Abstract
With optimal target antigen selection antibody-based therapeutics can be very effective agents for hematologic malignancies, but none have yet been approved for myeloma. Rituximab and brentuximab vedotin are examples of success for the naked antibody and antibody–drug conjugate classes, respectively. Plasma cell myeloma is an attractive disease for antibody-based targeting due to target cell accessibility and the complementary mechanism of action with approved therapies. Initial antibodies tested in myeloma were disappointing. However, recent results from targeting well-characterized antigens have been more encouraging. In particular, the CD38 and CD138 targeted therapies are showing single-agent activity in early phase clinical trials. Here we will review the development pipeline for naked antibodies and antibody–drug conjugates for myeloma. There is clear clinical need for new treatments, as myeloma inevitably becomes refractory to standard agents. The full impact is yet to be established, but we are optimistic that the first FDA-approved antibody therapeutic(s) for this disease will emerge in the near future.
Keywords: Multiple myeloma, Plasma cell myeloma, Antibody–drug conjugate, Monoclonal antibodies, Targeted cancer therapy, Immunotherapy
1. Introduction
The FDA approval of the monoclonal antibody (mAb) rituximab in 1997 was the harbinger of a significant change to the treatment of cancer. This single agent has become a component of first and subsequent line therapy in many subtypes of non-Hodgkin lymphoma [1, 2]. Central to efficacy of rituximab is the expression of its target antigen, CD20, on the cell surface. In solid tumors, the prototype for success is trastuzumab, a naked antibody that targets the human epidermal growth factor receptor 2 (HER2), which is approved for use in the treatment of breast cancer. Efforts to extend mAb therapy into other malignancies has been met with both resounding successes and costly failures, as only a small fraction of mAbs that have entered clinical trials in oncology have received FDA approval [3].
One potential way to improve upon the efficacy of mAbs is to use them as a targeted delivery system for chemotherapy. After years of research and development, antibody–drug conjugates (ADCs) have seen renewed excitement after the recent FDA approval for two new agents. The first is the anti-CD30 ADC brentuximab vedotin in Hodgkin lymphoma (HL) and anaplastic large cell lymphoma (ALCL). Early phase studies in patients with relapsed or refractory HL or ALCL have shown remarkable responses in the majority of patients, including significant numbers achieving complete response (CR), leading to accelerated FDA approval for these indications in 2011 [4,5]. Trastuzumab, targeting HER2, has also been utilized in this approach by linkage to another antitubulin cytotoxic (mertansine) to create ado-trastuzumab emtansine (T-DM1) [6]. T-DM1 is highly active in trastuzumab-resistant, HER2-positive breast cancer, leading to FDA approval in that setting [7]. Furthermore, T-DM1 was also found to be superior to trastuzumab in the first line setting, demonstrating the potential to improve upon the efficacy of naked antibodies [8]. Overall, the success of mAbs as novel cancer therapeutics has incited increasing efforts to broaden their application. Plasma cell myeloma (aka multiple myeloma) is one such disease where new therapy is needed, especially since this is an incurable disease and the development of resistance to current therapies is universal.
2. Rationale for developing antibody-based therapy for myeloma
Efforts to broaden the applicability of naked antibodies to myeloma by targeting antigens more specific to the disease are finally coming to fruition, after several years of mostly disappointing clinical trials. Extrapolating from established agents in other malignancies, there are several mechanisms by which an antibody therapeutic could potentially destroy myeloma cells [1]. Most mAbs function by binding to an appropriate cell surface antigen, where the “naked” antibody can direct the patients’ own immune system against the malignant cells, tagging them for elimination by antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) [9]. Many naked antibodies tested in vitro for myeloma have been shown to activate ADCC, but unfortunately this mechanism has demonstrated limited clinical activity by itself [2]. Inhibition of signal transduction is another mechanism that can contribute to the efficacy of clinically used antibodies. Thus, several antibodies were developed to target signaling pathways responsible for myeloma cell survival, proliferation and microenvironment interaction [3]. Efficacy can be accentuated by linkage of mAbs to cytotoxic small molecules (Fig. 1). These antibody–drug conjugates have the potential to be far more potent than their naked counterparts in tumor cell killing, when the target antigen is rapidly internalized. To date very few antibody–drug conjugates have been tested in myeloma. These “armed” antibodies may improve clinical efficacy and perhaps have the greatest promise for novel therapeutics in myeloma.
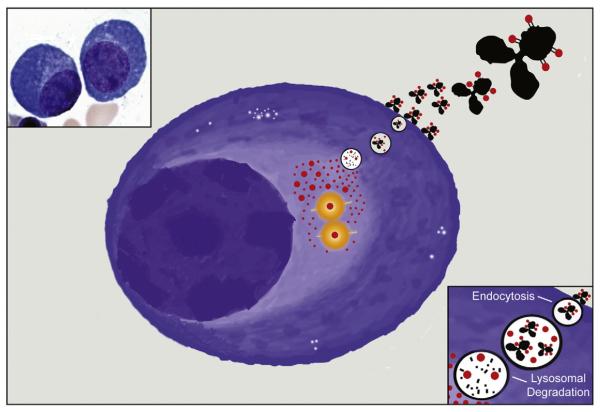
Fig. 1.
Illustration of a malignant plasma cell showing the mechanism of action for antibody–drug conjugates. ADC targets are ideally selected for endocytosis and trafficking into lysosome (upper right corner, magnified in lower right corner), where the antibodies are broken down (black), leaving the cytotoxic payloads (red) to diffuse out into the cytosol. In the case of commonly employed auristatin and maytansine derivatives, the payloads bind at their sites of action and induce microtubule catastrophe (yellow/orange) and lead to cell death. Upper left myeloma cell micrograph courtesy Kristie White, UCSF Hematopathology.
The treatment of myeloma has truly undergone a renaissance over the past 5–10 years. The use of proteasome inhibitors and IMiDs has drastically changed longevity for patients and the median overall survival now approaches a decade. Immunomodulatory drugs (IMiDs) have been thought to have pleiotropic immune effects. However, a critical mechanism of IMiD action was recently found to involve binding to Cereblon, a unique E3 ubiquitin ligase protein [10,11]. This interaction facilitates the degradation of Ikaros B-cell transcription factors [12]. The proteasome inhibitors also directly affect protein stability through inhibition of the chymotryptic site on the proteasome and producing a massive unfolded protein response [13]. The proteasome inhibitors and IMiDs have been used in combination with more traditional chemotherapy (alkylators and anthracyclines) and steroids to produce robust anti-myeloma effects in the frontline and relapse settings. However, despite these advances, resistance inevitably develops and the disease ultimately remains fatal. In addition, the disease can cause a debilitating course with a significant risk of skeletal disease (especially vertebral fractures), recurrent infections and/or kidney damage. Thus, there is still great need for novel therapeutics and new classes of drugs for this disease.
Antibody therapies provide exquisite targeting specificity and have the potential to greatly improve the outcome in this devastating disease. Malignant plasma cells (PCs) are primarily localized to the bone marrow (BM) and are readily accessible to intravenously infused antibody therapies through discontinuous capillaries (sinusoids) [14,15]. This contrasts to solid tumors, for which location and the capillary endothelium can present barriers to delivery [14,15]. The preclinical results for the many naked antibodies investigated for myeloma have been comprehensively reviewed previously [16]. Here, we will provide an update on a subset of the naked antibodies with emphasis on their clinical results, including CD38, signaling lymphocyte activation molecule family member 7 (SLAMF7/CS1), CD74, CD40 and insulin-like growth factor 1 receptor (IGF-IR/CD221). ADCs are now becoming the focus for this genre of drug development in myeloma. These will be emphasized here, with published targets consisting of CD138, CD56, Fc receptor-like 5 (FcRL5/CD307), CD74 and B-cell maturation antigen (BCMA).
3. Myeloma target antigens
One of the most important aspects of developing antibody-based therapeutic in myeloma is target antigen selection. Ideally the target should demonstrate selective overexpression on the malignant cells. HER2 is an analogous example, as the gene is amplified from 2 to greater than 20-fold and this is reflected in high cell surface expression in 30% breast cancer tumors [17]. Unfortunately, no marker has been identified to undergo consistent gene amplification in myeloma thus far. Toxicity is predicted by the target cell surface expression on non-malignant cells, and by taking into account the tissue distribution of the relatively large mAb molecules. It should be noted that the optimal level of target expression might differ for naked and “armed” antibodies. An example is brentuximab vedotin, where CD30 is expressed uniformly on malignant cells in HL, but not necessarily overexpressed [18,19]. Treatment with naked CD30 antibodies had little to no activity in Hodgkin lymphoma, whereas treatment with the ADC brentuximab vedotin has shown significant activity [20,21]. For ADCs, additional attributes of the target antigen’s biology are important for efficacy. These have been reviewed elsewhere [22]. In brief, internalization typically must occur for the payload to have specific cytotoxic effect on the target cell. Rapid internalization of antigen and recycling to the surface are ideal for toxin delivery. Furthermore, an intracellular pathway that delivers ADC to early endosomes and lysosomes is important for delivery and activation of the attached warhead [23,24]. This allows specific release of the warhead to its site of action.
