Abstract
Objective
Circulating cytokines are frequently cited as contributors to insulin resistance in children with obesity. This study examined whether circulating adipocytokines, independent of adiposity, predicted pubertal changes in insulin sensitivity (SI), insulin secretion (AIR), and β-cell function (BCF) in high-risk adolescents.
Design and Methods
158 overweight/obese Hispanic adolescents were followed for a median of 4 years. Adipocytokines were measured using Luminex technology. SI, AIR, and the disposition index (DI) were derived from an intravenous glucose tolerance test and Minimal Modeling. Total fat mass (TFM) was measured by DEXA and visceral adipose tissue (VAT) by MRI.
Results
Surprisingly, mean IL-8, IL-1β, IL-6 and TNF-α decreased between 5% and 6.5% per year from baseline (P<0.001). Despite the general temporal trends, gaining 1-SD of VAT was associated with a 2% and 5% increase in MCP-1 and IL-8 (P<0.05). In addition, a 1-SD higher MCP-1 or IL-6 concentration at baseline was associated with a 16% and 21% greater decline in SI during puberty vs. pre-puberty (P<0.05).
Conclusions
Several adipocytokines decreased during adolescence and were weakly associated with VAT and lower insulin sensitivity during puberty. Circulating adipocytokines have relatively limited associations with pubertal changes in diabetes risk, however the consistent findings with MCP-1 warrant further investigation.
Keywords: Inflammation, insulin resistance, circulating cytokines
Introduction
The prevalence of obesity in children and adolescents has risen over the past several decades and remains at an all time high, especially among Hispanics (1). As many as one-fourth of children with obesity present with impaired glucose tolerance, with some even progressing to type 2 diabetes (2). As with adults, elevated fat mass during childhood is thought to cause insulin resistance and subsequently increase diabetes risk (3). However, this pathophysiology is complicated by pubertal development, which is associated with dynamic increases in lean and fat masses, as well as a temporary fall in insulin sensitivity (SI) (4). Our group has shown that during this physiological period of insulin resistance, β-cell function (BCF) progressively declines during puberty in Hispanic youth who are overweight or obese and who have a family history of type 2 diabetes (5). In adults, the deterioration of BCF predicted the incidence of type 2 diabetes in both Pima Indians and Hispanic women with a history of gestational diabetes (6, 7). Although the transition to diabetes was rare in our cohort, persistence of pre-diabetes in these high-risk adolescents was associated with lower BCF (8). Therefore, understanding the physiological mediators of both insulin resistance and impaired BCF is critical to alleviating obesity-related co-morbidities in adolescents.
Obesity is a state of chronic low-grade inflammation (9), which is seen even among pediatric populations (10). In obesity, macrophages and other leukocytes are recruited into adipose tissue, and along with adipocytes, secrete an array of proinflammatory cytokines, including TNF-α, IL-6, and MCP-1 (11). Unlike general markers of inflammation such as Creactive protein (CRP), circulating levels of these adipose-derived cytokines, or “adipocytokines,” may more accurately reflect adipose tissue inflammation in obesity. In addition, these cytokines can also mediate inflammation-induced insulin resistance by directly impairing insulin signaling in adipocytes and skeletal muscle (12, 13). Several cross-sectional studies have reported increased adipocytokine levels in overweight or obese children (14–18), and these inflammatory factors were positively associated with fasting indices of insulin resistance (19–22). However, in studies that controlled for adiposity, the relationship between inflammatory biomarkers and insulin resistance largely disappears, suggesting that obesity-associated inflammation may not mediate insulin resistance in children and adolescents (19, 23). Furthermore, we are unaware of any longitudinal studies of circulating adipocytokines in pediatric populations.
