkits, and mobile pocket guidelines. Another educational barrier stems from the variety of information available about the NOACs, which can lead to confusion over their specific properties and use protocols. Access-related issues are primarily due to cost and also include other factors that have been discussed above.
Table 4.
Barriers to implementation of ESC 2012 guidelines
| Barrier |
|---|
| Practical |
|
| Educational |
|
| Access |
|
AF, atrial fibrillation; HAS-BLED, Hypertension, Abnormal liver/renal function, Stroke history, Bleeding predisposition, Labile INR, Elderly (age >65 years), Drug/alcohol use; NOAC, non-vitamin K antagonist oral anticoagulant; VKA, vitamin K antagonist; INR, international normalized ratio.
In light of these barriers, the Action for Stroke Prevention alliance writing committee recommends several best-practice strategies (summarized in Table 5) to improve adherence to the ESC guidelines (or their region-specific modification) and access to guideline-recommended therapies.
Table 5.
Best-practice strategies for implementation of the ESC 2012 guidelines and rationale for such strategies
| Strategy | Rationale |
|---|---|
| Practical | |
| Develop hospital and department protocols and checklists based on national/local guidelines and implement quality indicators | Provides clinical practical guidance for day-to-day management of patients with AF and allows measurement of guideline adherence |
| Regular multidisciplinary team meetings and local quality audits | Allows assessment of individual patients and can act as an internal check to ensure they are being managed in line with guideline recommendations Enhances peer-to-peer learning experience |
| Plan follow-up visits and laboratory check-ups | Ensures patients are compliant with guideline-recommended therapy and reduces the risk of complications |
| Provide clear practical guidance on the use of NOACs | Provides reassurance for physicians not experienced in the use of these drugs |
| Implement CHA2DS2-VASc and bleeding risk checklists before prescribing NOACs and at every follow-up visit | Ensures identification of patients suitable for antithrombotic therapy and those at increased risk of bleeding |
| Implement compliance checks, e.g. specific questions, pill ‘counting’, diary completion, SMS messages or alarm calls to take tablets | Ensures patients are compliant with guideline-recommended therapy, improves adherence and reduces the risk of complications |
| Educational | |
| Regularly disseminate ESC/national and local guideline information and updates | Raises awareness of guidelines |
| Develop timely country-specific/local guidelines based on the ESC recommendations | Allows recognition of country-specific requirements, such as access, so that guidelines are compatible with local conditions |
| Re-train/educate nurses currently involved in anticoagulation/warfarin clinics to take on a more general role in initiation and management of NOACs | Can provide an established point of contact through which patients can receive advice on anticoagulation with the NOACs |
| Develop simple algorithms for specific populations of patients with AF, as per Figure 2 (e.g. post-ischaemic stroke, post-haemorrhagic stroke, geriatric patients) | Provides guidance on when and how to start NOACs and for how long in these patients |
| Inform physicians on how to educate patients on the importance of adherence to therapy | Limits the likelihood of non-adherence to guideline-recommended protocols |
| Access | |
| Approach the responsible person within your healthcare system to: Highlight to key target groups (e.g. budget holders, policy makers, formulary gate keepers, the media, patient groups) the potential impact of not providing access to guideline-recommended therapies, from both financial and clinical perspectives |
Raises awareness that AF is a significant risk factor for stroke and that AF-related stroke is preventable |
| Perform country-specific cost-effectiveness analyses of the NOACs Educate payers/budget holders about better utilization of anticoagulants, including NOACs, highlighting potential long-term cost benefits |
Provides payers/budget holders with more robust evidence to consider the use of the NOACs as first-line therapy – |
| Inform politicians, patient groups and the media about differences in access to AF stroke prevention treatment within regions or countries Lobby parliamentary and healthcare bodies for equality of access to guideline-recommended therapies globally or across regions |
Puts pressure on policy makers to provide equality of care for stroke prevention in patients with AF with regards to medication |
AF, atrial fibrillation; CHA2DS2-VASc, Congestive heart failure/left ventricular dysfunction, Hypertension, Age ≥75 years (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65–74 years, Sex category (female); NOAC, non-vitamin K antagonist oral anticoagulant; SMS, short message service; ESC, European Society of Cardiology.