For myeloma, a standard panel of antigens has been used for immunohistochemical and flow cytometry identification and quantification of tumor cells. These antigens may also be appropriate targets for antibody-based therapy. Perhaps the best-known antigens are CD38, CD138 and CD56. The published frequency and specificity of expression for these markers in myeloma are summarized in Table 1. CD38 is expressed on nearly all PCs and myeloma cells, and is absent only rarely [25,26]. Staining of myeloma cells with fluorescently labeled anti-CD38 antibodies typically yields bright signals by flow cytometry [25]. CD38 is also expressed on immature B- and T-cells, NK cells, activated T-cells and monocytes [26]. CD138 is ubiquitously expressed on myeloma and normal PCs [27]. CD138 is also expressed on epithelial cells, immature B-cells and on cells involved in wound healing in mice [28,29]. CD138 expression has been found in breast and other carcinomas, possibly indicating other potential applications of CD138-targeted therapies [30]. CD56 is a unique marker for myeloma, in that it is expressed on malignant cells, but is absent or low on normal PCs [27,31]. Although 78% of myeloma patients express CD56 (n = 55), in one study it appears to be lost with progression to extramedullary disease and plasma cell leukemia [31–33]. CD56 is normally expressed on NK cells, some T-cells, and in neural and muscle tissue [34–36].
Table 1.
Summary of current antibody targets for myeloma.
| Antigen | Protein description | Notable functions | Normal expression | % Patients |
References |
|---|---|---|---|---|---|
| CD38 | Cyclic ADP ribose hydrolase | Ectoenzyme, immune cell adhesion, cell signaling |
Lymphocytes, monocytes, NK cells |
100% | Leo et al. [25], Funaro et al. [112] |
| CD138 | Heparin sulfate proteoglycan | Cell adhesion and signaling | Plasma cells, epithelial cells | 100% | Bataille et al. [26], Sanderson et al. [28], and Hayashi et al. [29] |
| CD56 | Neural cell adhesion molecule | Cell adhesion | NK cells, lymphocytes, neurons, muscle |
78% | Van Camp et al. [31], Abo et al. [34], Lanier et al. [35], and Mechtersheimer et al. [36] |
| CD74 | HLA-DR MHC-class II invariant chain | Antigen presentation | Lymphocytes, antigen presenting cells |
86% | Burton et al. [37], Wraight et al. [38], and Claesson-Welsh et al. [39] |
| CD40 | TNFR superfamily member | Antigen presentation, T-cell activation, B-cell growth and differentiation, cytokine production |
Antigen presenting cells, B-cells, endothelium, smooth muscle, fibroblasts |
70% | Uckun et al. [42], Mach et al. [43], Pellat- Decunynkck et al. [27], and Westendorf et al. [44] |
| IGF-1R | Insulin-like growth factor-1 receptor | Cell survival and proliferation | Ubiquitous | 84% | Bataille et al. [45], Mitsiades et al. [46] |
| SLAMF7 | Signaling lymphocyte activating- molecule related receptor family member |
Cell adhesion | NK cells, T-cells, monocytes, dendritic cells |
95% | Hsi et al. [48], Tai et al. [49], and Murphy et al. [50] |
| FcRL5 | Immunoglobulin superfamily member | Proliferation and isotype expression of antigen-primed B-cells |
B-lymphocytes | 100% | Davis et al. [51], Elkins et al. [52], and Ise et al. [53] |
Two other well-characterized differentiation antigens are CD74 and CD40. CD74 is the invariant chain of the human leukocyte antigen (HLA)-DR major histocompatibility complex (MHC) class II molecule. CD74 has been reported to be expressed on myeloma cells in 86% (n = 22) patients [37]. It is normally expressed on B-cells and antigen presenting cells such as Langerhans cells in the skin [38,39]. CD74 is an attractive ADC target due to rapid internalization and recycling to the surface of B-cells [40]. CD74 is also expressed on melanoma and colon cancer cell lines, potentially expanding the role of anti-CD74 therapy to those diseases [41]. CD40 is a tumor necrosis factor (TNF) receptor superfamily member that is expressed on antigen presenting cells, B-lymphocytes, endothelial, smooth muscle and fibroblasts [42,43]. CD40 has been found to be expressed in ~70% myeloma patients (n = 37) [27,44]. Though expressed at a seemingly lower level as measured by flow cytometry than CD38, its expression can increase with disease progression [27].
Other B-cell specific targets and those important to the pathogenesis of myeloma include IGF-1R, IL6, SLAMF7 (aka CS1), FcRL5 and BCMA. IGF-1R and IL6 are principle components of growth factor signal transduction for myeloma. IGF-1R was expressed in 84% myeloma patients cumulatively from two studies (n = 91) [45,46]. IGF-1R overexpression is correlated with the t(4;14) translocation, disease progression and lack of CD45 [45]. Potentially problematic for selectivity, IGF-1R is widely expressed in normal tissue [47]. SLAMF7 is expressed on myeloma cells from 95% of patients (n = 20), albeit at relatively lower levels than CD38 and CD138 [48,49]. The normal expression pattern of SLAMF7 is restricted to PCs, NK cells, activated lymphocytes, monocytes and dendritic cells [48–50]. FcrL5 protein is specifically expressed on B-cells and PCs [51]. FcrL5 was proposed as a target for myeloma based on this high degree of specificity and that expression levels were >3-fold higher on myeloma cells compared to normal PCs, by median fluorescence intensity (MFI) [52]. This antigen has been found to be expressed on 100% (n = 24) of cumulative myeloma cases examined, although the expression level appears low compared to that of CD38 or CD138 [52, 53]. FcrL5 was observed to internalize within 2 h of mAb binding, and delivered to lysosomes within 13 h by colocalization with Lysosomal-associated membrane protein 1 (LAMP1) by immunocytochemistry, making it an reasonable candidate for ADC development [52]. BCMA is expressed specifically in the plasma cells, with expression on 92% of myeloma patient samples tested across four studies (n = 27), though cell surface expression level was heterogeneous [54–57]. As seen in Table 1, the current myeloma antigens mostly have good specificity for myeloma and PCs. Thus, the on-target side effects are often expected be immune, especially B-cell mediated deficiency.
4. Myeloma antibody–drug conjugate construction
Antibody–drug conjugates utilize the cell specificity of a monoclonal antibody to preferentially deliver their cytotoxic payload to cells with higher surface expression levels of the target antigen. In addition to the previously mentioned choice of antigen, the selection of a proper linker and drug are also important. To prevent premature release of the drug in the circulation, the linker must have a half-life in blood comparable to that of the antibody. The first ADC approved by the FDA, gemtuzumab ozogamicin (GO), utilized an acid sensitive acyl hydrazone linker that was designed for hydrolysis in the low pH environment of the lysosome. Unfortunately this class of linker has been shown to have a significant rate of drug release in plasma, and GO was eventually withdrawn from the US market in 2010 [58]. Significant hepatotoxicity was seen perhaps due to nonspecific drug release. Despite this, recent meta-analysis of randomized trials has reported that GO improves outcomes for a subset of patients with acute myeloid leukemia and efforts are ongoing to potentially revive its clinical use [59].
Newer linkers have shown improved serum stability while maintaining proper release function after internalization into cells. Brentuximab vedotin utilizes a valine-citrulline dipeptide linker that can be cleaved by lysosomal proteases such as cathepsin B [60]. Numerous experiments have demonstrated that this linker has excellent serum stability, but is readily cleaved once internalized into cells. In contrast, T-DM1 contains a non-cleavable linker for the covalent attachment of the cytotoxic drug to the antibody [6]. This ADC relies on lysosomal digestion of the antibody component to liberate the drug metabolite consisting of the drug still attached to the amino acid to which it was conjugated (Fig. 2). Yet another linker is the sterically-hindered disulfide linker. The disulfide is readily cleaved once introduced into the reducing environment of the cell, but methyl groups adjacent to the disulfide bond are necessary to slow the rate of premature release in circulation. Though this linker has not yet been used in any approved ADCs, it has been used in several conjugates in clinical trials, including the anti-CD138 ADC [61].
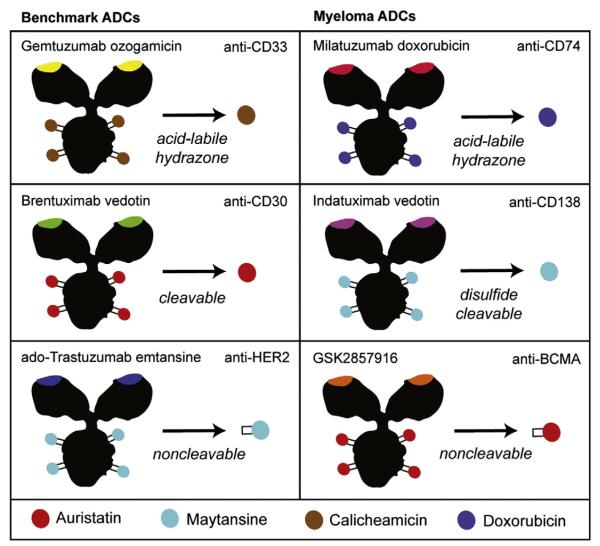
Fig. 2.
Mix-and-match antibody–drug conjugate construction. FDA-approved ADCs (left column) have consisted of gemtuzumab ozogamicin for AML (later withdrawn), brentuximab vedotin for Hodgkin lymphoma and ado-trastuzumab emtansine for breast cancer. The linkers and drugs used for this approach have evolved and diversified over this time. GO used a labile hydrazone linker connected to a DNA-damaging agent, whereas newer ADCs mostly utilize cleavable or non-cleavable linkers with potent antitubulins that optimize intracellular delivery. These technologies, which were pioneered by Seattle Genetics and Immunogen, have been adapted for other malignancies such as myeloma. Examples of ADCs developed for use in myeloma are shown in the right column, showing a mix of constructions similar to the previously FDA-approved agents.