The aims of this study were: 1) to model the temporal trends of 5 relevant adipocytokines (MCP-1, TNF-α, IL-1β, IL-6, and IL-8) from childhood to adolescence, 2) to determine whether changes in total fat mass (TFM) and visceral fat (VAT) were differentially associated with circulating adipocytokines, and 3) to test whether higher serum adipocytokines were associated with greater declines in insulin sensitivity, insulin secretion, and BCF independent of total or visceral adiposity. Because inflammatory cytokines are involved in cardiovascular disease, we have also analyzed changes in certain blood lipids during the same period to provide additional context for the changes in cytokine levels.
Design and Methods
Data were obtained from the Study of Latino Adolescents at Risk (SOLAR) project at the University of Southern California (USC), which is a longitudinal cohort from 2001 to 2013 that aimed to study the progression of T2D risk in susceptible youth across the pubertal transition. Details of the cohort have been described previously (24, 25). Briefly, inclusion criteria for the SOLAR study were as follows: 1) age 8–13 years of age, 2) body mass index (BMI) ≥ 85th percentile for age and sex according to the Centers for Disease Control and Prevention (CDC) guidelines, 3) Hispanic ancestry (all four grandparents self-reported Hispanic ancestry), and 4) family history of type 2 diabetes in at least one parent, sibling, or grandparent. On an annual basis, participants in this analysis completed an inpatient overnight visit for DEXA, MRI, and a frequently sampled intravenous glucose tolerance test (FSIVGTT). This current analysis consists of a total of 158 children (83 males, 75 females) who completed at least 2 annual visits. The Institutional Review Board, Health Science Campus, at USC approved the SOLAR study. Informed consent and assent were obtained from all parents and children, respectively.
Adiposity and Metabolic Measures
Adiposity and metabolic measurements were described previous. Briefly, a DEXA scan was performed to determine whole body composition using a Hologic QDR 4500W (Hologic, Bedford, MA). Visceral Adipose Tissue (VAT) cross-sectional area was measured using a 1.5 Signa LX-Ecospeed 1.5 Tesla magnet (Waukesha, Wisconsin, General Electric) with a single-slice axial view at the level of the umbilicus (24).
A FSIVGTT was performed in the morning following an overnight visit in the General Clinical Research Center. Glucose was measured by the glucose oxidase method on a Yellow Springs Instrument 2700 (Yellow Springs, OH) and insulin by ELISA (Linco, St. Charles, MO), and these data were analyzed with the MINMOD MILLENIUM 2002 computer program (Version 5.16, Richard N. Bergman) to determine insulin sensitivity (SI), the acute insulin response to glucose (AIR), and the disposition index (DI), which is the product of SI and AIR and measures BCF (26). Fasting lipids were assessed using Vitros Chemistry DT Slides (Johnson and Johnson Clinical Diagnostics, Inc., Rochester, New York).
Adipocytokines
Baseline serum samples from the FSIVGTT (−15min and (−5min) were pooled in order to minimize fluctuations in analyte concentrations resulting from pulsatile secretion and stored at –80°C. Adipocytokines (MCP-1, TNF-α, IL-1β, IL-6, and IL-8) were assayed in duplicate using a commercially available magnetic bead-based multiplex ELISA (#HADK2MAG-61K-08, EMD-Millipore) on a Luminex MAGPIX according to the manufacturer’s instructions. At the concentrations detected in our population, the intra-assay variations were 4.1%, 2.8%, 4.0%, 4.3%, 3.9%, and the inter-assay variations were 2.9%, 6.3%, 4.4%, 4.8%, and 2.4% for MCP-1, TNF-α, IL-1β, IL-6, and IL-8, respectively. All samples from a single subject were assayed on the same plate.
Statistical Analysis
1st Model Series: Changes in adipocytokines with age/puberty and relationships to fat distribution
Linear mixed effects regression models were used to analyze the changes in adipocytokines across time. A linear age-as-time model was used because there was no evidence for a quadratic trend (PAge2≥0.10). TFM and VAT are time-varying covariates and were therefore modeled as baseline (TFMBaseline, VATBaseline,) and change-from-baseline (ΔTFM, and ΔVAT). The baseline estimate represents a standard cross-sectional estimate of the association between x and y at baseline. Change-from-baseline is a longitudinal estimate that is not confounded by genetic make-up or other unmeasured time-invariant covariates, and is interpreted as the association between a change in x from baseline and the concomitant change in y over the same period (27). Each model was also adjusted for sex. Box-Cox transformations were performed on each adipocytokine to better approximate normality and homoscedasticity. The correlation structure of these models included random intercepts and an exponentially decaying serial correlation, which outperformed random intercept and slope models. A plate-specific random intercept was also included in the models. Triglycerides, HDL-C, and LDL-C were modeled in the same manner.