Measuring healthcare quality for stroke prevention: potential role in guideline implementation
Registries provide information on whether guidelines have been implemented. However, could healthcare quality measures be used to drive implementation of, and adherence to, guidelines as they are issued? Unfortunately, data on the use of healthcare quality measures to improve stroke prevention in patients with AF are generally limited, although national audit data are available in some countries, highlighting gaps in care for stroke prevention.76,77
In the USA, there are several initiatives aimed at assessing the quality of stroke care, including the Stroke Practice Improvement Network, The Paul Coverdell National Acute Stroke Registry, and the ‘Get with the Guidelines' programme. Likewise, in Australia, the national prescribing service MedicineWise is a programme that aims to improve cardiovascular management in primary care. However, although the use of healthcare quality measures has shown improvements in care and adherence to guidelines in some cases, methods for implementing these improvements have varied and the results have not been consistent.78–80 In Japan, the Japanese Stroke Databank is a patient oriented, academically controlled database aimed at establishing rigorous evidence for the quality improvement of stroke care (http://cvddb.med.shimane-u.ac.jp). Physicians register data on their own academic incentive and the databank is endorsed by the representative of stroke patients, providing a successful collaboration between patients and physicians to overcome stroke. In the UK, the Quality and Outcomes Framework incentivizes providers for the provision of high-quality care, against explicit clinical indicators and defined targets, and helps to standardize improvements in the delivery of primary medical services. This system is currently being used to improve records of patients with AF and their ongoing management and could be applied to improving the use of NOACs for stroke prevention in patients with AF.
Until validated and standardized healthcare quality measures for stroke prevention in patients with AF are developed, the role of such measures in driving ESC guideline implementation is limited. However, measures developed at a local or hospital level could be of benefit and warrant further investigation.
Limitations of these recommendations
It should be noted that, although the majority of the evidence cited in this document comes from published clinical studies or real-world investigations, some of the country-specific information, and the barriers identified as a result, are based on personal evidence provided by writing committee members. The recommendations provided represent the opinion of the group, and would require practical implementation to test their robustness and applicability to different healthcare scenarios. As noted above, the lack of certain standardized structures may prevent some recommendations from being implemented in practice at this time.
Conclusions
Atrial fibrillation-related stroke is potentially preventable and, through better implementation of the 2012 ESC guidelines, its impact on patients, their families, and carers, and healthcare systems can be reduced. However, this cannot be achieved without increased detection of patients with AF and the increased use of anticoagulation in those assessed to be at moderate or high stroke risk, using either well-controlled warfarin or, preferably, NOACs. Providing patients with a choice of therapy and allowing physicians to prescribe the most appropriate anticoagulant for their patients will only be possible if there is access to all guideline-recommended therapies. In outlining practical measures that physicians and medical health societies working in this field can instigate to improve guideline implementation, we believe that substantial benefits, in terms of improved patient outcomes and reduced healthcare burden, can be achieved.
Funding
This work was supported by editorial support funded by Bayer HealthCare Pharmaceuticals and Janssen Scientific Affairs, LLC. The sponsors provided suggestions and comments, but the content of this document represents the work of the authors.
Conflict of interest: A.J.C.: Speaker/consulting fees from Sanofi, Bayer, Daiichi Sankyo, Boehringer Ingelheim, Pfizer, and Bristol-Myers Squibb. F.J.P.: Speaker/consulting fees from AstraZeneca, Bayer, and Boehringer Ingelheim. G.J.H.: Speaker fees from Bayer. F.A.: Speaker/consulting fees from Amgen, Bayer, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, and Eli-Lilly. F.D.R.H.: Speaker/consulting fees from Bristol-Myers Squibb/Pfizer, Bayer, and Boehringer Ingelheim; grants/sponsorship from Bayer and Daiichi Sankyo. László Csiba: Speaker/consulting fees from Bayer, Boehringer Ingelheim, MSD, sanofi-aventis, and Egis. Gabriel R. de Freitas: Speaker fees from Bayer and Boehringer Ingelheim. Shinya Goto: Honoraria from Eisai, sanofi-aventis, Otsuka, Bayer, Novartis, AstraZeneca, Astellas, Pfizer, Medtronics-Japan, Tanabe-Mitsubishi, Takeda, Daiichi Sankyo, Mochida, and MSD. Werner Hacke: Speaker/consulting fees from Bayer and Daiichi Sankyo. Lorenzo Mantovani: Consulting fees from Bayer; grants from Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, and Daiichi Sankyo.