The proper drug selection is also important for creating an effective antibody–drug conjugate. Many steps are necessary for the successful delivery of an ADC drug component to its intracellular target (ADC tumor localization in vivo, cell internalization, cleavage and lysosome escape), so only a low percentage of the injected payload eventually reaches the desired target [62]. For this reason, most successful ADCs utilize extremely potent drugs. Monomethyl auristatin E (MMAE), a synthetic derivative of the natural product dolastatin, is the drug used in brentuximab vedotin and in several other ADCs entering clinical trials. Average EC50 (half maximal effective concentration) of 1–5 nM on a wide range of cancer cells has been observed with this drug which was approximately 50- and 200-fold more potent than vinblastine and doxorubicin, respectively [58]. Derivatives of the natural product maytansine have similar potency as the auristatins and have found use in T-DM1, as well as a number of other ADCs entering clinical trials.
The antibody–drug conjugates being tested as myeloma therapeutics utilize a variety of these drugs and linkers (Fig. 2). Indatuximab ravtansine consists of an antibody targeting CD138, a maytansine analog (DM4), and a sterically-hindered disulfide bond linker. This ADC composition was the most potent after testing multiple cleavable and non-cleavable versions in preclinical myeloma models [61]. The drugs are attached to lysine side chains by titrating the concentration of drug during conjugation and with this strategy an average ratio of 3.5 drugs per antibody is achieved. Similarly, lorvotuzumab mertansine (IMG901) utilizes the same drug but with a less hindered disulfide bond linker (DM1) [63]. The drug is attached to the lysine side chains of an antibody targeting CD56, in the same manner as indatuximab ravtansine. The recently published GSK2857916 anti-BCMA ADC utilizes a non-cleavable linker conjugated to another auristatin derivative, monomethyl auristatin F (MMAF) [57].
A distinctly different antibody–drug conjugate is milatuzumab–doxorubicin, which consists of an anti-CD74 antibody conjugated to doxorubicin [64]. Possible disadvantages to this ADC are that doxorubicin is a much less potent drug than either the maytansines or auristatins and the acid labile hydrazone linker, which may result in premature release of the drug while still in circulation. While premature drug release is undesirable, this may pose less of a problem than with gemtuzumab ozogamicin because of the diminished potency of the doxorubicin. One topic of debate is whether drugs that have efficacy in myeloma treatment as chemotherapies (like doxorubicin) would be more effective payloads, given that antitubulin agents are not useful alone in myeloma therapy. However, this argument may not be applicable to ADCs utilizing maytansines or auristatins due to their much higher potency. It will be interesting to see which of these strategies results in the most effective myeloma treatment.
Since it is often difficult to predict which linker and drug combination will be most effective, some companies will develop multiple ADCs during for preclinical evaluation. Genentech, for example, tested several versions of its anti-FcRL5 ADC [52]. Two versions were prepared using auristatin derivatives, one with a dipeptide cleavable linker and the other with a non-cleavable linker. In addition, they also tested two maytansine derivative ADCs with either a cleavable disulfide linker or a non-cleavable linker. There were significant differences between the in vivo potency of these ADCs in xenograft mouse models, thus demonstrating the importance of testing different combinations of drugs and linkers [52]. As more ADCs are tested in clinical trials it will hopefully become possible to better predict which combination of drug and linker is best suited for a particular type of disease.
5. Myeloma clinical response criteria
For the discussion herein, the updated response criteria by European Group for Blood and Marrow Transplant (EBMT) were used to assess response [65]. In general, these criteria attempt to assess myeloma disease burden by the amount of monoclonal proteins present, and the amount of PCs found in the marrow or in isolated tumors (plasmacytomas). In review, normal PCs have an important role in immune function by manufacturing and secreting diverse antibody proteins. When a monoclonal PC population persists and expands inappropriately, the monotypic antibody produced can be readily detected as a monoclonal “M-spike” on serum protein electrophoresis (SPEP), urine protein electrophoresis (UPEP) and/or through serum free light chain assays.
Disease progression, and response to therapy, can be followed quantitatively by the trend of M-spike and corresponding serum free light chain levels. The EBMT criteria categorizes responses as minimal response (MR) with 25–49% decrease in M-spike concentration, partial response (PR) with greater than 50%, very good partial response (VGPR) with greater than 90%, CR with negative SPEP immunofixation and BM PCs are less than 5%, and stringent CR (sCR) when light chain ratio is normal and multiparametric BM flow cytometry does not detect a clonal PC population [65,66]. The stable disease (SD) category consists of nonresponders who have less than 25% decrease or increase of the M-spike from baseline. A caveat is that SD can be misleading because it does not accurately reflect time to progression (TTP) [65]. However, SD is included in the trial descriptions here, since the phase I trials are not designed to assess TTP. Of note, MR was not included in the EBMT update, but is also included here since many trials report these responses.
6. Anti-myeloma naked antibodies: preclinical & clinical results
Numerous naked antibodies have been tested in preclinical myeloma models. Antibodies raised against six antigens have been tested clinically: CD38, SLAMF7, CD74, CD40, IL-6 and IGF1R. It should be emphasized that the phase I trials discussed herein cannot be compared to each other directly, as they are designed to assess safety alone. Furthermore, patients enrolled vary according to disease characteristics and prior therapies. The response rate in these trials is generally an underestimate, as significant (and variable) numbers of patients in a dose-escalation scheme are likely to be treated with small doses that do not achieve enough target occupancy for effect. Still, the ease of monitoring for response in myeloma allows for observation of activity, when present. A summary of the phase I dose-escalation trials of antibody therapeutics in myeloma is shown in Table 2.
Table 2.
Summary of antibody therapeutics developed for the treatment of myeloma.
| Agent | Company | Target | Description | Dose range | Phase I activity* | Notable AEs | Current status | References |
|---|---|---|---|---|---|---|---|---|
| Naked antibodies | ||||||||
| Daratumumab | Genmab, Janssen | CD38 | Human IgG | 0.005–16 mg/kg Q1W | ORR 24%, SD NR | Infusion-related events (G3, 6%) | Phase II (open) | Plesner et al. [68] |
| SAR650984 | Sanofi | CD38 | Humanized IgG1 |
1–10 mg/kg Q1–2 W | ORR 28%, SD 11% | Pneumonia (G3, 6.3%), hyperglycemia (G3, 3.1%), infusion reaction (G2, 3.1%) |
Phase I, Ib (open) | Martin et al. [69,70] |
| Elotuzumab | Bristol-Myers Squibb |
SLAMF7 | Humanized IgG1 |
10–20 mg/kg Q2W | ORR 0%, SD 26% | Acute kidney injury (G4, 3%), hypersensitivity (G3, 3%), bradycardia (G2, 3%), chest pain (G2, 3%), pyrexia (G2, 3%), chills (G2, 3%) |
Phase III (results awaited) |
Zonder et al. [73] |
| Dacetuzumab | Seattle Genetics | CD40 | Humanized IgG |
0.5–12 mg/kg Q1W | ORR 0%, SD 20% | Aseptic meningitis (G3–4, 5%), ocular inflammation (G1–2 21%), cytokine release syndrome (G3, 5%), transaminitis (G2–3, 9%) |
No current myeloma trials |
Hussein et al. [77] |
| Lucatumumab | Novartis | CD40 | Human IgG | 1–6 mg/kg Q1W | ORR 4%, SD 43% | Thrombocytopenia (G4, 4%), transaminitis (G3, 4%), and increased lipase (G4, 1%) |
No current myeloma trials |
Bensinger et al. [79] |
| Figitumumab | Pfizer | IGF-1R | Human IgG2 |
0.025–20 mg/kg Q4W | ORR 0%, SD 59% | Anemia (G2–3, 9%), hyperglycemia (G3, 2%) | No current myeloma trials |
Lacy et al. [82] |
| AVE1642 | Sanofi | IGF-1R | Humanized IgG |
3–18 mg/kg Q2W | ORR 7%, SD 47% | Hyperglycemia (G3, 7%) | DC in myeloma | Moreau et al. [83] |
| Siltuximab | Janssen | IL6 | Chimeric IgG |
5–40 mg daily × 14 days (Ref 87), 3–12 mg/kg Q1–3 W (Ref 88) |
ORR 0%, SD NR (Ref 87), ORR 15%, SD 61% (Ref 88) |
Neutropenia (Ref 60, G1–4, 46%), thrombocytopenia (Ref 60, G1–4, 46%), infection (Ref 60, G1–4, 66%) |
No current myeloma trials |
Van Zaanen et al. [87], Kurzrock et al. [88] |
| Milatuzumab | Immunomedics | CD74 | Humanized IgG |
1.5–16 mg/kg BIW | ORR 0%, SD 26% | Anemia (G3, 4%), infusion reaction (G3 4%) | No current myeloma trials |
Kaufman et al. [80] |
| Antibody–drug conjugates | ||||||||
| Indatuximab Ravtansine | Biotest | CD138 | Chimeric IgG-DM4 |
10–200 mg/m2 Q3W | ORR 11%, SD 41% | Mucositis, stomatitis or hand–foot syndrome (G2–3), dry eyes or blurred vision (G1–2) |
Phase I/Iia (open) | Jagannath et al. [90] |
| Lorvotuzumab mertansine |
ImmunoGen | CD56 | Humanized IgG-DM1 |
40 to 140 mg/m2 Q1W | ORR 18%, SD 28% (all doses) |
Fatigue, renal failure, weakness, and absence of deep tendon reflexes (each G3, 4%) |
DC in myeloma | Chanan-Khan et al. [92] |
Cross-comparison of Phase I activity is not possible due to multiple variables, including varying dose-escalation schemes, patient disease characteristics and prior treatment regimens. AE = adverse events, BIW = dose twice weekly, DC = discontinued. G = adverse event grade, NR = not reported, ORR = overall response rate, Q1W = dose weekly, Q2W = dose every 2 weeks, Q4W = dose every four weeks, Q1–2 W=dose every 1–2 weeks, Q1–3 W = dose every 1–3 weeks, SD = stable disease.
