Abstract
AMPA receptor GluA2 subunits are strongly implicated in cognition, and prior work suggests that these subunits may be regulated by atypical protein kinase C (aPKC) isoforms. The present study assessed whether hippocampal and cortical AMPA receptor GluA2 subunit regulation may be an underlying factor in known age-related differences to cognitive-impairing doses of ethanol, and if aPKC isoforms modulate such responses. Hippocampal AMPA receptor GluA2 subunit, PKMζ, and PKCı/λ expression were elevated during adolescence compared to adults. 1 hour following a low dose (1.0 g/kg) ethanol exposure, hippocampal AMPA receptor GluA2 subunit serine 880 phosphorylation was decreased in adolescents, but was increased in adults. Age-dependent changes in GluA2 subunit phosphorylation were paralleled by alterations in aPKC isoforms, and zeta inhibitory peptide (ZIP) administration prevented ethanol-induced increases in both in adults. Ethanol-induced changes in GluA2 subunit phosphorylation were associated with delayed regulation in synaptosomal GluA2 subunit expression 24 hours later. A higher ethanol dose (3.5 g/kg) failed to elicit changes in most measures in the hippocampus at either age. Similar to the hippocampus, analysis of cerebral cortical tissue also revealed age-related declines. However, no demonstrable effects were found following a low dose ethanol exposure at either age. High dose ethanol exposure reduced adolescent GluA2 subunit phosphorylation and aPKC isoform expression that were again accompanied by delayed reductions in synaptosomal GluA2 subunit expression. Together, these results suggest that GluA2-containing AMPA receptor modulation by aPKC isoforms is age-, region- and dose-dependently regulated, and may potentially be involved in developmentally regulated ethanol-induced cognitive impairment and other ethanol behaviors.
Keywords: Adolescence, Ethanol, Protein Kinase C (PKC), PKMζ, PKCı/λ, AMPA receptors GluA2 subunits
1. INTRODUCTION
Long-term potentiation (LTP) is essential for cognitive processes and intricately involves the regulation of synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors. In particular, AMPA receptor GluA2 subunit expression (formerly GluR2) is a predictor of memory performance and is imperative for the maintenance of long-term memories (Takahashi et al., 2003, McCormack et al., 2006, Kessels and Malinow, 2009). Reductions in AMPA receptor GluA2 containing receptors have been directly linked to, or associated with, cognitive performance in a number of behavioral paradigms, including hippocampal dependent tasks, fear, and drug-associated memories (Wiltgen et al., 2010, Venkitaramani et al., 2011, Liu et al., 2010, Yu et al., 2013, Liu et al., 2012b).
Phosphorylation involving protein kinase C (PKC) is implicated in synaptic AMPA receptor trafficking, and the serine 880 (ser880) residue has been identified as the major phosphorylation site for PKC (McDonald et al., 2001). Numerous mammalian PKC isoforms exist that are grouped into either conventional (c), novel (n) or atypical (a) PKC isoforms. Recent work postulates that aPKC isoforms may be involved in GluA2 subunit phosphorylation as supported by pharmacological investigation using partially-selective PKC inhibitors (States et al., 2008). The aPKC family includes PKCζ, PKMζ and PKCı/λ. Notably, PKMζ is a constitutively active PKC isoform that consists of only the catalytic domain of PKCζ generated from internal promoters within the PRKCZ gene (Sacktor, 2012), and only PKMζ and PKCı/λ are found prominently expressed in cortical and hippocampal brain regions (Oster et al., 2004, Hernandez et al., 2003, Lee et al., 2013).
Multiple studies employing viral overexpression, knockdown, or inhibition by ZIP demonstrate that PKMζ aides in GluA2 subunit cell surface stability, LTP, as well as the maintenance of spatial orientation, taste, fear, and memories related to drugs of abuse (Yao et al., 2008, Migues et al., 2010, Li et al., 2011, Parsons and Davis, 2011, Shema et al., 2007, Pastalkova et al., 2006). PKMζ has been shown to increase GluA2 stability, but such effects are suggested to be indirect by promoting interactions with N-ethylmaleimide sensitive factor (NSF) and blocking PICK1-mediated internalization (Yao et al., 2008). However, direct aPKC involvement in ser880 phosphorylation has yet to be investigated. Furthermore, although PKMζ‘s involvement in GluA2 regulation and memory is promising, recent reports call into question the role of PKMζ in cognition as PRKCZ knockout mice display normal learning and memory compared to controls (Lee et al., 2013, Volk et al., 2013). Adding to this controversy, ZIP has been reported to have equal efficacy at both PKMζ and PKCı/λ (Lee et al., 2013), and PKCı/λ may also regulate AMPA receptor phosphorylation, synaptic incorporation, and LTP, as has been shown for GluA1 (Ren et al., 2013). Clearly, more studies are needed to delineate the relationship between aPKC isoforms and GluA2-containing receptors.
Naturally occurring differences, such as age-related responses to ethanol, may better illuminate the relationship between AMPA receptor GluA2 subunits and aPKC isoforms. Adolescence is a critical period of development marked by increases in impulsivity that overlap with initial drug use (Spear, 2010). Multiple behavioral studies demonstrate that adolescents and adults differ in ethanol behavioral sensitivity. Remarkably, they display greater sensitivity to ethanol’s amnestic effects, predominantly in hippocampal-dependent tasks (Land and Spear, 2004, Markwiese et al., 1998), but are largely unimpaired in memory tasks that co-involve the cerebral cortex (Swartzwelder et al., 2012, Rajendran and Spear, 2004). Such behavioral results suggest region-specific modulation by ethanol. Conversely, as working memory and decision-making are enhanced and refined during adolescence (Best and Miller, 2010, Brenhouse and Andersen, 2011), increased ethanol impairment is of particular importance, as neurobiological modifications may impact cognitive functioning well after ethanol is cleared. In fact, chronic ethanol exposure during adolescence has been shown to disrupt cognitive flexibility into adulthood in rats (Semenova, 2012), and recently, sustained effects were noted in human adolescents (Peeters et al., 2014). In addition to impaired cognitive performance, exposure during earlier developmental periods also affects AMPA receptor GluA2 subunit-related LTP and expression (An et al., 2013, Dettmer et al., 2003, Hsiao and Frye, 2003, Bellinger et al., 2002, Wang et al., 2012). Taken together, it is highly possible that aPKC isoform regulation of AMPA receptor GluA2 subunits may be a major factor in differential ethanol effects across age.
Recent evidence from our lab further supports ontogenetic PKC involvement. Not only does expression of cortical PKC isoforms vary across adolescence, but alterations in n- and aPKC isoforms following high-dose ethanol exposure only occurred in adolescents (Santerre et al., 2013). In the present study, we investigated whether ethanol dose-dependent regulation of AMPA receptor GluA2 subunits by aPKCs may account for age-related ethanol disparities. We examined hippocampal and cortical synaptosomal AMPA receptor GluA2 subunit and aPKC isoform expression across adolescence. AMPA receptor GluA2 subunit levels and ser880 phosphorylation as well as aPKC isoform expression was subsequently assessed following low- or high-dose ethanol exposure in adolescents and adults. ZIP was then employed to determine whether ZIP-sensitive kinases modulated AMPA receptor GluA2 phosphorylation. Finally, AMPA receptor GluA2 subunit levels were assessed 24 hours later to determine if ser880 phosphorylation subsequently affected subunit regulation after ethanol elimination.