Acknowledgements
The authors would like to acknowledge Sarah Atkinson, who provided editorial support for this manuscript.
ASP Writing Committee Members
A. John Camm, Fausto J. Pinto, Graeme J. Hankey, Felicita Andreotti, F.D. Richard Hobbs, László Csiba, Gabriel R. de Freitas, Shinya Goto, Carlos Cantú, Jorge Gonzalez-Zuelgaray, Werner Hacke, Han Hwa Hu, Lorenzo Mantovani, Byung-Woo Yoon, Dayi Hu, and Kui-Hian Sim.
Contributor Information
Collaborators: on behalf of the Writing Committee of the Action for Stroke Prevention alliance, A. John Camm, Fausto J. Pinto, Graeme J. Hankey, Felicita Andreotti, F.D. Richard Hobbs, László Csiba, Gabriel R. de Freitas, Shinya Goto, Carlos Cantú, Jorge Gonzalez-Zuelgaray, Werner Hacke, Han Hwa Hu, Lorenzo Mantovani, Byung-Woo Yoon, Dayi Hu, and Kui-Hian Sim
References
- 1.World Health Organization. The top 10 causes of death (Fact sheet no 310). 2014. http://www.who.int/mediacentre/factsheets/fs310/en/index.html (6 January 2015, date last accessed).
- 2.World Heart Federation. The global burden of stroke. 2014. http://www.world-heart-federation.org/cardiovascular-health/stroke/ (6 January 2015, date last accessed).
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am 2008;92:17–40, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace 2011;13:1110–7. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics - 2010 update: a report from the American Heart Association. Circulation 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 7.Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol 2008;52:865–8. [DOI] [PubMed] [Google Scholar]
- 8.Lip GYH, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest 2012;142:1489–98. [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke 1996;27:1765–9. [DOI] [PubMed] [Google Scholar]
- 10.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760–4. [DOI] [PubMed] [Google Scholar]
- 11.Brüggenjürgen B, Rossnagel K, Roll S, Andersson FL, Selim D, Müller-Nordhorn J, et al. The impact of atrial fibrillation on the cost of stroke: the Berlin Acute Stroke Study. Value Health 2007;10:137–43. [DOI] [PubMed] [Google Scholar]
- 12.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Europace 2012;14:1385–413. [DOI] [PubMed] [Google Scholar]
- 13.Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD Registry. PLoS One 2013;8:e63479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 16.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 17.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 18.Taillandier S, Olesen JB, Clémenty N, Lagrenade I, Babuty D, Lip GYH, et al. Prognosis in patients with atrial fibrillation and CHA(2)DS(2)-VASc score = 0 in a community-based cohort study. J Cardiovasc Electrophysiol 2012;23:708–13. [DOI] [PubMed] [Google Scholar]
- 19.Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 2012;11:315–22. [DOI] [PubMed] [Google Scholar]
- 20.Diener HC, Connolly SJ, Ezekowitz MD, Wallentin L, Reilly PA, Yang S, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol 2010;9:1157–63. [DOI] [PubMed] [Google Scholar]
- 21.Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012;11:503–11. [DOI] [PubMed] [Google Scholar]
- 22.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:227–76. [DOI] [PubMed] [Google Scholar]
- 23.You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141:e531S–75S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Europace 2006;8:651–745. [DOI] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Jr, Cigarroa JE, et al. 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 26.Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 2013;15:625–51. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Health and Care Excellence. Clinical guideline 180: the management of atrial fibrillation. 2014. http://www.nice.org.uk/guidance/CG180 (6 January 2015, date last accessed). [PubMed]