6.1. Anti-CD38: daratumumab and SAR650984
Two anti-CD38 mAbs are currently in clinical trials. Daratumumab is a CD38 mAb that was shown to exhibit in vitro anti-myeloma cell proliferation, ADCC, CDC and in vivo activity against myeloma cell line xenografts [67]. In phase I dose-escalation trials, Daratumumab was well-tolerated and has shown single-agent activity, with an ORR of 24% (n = 29) [68]. A phase II study of daratumumab in proteasome inhibitor and IMiD refractory myeloma and a phase I/II in combination with lenalidomide and dexamethasone are currently open (clinicaltrials.gov ID NCT01985126 and NCT1615029). The second CD38 mAb, SAR650984, is currently being tested in a phase I dose-escalation and phase Ib combination study with lenalidomide and dexamethasone (clinicaltrials.gov ID NCT01749969 and NCT01084252). The phase I single-agent trial of SAR650984 has been completed and presented at the American Society of Hematology (ASH) 2013 annual meeting, showing good tolerance and promising activity, with an ORR 28% (n = 18, clinicaltrials.gov ID NCT01084252) [69]. The Phase I combination study of SAR650984 with lenalidomide and dexamethasone was recently reported at the American Society of Clinical Oncology (ASCO) 2014 annual meeting, demonstrating an ORR of 58% in heavily pre-treated patients (clinicaltrials.gov ID NCT01749969) [70]. Preclinical study of SAR650984 found this antibody to antagonize ADP-ribosyl cyclase activity of CD38 and induce apoptosis in primary myeloma patient cells ex vivo in addition to ADCC and CDC induction [71]. Thus, both anti-CD38 antibodies appear active and the FDA has designated breakthrough drug status for daratumumab in relapsed/refractory myeloma.
6.2. Anti-SLAMF7: elotuzumab
Elotuzumab, an anti-SLAMF7 antibody, is the most extensively studied naked antibody tested clinically for myeloma thus far [72]. Preclinical study of elotuzumab showed induction of ADCC in vitro and activity in myeloma xenografts into immunocompromised mice [48,49]. However, in phase 1 clinical trial of elotuzumab, this antibody was shown to be safe, but no objective responses were observed and only a portion had SD [73]. Thus, the clinical testing shifted to focus on combination regimens [74,75]. In a phase II trial, the combination of elotuzumab with lenalidomide and dexamethasone resulted in an ORR of 84% (n = 73) in relapsed myeloma [75]. This high response rate led to recent breakthrough designation by the FDA. Two randomized trials in both newly diagnosed and relapsed/refractory myeloma have been done to examine whether addition of elotuzumab to steroids, IMiDs and/or proteasome inhibitors increases the efficacy of those regimens (clinicaltrials.gov ID NCT01239797, NCT01891643, NCT01478048). These phase III trials are now complete and report of their results is awaited.
6.3. Anti-CD40 and Anti-CD74 antibodies
Other differentiation antigens on myeloma cells that have been targeted by mAbs in clinical trials include CD40 and CD74. Dacetuzumab (SGN-40) is an anti-CD40 antibody that shows both ADCC and signal transduction-mediated apoptosis in myeloma cells [76]. Again, a single-agent phase I trial showed no objective responses [77]. Studies have since been completed with dacetuzumab in combination with lenalidomide and dexamethasone, showing some mild activity in heavily pre-treated patients [78]. No current trials with dacetuzumab are open in myeloma. A second CD40 monoclonal antibody lucatumumab (HCD122) has also been studied in dose-escalation phase I trial in myeloma, with similar activity [79]. CD74 has been evaluated as a potential therapeutic target in myeloma with the antibody milatuzumab. Phase I trial with milatuzumab showed stable disease in a portion of patients, but no objective responses [80]. Thus, CD40 and CD74 do not appear to be fruitful naked mAb targets in myeloma.
6.4. Anti-IGF-1R and Anti-IL6 Antibodies
Antigens important for myeloma pathogenesis that have been evaluated as targets for naked antibodies are IGF-1R and IL6. There are two antibodies to IGF-1R that have reached early clinical trials for myeloma. Preclinical evidence showed sensitivity of myeloma cell lines and primary samples to IGF-1R inhibition [46,81]. Figitumumab (CP-751871) is an IGF-1R antibody tested in phase 1. Partial responses were observed in combination with steroids, but not as a single agent [82]. AVE1642 is the other clinically tested anti-IGF-1R antibody, for which no responses were observed as a single agent and unclear contribution evident when combined with bortezomib. These results led to cessation of testing for myeloma [83]. One lesson taken from AVE1642 may be the importance for biomarker use in patient selection. IGF-1R expression and low CD45 have been shown in vitro to be critical to sensitivity for IGF-1R inhibition [83]. Those markers could have helped to pick patients most likely to respond. The antibody siltuximab utilized a different approach than targeting a cell surface protein. Siltuximab targets the secreted cytokine IL6. Multiple reports have shown that IL6 contributes to myeloma cell survival and proliferation, and confers resistance to bortezomib and steroids [84–86]. In phase I studies a small portion of patients responded to single-agent siltuximab in myeloma, with 2 of 13 patients achieving CR in one trial [87,88]. Unfortunately, single-agent activity was not confirmed in phase II, although activity was seen when combined with steroids [89]. Siltuximab is not currently undergoing further clinical study in myeloma, but rather is being pursued in Castleman’s disease. Thus, naked antibodies targeting IGF-1R and IL6 have been disappointing in clinical trials for myeloma. However, in light of the documented importance of IGF-1R and IL6 to myeloma pathogenesis, they remain possible targets to explore with other modalities.
Overall, serious adverse events have been infrequent for mAbs tested in myeloma, with infusion-related reactions being the most common. These have been manageable with steroid premedication and administration adjustments. Unfortunately, naked antibody therapies based on pathogenically altered pathways in myeloma have thus far been disappointing. CD38 has emerged as the most promising mAb target thus far, with 2 independent agents producing substantial single-agent responses. There appear to be multiple mechanisms for the anti-myeloma activity of these naked anti-CD38 antibodies [67,71]. Although single-agent activity for mAb targeting SLAMF7 was modest, phase III trials to elucidate possible synergism with other effective myeloma treatments have recently reached completion and results are awaited.
7. Myeloma antibody–drug conjugates: preclinical & clinical results
7.1. Antibody–drug conjugates in clinical trials
Antibody–drug conjugates for myeloma are earlier in the pipeline than naked antibodies, and are garnering attention from recent successful examples of brentuximab vedotin and T-DM1. The furthest along is the anti-CD138 ADC indatuximab ravtansine (BT062). In preclinical testing, the EC50 in vitro was ~1 nM from negatively selected CD138-positive cells from one myeloma patient [61]. Cytotoxicity for PMNCs was reported not present at the relatively low concentration of 12 nM [61]. The first-in-man, single-agent phase I study of indatuximab ravtansine in myeloma showed an ORR of 11%, with 41% achieving SD (n = 27) [90]. A phase I/IIa trial of indatuximab ravtansine in combination with lenalidomide and dexamethasone in 9 evaluable patients was recently reported at the annual ASH 2013 meeting, with 78% ORR [91]. The anti-CD56 ADC, lorvotuzumab mertansine, showed an EC50 in the range of 10–50 nM in 3 CD56 expressing myeloma cell lines, with maximum effect observed at 96 h [63]. Interestingly, cell surface expression did not correlate with sensitivity in the lines tested, possibly bringing in question the concept that expression level will always predict efficacy. Besides intrinsic sensitivity differences to mertansine, internalization could be another explanation for such variability in ADC efficacy. A phase I study presented for Lorvotuzumab mertansine in myeloma patients selected for CD56 expression produced an ORR 17%, with 28% SD [92]. Unfortunately, ImmunoGen later announced that the phase II study in myeloma and small cell lung cancer was discontinued due to lack of efficacy and infection-related adverse events. In sum, while clinical study of these new agents for myeloma is in its infancy, preliminary presentations have indicated single-agent activity is present for anti-CD138 and -CD56-ADCs, although indatuximab ravtansine is the sole agent being taken forward in further trials.
7.2. Preclinical antibody–drug conjugates
The antibody–drug conjugates in preclinical development target CD74, FcRL5 and BCMA. The preclinical characteristics of ADCs developed for myeloma are summarized in Table 3. Milatuzumab, anti-CD74 antibody conjugated to doxorubicin showed in vitro EC50 of 900 nM at 4 h with MC/CAR cells and in vivo activity against MC/CAR xenografts in SCID mice [64]. A phase I clinical trial for milatuzumab–doxorubicin is registered, but currently on hold (clinicaltrials.gov ID NCT01101594). Recently, Genentech developed ADCs targeting the FcRL5 protein. While expressed on myeloma cells from primary samples, FcRL5 was surprisingly not expressed on myeloma cell lines tested [52]. When stably expressed in the OPM2 myeloma cell line, anti-FcRL5 DM4, MMAF and MMAE conjugates each had EC50 of 50 ng/ml (0.33 nM) [52]. In vivo, DM4 and MMAE conjugates with cleavable linkers effectively inhibited tumor growth (by volume) in SCID mouse subcutaneous xeno-grafts of OPM2-FcRL5 and EJM-FcRL5 cells, showing similar activity as biweekly bortezomib [52]. Testing in monkeys was also performed to demonstrate tolerability prior to human testing [52]. Human testing for anti-FcRL5 ADC has not yet been registered.
Table 3.