2. EXPERIMENTAL PROCEDURES
2.1. Animals
Experiments were conducted in accordance with the National Institute of Health Guidelines under Institutional Animal Care and Use Committee-approved protocols at Binghamton University, State University of New York. Adolescent and adult male Sprague-Dawley rats were bred in house at Binghamton University. Postnatal day (P) 28, 35 and 42 correspond with early-, mid- and late-adolescence, respectively whereas P75 was considered adulthood. Rats were maintained on a standard 12 h light–dark schedule with lights on at 7:00 AM. Animals were pair-housed post weaning and had ad libitum access to rat chow and water. All subjects had environmental enrichment consisting of crinkle paper and a wooden block.
2.2. Surgical Procedures
Animals were unilaterally cannulated just dorsal to the anterior hippocampus similar to our previous studies (Gigante et al., 2014, Santerre et al., 2013). Briefly, adult (P68) subjects were anesthetized with 2.5–3.0% isoflurane and placed into a stereotaxic device. A 12.5 mm steel cannula (Plastics One) was positioned using the following coordinates relative to bregma: −3.5 AP, +/− 2.6 ML as noted in Paxinos and Watson’s stereotaxic atlas (2007). Subjects were given Buprenex as an analgesic once immediately post-surgery and second post-operative dose 24 hours later. All subjects were singly housed with added environmental enrichment consisting of a wooden block and crinkle paper. Animals were allowed to recover for one week and were handled daily to check body weights, and cannula patency.
2.3. Drugs and Reagents
Zeta inhibitory peptide (ZIP, Tocris, now R&D Systems, Minneapolis, MN) was administered at 10 nmol per rat at a flow rate of 0.5 μL per min over a two min period. Consistent with prior studies aCSF was utilized as a control (Velez-Hernandez et al., 2013, Howell et al., 2014). Ethanol (Pharmco, Brookfield, CT) was administered intraperitoneally (i.p.) as 20% ethanol in saline.
2.4. Tissue Collection
For assessment of PKMζ and GluR2 subunit expression across ontogeny, rats were sacrificed at predetermined ages (P28, P35, P42 and P75). For acute ethanol studies, rats were injected with ethanol (1.0 or 3.5 g/kg) or saline, and sacrificed at predetermined time points (1 and 24 hours). Time points were based off our prior work demonstrating changes in adolescent PKC isoforms 60 min following ethanol injection (Santerre et al., 2013), as well as studies showing changes in receptor subunit regulation 24-hours following ethanol exposure (Liang et al., 2007), when ethanol is systemically eliminated (Buck et al., 2011). For both studies, the brain was rapidly removed from the skull, flash frozen, and stored at −80°C.
2.5. Sample Preparations
Total cortical and hippocampal tissue were used for assessment. For whole cell samples, following dissection, samples were homogenized in a mixture of 1% sodium dodecyl sulfate (SDS), 1mM ethylenediaminetetraacetic acid (EDTA), and 10mM of Tris (Santerre et al., 2013, Gigante et al., 2014). For P2 synaptosomal samples, following dissection, samples were homogenized in 0.32M sucrose/PBS solution and spun at low speed centrifugation (1,000×g) followed by centrifuging the resulting supernatant at 12,000 × g for 20 min. The pellet (P2 fraction) was resuspended in phosphate buffered saline (PBS). Phosphatase inhibitors were obtained from Sigma-Aldrich (St. Louis, MO) and added to tissue samples at a final concentration of 1%. Protein concentrations of all samples were quantified using a bicinchoninic acid protein assay kit as we have done previously (Thermo Fisher Scientific, Waltham, MA) (Santerre et al., 2013).
2.6. Western blot analysis
Protein samples were subjected to SDS polyacrylamide gel electrophoresis (SDS-PAGE) using Novex Tris–Glycine gels (8–16%) and transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen, Carlsbad, CA, USA). Membranes were probed with antibodies for the following proteins: PKCζ (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), phospho-GluA2 (Ser880) and GluA2 (Millipore, Billerica, MA). The PKCζ antibody used shares a conserved binding residue to PKCı/λ and the smaller kDa protein PKMζ, thus allowing for the quantification of both proteins simultaneously. All blots were subsequently exposed to a second primary antibody directed against β-actin (Millipore) to verify equivalent protein loading and transfer. Samples were run in duplicate or triplicate and averaged. All bands were detected by enhanced chemiluminescence and exposed to autoradiography film under non-saturating conditions (GE Healthcare, Piscataway, NJ, USA) and analyzed using NIH Image J.
2.7. Statistical analysis
All comparisons were made within blots. For age-dependent studies, each group was compared to adult samples (P75). Analyses were conducted using one-way ANOVA with Dunnett’s post hoc test when appropriate. For ethanol exposure time dependent studies, each group was compared to age-matched saline controls at each time point using Student’s t-test. For hippocampal ZIP-associated kinase inhibition studies, data were analyzed by 2-way ANOVA with Fisher’s LSD host hoc. For all experiments, p<0.05 (α=0.05) was considered significant.
3. RESULTS
3.1. Hippocampal AMPA receptor GluA2 subunit and aPKC expression decreases throughout adolescence
We first examined total hippocampal GluA2 subunit expression during early-, mid- and late-adolescence (P28, 35 and 42) relative to adults (P75). Analysis revealed an effect of age for GluA2 in the hippocampus [F3,26=4.210, p<0.05]. Further post-hoc analysis revealed that hippocampal GluA2 expression was elevated by 32.2% and 26.3% during early and mid adolescence, relative to adults (Figure 1A). In tandem, analysis of PKMζ expression revealed an effect of age [F3,27=5.944, p<0.01] with post-hoc analysis also revealing that PKMζ expression was higher during early- and mid-adolescence by 71.3% and 50.2% (Figure 1B). For PKCı/λ, analysis revealed an effect of age [F3,26=3.949, p<0.05], with expression higher during early adolescence by 53.7% relative to adults (Figure 1C).
Figure 1.