- 28.Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol 2012;28:125–36. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa S, Aonuma K, Tse HF, Huang D, Huang JL, Kalman J, et al. The APHRS's 2013 statement on antithrombotic therapy of patients with nonvalvular atrial fibrillation. J Arrhythmia 2014;29:190–200. [Google Scholar]
- 30.Cappato R, Ezekowitz MD, Klein AL, Camm AJ, Ma CS, Le Heuzey JY, et al. Rivaroxaban versus vitamin K antagonists for cardioversion in atrial fibrillation. Eur Heart J 2014; doi:10.1093/eurheartj/ehu367. [DOI] [PubMed] [Google Scholar]
- 31.Pisters R, van Oostenbrugge RJ, Knottnerus IL, de Vos CB, Boreas A, Lodder J, et al. The likelihood of decreasing strokes in atrial fibrillation patients by strict application of guidelines. Europace 2010;12:779–84. [DOI] [PubMed] [Google Scholar]
- 32.Lip GYH, Noack H, Ferreira J, Connolly SJ, Yusuf S. Patient outcomes using the European label for dabigatran. A post-hoc analysis from the RE-LY database. Thromb Haemost 2014;111:933–42. [DOI] [PubMed] [Google Scholar]
- 33.Oldgren J, Healey JS, Ezekowitz M, Commerford P, Avezum A, Pais P, et al. Variations in cause and management of atrial fibrillation in a prospective registry of 15,400 emergency department patients in 46 countries: the RE-LY Atrial Fibrillation Registry. Circulation 2014;129:1568–76. [DOI] [PubMed] [Google Scholar]
- 34.Huisman MV, Lip GYH, Diener HC, Dubner SJ, Halperin JL, Ma CS, et al. Design and rationale of Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J 2014;167:329–34. [DOI] [PubMed] [Google Scholar]
- 35.Nieuwlaat R, Capucci A, Camm AJ, Olsson SB, Andresen D, Davies DW, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J 2005;26:2422–34. [DOI] [PubMed] [Google Scholar]
- 36.Breithardt G, Dobrev D, Doll N, Goette A, Hoffmann B, Kirchhof P, et al. The German Competence Network on Atrial Fibrillation (AFNET). Herz 2008;33:548–55. [DOI] [PubMed] [Google Scholar]
- 37.Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol 2011;100:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosch RF, Kirch W, Theuer JD, Pittrow D, Kohlhaussen A, Willich SN, et al. Atrial fibrillation management, outcomes and predictors of stable disease in daily practice: prospective non-interventional study. Int J Cardiol 2013;167:750–6. [DOI] [PubMed] [Google Scholar]
- 39.Zoni-Berisso M, Filippi A, Landolina M, Brignoli O, D'Ambrosio G, Maglia G, et al. Frequency, patient characteristics, treatment strategies, and resource usage of atrial fibrillation (from the Italian Survey of Atrial Fibrillation Management [ISAF] study). Am J Cardiol 2013;111:705–11. [DOI] [PubMed] [Google Scholar]
- 40. doi: 10.1093/europace/euu225. Olesen JB, Sørensen R, Hansen ML, Lamberts M, Weeke P, Mikkelsen AP et al. Non-vitamin K antagonist oral anticoagulation agents in anticoagulant naïve atrial fibrillation patients: Danish nationwide descriptive data 2011–2013. Europace 2015;17:187–93. [DOI] [PubMed] [Google Scholar]
- 41. doi: 10.1093/europace/eut292. Lip GY, Bongiorni MG, Dobreanu D, Lewalter T, Hastrup Svendsen J, Blomström-Lundqvist C et al. Novel oral anticoagulants for stroke prevention in atrial fibrillation: results of the European Heart Rhythm Association survey. Europace 2013;15:1526–32. [DOI] [PubMed] [Google Scholar]
- 42.Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events--European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014;16:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimetbaum PJ, Thosani A, Yu HT, Xiong Y, Lin J, Kothawala P, et al. Are atrial fibrillation patients receiving warfarin in accordance with stroke risk? Am J Med 2010;123:446–53. [DOI] [PubMed] [Google Scholar]