Summary of preclinical results for antibody–drug conjugates developed for myeloma.
| Company | Target | Linker | Payload | MM cell EC50* |
Pros | Cons | Trial status | |
|---|---|---|---|---|---|---|---|---|
| Indatuximab Ravtansine |
Biotest | CD138 | Cleavable | DM4 | 1 nM | Good target cell specificity | Internalization efficiency unknown |
Phase I/Iia (open) |
| Lorvotuzumab mertansine |
ImmunoGen | CD56 | Cleavable | DM1 | 10–50 nM | Upregulated expression on MM cells |
Expression on other cell types |
Development discontinued |
| Milatuzumab–DOX | Immunomedics | CD74 | Acid labile hydrazone |
Doxorubicin | 900 nM | Rapid internalization and recycling |
Lower potency, labile linker |
Phase I (not open) |
| anti-FcrL5 ADC | Genetech | FcrL5 | Cleavable & non- cleavable |
DM4, MMAF and MMAE |
0.33 nM | High target cell specificity | Low expression level? | Not yet registered |
| GSK2857916 | GlaxoSmith Kline |
BCMA | Non-cleavable | MMAF | 0.08–6.6 nM | High target cell specificity | Variable expression level |
Phase I (not open) |
Cross-comparison of agent EC50s is limited due to varying assay conditions and cell lines used in the study.
A third target evaluated for antibody–drug conjugate development is BCMA. Preclinical presentation at ASH 2013 for an anti-BCMA antibody conjugated to MMAF, reported rapid internalization, trafficking of antibody to lysosomes and antigen recycling by 6 h [93]. They reported ADC cytotoxicity from 500–1000 ng/ml (3.3–6.6 nM) with primary myeloma cells [93]. A recently published report of anti-BCMA-ADC GSK2857916 further showed in vitro myeloma cell line EC50 ranging from 11.5–1000 ng/ml (0.08–6 nM) and potent elimination of myeloma cell line xenografts [57]. BCMA-ADC phase I trial is planned, but not yet open (clinicaltrials.gov ID NCT02064387). Also of interest, brentuximab vedotin is also in clinical trials for CD30-positive myeloma, although this is likely to be a minority of patients (clinicaltrials.gov ID NCT01461538). On a limited number of samples (n = 7), plasma cells from 43% myeloma patients express CD30 [94]. Of ADC targets under study in myeloma, CD74, FcRL5 and BCMA have been shown to rapidly internalize bound antibody, whereas this has not been specifically addressed for CD138 or CD56 [40,52,93]. However, all of the discussed ADCs for myeloma appear potent in vitro and active by in vivo preclinical models. The phase I trials with these agents will likely open soon and hopefully will show promise for patients with relapsed or refractory disease.
8. Other antibody-related approaches to myeloma
8.1. Chimeric antigen receptor t-cell therapy
A discussion of immunotherapy for any hematologic malignancy nowadays must also mention the potential for chimeric antigen receptor T-cell (CAR-T) therapy and checkpoint inhibitors. CAR-T therapy genetically engineers a patient’s collected T-cells to express a chimeric protein composed of an antibody variable domain and the T-cell receptor (TCR) signaling domain (CD3z), along with a fused or coexpressed coactivator protein. These T-cells are then re-infused and home to target antigen, where binding leads to TCR engagement, coactivator signaling and a potent proliferative and cytotoxic immune response. This approach has been surprisingly effective in producing long-term disease control in small clinical trials in acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL) [95,96]. Complete response rate as high as 88% (n = 16) has been seen in ALL [97]. The possibility of CAR-T as a salvage strategy in myeloma has also begun to receive attention [56,98]. In the case of CAR-T therapy, antigen selection it is critically important to avoid even low level expression on normal cells, which has been shown to cause serious on-target organ toxicity from the potent T-cell activity [98]. For the CAR-T approach in myeloma, the only published construct thus far targets the BCMA receptor that is strictly confined to the B-cell lineage [56]. There is also a clinical trial in China currently recruiting to evaluate CD138 as the target for CAR-T therapy for resistant myeloma patients (clinicaltrials.gov ID NCT01638936). The CAR-T approach is a high-risk, high-reward strategy with potential for long-term disease control or cure at the expense of significant cytokine storm that often necessitates ICU-level care [96]. Plans to scale up this approach to increasing numbers of patients and new target malignancies are underway at select centers.
8.2. Immune checkpoint blockade
A second strategy for immunotherapy of cancer gaining interest is immune checkpoint blockade. This can be achieved through antibodies targeting surface receptors or ligands responsible for silencing the immune response. In theory disruption of these checkpoints may help the immune system recognize malignant cells. This is being validated by remarkable responses seen in solid tumors such as metastatic melanoma with the programmed death 1 (PD1) antibody nivolumab [99]. PD1-ligand (PD-L1) has been shown to be expressed on myeloma cells isolated from patients (n = 82), but was only present on a fraction (median 23%) of CD138-positive cells [100]. When present it is likely to silence prospective T-cell attack. Interestingly, in phase I dose-escalation study of the anti-PD1 antibody pidilizumab (CT-011) in hematologic malignancies, one myeloma patient was included and had SD for over one year [101]. Two clinical trials are now recruiting for myeloma using PD1 antibodies nivolumab and pidilizumab (clinicaltrials.gov ID NCT02077959 and NCT01592370). One group has shown antibody blockade of PD1 with pidilizumab augments T-cell cytotoxicity of a dendritic anti-myeloma vaccine ex vivo [102]. A clinical trial is now recruiting patients to test antibody alone or in combination with vaccine after autologous stem cell transplant (clinicaltrials.gov ID NCT01067287). It is currently unclear whether the level of expression of PD-L1 in myeloma will be sufficient to translate to responses to this approach. Thus, we are eager to see clinical results from this immune cell-activating strategy for myeloma.
9. Conclusion and future directions
Antibody therapies are poised to aid in the treatment of myeloma. CD38 and CD138 were among the earliest markers used to identify myeloma cells and appear so far to translate to suitable antigens for targeted antibody therapy for this disease. Just recently, clinical trial abstracts for CD38 naked mAbs and CD138 ADC have showed encouraging single-agent ORR of 24–28% and 11%, respectively. By comparison, the phase I study of bortezomib in myeloma showed responses in 33% patients (n = 9) [103]. In the IMiD class, the lenalidomide phase I trial found ORR of 71% (n = 24) [104]. Elotuzumab has just completed two large Phase III trials, and has the potential of being the first FDA-approved mAb in myeloma. In the cases of CD38 mAb and CD138 ADC, single activity is clearly being seen, as it was at similar phase of development for proteasome inhibitors and IMiDs. As evidenced by the FDA breakthrough designation for daratumamab and elotuzumab, the enthusiasm for the new agents is widespread.
Important considerations remain in developing the future of antibody-based therapy in myeloma. These potential translational challenges are outlined in Table 4. Current myeloma treatments can induce deep remission, but relapse inevitably develops due to incomplete elimination of tumor cells and regrowth of resistant clones. Operationally speaking, those residual tumor cells with self-renewal potential may be considered myeloma initiating cells (M-IC). These cells may have different surface antigen expression than terminally differentiated myeloma cells. One possible limitation of targeting markers of terminal plasma cell differentiation, such as CD138, is the possible lack of their expression on M-IC [105,106]. Whether many of the markers discussed here, such as CD38, are expressed on M-IC is unknown. The lack of progress is due in part to the lack of appropriate markers that precisely de-fine M-IC. Thus, the reproducible characterization of cell surface markers on M-IC has clear implications on the curative potential of antibody-based therapies in this disease.
Table 4.
Future challenges to the application of antibody-based therapies to myeloma.
| Challenges | Clinical relevance | Research objectives to be addressed |
|---|---|---|
| Target expression on M-IC |
Finding curative potential |
|
| Ideal drug combinations |
Finding synergism and avoiding antagonism |
|
| Disease setting | Utility in relpased, refractory or newly diagnosed disease |
|
| Optimization of ADCs | Maximizing efficacy |
|
ADC = antibody–drug conjugate, M-IC = myeloma initiating cells.
Another important issue is how these new antibodies, either naked or armed, will be integrated with currently used myeloma treatments. Combination therapy with steroids, proteasome inhibitors and IMiDs reduce ability of malignant cells to develop resistance to each individual agent. Combinations may also provide synergism in some cases. Of note, it has been shown that bortezomib influences the expression level of cell surface markers [107]. In the case of CD138 and CD38, both have been shown to have decreased expression after bortezomib treatment [107]. Other surface antigens may be unaffected or upregulated by bortezomib. Thus, the influence of proteasome inhibitors on-target antigen expression is an important consideration for antibody directed therapies that will warrant investigation for each antigen. As these agents have widely different mechanisms of action, it may be anticipated that they will work well together and be synergistic in eliminating myeloma cells.
All together, the future treatment landscape for myeloma is taking shape rapidly. Whereas CD38 has emerged as the most promising naked antibody target, several ADCs have potential to improve the efficacy of naked antibody treatments. In addition to indatuximab ravtansine and lorvotuzumab mertansine, more ADCs are sure to undergo clinical testing in myeloma soon. These can conceivably improve on naked mAb efficacy as they have done with the examples discussed of HER2-positive breast cancer and Hodgkin lymphoma. Also, new technologies such as site-specific drug conjugation could further improve therapeutic index of current ADCs [108,109]. CAR-T approaches may also be another potent way to take advantage of the known myeloma antigens for improved therapies. Already in clinic, the new proteasome inhibitor carfilzomib and IMiD pomalidomide have improved the established drug classes and have good activity in bortezomib and lenalidomide refractory patients [110,111]. Combination of these next-generation agents with mAbs or ADCs may further improve response rate and depth of response. The full extent of benefit antibody therapies will contribute to this therapeutic armamentarium awaits randomized studies in the near future. These have exciting potential to enhance efficacy in combination regimens and importantly, to provide additional options to patients with proteasome inhibitor and IMiD refractory myeloma, a population without approved therapies available.