2nd models series: Changes in diabetes risk across pubertal status and relationships to adipocytokines
SI, AIR, DI and fasting measures were modeled using puberty-as-time to explicitly test whether adipocytokine concentrations modified the effect of puberty on metabolic outcomes (i.e. an adipocytokine-by-pubertal status interaction). Pubertal status was defined as pre-pubertal (Tanner 1), pubertal (Tanners 2–4), and post-pubertal (Tanner 5). Because pubertal status is categorical, these models were analyzed by ANCOVA to test for a significant pubertal status-by-adipocytokine interaction. When significant, post-hoc comparisons across pubertal status at low (−1SD) and high (+1SD) levels of the adipocytokine were performed in order to interpret the interaction. These models were adjusted for sex, TFMBaseline, VATBaseline, ΔTFM, and ΔVAT. SI, AIR, DI and fasting measures were log transformed to meet model assumptions. AIR, DI, and fasting glucose and insulin were modeled using a random-intercept and an exponentially decaying serial correlation; an estimate of measurement error was also included for SI.
Restricted Maximum Likelihood was used to estimate model parameters with statistical significance set a P<0.05. Given the relatively few adipocytokines analyzed, and that findings for each adipocytokine would have their own biological interpretations, P-values were not adjusted for multiple comparisons. Data were analyzed using R 2.15.2 with the nlme, car, and effects packages.
Results
Fifty-three percent of participants were male. The average age at baseline was 11.3 ± 1.8 years. Over half of the children were in Tanner stages 1 or 2 at baseline, and the remaining 33% were between stages 3 and 5. The mean baseline age- and sex-adjusted BMI percentile was 96.3 ± 5.6 and waist circumference was 87.1 ± 13.0cm. Adiposity variables from DEXA and MRI, fasting glucose and insulin, metabolic outcomes, and serum adipocytokine values are provided in Table 1. There was a median of 4 years of follow-up per subject with a range from 2 to 7 visits.
Table 1.
Baseline Characteristics of the SOLAR cohort
| Variable | Value |
|---|---|
| Male, n (%) | 83 (53) |
| Female | 75 (47) |
| Tanner 1 | 60 (38) |
| 2 | 45 (28) |
| 3 | 15 (9) |
| 4 | 23 (15) |
| 5 | 15 (9) |
| Age (y) | 11.3 ± 1.8 |
| BMI (kg/m2) | 28.2 ± 5.3 |
| BMI -Percentile | 96.3 ± 5.6 |
| BMI -Z Score | 2.0 ± 0.5 |
| WC (cm) | 87.1 ± 13 |
| Body Fat (%) | 38.1 ± 5.9 |
| Lean Mass (kg) | 37.5 ± 10.7 |
| Fat Mass (kg) | 25.0 ± 10.2 |
| VAT (cm2) | 46.9 ± 21 |
| Fasting Glucose (mg/dl) | 93.1 ± 5.7 |
| Fasting Insulin (µU/ml) | 21.1 ± 12.4 |
| SI (104min−1/µU/ml) | 2.1 ± 1.5 |
| AIR (µU/ml×10min) | 1740 ± 1260 |
| DI | 2600 ± 1400 |
| Triglycerides (mg/dl) | 105.5 ± 48.3 |
| HDL-C (mg/dl) | 38.2 ± 8.9 |
| LDL-C (mg/dl) | 94.2 ± 23 |
| MCP-1 (pg/ml) | 223.5 ± 80.4 |
| TNF-α (pg/ml) | 7.4 ± 2.9 |
| IL-6 (pg/ml) | 6.9 ± 7.9 |
| IL-1β (pg/ml) | 4.2 ± 3.7 |
| IL-8 (pg/ml) | 3.7 ± 2.3 |
Unless specified otherwise, values are reported as mean ± standard deviation.