- 44.Cullen MW, Kim S, Piccini JP, Sr, Ansell JE, Fonarow GC, Hylek EM, et al. Risks and benefits of anticoagulation in atrial fibrillation: insights from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circ Cardiovasc Qual Outcomes 2013;6:461–9. [DOI] [PubMed] [Google Scholar]
- 45.Goto S, Ikeda Y, Chan JCN, Wilson PWF, Cheng Yeo T, Liau CS, et al. Risk-factor profile, drug usage and cardiovascular events within a year in patients with and at high risk of atherothrombosis recruited from Asia as compared with those recruited from non-Asian regions: a substudy of the REduction of Atherothrombosis for Continued Health (REACH) registry. Heart Asia 2011;3:93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Brien EC, Simon DN, Allen LA, Singer DE, Fonarow GC, Kowey PR, et al. Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J 2014;168:487–94. [DOI] [PubMed] [Google Scholar]
- 47.The ACTIVE Investigators. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med 2009;360:2066–78. [DOI] [PubMed] [Google Scholar]
- 48.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. [DOI] [PubMed] [Google Scholar]
- 49.Deplanque D, Leys D, Parnetti L, Schmidt R, Ferro J, De Reuck J, et al. Secondary prevention of stroke in patients with atrial fibrillation: factors influencing the prescription of oral anticoagulation at discharge. Cerebrovasc Dis 2006;21:372–9. [DOI] [PubMed] [Google Scholar]
- 50.Lugtenberg M, Burgers JS, Besters CF, Han D, Westert GP. Perceived barriers to guideline adherence: a survey among general practitioners. BMC Fam Pract 2011;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Japanese Circulation Society Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2008): digest version. Circ J 2010;74:2479–500. [DOI] [PubMed] [Google Scholar]
- 52.Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study. Circ J 2012;76:2104–11. [DOI] [PubMed] [Google Scholar]
- 53.Scottish Medicines Consortium. Rivaroxaban 15 and 20 mg film-coated tablets (Xarelto®) SMC No. (756/12). 2012. http://www.scottishmedicines.org.uk/files/advice/rivaroxaban_Xarelto_for_AF_FINAL_Jan_2012_for_website.pdf (6 January 2015, date last accessed).
- 54.Northamptonshire Prescribing Advisory Group. Implementation of NICE TAs 249, 256 and 275. 2013. http://www.neneccg.nhs.uk/resources/uploads/files/implementation-of-nice-tas-249-256-and-275-dabigatran-rivaroxaban-and-apixaban-april-2013-final.pdf (6 January 2015, date last accessed).
- 55.NHS Dorset Clinical Commissioning Group. Position statement on oral anticoagulants in atrial fibrillation. 2014. http://www.dorsetccg.nhs.uk/Downloads/aboutus/medicines-management/Other%20Guidelines/NOACs%20in%20AF.pdf (December 2014, date last accessed).
- 56.Lenti L, Brainin M, Titianova E, Morovic S, Demarin V, Kalvach P, et al. Stroke care in Central Eastern Europe: current problems and call for action. Int J Stroke 2013;8:365–71. [DOI] [PubMed] [Google Scholar]
- 57.The European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 58.Agencia Española de Medicamentos y Productos Sanitarios. Criterios y recomendaciones generales para el uso de nuevos anticoagulantes orales en la prevención del ictus y la embolia sistémica en pacientes con FA no valvular. Informe de utilidad terapéutica 24 de septiembre de 2012 2012. http://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/criterios-anticoagulantes-orales.pdf (8 January 2015, date last accessed).
- 59.Biskupiak J, Ghate SR, Jiao T, Brixner D. Cost implications of formulary decisions on oral anticoagulants in nonvalvular atrial fibrillation. J Manag Care Pharm 2013;19:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Institute for Health and Clinical Excellence. Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism. Technology appraisal 261 2012. http://www.nice.org.uk/ta261 (26 January 2015, date last accessed).