Practice points.
Although myeloma treatment has seen great advances with the emergence of proteasome inhibitors and IMiDs, myeloma remains an incurable disease and development of resistance to standard agents remains inevitable.
Efforts to broaden the applicability of antibody-based therapies to myeloma are approaching realization. One or more agents in this class may become FDA-approved in the next 1–2 years.
Antibody–drug conjugates have entered early phase clinical study and hold promise to improve anti-myeloma activity over their naked antibody predecessors.
Research agenda.
Improved description of cell surface antigens present on myeloma initiating cells for targeting with antibody-based therapeutics with potential to eliminate the regenerative potential of the disease.
Preclinical and clinical combination studies of antibody-based therapies with standard agents to find how these therapies interact and describe how they can best work together.
Relapsed, high risk (e.g. loss of chromosome 17p containing p53) and newly diagnosed settings may each derive unique benefits and warrant studies to specifically address each.
The technology for linkers and payloads used in antibody–drug conjugates continues to evolve. An important ongoing effort will be to incorporate the cutting-edge approaches into novel conjugates for clinical application.
Acknowledgments
We thank the following agencies for financial support: the UCSF Stephen and Nancy Grand Multiple Myeloma Translational Initiative (a research grant to BL and a Young Investigator award to DS), and the National Institutes of Health/National Cancer Institute (R01 CA 118919, R01 CA129491, and R01 CA 171315 to BL). The plasma cell micrograph inset for Fig. 1 was kindly provided by Kristie White, MD, University of California San Francisco Hematopathology. We also wish to thank Professor Marc A. Shuman. MD of UCSF for his helpful guidance on this subject.
Abbreviations
- ASCO
American Society of Clinical Oncology
- ASH
American Society of Hematology
- ALL
acute lymphoblastic leukemia
- ALCL
anaplastic large cell lymphoma
- ADCC
antibody-dependent cellular cytotoxicity
- ADC
antibody–drug conjugate
- BM
bone marrow
- CAR-T
chimeric antigen receptor T-cell
- CLL
chronic lymphocytic leukemia
- CDC
complement-dependent cytotoxicity
- CR
complete response
- EC50
half maximal effective concentration
- EBMT
European Group for Blood and Marrow Transplant
- GO
gemtuzumab ozogamicin
- FcrL5
Fc receptor-like 5
- HLA
human leukocyte antigen
- HER2
human epidermal growth factor receptor 2
- HL
Hodgkin lymphoma
- IMiDs
immunomodulatory drugs
- IGF-IR
insulin-like growth factor 1 receptor
- LAMP1
lysosomal-associated membrane protein 1
- MHC
major histocompatibility complex
- mAb
monoclonal antibody
- MFI
median fluorescence intensity
- MMAE
monomethyl auristatin E
- MMAF
monomethyl auristatin F;
- M-IC
myeloma initiating cells;
- ORR
overall response rate;
- MR
minor response
- PR
partial response
- PD-L1
PD1-ligand
- PC
plasma cell
- PD1
programmed death 1
- SPEP
serum protein electrophoresis
- SLAMF7
signaling lymphocyte activation molecule family member 7
- SD
stable disease
- T-DM1
ado-trastuzumab emtansine
- TCR
T-cell receptor
- TTP
time to progression
- TNF
tumor necrosis factor
- VGPR
very good partial response
Footnotes
Conflicts of interest statement
The authors declare no conflicts of interest.
References
- [1].Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin’s lymphoma. Annu Rev Med. 2008;59:237–50. doi: 10.1146/annurev.med.59.060906.220345. [DOI] [PubMed] [Google Scholar]
- [2].Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63(8):803–43. doi: 10.2165/00003495-200363080-00005. [DOI] [PubMed] [Google Scholar]
- [3].Nelson AL, Dhimolea E, Reichert JM. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov Oct. 2010;9(10):767–74. doi: 10.1038/nrd3229. [DOI] [PubMed] [Google Scholar]
- [4].Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2012 Jun 20;30(18):2183–9. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol Off J Am Soc Clin Oncol. 2012 Jun 20;30(18):2190–6. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- [6].Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 2008 Nov 15;68(22):9280–90. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- [7].Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012 Nov 8;367(19):1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013 Mar 20;31(9):1157–63. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]
- [9].Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012 Apr;12(4):278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- [10].Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012 Nov;26(11):2326–35. doi: 10.1038/leu.2012.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhu YX, Braggio E, Shi C-X, Bruins LA, Schmidt JE, Wier SV, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011 Nov 3;118(18):4771–9. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014 Jan 17;343(6168):305–9. doi: 10.1126/science.1244917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006 Jun 15;107(12):4907–16. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res. 1990 Feb 1;50(Suppl. 3):814s–9s. [PubMed] [Google Scholar]
- [15].Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2009 Nov 19;12(1):33–43. doi: 10.1208/s12248-009-9157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tai Y-T, Anderson KC. Antibody-based therapies in multiple myeloma. Bone Marrow Res. 2011;2011:924058. doi: 10.1155/2011/924058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- [18].Dürkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin’s disease. Cell. 1992 Feb 7;68(3):421–7. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- [19].Falini B, Pileri S, Pizzolo G, Dürkop H, Flenghi L, Stirpe F, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995 Jan 1;85(1):1–14. [PubMed] [Google Scholar]
- [20].Ansell SM, Horwitz SM, Engert A, Khan KD, Lin T, Strair R, et al. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-060) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2007 Jul 1;25(19):2764–9. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- [21].Forero-Torres A, Leonard JP, Younes A, Rosenblatt JD, Brice P, Bartlett NL, et al. A phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009 Jul;146(2):171–9. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- [22].Bander NH. Antibody–drug conjugate target selection: critical factors. Methods Mol Biol Clifton NJ. 2013;1045:29–40. doi: 10.1007/978-1-62703-541-5_2. [DOI] [PubMed] [Google Scholar]
- [23].Erickson HK, Park PU, Widdison WC, Kovtun YV, Garrett LM, Hoffman K, et al. Antibody–maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 2006 Apr 15;66(8):4426–33. doi: 10.1158/0008-5472.CAN-05-4489. [DOI] [PubMed] [Google Scholar]
- [24].Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced activity of monomethylauristatin f through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006 Jan;17(1):114–24. doi: 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- [25].Leo R, Boeker M, Peest D, Hein R, Bartl R, Gessner JE, et al. Multiparameter analyses of normal and malignant human plasma cells: CD38++, CD56+, CD54+, cIg+ is the common phenotype of myeloma cells. Ann Hematol. 1992 Mar;64(3):132–9. doi: 10.1007/BF01697400. [DOI] [PubMed] [Google Scholar]
- [26].Bataille R, Jégo G, Robillard N, Barillé-Nion S, Harousseau J-L, Moreau P, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica. 2006 Sep;91(9):1234–40. [PubMed] [Google Scholar]
- [27].Pellat-Deceunynck C, Bataille R, Robillard N, Harousseau JL, Rapp MJ, Morineau NJ, et al. Expression of CD28 and CD40 in human myeloma cells: a comparative study with normal plasma cells. Blood. 1994 Oct 15;84(8):2597–603. [PubMed] [Google Scholar]
- [28].Sanderson RD, Lalor P, Bernfield M. B lymphocytes express and lose syndecan at specific stages of differentiation. Cell Regul. 1989 Nov;1(1):27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hayashi K, Hayashi M, Jalkanen M, Firestone JH, Trelstad RL, Bernfield M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J Histochem Cytochem Off J Histochem Soc. 1987 Oct;35(10):1079–88. doi: 10.1177/35.10.2957423. [DOI] [PubMed] [Google Scholar]
- [30].Nguyen TL, Grizzle WE, Zhang K, Hameed O, Siegal GP, Wei S. Syndecan-1 overexpression is associated with nonluminal subtypes and poor prognosis in advanced breast cancer. Am J Clin Pathol. 2013 Oct;140(4):468–74. doi: 10.1309/AJCPZ1D8CALHDXCJ. [DOI] [PubMed] [Google Scholar]
- [31].Van Camp B, Durie BG, Spier C, De Waele M, Van Riet I, Vela E, et al. Plasma cells in multiple myeloma express a natural killer cell-associated antigen: CD56 (NKH-1; Leu-19) Blood. 1990 Jul 15;76(2):377–82. [PubMed] [Google Scholar]
- [32].Pellat-Deceunynck C, Barillé S, Puthier D, Rapp MJ, Harousseau JL, Bataille R, et al. Adhesion molecules on human myeloma cells: significant changes in expression related to malignancy, tumor spreading, and immortalization. Cancer Res. 1995 Aug 15;55(16):3647–53. [PubMed] [Google Scholar]
- [33].Pellat-Deceunynck C, Barillé S, Jego G, Puthier D, Robillard N, Pineau D, et al. The absence of CD56 (NCAM) on malignant plasma cells is a hallmark of plasma cell leukemia and of a special subset of multiple myeloma. Leukemia. 1998 Dec;12(12):1977–82. doi: 10.1038/sj.leu.2401211. [DOI] [PubMed] [Google Scholar]