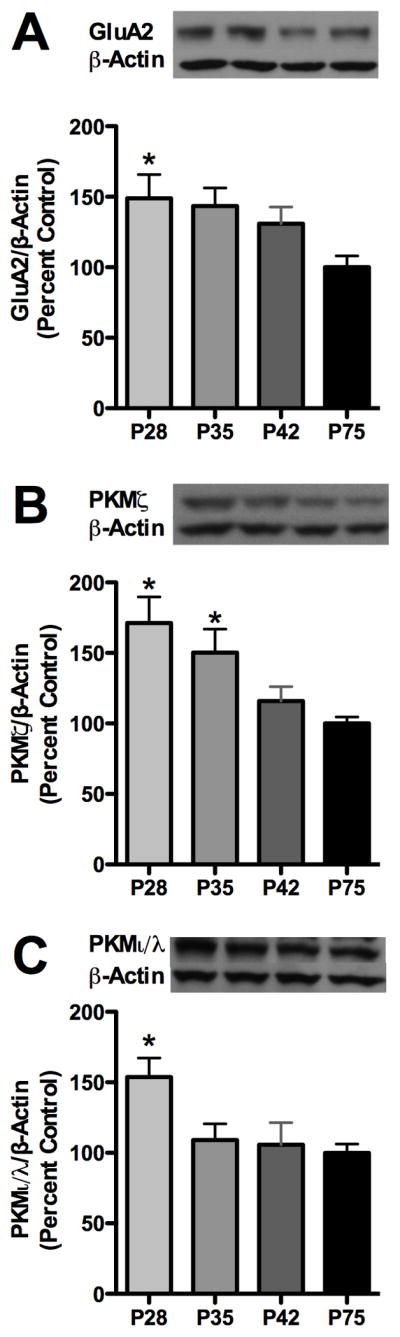
Hippocampal AMPA receptor GluA2 subunit and aPKC isoform synaptosomal expression decreases across adolescence. Graphs depict: (A) GluA2, (B) PKMζ, and (C) PKCι/λ expression. Representative immunoblot images are shown for each. Data is presented as mean ± SEM. *p < 0.05 compared to P75, n = 7–8/group.
3.2 Hippocampal P2 synaptosomal AMPA receptor GluA2 subunit phosphorylation and aPKC expression are age- and dose-dependently regulated following ethanol exposure
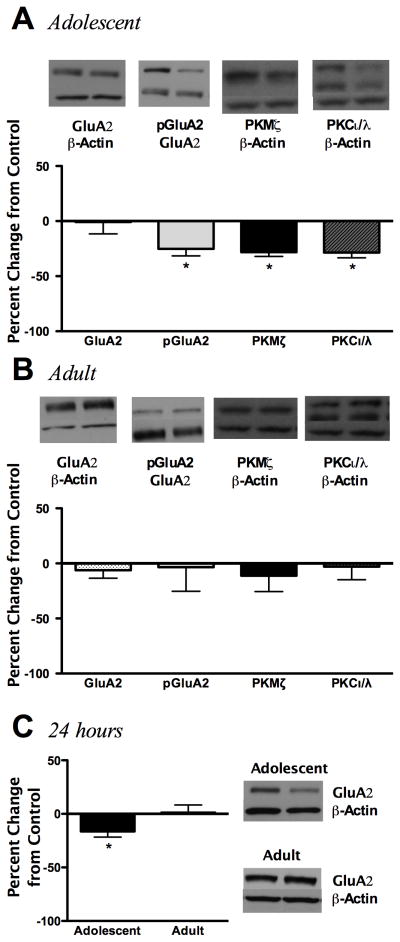
Given that adolescents display greater ethanol-induced cognitive impairment compared to adults, and that AMPA receptor GluA2 subunits play a major role in cognitive behavior, we next investigated whether synaptosomal AMPA receptor GluA2 subunit levels were altered one hour following an low dose ethanol (1.0 g/kg) exposure. Although GluA2 subunit expression did not differ from age-matched controls following ethanol exposure, bidirectional differences were noted for GluA2 ser880 phosphorylation. Adolescents had reduced GluA2 phosphorylation (p<0.01, 39.8%) whereas adults had increased phosphorylation (p<0.05, 57.9%) following ethanol exposure (Figure 2A, 2B). Interestingly, adolescent PKMζ and PKCı/λ also displayed age-related bidirectional changes. Ethanol exposure reduced adolescent PKMζ (p<0.05, 16.3%) and PKCı/λ (p < 0.01, 44.5%), while increasing PKMζ expression, but not PKCı/λ, in adults (p<0.05, 26.6%) (Figure 2A, 2B). To further investigate if hippocampal GluA2 phosphorylation and aPKC expression were ethanol dose-dependent, hippocampal expression patterns were assessed following a high dose ethanol exposure (3.5 g/kg). With the exception of increases in adolescent PKCı/λ (p<0.05, 30.7%), high dose ethanol exposure had no effect on hippocampal AMPA receptor GluA2 subunit expression, phosphorylation or PKMζ expression in adolescents or adults 1 hour following ethanol exposure (Table 1).
Figure 2.
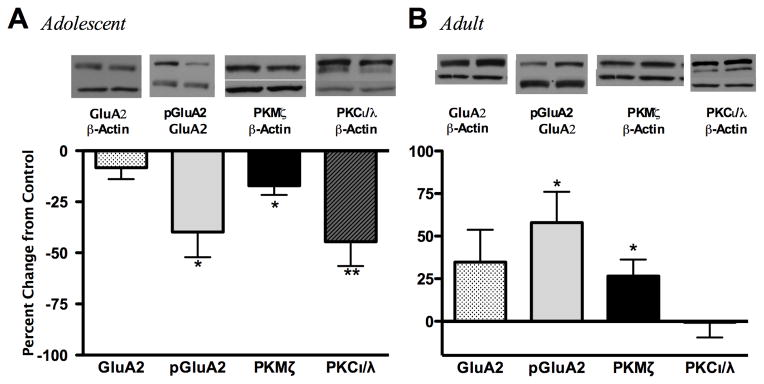
Effects of low dose (1.0 g/kg) ethanol exposure on hippocampal synaptosomal AMPA receptor GluA2 subunit expression and phosphorylation, as well as aPKC isoform expression 1 hour following ethanol administration. Graphs represent adolescents (A) and adults (B). Representative immunoblot images are shown for AMPA receptor GluA2 subunit expression, pGluA2 (serine 880), and PKMζ, PKCı/λ. Data is presented as mean ± SEM. *p < 0.05 compared with age-matched saline controls, n = 8/group.
Table 1.
Hippocampal AMPA receptor GluA2 subunit and aPKC isoform expression 1 or 24 hours following high dose ethanol. Values are expressed as percent control. Data are presented as mean ± S.E.M. n = 8 per group
| Hippocampus (3.5 g/kg) | ||
|---|---|---|
| Adolescent | Adult | |
| GluA2 | −14.5 ± 13.1 | 0.8 ± 12.8 |
| pGluA2 | 3.5 ± 18.44 | −5.4 ± 8.9 |
| PKMζ | 2.4 ± 6.8 | −8.8 ± 4.1 |
| PKMι/λ | 31.9 ± 12.5a | −19.0 ± 9.7 |
| GluA2 24h | −6.3 ± 16.0 | −7.1 ± 7.8 |
p < 0.05 compared with age-matched saline controls.