BMI = body mass index, WC = waist circumference, VAT = visceral adipose tissue area, SI = insulin sensitivity, AIR = acute insulin response to glucose, DI = disposition index
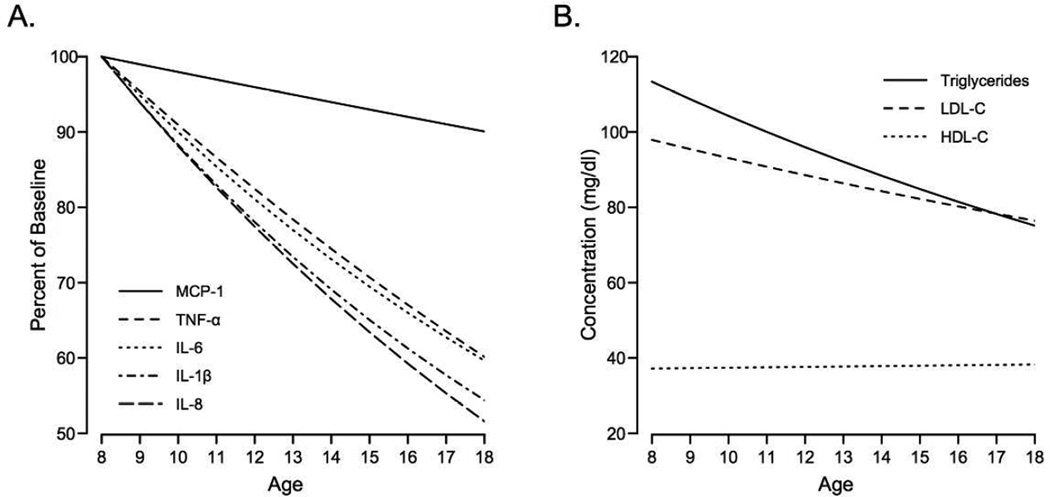
In the first series of models, each adipocytokine was regressed onto age, TFMBASELINE, VATBASELINE, ΔTFM, ΔVAT, and sex, using linear mixed effects models. Independent of changes in adiposity, TNF-α, IL-6, IL-1β, and IL-8 decreased at annual rates of 0.39pg/ml (6.5%), 0.28pg/ml (6.5%), 0.25pg/ml (5%), and 0.23pg/ml (5%), respectively (p<0.001, each; Table 2). However, there was no statistically significant change in MCP-1 over time (P=0.09). As shown in Figure 1A, average plasma levels of adipocytokines TNF-α, IL-6, IL-1β, and IL-8 decreased from 8 to 18 years of age. Sex did not statistically significantly modify the changes in any adipocytokines over time (i.e. βSex×βAge), although there was a trend (PINTERACTION=0.054) for a 4.1pg/ml per year decline in MCP-1 for girls with no change in MCP-1 over time in boys. TNF-α was the only adipocytokine associated with sex and was 1.12pg/ml higher, on average, in boys than girls (P <0.001).
Table 2.