- [34].Abo T, Balch CM. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J Immunol. 1981 Sep;127(3):1024–9. (Baltim Md 1950) [PubMed] [Google Scholar]
- [35].Lanier LL, Le AM, Ding A, Evans EL, Krensky AM, Clayberger C, et al. Expression of Leu-19 (NKH-1) antigen on IL 2-dependent cytotoxic and non-cytotoxic T cell lines. J Immunol. 1987 Apr 1;138(7):2019–23. (Baltim Md 1950) [PubMed] [Google Scholar]
- [36].Mechtersheimer G, Staudter M, Möller P. Expression of the natural killer cell-associated antigens CD56 and CD57 in human neural and striated muscle cells and in their tumors. Cancer Res. 1991 Feb 15;51(4):1300–7. [PubMed] [Google Scholar]
- [37].Burton JD, Ely S, Reddy PK, Stein R, Gold DV, Cardillo TM, et al. CD74 is expressed by multiple myeloma and is a promising target for therapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2004 Oct 1;10(19):6606–11. doi: 10.1158/1078-0432.CCR-04-0182. [DOI] [PubMed] [Google Scholar]
- [38].Wraight CJ, van Endert P, Möller P, Lipp J, Ling NR, MacLennan IC, et al. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J Biol Chem. 1990 Apr 5;265(10):5787–92. [PubMed] [Google Scholar]
- [39].Claesson-Welsh L, Scheynius A, Tjernlund U, Peterson PA. Cell surface expression of invariant gamma-chain of class II histocompatibility antigens in human skin. J Immunol. 1986 Jan;136(2):484–90. (Baltim Md 1950) [PubMed] [Google Scholar]
- [40].Hansen HJ, Ong GL, Diril H, Valdez A, Roche PA, Griffiths GL, et al. Internalization and catabolism of radiolabelled antibodies to the MHC class-II invariant chain by B-cell lymphomas. Biochem J. 1996 Nov 15;320(Pt 1):293–300. doi: 10.1042/bj3200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ong GL, Goldenberg DM, Hansen HJ, Mattes MJ. Cell surface expression and metabolism of major histocompatibility complex class II invariant chain (CD74) by diverse cell lines. Immunology. 1999 Oct;98(2):296–302. doi: 10.1046/j.1365-2567.1999.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Uckun FM, Gajl-Peczalska K, Myers DE, Jaszcz W, Haissig S, Ledbetter JA. Temporal association of CD40 antigen expression with discrete stages of human B-cell ontogeny and the efficacy of anti-CD40 immunotoxins against clonogenic B-lineage acute lymphoblastic leukemia as well as B-lineage non-Hodgkin’s lymphoma cells. Blood. 1990 Dec 15;76(12):2449–56. [PubMed] [Google Scholar]
- [43].Mach F, Schönbeck U, Sukhova GK, Bourcier T, Bonnefoy J-Y, Pober JS, et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40–CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci. 1997 Mar 4;94(5):1931–6. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Westendorf JJ, Ahmann GJ, Armitage RJ, Spriggs MK, Lust JA, Greipp PR, et al. CD40 expression in malignant plasma cells. Role in stimulation of autocrine IL-6 secretion by a human myeloma cell line. J Immunol. 1994 Jan 1;152(1):117–28. (Baltim Md 1950) [PubMed] [Google Scholar]
- [45].Bataille R, Robillard N, Avet-Loiseau H, Harousseau J-L, Moreau P. CD221 (IGF-1R) is aberrantly expressed in multiple myeloma, in relation to disease severity. Haematologica. 2005 May;90(5):706–7. [PubMed] [Google Scholar]
- [46].Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004 Mar;5(3):221–30. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- [47].LeRoith D, Werner H, Beitner-Johnson D, Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995 Apr;16(2):143–63. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- [48].Hsi ED, Steinle R, Balasa B, Szmania S, Draksharapu A, Shum BP, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res Off J Am Assoc Cancer Res. 2008 May 1;14(9):2775–84. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tai Y-T, Dillon M, Song W, Leiba M, Li X-F, Burger P, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008 Aug 15;112(4):1329–37. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Murphy JJ, Hobby P, Vilarino-Varela J, Bishop B, Iordanidou P, Sutton BJ, et al. A novel immunoglobulin superfamily receptor (19A) related to CD2 is expressed on activated lymphocytes and promotes homotypic B-cell adhesion. Biochem J. 2002 Feb 1;361(Pt 3):431–6. doi: 10.1042/0264-6021:3610431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Davis RS, Wang YH, Kubagawa H, Cooper MD. Identification of a family of Fc receptor homologs with preferential B cell expression. Proc Natl Acad Sci U S A. 2001 Aug 14;98(17):9772–7. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Elkins K, Zheng B, Go M, Slaga D, Du C, Scales SJ, et al. FcRL5 as a target of antibody–drug conjugates for the treatment of multiple myeloma. Mol Cancer Ther. 2012 Oct;11(10):2222–32. doi: 10.1158/1535-7163.MCT-12-0087. [DOI] [PubMed] [Google Scholar]
- [53].Ise T, Nagata S, Kreitman RJ, Wilson WH, Wayne AS, Stetler-Stevenson M, et al. Elevation of soluble CD307 (IRTA2/FcRH5) protein in the blood and expression on malignant cells of patients with multiple myeloma, chronic lymphocytic leukemia, and mantle cell lymphoma. Leukemia. 2007 Jan;21(1):169–74. doi: 10.1038/sj.leu.2404445. [DOI] [PubMed] [Google Scholar]
- [54].Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, et al. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 2004 Jan 15;103(2):689–94. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- [55].Neri P, Kumar S, Fulciniti MT, Vallet S, Chhetri S, Mukherjee S, et al. Neutralizing B-cell-activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res. 2007 Oct 1;13(19):5903–9. doi: 10.1158/1078-0432.CCR-07-0753. [DOI] [PubMed] [Google Scholar]
- [56].Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, et al. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res Off J Am Assoc Cancer Res. 2013 Apr 15;19(8):2048–60. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tai Y-T, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, et al. Novel afucosylated anti-B cell maturation antigen-monomethyl auristatin F antibody–drug conjugate (GSK2857916) induces potent and selective anti-multiple myeloma activity. Blood. 2014 May 15;123(20):3128–38. doi: 10.1182/blood-2013-10-535088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003 Jul;21(7):778–84. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- [59].Hills RK, Petersdorf S, Estey EH, Othus M, Appelbaum FR, Castaigne S, et al. The addition of gemtuzumab ozogamicin (GO) to induction chemotherapy reduces relapse and improves survival in patients without adverse risk karyotype: results of an individual patient meta-analysis of the five randomised trials. Blood. 2013 Nov 15;122(21) (Abstract 356) [Google Scholar]
- [60].Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012 Jul;30(7):631–7. doi: 10.1038/nbt.2289. [DOI] [PubMed] [Google Scholar]
- [61].Ikeda H, Hideshima T, Fulciniti M, Lutz RJ, Yasui H, Okawa Y, et al. The monoclonal antibody nBT062 conjugated to cytotoxic Maytansinoids has selective cytotoxicity against CD138-positive multiple myeloma cells in vitro and in vivo. Clin Cancer Res Off J Am Assoc Cancer Res. 2009 Jun 15;15(12):4028–37. doi: 10.1158/1078-0432.CCR-08-2867. [DOI] [PubMed] [Google Scholar]
- [62].Teicher BA, Chari RVJ. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011 Oct 15;17(20):6389–97. doi: 10.1158/1078-0432.CCR-11-1417. [DOI] [PubMed] [Google Scholar]
- [63].Tassone P, Gozzini A, Goldmacher V, Shammas MA, Whiteman KR, Carrasco DR, et al. In vitro and in vivo activity of the maytansinoid immunoconjugate huN901-N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine against CD56+ multiple myeloma cells. Cancer Res. 2004 Jul 1;64(13):4629–36. doi: 10.1158/0008-5472.CAN-04-0142. [DOI] [PubMed] [Google Scholar]
- [64].Sapra P, Stein R, Pickett J, Qu Z, Govindan SV, Cardillo TM, et al. Anti-CD74 antibody–doxorubicin conjugate, IMMU-110, in a human multiple myeloma xenograft and in monkeys. Clin Cancer Res Off J Am Assoc Cancer Res. 2005 Jul 15;11(14):5257–64. doi: 10.1158/1078-0432.CCR-05-0204. [DOI] [PubMed] [Google Scholar]
- [65].Durie BGM, Harousseau J-L, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006 Sep;20(9):1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- [66].Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT European Group for Blood and Marrow Transplant. Br J aematol. 1998 Sep;102(5):1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- [67].De Weers M, Tai Y-T, van der Veer MS, Bakker JM, Vink T, Jacobs DCH, et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol. 2011 Feb 1;186(3):1840–8. doi: 10.4049/jimmunol.1003032. (Baltim Md 1950) [DOI] [PubMed] [Google Scholar]
- [68].Plesner T, Lokhorst H, Gimsing P, Nahi H, Lisby S, Richardson PG. Daratumumab, a CD38 monoclonal antibody in patients with multiple myeloma — data from a dose-escalation phase I/II study. Blood. 2012 Nov 16;120(21) (Abstract 73) [Google Scholar]
- [69].Martin TG, Strickland SA, Glenn M, Zheng W, Daskalakis N, Mikhael JR. SAR650984, a CD38 monoclonal antibody in patients with selected CD38+ hematological malignancies — data from a dose-escalation phase I study. Blood. 2013 Nov 15;122(21) (Abstract 284) [Google Scholar]
- [70].Martin TG, Hsu K, Charpentier E, Vij R, Baz RC, Benson DM, et al. A phase Ib dose escalation trial of SAR650984 (anti-CD-38 mAb) in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma. ASCO Meet Abstr; Jun 11 2014; (Abstract 8512) [Google Scholar]