3.3. ZIP-sensitive kinases occlude ethanol-induced increases in adult hippocampal AMPA receptor GluA2 subunit phosphorylation
As synaptosomal AMPA receptor GluA2 subunit phosphorylation associated with aPKC isoform expression, we next determined whether a ZIP-sensitive kinase modulated GluA2 subunit phosphorylation following ethanol exposure. Since ethanol increased GluA2 subunit phosphorylation in adults, ZIP or vehicle was directly administered to the anterior hippocampus prior to ethanol or saline administration. Analysis revealed an interaction of ZIP pretreatment and ethanol exposure (F1,23=10.23, p<0.01), and a potential main effect of ZIP pretreatment (F1,23=3.98, p=0.058). Further post-hoc analysis revealed that ethanol alone again increased AMPA receptor GluA2 subunit phosphorylation (p<0.05, Figure 3A), but that ZIP administration reduced increases in ethanol-exposed animals (p<0.05). GluA2 subunit phosphorylation did not differ between control and ethanol exposed subjects following ZIP treatment. Synaptosomal PKMζ was assessed to evaluate ZIP efficacy. Analysis revealed a main effect of ZIP pretreatment (F1,22=20.59, p < 0.01) and an interaction of ZIP pretreatment and ethanol exposure that approached significance (F1,22=10.31, p=0.05). Further post-hoc analysis revealed that ZIP pretreatment reduced synaptosomal PKMζ expression (p<0.01, Figure 3B) compared to vehicle treated subjects. Similar to a lack of ethanol-exposure on PKCı/λ in adults, analysis revealed null effects of ZIP pretreatment, ethanol exposure, or an interaction on PKCι/λ expression (Figure 3C).
Figure 3.
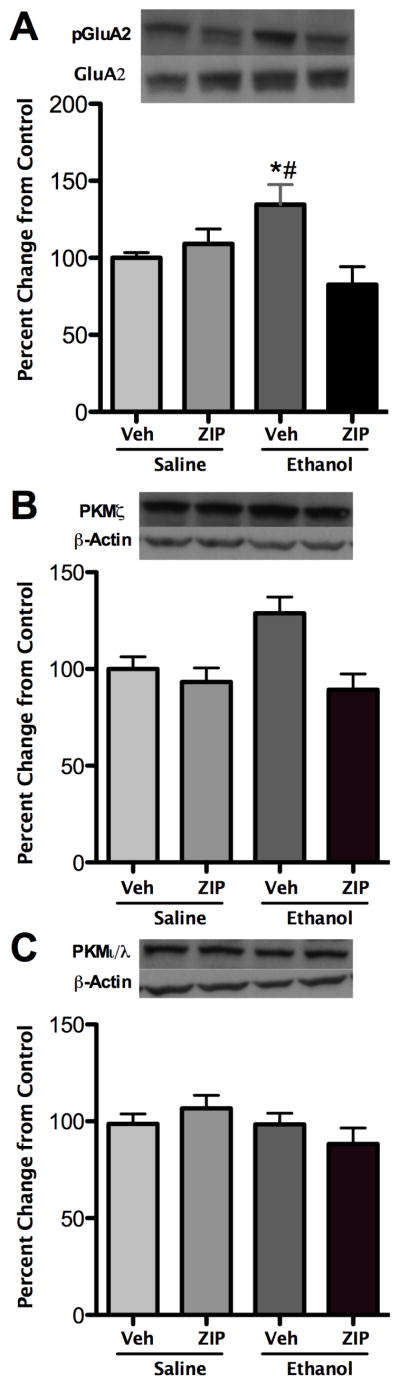
Effects of zeta inhibitory peptide (ZIP) on synaptosomal AMPA receptor GluA2 subunit ser880 phosphorylation and aPKC isoform expression 1 hour following low dose (1.0 g/kg) ethanol exposure. (A) Ethanol exposure increased GluA2 phosphorylation that was prevented by zeta inhibitory peptide (ZIP, 10 nmol) administration (*, p < 0.05, compared to vehicle; #, p < 0.05, compared to ZIP + ethanol). (B) PKMζ expression was reduced overall following ZIP administration (p < 0.01). (C) PKCι/λ expression did not differ following ZIP administration. Representative immunoblot images are shown. Data are presented as mean ± SEM, n = 6–8/group.
3.4. Ethanol-induced alterations in hippocampal AMPA receptor GluA2 subunit phosphorylation associate with delayed regulation in synaptosomal GluA2 subunit expression
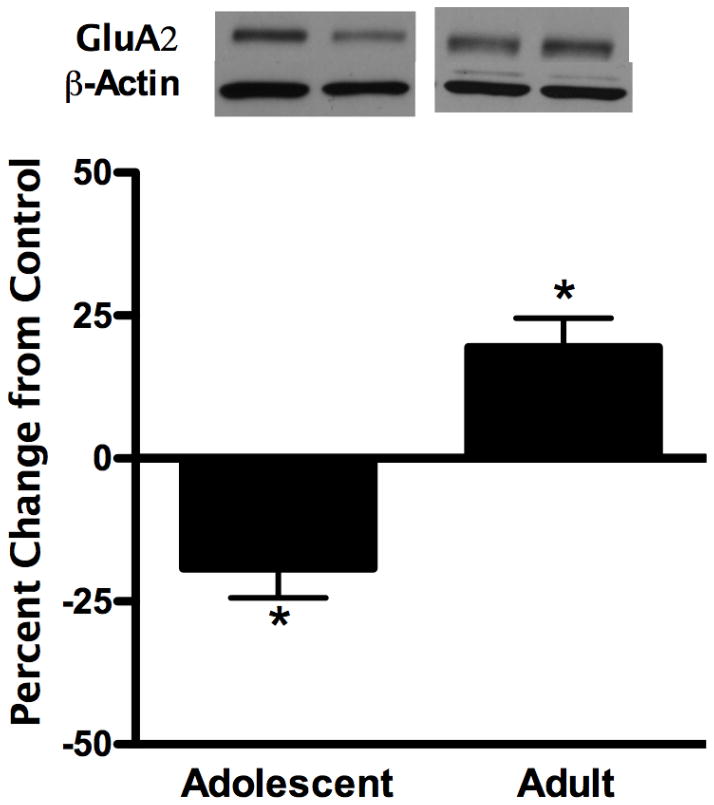
We next examined whether ethanol-induced changes in hippocampal AMPA receptor GluA2 phosphorylation elicit changes in synaptosomal GluA2 subunit levels 24 hours later when ethanol is pharmacokinetically eliminated. 1.0 g/kg ethanol decreased GluA2 subunit levels in adolescents (p<0.05, 19.2%), whereas expression was increased in adults (p<0.01, 20.3%, Figure 4). Similar to the lack of changes in GluA2 phosphorylation for both ages, hippocampal GluA2 subunit levels were unaffected 24 hours later following high dose ethanol exposure (see Table 1).
Figure 4.
Hippocampal AMPA receptor GluA2 subunit synaptosomal expression 24 hours following low dose (1.0 g/kg) ethanol exposure. Representative immunoblot images are shown. Data are presented as mean ± SEM. *p < 0.05 compared with age-matched saline controls, n = 8/group.