Adipocytokines Across Adolescence: Coefficients from Linear Mixed Effects Models
| TFM and VAT covariates included togetder into tde model | ||||||||||
| MCP-1 | TNF-α | IL-6 | IL-1β | IL-8 | ||||||
| β* | P | β | P | β | P | β | P | β | P | |
| Age | −2.31 | 0.09 | −0.39 | <0.001 | −0.28 | 0.001 | −0.25 | <0.001 | −0.23 | <0.001 |
| TFMBASELINE | −0.32 | NS | 0.04 | NS | 0.07 | NS | 0.05 | NS | 0.01 | NS |
| ΔTFM | 0.96 | 0.071 | 0.02 | NS | 0.00 | NS | 0.02 | NS | −0.01 | NS |
| VATBASELINE | 0.45 | NS | 0.00 | NS | 0.00 | NS | −0.01 | NS | 0.00 | NS |
| ΔVAT | 0.29 | 0.040 | 0.01 | NS | 0.00 | NS | 0.00 | NS | 0.01 | 0.027 |
| Sex† | 10.37 | 0.077 | 1.12 | <0.001 | −0.41 | NS | −0.09 | NS | 0.25 | NS |
| TFM and VAT covariates included separately into the model | ||||||||||
| TFMBASELINE | 0.54 | NS | 0.04 | 0.04 | −0.002 | 0.055 | −0.002 | 0.052 | 0.00 | NS |
| ΔTFM | 2.01 | 0.008 | 0.03 | NS | 0.00 | NS | 0.00 | NS | 0.00 | NS |
| VATBASELINE | 0.52 | NS | 0.01 | NS | 0.00 | NS | 0.00 | NS | 0.00 | NS |
| ΔVAT | 0.53 | 0.007 | 0.01 | 0.026 | 0.00 | NS | 0.00 | NS | 0.002 | 0.032 |
Coefficients are reported back-transformed from the Box-Cox transformations.
Coded as male=1 and female=0. ΔTFM and ΔVAT are change-from-baseline in total fat mass and visceral adipose tissue area, respectively.
Figure 1.
A: Mean change in adipocytokines across age, adjusted for sex, and baseline and change in TFM and VAT. Re-scaled as percent from baseline (age 8). The effect of age was statistically significant for all adipocytokines other than MCP-1. B: Mean change in blood lipids across age, adjusted as in A. The effect of age was significant for triglycerides and LDL-C.
Baseline and change in TFM and VAT were also examined in the first series of models to determine the effects of fat distribution on these circulating adipocytokines. Overall, ΔVAT was positively associated with both MCP-1 (β=0.29, P =0.040) and IL-8 (β=0.010, P =0.027); a 1-SD gain in VAT from baseline was associated with a 2% and 5% increase in MCP-1 and IL-8, respectively. However, none of the adipocytokines were associated with TFMBASELINE, VATBASELINE, or ΔTFM. Neither baseline nor change-from-baseline in TFM or VAT modified the change in any adipocytokine across the study period, as there were no statistically significant interactions between adiposity covariates and age. Because TFM and VAT are positively correlated, particularly TFMBASELINE and VATBaseline (r=0.603), we also analyzed models with TFM and VAT variables entered separately in order to mitigate potential multicollinearity. As seen in Table 2, these models expanded the number of positive associations. TFMBASELINE and ΔVAT correlated with TNF-α, so that a 1-SD increase in either variable predicted a 5% and 2% increase in TNF-α. 1-SD increase in ΔTFM was associated with a 4% increase in MCP-1. All coefficients are provided in Table 2.
Fasting triglycerides, HDL-C, and LDL-C were also monitored across age to determine if they shared similar temporal trends as adipocytokines. After adjusting for age and adiposity, triglycerides and LDL-C decreased at rates of 4.7mg/dl and 2.4mg/dl per year (P<0.001), whereas HDL-C was unchanged (Figure 1B). Higher TFMBASELINE was associated with lower HDL-C (β=−0.227, P=0.001), whereas a 1-SD gain in TFM was associated with a 1.5mg/dl decrease in HDL-C (P<0.001), and an increase of 11.9mg/dl (P<0.05) and 2.6mg/dl (P<0.05), respectively. ΔVAT was also positively associated with TG and LDL-C, so that a 1-SD gain in VAT associated with a 5.1mg/dl (P<0.05) and 3.4mg/dl (P<0.001) increase in triglycerides and LDL-C during the same time. All model coefficients are provided in Table 3.
Table 3.