- 61.Kleintjens J, Li X, Simoens S, Thijs V, Goethals M, Rietzschel ER, et al. Cost-effectiveness of rivaroxaban versus warfarin for stroke prevention in atrial fibrillation in the Belgian healthcare setting. Pharmacoeconomics 2013;31:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014;34:9–17. [DOI] [PubMed] [Google Scholar]
- 63.Porsdal V, Boysen G. Direct costs during the first year after intracerebral hemorrhage. Eur J Neurol 1999;6:449–54. [DOI] [PubMed] [Google Scholar]
- 64.Ghate SR, Biskupiak J, Ye X, Kwong WJ, Brixner DI. All-cause and bleeding-related health care costs in warfarin-treated patients with atrial fibrillation. J Manag Care Pharm 2011;17:672–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.National Institute for Health and Clinical Excellence. Rivaroxaban for the prevention of stroke and systemic embolism in people with atrial fibrillation. Technology appraisal TA256. 2012. http://www.nice.org.uk/ta256 (6 January 2015, date last accessed).
- 66.National Institute for Health and Clinical Excellence. Dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation. Technology appraisal TA249 2012. http://www.nice.org.uk/ta249 (6 January 2015, date last accessed). [DOI] [PubMed]
- 67.National Institute for Health and Care Excellence. Apixaban for preventing stroke and systemic embolism in people with nonvalvular atrial fibrillation. Technology appraisal TA275 2013. http://www.nice.org.uk/guidance/TA275 (6 January 2015, date last accessed).
- 68.Harrington AR, Armstrong EP, Nolan PE, Jr, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 2013;44:1676–81. [DOI] [PubMed] [Google Scholar]
- 69.Lee S, Anglade MW, Pham D, Pisacane R, Kluger J, Coleman CI. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol 2012;110:845–51. [DOI] [PubMed] [Google Scholar]
- 70.Kansal AR, Sorensen SV, Gani R, Robinson P, Pan F, Plumb JM, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 2012;98:573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1–11. [DOI] [PubMed] [Google Scholar]
- 72.Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011;123:2562–70. [DOI] [PubMed] [Google Scholar]
- 73.Sorensen SV, Kansal AR, Connolly S, Peng S, Linnehan J, Bradley-Kennedy C, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation: a Canadian payer perspective. Thromb Haemost 2011;105:908–19. [DOI] [PubMed] [Google Scholar]
- 74.Adcock AK, Lee-Iannotti JK, Aguilar MI, Hoffman-Snyder CR, Wingerchuk DM, Wellik KE, et al. Is dabigatran cost effective compared with warfarin for stroke prevention in atrial fibrillation?: a critically appraised topic. Neurologist 2012;18:102–7. [DOI] [PubMed] [Google Scholar]
- 75.Schulman S, Anderson DR, Bungard TJ, Jaeger T, Kahn SR, Wells P, et al. Direct and indirect costs of management of long-term warfarin therapy in Canada. J Thromb Haemost 2010;8:2192–200. [DOI] [PubMed] [Google Scholar]
- 76.Royal College of Physicians. Stroke improvement national audit programme (SINAP). 2012. http://www.rcplondon.ac.uk/sites/default/files/sinap-comprehensive-public-report-2012.pdf (6 January 2015, date last accessed).
- 77.Canadian Stroke Network. The quality of stroke care in Canada. 2011. http://www.strokebestpractices.ca/wp-content/uploads/2011/06/QoSC-EN.pdf (6 January 2015, date last accessed).
- 78.Gadzhanova SV, Roughead EE, Bartlett MJ. Improving cardiovascular disease management in Australia: NPS MedicineWise. Med J Aust 2013;199:192–5. [DOI] [PubMed] [Google Scholar]
- 79.Hinchey JA, Shephard T, Tonn ST, Ruthazer R, Hermann RC, Selker HP, et al. The Stroke Practice Improvement Network: a quasiexperimental trial of a multifaceted intervention to improve quality. J Stroke Cerebrovasc Dis 2010;19:130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwamm LH, Fonarow GC, Reeves MJ, Pan W, Frankel MR, Smith EE, et al. Get With the Guidelines-Stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation 2009;119:107–15. [DOI] [PubMed] [Google Scholar]