3.5. Cortical AMPA receptor GluA2 subunit and aPKC expression decreases throughout adolescence
AMPA receptor GluA2 receptors are robustly expressed in other structures that associate with cognitive performance; therefore, we next examined total cortical GluA2 subunit expression during adolescence relative to adults. Analysis revealed an effect of age for GluA2 [F3,27=3.161, p<0.05], with post-hoc analysis revealed that cortical GluA2 expression was 49.1% greater during early adolescence relative to adults (Figure 5A). Analysis of PKMζ expression also revealed an effect of age [F3,56=4.307, p<0.01], with post-hoc analysis revealing that PKMζ expression was higher during early- and mid-adolescence by 45.9% and 39.3% (Figure 5B). PKC ι/λ levels are not shown as we previously reported increased cortical PKCı/λ during mid-adolescence (Santerre et al., 2013).
Figure 5.
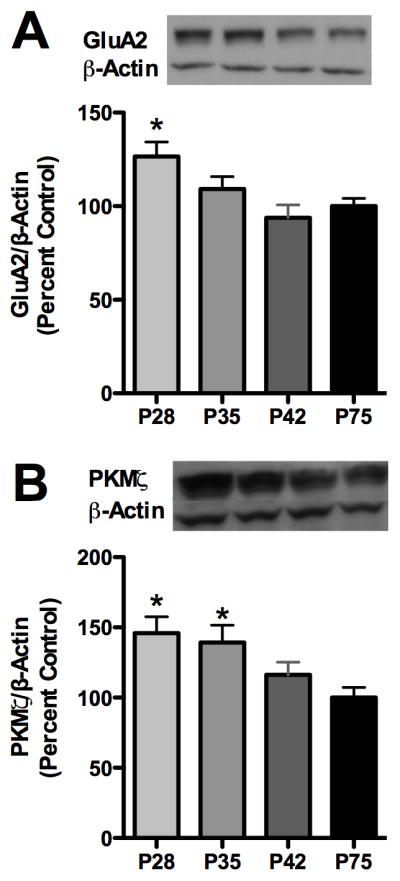
Cortical AMPA receptor GluA2 subunit and aPKC isoform synaptosomal expression decreases across adolescence. Graphs depict: (A) GluA2, and (B) PKMζ expression. Cortical PKCı/λ was previously published elsewhere (Santerre et al., 2013). Representative immunoblot images are shown. Data are presented as mean ± SEM. *p < 0.05 compared to P75, n = 7 – 8/group for GluA2 and 14–16/group for PKMζ.
3.6. Cortical P2 synaptosomal AMPA receptor GluA2 subunit phosphorylation and aPKC expression are age and dose-dependently regulated following ethanol exposure
In order to ascertain whether ethanol-induced changes observed in the hippocampus were conserved in the cerebral cortex, analyses were also conducted following low (1.0 g/kg) and high (3.5 g/kg) ethanol exposure. Low dose ethanol exposure had no effect on cortical synaptosomal AMPA receptor GluA2 subunit expression, phosphorylation, or aPKC expression in either age after 1 hour or GluA2 subunit expression after 24 hours (Table 2). Interestingly, following high-dose ethanol-exposure, adolescent cortical AMPA receptor GluA2 subunit phosphorylation was reduced at 1 hour (p<0.05, 25.2%) and paralleled by reductions in PKMζ expression (p<0.01, 28.2%) (Figure 6A). No changes were found in adults (Figure 6B). Reductions in adolescent GluA2 phosphorylation associated with decreases in AMPA receptor GluA2 subunit expression 24 hours later (p<0.05, 17%, Figure 6C). Adult GluA2 subunit expression remained unaltered 24 hours later.
Table 2.
Cortical AMPA receptor GluA2 subunit and aPKC isoform expression 1 or 24 hours following low dose ethanol exposure. Values are expressed as percent control. Data are presented as mean ± S.E.M. n = 8 per group.
| Cortex (1.0 g/kg) | ||
|---|---|---|
| Adolescent | Adult | |
| GluA2 | −5.0 ± 3.0 | 6.1 ± 13.4 |
| PGluA2 | −12.4 ± 4.7 | −12.9 ± 8.3 |
| PKMζ | −0.1 ± 5.1 | −2.2 ± 9.2 |
| PKMι/λ | 2.6 ± 4.5 | 8.6 ± 11.3 |
| GluA2 24h | 9.4 ± 10.0 | 20.5 ± 15.5 |
Figure 6.
Effects of high dose (3.5 g/kg) ethanol exposure on cortical synaptosomal AMPA receptor GluA2 subunit expression phosphorylation expression as well as PKMζ isoform expression. Analysis of adolescent (A) and adult (B) AMPA receptor GluA2 subunit expression, serine 880 phosphorylation and aPKC isoform expression at 1 hour following ethanol exposure. (C) Adolescent and adult AMPA receptor GluA2 subunit expression 24 hours following ethanol exposure. Adolescent and adult PKCι/λ results were previously reported in Santerre et al., 2013, but are shown again for completeness. Representative blots are shown for each. Data represent mean ± SEM. *p < 0.05 compared with age-matched saline controls, n = 8/group.
4. DISCUSSION
AMPA receptor GluA2 subunits are critical for LTP and cognitive behavior. The present study demonstrates that hippocampal and cortical AMPA receptor GluA2 subunits and aPKC isoforms are developmentally regulated, such that both are elevated during adolescence and progressively decrease with age. Furthermore, these results demonstrate that cognitive-impairing low doses of ethanol induce age-dependent opposing effects in hippocampal AMPA receptor GluA2 subunit phosphorylation and delayed subunit regulation. Effects were dose dependent, as a higher soporific dose had no effect on hippocampal GluA2 subunit levels. Effects were also regionally selective, as a low dose failed to elicit cortical changes whereas a high dose ethanol administration altered only adolescent GluA2 and aPKC expression. Importantly, our results not only indicate that ethanol-induced changes in synaptosomal aPKC isoform expression are accompanied by GluA2 phosphorylation, but that a ZIP-sensitive kinase, likely an aPKC isoform, influences GluA2 subunit phosphorylation.
Elevation of AMPA receptor GluA2 subunit levels and aPKC expression during adolescence was not unexpected. Both are implicated in modulating cognitive tasks, and the adolescent period is critical for enhancing working memory and decision-making. Such results extend prior work. aPKC isoforms are present at birth and do not decrease compared to other PKC isoforms that display lower levels of expression and steadily increase during early postnatal stages (Jiang et al., 1994). Age-related declines may be related to synaptic pruning, neuronal reorganization and cell death, particularly within cortical regions. However, this explanation isn’t entirely satisfactory as hippocampal organization occurs earlier in development (Soriano et al., 1994). Despite higher GluA2 subunit levels, adolescents display greater cognitive impairment following ethanol exposure (Markwiese et al., 1998, although see: Rajendran and Spear, 2004), and ethanol doses analogous to our study suppress the induction of hippocampal LTP in adolescents, but not adults (Pyapali et al., 1999, Swartzwelder et al., 1995). Thus, our observed regulatory differences in GluA2 subunit phosphorylation following ethanol exposure more likely account for age-related ethanol behavioral differences rather than total GluA2 subunit expression.