Blood Lipids Across Adolescence: Coefficients from Linear Mixed Effects Models
| Triglycerides | HDL-C | LDL-C | ||||
|---|---|---|---|---|---|---|
| β* | P | β | β | β | β | |
| Age | −4.650 | <0.001 | 0.061 | NS | −2.44 | <0.001 |
| TFMBASELINE | 0.700 | NS | −0.227 | 0.001 | 0.33 | NS |
| ΔTFM | 2.007 | <0.001 | −0.255 | <0.001 | 0.44 | 0.021 |
| VATBASELINE | 0.234 | NS | −0.014 | NS | −0.04 | NS |
| ΔVAT | 0.284 | 0.013 | −0.007 | NS | 0.19 | 0.001 |
| Sex† | 5.351 | NS | −1.182 | 0.019 | 1.04 | NS |
Coefficients are reported back-transformed from the Box-Cox transformations.
Coded as male=1 and female=0. ΔTFM and ΔVAT are change-from-baseline in total fat mass and visceral adipose tissue area, respectively.
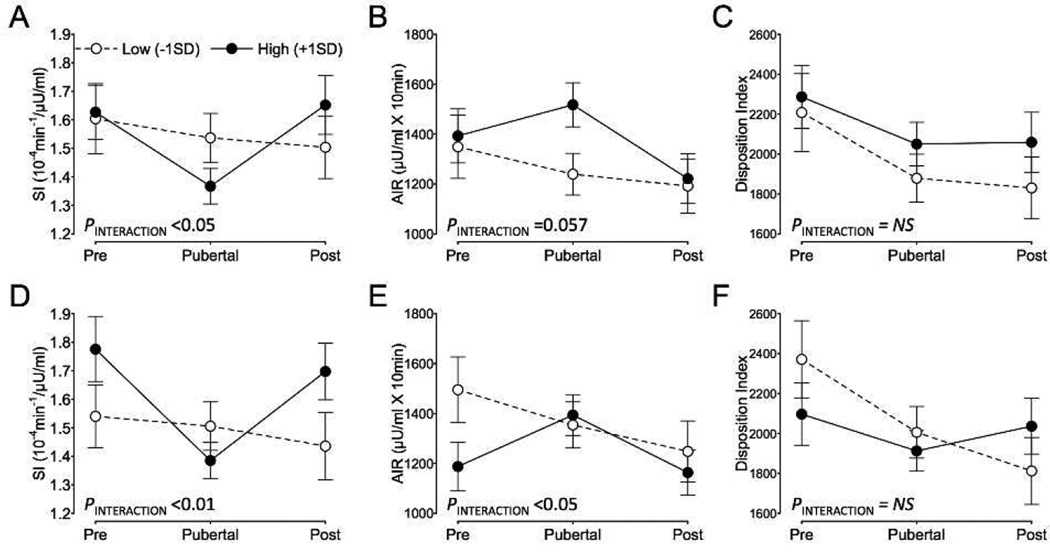
Since SI and BCF are known to decrease with sexual maturation, we examined changes in metabolic parameters across puberty to determine whether greater adipocytokine levels exacerbated pubertal-declines in SI and BCF. Indeed, higher levels of MCP-1BASELINE (P<0.05) and IL-6BASELINE (P<0.01) resulted in greater declines in SI during puberty, which returned to pre-pubertal levels during post-puberty. Specifically, 1-SD higher concentrations of MCP1BASELINE or IL-6BASELINE were associated with a 16% or 22% decrease, respectively, in SI during puberty compared to pre-puberty (P<0.05, each; Fig. 2A and 2C). For AIR, elevated IL-6 BASELINE further increased the AIR seen during puberty (P<0.05) while higher MCP-1BASELINE showed a tendency for a similar effect (P=0.057), and in both cases, AIR returned to pre-pubertal levels during post-puberty. Specifically, each 1-SD increase in MCP-1BASELINE or IL-6BASELINE was associated with a 9% and 17% rise in AIR from pre-puberty to puberty (Fig. 2B and 2D). Neither MCP-1BASELINE nor IL-6BASELINE exacerbated the decline in DI that occurred during puberty (Fig. 2C and 2D). In addition, there were no statistically significant interactions between adipocytokine levels and pubertal status for either fasting glucose or fasting insulin. However, TNF-αBASELINE was positively associated with fasting insulin (β=0.020, P=0.032), where 1-SD higher TNF-α at baseline was associated with 6% higher fasting insulin. There was no evidence that the effects of adipocytokines on changes in metabolic parameters during puberty were modified by sex (i.e. PPubertal-status X Adipocytokine X Sex >0.05).