- [71].Wetzel M-C, Nicolazzi C, Vallee F, Deckert J, Dumontet C, Plesa A, et al. Abstract 4735: SAR650984: characterization of a potent phase I humanized anti-CD38 antibody for the treatment of multiple myeloma and other hematologic malignancies. Cancer Res. 2013 Aug 14;73(Suppl. 8) (Abstract 4735) [Google Scholar]
- [72].Veillette A, Guo H. CS1, a SLAM family receptor involved in immune regulation, is a therapeutic target in multiple myeloma. Crit Rev Oncol Hematol. 2013 Oct;88(1):168–77. doi: 10.1016/j.critrevonc.2013.04.003. [DOI] [PubMed] [Google Scholar]
- [73].Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012 Jul 19;120(3):552–9. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jakubowiak AJ, Benson DM, Bensinger W, Siegel DSD, Zimmerman TM, Mohrbacher A, et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol Off J Am Soc Clin Oncol. 2012 Jun 1;30(16):1960–5. doi: 10.1200/JCO.2011.37.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lonial S, Jagannath S, Moreau P, Jakubowiak AJ, Raab MS, Facon T, et al. Phase (Ph) I/II study of elotuzumab (Elo) plus lenalidomide/dexamethasone (Len/dex) in relapsed/refractory multiple myeloma (RR MM): Updated Ph II results and Ph I/II long-term safety. ASCO Meet Abstr; Jun 17 2013; (Abtract 8542) [Google Scholar]
- [76].Tai Y-T, Catley LP, Mitsiades CS, Burger R, Podar K, Shringpaure R, et al. Mechanisms by which SGN-40, a humanized anti-CD40 antibody, induces cytotoxicity in human multiple myeloma cells: clinical implications. Cancer Res. 2004 Apr 15;64(8):2846–52. doi: 10.1158/0008-5472.can-03-3630. [DOI] [PubMed] [Google Scholar]
- [77].Hussein M, Berenson JR, Niesvizky R, Munshi N, Matous J, Sobecks R, et al. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010 May;95(5):845–8. doi: 10.3324/haematol.2009.008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Agura E, Niesvizky R, Matous J, Munshi N, Hussein M, Parameswaran RV, et al. Dacetuzumab (SGN-40), lenalidomide, and weekly dexamethasone in relapsed or refractory multiple myeloma: multiple responses observed in a phase 1b study. Blood. 2009 Nov 20;114(22) (Abstract 2870) [Google Scholar]
- [79].Bensinger W, Maziarz RT, Jagannath S, Spencer A, Durrant S, Becker PS, et al. A phase 1 study of lucatumumab, a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br J Haematol. 2012 Oct;159(1):58–66. doi: 10.1111/j.1365-2141.2012.09251.x. [DOI] [PubMed] [Google Scholar]
- [80].Kaufman JL, Niesvizky R, Stadtmauer EA, Chanan-Khan A, Siegel D, Horne H, et al. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br J Haematol. 2013 Nov;163(4):478–86. doi: 10.1111/bjh.12565. [DOI] [PubMed] [Google Scholar]
- [81].Sprynski AC, Hose D, Caillot L, Réme T, Shaughnessy JD, Jr, Barlogie B, et al. The role of IGF-1 as a major growth factor for myeloma cell lines and the prognostic relevance of the expression of its receptor. Blood. 2009 May 7;113(19):4614–26. doi: 10.1182/blood-2008-07-170464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol Off J Am Soc Clin Oncol. 2008 Jul 1;26(19):3196–203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- [83].Moreau P, Cavallo F, Leleu X, Hulin C, Amiot M, Descamps G, et al. Phase I study of the anti insulin-like growth factor 1 receptor (IGF-1R) monoclonal antibody, AVE1642, as single agent and in combination with bortezomib in patients with relapsed multiple myeloma. Leukemia. 2011 May;25(5):872–4. doi: 10.1038/leu.2011.4. [DOI] [PubMed] [Google Scholar]
- [84].Voorhees PM, Chen Q, Kuhn DJ, Small GW, Hunsucker SA, Strader JS, et al. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clin Cancer Res Off J Am Assoc Cancer Res. 2007 Nov 1;13(21):6469–78. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- [85].Voorhees PM, Chen Q, Small GW, Kuhn DJ, Hunsucker SA, Nemeth JA, et al. Targeted inhibition of interleukin-6 with CNTO 328 sensitizes pre-clinical models of multiple myeloma to dexamethasone-mediated cell death. Br J Haematol. 2009 May;145(4):481–90. doi: 10.1111/j.1365-2141.2009.07647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Xiao H, et al. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994 Nov 1;84(9):3063–70. [PubMed] [Google Scholar]
- [87].Van Zaanen HC, Lokhorst HM, Aarden LA, Rensink HJ, Warnaar SO, van der Lelie J, et al. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. Br J Haematol. 1998 Aug;102(3):783–90. doi: 10.1046/j.1365-2141.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- [88].Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, Lonial S, et al. A phase I, open-label study of siltuximab, an anti-IL-6 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma, multiple myeloma, or Castleman disease. Clin Cancer Res Off J Am Assoc Cancer Res. 2013 Jul 1;19(13):3659–70. doi: 10.1158/1078-0432.CCR-12-3349. [DOI] [PubMed] [Google Scholar]
- [89].Voorhees PM, Manges RF, Sonneveld P, Jagannath S, Somlo G, Krishnan A, et al. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2013 May;161(3):357–66. doi: 10.1111/bjh.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Jagannath S, Chanan-Khan A, Heffner LT, Avigan D, Zimmerman TM, Lonial S, et al. BT062, an antibody–drug conjugate directed against CD138, shows clinical activity in patients with relapsed or relapsed/refractory multiple myeloma. Blood. 2011 Nov 18;118(21) (Abstract 305) [Google Scholar]
- [91].Kelly KR, Chanan-Khan A, Somlo G, Heffner LT, Siegel DS, Zimmerman TM, et al. Indatuximab ravtansine (BT062) in combination with lenalidomide and low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma: clinical activity in len/dex-refractory patients. Blood. 2013 Nov 15;122(21) (Abstract 758) [Google Scholar]
- [92].Chanan-Khan A, Wolf JL, Garcia J, Gharibo M, Jagannath S, Manfredi D, et al. Efficacy analysis from phase i study of lorvotuzumab mertansine (IMGN901), used as mono-therapy, in patients with heavily pre-treated CD56-positive multiple myeloma — a preliminary efficacy analysis. Blood. 2010 Nov 19;116(21) (Abstract 1962) [Google Scholar]
- [93].Yong KL, Germaschewski FM, Rodriguez-Justo M, Bounds D, Lee L, Mayes PA, et al. Evaluation Of BCMA as a therapeutic target in multiple myeloma using an antibody–drug conjugate. Blood. 2013 Nov 15;122(21):4447. [Google Scholar]
- [94].Gattei V, Degan M, Gloghini A, Iuliis AD, Improta S, Rossi FM, et al. CD30 ligand is frequently expressed in human hematopoietic malignancies of myeloid and lymphoid origin. Blood. 1997 Mar 15;89(6):2048–59. [PubMed] [Google Scholar]
- [95].Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011 Aug 25;365(8):725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014 Feb 19;6(224) doi: 10.1126/scitranslmed.3008226. (224ra25-224ra25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Maus MV, June CH. Zoom Zoom: racing CARs for multiple myeloma. Clin Cancer Res Off J Am Assoc Cancer Res. 2013 Apr 15;19(8):1917–9. doi: 10.1158/1078-0432.CCR-13-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol Off J Am Soc Clin Oncol. 2014 Mar 3;32(10):1020–30. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007 Jul 1;110(1):296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- [101].Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008 May 15;14(10):3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- [102].Rosenblatt J, Glotzbecker B, Mills H, Vasir B, Tzachanis D, Levine JD, et al. PD-1 blockade by CT-011, anti PD-1 antibody, enhances ex-vivo T cell responses to autologous dendritic/myeloma fusion vaccine. J Immunother. 2011 Jun;34(5):409–18. doi: 10.1097/CJI.0b013e31821ca6ce. (Hagerstown Md 1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Orlowski RZ, Stinchcombe TE, Mitchell BS, Shea TC, Baldwin AS, Stahl S, et al. Phase I trial of the proteasome inhibitor PS-341 in patients with refractory hematologic malignancies. J Clin Oncol Off J Am Soc Clin Oncol. 2002 Nov 15;20(22):4420–7. doi: 10.1200/JCO.2002.01.133. [DOI] [PubMed] [Google Scholar]
- [104].Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002 Nov 1;100(9):3063–7. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- [105].Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004 Mar 15;103(6):2332–6. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Huff CA, Matsui W. Multiple myeloma cancer stem cells. J Clin Oncol Off J Am Soc Clin Oncol. 2008 Jun 10;26(17):2895–900. doi: 10.1200/JCO.2007.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Tagoug I, Plesa A, Dumontet C. Bortezomib influences the expression of malignant plasma cells membrane antigens. Eur J Pharmacol. 2013 Apr 15;706(1–3):11–6. doi: 10.1016/j.ejphar.2013.02.002. [DOI] [PubMed] [Google Scholar]
- [108].Panowksi S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014 Jan 1;6(1):34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Behrens CR, Liu B. Methods for site-specific drug conjugation to antibodies. mAbs. 2014 Jan-Feb;6(1):46–53. doi: 10.4161/mabs.26632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012 Jun 14;119(24):5661–70. doi: 10.1182/blood-2012-03-414359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Schey SA, Fields P, Bartlett JB, Clarke IA, Ashan G, Knight RD, et al. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol Off J Am Soc Clin Oncol. 2004 Aug 15;22(16):3269–76. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- [112].Funaro A, Spagnoli GC, Ausiello CM, Alessio M, Roggero S, Delia D, et al. Involvement of the multilineage CD38 molecule in a unique pathway of cell activation and proliferation. J Immunol. 1990 Oct 15;145(8):2390–6. (Baltim Md 1950) [PubMed] [Google Scholar]