AMPA receptor GluA2 subunit phosphorylation is strongly linked to synaptosomal and membrane expression, but disagreement exists as to whether ser880 phosphorylation results in increased membrane stability or internalization. Ser880 phosphorylation has been shown to increase PICK1-mediated internalization and long-term depression by interfering with GRIP/ABP association in cultured hippocampal and cerebellar Purkinje neurons (e.g., Chung et al., 2000, Xia et al., 2000). However, recent work in hippocampal neurons has challenged these results by demonstrating that subpopulations of GluA2 subunits do not require GRIP/ABP for synaptic anchoring, and that PKC-dependent phosphorylation increased synaptic stability and is likely not associated with internalized subunits (States et al., 2008). The present study demonstrates that phosphorylation results in parallel changes in synaptosomal GluA2 subunit levels 24 hours later and therefore supports the involvement of phosphorylation in synaptic stability. One possibility for the differences with prior reports is that ethanol regulation of synaptic GluA2-containing AMPA receptors may be GRIP/ABP independent. Alternatively, aPKC isoforms may elicit opposing actions on GluA2 subunit trafficking than conventional PKC isoforms. PKCα-mediated activity as well as CamKII co-involvement contribute to GluA2 subunit internalization (Terashima et al., 2004, Perez et al., 2001), likely through interactions with PDZ proteins (Xia et al., 2000, Kim et al., 2001). Given these discordant effects, further studies are clearly needed to compare the contribution of specific PKC isoforms and GRIP/ABP in ethanol action. Nonetheless, our resultant increases in adult hippocampal GluA2 subunit levels are consistent with elevations following ethanol exposure elsewhere (Wang et al., 2012, Chandler et al., 1999).
Changes in aPKC isoform levels paralleled AMPA receptor GluA2 subunit phosphorylation. Using a naturally occurring model with differences in ethanol’s cognitive impairment, age-related divergent responses in both aPKC isoform expression and GluA2 subunit phosphorylation strengthens prior studies implicating aPKC activity in LTP and cognitive function (Shema et al., 2007, Ren et al., 2013). Recent knockout data along with studies assessing the specificity of ZIP have questioned the role of PKMζ in cognition. Taking the present results into consideration further suggests that redundancy in aPKC isoform activity may underlie GluA2 subunit regulation (see also: Howell et al., 2014), particularly as ZIP is now known to inhibit PKMζ and PKCı/λ equally (Lee et al., 2013). ZIP administration occluded ethanol-induced increases in adult GluA2 subunit phosphorylation. While we are confident that ZIP acts primarily to inhibit atypical PKC isoforms (Yao et al., 2008), the possibility that ZIP acts in a yet unidentified manner cannot be completely ruled out. Nonetheless, to our knowledge, this is the first study implicating aPKC isoforms in AMPA receptor GluA2 subunit phosphorylation. It is unknown whether PKMζ or PKCı/λ is primarily responsible for GluA2 subunit phosphorylation and subsequent expression, but PKMζ may have a more prominent role than PKCı/λ. First, PKMζ expression across early- and mid-adolescence more closely mirrors GluA2 subunit levels. Second, following a low dose ethanol exposure when adult hippocampal PKMζ expression and GluA2 subunit phosphorylation were elevated, PKCı/λ was unchanged. Third, following high dose ethanol exposure, adolescent hippocampal PKCı/λ levels were increased but PKMζ levels and GluA2 phosphorylation did not differ, nor were GluA2 subunit levels elevated 24 hours later. Immunoprecipitation studies will better determine if aPKC isoforms directly interact with GluA2 subunits, particularly given that prior work indicates that PKMζ may work indirectly by enhancing NSF-mediated interactions or PSD95 clustering (Yao et al., 2008).
Adolescents displayed decreases in aPKC isoforms and GluA2 phosphorylation that were ethanol dose- and region-dependent. Importantly, decrements at amnestically relevant ethanol doses were only observed in the hippocampus, while higher doses were cortically restricted. Such regional differences mirror low ethanol doses that impair only adolescent hippocampal-mediated spatial memory tasks while higher doses result in more widespread effects. Apart from this, one interesting observation from the current study is ethanol’s ability to decrease PKMζ expression. PKMζ protein levels are suggested to be stable (Sacktor, 2012) and other drugs of abuse and stress increase its expression (Li et al., 2011, Parsons and Davis, 2011). Again, these effects are likely age-dependent as ethanol increased adult PKMζ levels. Adolescent aPKC levels may also be attributable to cognitive processes during the experiment itself (i.e., cues related to novel environment) (Liu et al., 2012a), such that control subjects may be exhibiting increased PKMζ, rather than decreases in the ethanol-exposed animals. Therefore, it must also be acknowledged that environmental and social enrichment (pair-housing) were provided to animals, which may also impact ethanol regulation of aPKCs and GluA2 subunits. However, single-housed subjects exhibited similar changes in GluA2 and PKMζ as pair-housed animals, thus minimizing social enrichment effects.
Although our biochemical analysis associates nicely with cognitive-impairments captured in the literature, a major limitation of the current study is the inability to assess GluA2 phosphorylation and regulation in age-related cognitive responses. Moreover, it is not clear if known age-related ethanol-induced behavioral impairments are primarily due to aPKC-GluA2 or broader ethanol activity. Methodological barriers have made direct behavioral investigation difficult. Adolescents already display elevated GluA2 subunit and aPKC isoform expression. Our results further suggest ethanol age-related action appears to be more related to regulation than total expression, thereby reducing enthusiasm for overexpression studies. Conversely, inhibition or knockdown of either aPKC isoforms or GluA2 subunit levels both result in memory impairments. Such an approach would further confound ethanol impairment. In vivo site-directed mutations would ultimately be needed to better elucidate the role of ser880 phosphorylation but are well beyond the current scope. Such an approach would also be critical to further understand GluA2 phosphorylation in electrophysiological responses. Of note, PKC activation alone increases miniature excitatory postsynaptic current frequency and amplitude (Carroll et al., 1998), and ethanol-induced LTD is found only in adolescents (Pyapali et al., 1999), both of which are in line with the current results.
The timing of receptor trafficking related to LTP formation as well as future susceptibility to excitotoxicity should also be considered. Temporary removal of GluA2-containing AMPA receptors coupled with increases in GluA1 facilitates LTP formation that is then stabilized by reinsertion of GluA2-containg AMPA receptors in a PKC activity dependent manner (Plant et al., 2006). Therefore, removal of GluA2 subunits in adolescents following ethanol exposure could enhance initial LTP formation whereas LTP is stabilized in adults. Alternatively, LTP-related events may be occurring earlier in adults than adolescents. Aside from LTP, removal of adolescent GluA2-containing AMPA receptors may result in increased Ca2+ permeable AMPA receptors that are linked to excitotoxic cell death (Ferguson et al., 2008). Future time course studies characterizing AMPA receptor GluA1 subunit expression and electrophysiological responses in both ages following ethanol exposure will begin to address both issues.
Finally, the present study extends our previous results in understanding the contribution of the developing kinome (Santerre et al., 2013). Importantly, fluctuations in PKC isoforms across adolescence occur in multiple brain regions. While the current study focused on AMPA receptor GluA2 subunits, given PKC’s ability to regulate various ligand-gated ion channel families, these results further strengthen that differences in the adolescent kinome should be a major consideration in future age-related neurotransmitter system investigations as well as their relation to behavioral responses. It is unclear as to which upstream signaling mechanisms initiate age-related divergent responses in aPKC isoforms. Interestingly, GluA2 subunit phosphorylation is modulated by cytoplasmic phospholipase A2 (cPLA2) activity, a known pro-inflammatory pathway in the brain (Gentile et al., 2012, Menard et al., 2005). This is of particular importance as cPLA2 is not only implicated in activating aPKC and nPKC isoforms via arachidonic acid production (Gailly et al., 1997), but inhibiting PKC prevents cPLA2 modulation of GluA2 subunit phosphorylation (Menard et al., 2005). cPLA2 activity is elevated in a diagnosed Alzheimer’s disease tissue as well as a synonymous transgenic mice line, and reducing such activity decreased learning and memory deficits (Sanchez-Mejia et al., 2008). Our recent work further supports cPLA2 in age-dependent ethanol responses, as inhibition increased ethanol’s soporific effects only in adolescents (Santerre et al., 2013) at doses commensurate with changes in cortical aPKC isoform and GluA2 phosphorylation. Clearly more work is needed to investigate cPLA2 involvement in adolescent ethanol responses. Other upstream factors may be involved such as those involved with stress responsivity. Ethanol increases in corticosterone, modulate AMPA receptor GluA2 subunit levels (Martin et al., 2009) and could impact ethanol age-related responses as recent work indicates decreased ethanol induction of corticosterone in female adolescents, but less so in males (Willey et al., 2012).
In summary, the present study demonstrates that aPKC isoform expression decreases through adolescence and parallels AMPA receptor GluA2 subunit expression. Furthermore, this study suggests that age-related differences in activation of aPKC isoforms are responsible for regulating AMPA receptor GluA2 subunits following ethanol exposure and may underlie age-related differences in hippocampal cognitive impairment from ethanol.
Article Highlights.
AMPA receptor GluA2 subunits and atypical PKC isoforms are elevated during adolescence.
GluA2-containing receptors and atypical PKC regulation may contribute to ethanol cognitive sensitivity across age.
Demonstrates a ZIP-sensitive kinase modulates GluA2 subunit phosphorylation in the presence of ethanol.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism AA019367 (Linda P. Spear – faculty recruitment of David F. Werner) AA017823 (DFW – pilot project; Developmental Exposure Alcohol Research Center) and the Binghamton University Center for Development and Behavioral Neuroscience.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- An L, Yang Z, Zhang T. Imbalanced synaptic plasticity induced spatial cognition impairment in male offspring rats treated with chronic prenatal ethanol exposure. Alcohol Clin Exp Res. 2013;37:763–70. doi: 10.1111/acer.12040. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Davidson MS, Bedi KS, Wilce PA. Neonatal ethanol exposure reduces AMPA but not NMDA receptor levels in the rat neocortex. Brain Res Dev Brain Res. 2002;136:77–84. doi: 10.1016/s0165-3806(02)00363-2. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH. A developmental perspective on executive function. Child Dev. 2010;81:1641–60. doi: 10.1111/j.1467-8624.2010.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck HM, Hueston CM, Bishop C, Deak T. Enhancement of the hypothalamicpituitary- adrenal axis but not cytokine responses to stress challenges imposed during withdrawal from acute alcohol exposure in Sprague-Dawley rats. Psychopharmacology (Berl) 2011;218:203–15. doi: 10.1007/s00213-011-2388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Nicoll RA, Malenka RC. Effects of PKA and PKC on miniature excitatory postsynaptic currents in CA1 pyramidal cells. J Neurophysiol. 1998;80:2797–800. doi: 10.1152/jn.1998.80.5.2797. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Norwood D, Sutton G. Chronic ethanol upregulates NMDA and AMPA, but not kainate receptor subunit proteins in rat primary cortical cultures. Alcohol Clin Exp Res. 1999;23:363–70. [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–67. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer TS, Barnes A, Iqbal U, Bailey CD, Reynolds JN, Brien JF, Valenzuela CF. Chronic prenatal ethanol exposure alters ionotropic glutamate receptor subunit protein levels in the adult guinea pig cerebral cortex. Alcohol Clin Exp Res. 2003;27:677–81. doi: 10.1097/01.ALC.0000060521.32215.E9. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Christensen RN, Gensel JC, Miller BA, Sun F, Beattie EC, Bresnahan JC, Beattie MS. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J Neurosci. 2008;28:11391–400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P, Gong MC, Somlyo AV, Somlyo AP. Possible role of atypical protein kinase C activated by arachidonic acid in Ca2+ sensitization of rabbit smooth muscle. J Physiol. 1997;500 (Pt 1):95–109. doi: 10.1113/jphysiol.1997.sp022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile MT, Reccia MG, Sorrentino PP, Vitale E, Sorrentino G, Puca AA, Colucci-D’amato L. Role of cytosolic calcium-dependent phospholipase A2 in Alzheimer’s disease pathogenesis. Mol Neurobiol. 2012;45:596–604. doi: 10.1007/s12035-012-8279-4. [DOI] [PubMed] [Google Scholar]
- Gigante ED, Santerre JL, Carter JM, Werner DF. Adolescent and adult rat cortical protein kinase A display divergent responses to acute ethanol exposure. Alcohol. 2014 doi: 10.1016/j.alcohol.2014.01.011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase M zeta synthesis from a brain mRNA encoding an independent protein kinase C zeta catalytic domain. Implications for the molecular mechanism of memory. J Biol Chem. 2003;278:40305–16. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Howell KK, Monk BR, Carmack SA, Mrowczynski OD, Clark RE, Anagnostaras SG. Inhibition of PKC disrupts addiction-related memory. Front Behav Neurosci. 2014;8:70. doi: 10.3389/fnbeh.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao SH, Frye GD. AMPA receptors on developing medial septum/diagonal band neurons are sensitive to early postnatal binge-like ethanol exposure. Brain Res Dev Brain Res. 2003;142:89–99. doi: 10.1016/s0165-3806(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Jiang X, Naik MU, Hrabe J, Sacktor TC. Developmental expression of the protein kinase C family in rat hippocampus. Brain Res Dev Brain Res. 1994;78:291–5. doi: 10.1016/0165-3806(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–50. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Chung HJ, Lee HK, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci U S A. 2001;98:11725–30. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land C, Spear NE. Ethanol impairs memory of a simple discrimination in adolescent rats at doses that leave adult memory unaffected. Neurobiol Learn Mem. 2004;81:75–81. doi: 10.1016/j.nlm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, Mcmahon T, Dadgar J, Fischbach-Weiss SC, Messing RO. Prkcz null mice show normal learning and memory. Nature. 2013;493:416–9. doi: 10.1038/nature11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YQ, Xue YX, He YY, Li FQ, Xue LF, Xu CM, Sacktor TC, Shaham Y, Lu L. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–46. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci. 2007;27:12367–77. doi: 10.1523/JNEUROSCI.2786-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, He S, Yu X. Early natural stimulation through environmental enrichment accelerates neuronal development in the mouse dentate gyrus. PLoS One. 2012a;7:e30803. doi: 10.1371/journal.pone.0030803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Formisano L, Savtchouk I, Takayasu Y, Szabo G, Zukin RS, Liu SJ. A single fear-inducing stimulus induces a transcription-dependent switch in synaptic AMPAR phenotype. Nat Neurosci. 2010;13:223–31. doi: 10.1038/nn.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhou QX, Hou YY, Lu B, Yu C, Chen J, Ling QL, Cao J, Chi ZQ, Xu L, Liu JG. Actin polymerization-dependent increase in synaptic Arc/Arg3.1 expression in the amygdala is crucial for the expression of aversive memory associated with drug withdrawal. J Neurosci. 2012b;32:12005–17. doi: 10.1523/JNEUROSCI.0871-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–21. [PubMed] [Google Scholar]
- Martin S, Henley JM, Holman D, Zhou M, Wiegert O, Van Spronsen M, Joels M, Hoogenraad CC, Krugers HJ. Corticosterone alters AMPAR mobility and facilitates bidirectional synaptic plasticity. PLoS One. 2009;4:e4714. doi: 10.1371/journal.pone.0004714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccormack SG, Stornetta RL, Zhu JJ. Synaptic AMPA receptor exchange maintains bidirectional plasticity. Neuron. 2006;50:75–88. doi: 10.1016/j.neuron.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Mcdonald BJ, Chung HJ, Huganir RL. Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology. 2001;41:672–9. doi: 10.1016/s0028-3908(01)00129-0. [DOI] [PubMed] [Google Scholar]
- Menard C, Patenaude C, Massicotte G. Phosphorylation of AMPA receptor subunits is differentially regulated by phospholipase A2 inhibitors. Neurosci Lett. 2005;389:51–6. doi: 10.1016/j.neulet.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–4. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- Oster H, Eichele G, Leitges M. Differential expression of atypical PKCs in the adult mouse brain. Brain Res Mol Brain Res. 2004;127:79–88. doi: 10.1016/j.molbrainres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Davis M. Temporary disruption of fear-potentiated startle following PKMzeta inhibition in the amygdala. Nat Neurosci. 2011;14:295–6. doi: 10.1038/nn.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–4. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CH. The Rat Brain in Stereotaxic Coordinates. London, UK: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- Peeters M, Monshouwer K, Janssen T, Wiers RW, Vollebergh WA. Working memory and alcohol use in at-risk adolescents: a 2-year follow-up. Alcohol Clin Exp Res. 2014;38:1176–83. doi: 10.1111/acer.12339. [DOI] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–28. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, Mcbain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–4. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS. Age and dosedependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol. 1999;19:107–11. doi: 10.1016/s0741-8329(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci. 2004;1021:441–4. doi: 10.1196/annals.1308.060. [DOI] [PubMed] [Google Scholar]
- Ren SQ, Yan JZ, Zhang XY, Bu YF, Pan WW, Yao W, Tian T, Lu W. PKClambda is critical in AMPA receptor phosphorylation and synaptic incorporation during LTP. EMBO J. 2013;32:1365–80. doi: 10.1038/emboj.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC. Memory maintenance by PKMzeta--an evolutionary perspective. Mol Brain. 2012;5:31. doi: 10.1186/1756-6606-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mejia RO, Newman JW, Toh S, Yu GQ, Zhou Y, Halabisky B, Cisse M, Scearce-Levie K, Cheng IH, Gan L, Palop JJ, Bonventre JV, Mucke L. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat Neurosci. 2008;11:1311–8. doi: 10.1038/nn.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre JL, Gigante ED, Landin JD, Werner DF. Molecular and behavioral characterization of adolescen protein kinase C following high dose ethanol exposure. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3267-6. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenova S. Attention, impulsivity, and cognitive flexibility in adult male rats exposed to ethanol binge during adolescence as measured in the five-choice serial reaction time task: the effects of task and ethanol challenges. Psychopharmacology (Berl) 2012;219:433–42. doi: 10.1007/s00213-011-2458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science. 2007;317:951–3. doi: 10.1126/science.1144334. [DOI] [PubMed] [Google Scholar]
- Soriano E, Del Rio JA, Martinez A, Super H. Organization of the embryonic and early postnatal murine hippocampus. I. Immunocytochemical characterization of neuronal populations in the subplate and marginal zone. J Comp Neurol. 1994;342:571–95. doi: 10.1002/cne.903420406. [DOI] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. W.W. Norton & Company, Inc; New York, NY: 2010. [Google Scholar]
- States BA, Khatri L, Ziff EB. Stable synaptic retention of serine-880- phosphorylated GluR2 in hippocampal neurons. Mol Cell Neurosci. 2008;38:189–202. doi: 10.1016/j.mcn.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of longterm potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–5. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swartzwelder NA, Risher ML, Abdelwahab SH, D’abo A, Rezvani AH, Levin ED, Wilson WA, Swartzwelder HS, Acheson SK. Effects of ethanol, Delta(9)- tetrahydrocannabinol, or their combination on object recognition memory and object preference in adolescent and adult male rats. Neurosci Lett. 2012;527:11–5. doi: 10.1016/j.neulet.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–8. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;24:5381–90. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez-Hernandez ME, Vazquez-Torres R, Velasquez-Martinez MC, Jimenez L, Baez F, Sacktor TC, Jimenez-Rivera CA. Inhibition of Protein kinase Mzeta (PKMzeta) in the mesolimbic system alters cocaine sensitization in rats. J Drug Alcohol Res. 2013;2:235669. doi: 10.4303/jdar/235669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaramani DV, Moura PJ, Picciotto MR, Lombroso PJ. Striatal-enriched protein tyrosine phosphatase (STEP) knockout mice have enhanced hippocampal memory. Eur J Neurosci. 2011;33:2288–98. doi: 10.1111/j.1460-9568.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Bachman JL, Johnson R, Yu Y, Huganir RL. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493:420–3. doi: 10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ben Hamida S, Darcq E, Zhu W, Gibb SL, Lanfranco MF, Carnicella S, Ron D. Ethanol-mediated facilitation of AMPA receptor function in the dorsomedial striatum: implications for alcohol drinking behavior. J Neurosci. 2012;32:15124–32. doi: 10.1523/JNEUROSCI.2783-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46:29–36. doi: 10.1016/j.alcohol.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Royle GA, Gray EE, Abdipranoto A, Thangthaeng N, Jacobs N, Saab F, Tonegawa S, Heinemann SF, O’dell TJ, Fanselow MS, Vissel B. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domaincontaining proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. PKM zeta maintains late long-term potentiation by N-ethylmaleimidesensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008;28:7820–7. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YJ, Chang CH, Gean PW. AMPA receptor endocytosis in the amygdala is involved in the disrupted reconsolidation of Methamphetamine-associated contextual memory. Neurobiol Learn Mem. 2013;103:72–81. doi: 10.1016/j.nlm.2013.04.004. [DOI] [PubMed] [Google Scholar]