Figure 2.
A–C: Estimated marginal means for insulin sensitivity (SI; A), acute insulin response (AIR; B), and the disposition index (C) at each pubertal stage for high and low levels of baseline MCP-1. D–F: SI (D), AIR (E), and the disposition index (F) for high and low levels of baseline IL-6. Back-transformed estimates are adjusted for years-elapsed, gender, baseline and changefrom- baseline in TFM and VAT. N=158 in total, with 97, 286 and 180 measurements at pre-, puberty, and post-puberty. 1-SD corresponds to 84 and 8pg/ml for baseline MCP-1 and IL-6, respectively.
In addition to the population average trends described above, we also examined whether changes-from-baseline of circulating adipocytokines within individuals tracked with changes in metabolic parameters across time. ΔMCP-1, but not ΔIL-6 or Δ TNF-α, was inversely associated with SI (β=−0.0016, P<0.001) and positively associated with AIR (β=1.711, P=0.003). Specifically, a 1-SD increase in MCP-1 from baseline was associated with a 9% fall in SI and a 7% rise in AIR during the same period. Consistent with these relationships, ΔMCP-1 was not associated with DI. ΔMCP-1 was also positively associated with fasting insulin, whereby fasting insulin increased by 5% per 1-SD increase in MCP-1 from baseline. Fasting glucose was unrelated to baseline or changes-from-baseline in adipocytokine levels.
Discussion
Inflamed adipose tissue in obesity secretes an array of adipocytokines that perpetuate inflammation and induce insulin resistance (11). Studies in adults have shown that chronic lowgrade inflammation in obesity increases the risk for developing type 2 diabetes (9, 28). Inflammatory biomarkers are also elevated in children with obesity (10, 14–18), but there is limited evidence that inflammation mediates, or at least is an independent predictor of, insulin resistance in youth. In the current study we found that TNF-α, IL-6, IL-1β, and IL-8 declined, while MCP-1 was unchanged during adolescence in this cohort. Contrary to our hypothesis, only MCP-1 and IL-6 were weakly associated with increases in VAT. At the same time, higher baseline levels of MCP-1 and IL-6, as well as longitudinal increases in MCP-1, were associated with decreases in SI during puberty. This insulin resistance was met with appropriate β-cell compensation, as these same adipocytokines were associated with similar increases in AIR.
Higher levels of circulating inflammatory markers predict the incidence of type 2 diabetes in adults (29). Given that BCF, a predictor of type 2 diabetes incidence, is known to decline in our cohort (5), the most striking finding of this analysis is that 4 of the 5 adipocytokines decreased with increasing age, while MCP-1 did not, on average, change over time. The different trajectory of MCP-1 is likely explained by its inverse relationship with SI, which itself decreased with increasing age. The general decline in these inflammatory markers is consistent with a serial-cross-sectional study from NHANES suggesting that the obesityassociated elevations in CRP and absolute neutrophil count peak during early adolescence but begin to decline in the later teen years (30). We observed similar trends with blood lipids: fasting triglycerides and LDL-C both decreased with age independent of changes in adiposity. The observations with lipids corroborate our findings with inflammation: maturation during adolescence per se appears to at least partially mitigate some pathophysiological factors associated with childhood obesity. Understanding the mechanism behind the age related improvements in inflammatory and lipid parameters may help to identify novel therapeutic targets for mitigating obesity associated inflammation and dyslipidemia.
The greater abundance of adipose tissue itself, particularly VAT, is thought to contribute to greater circulating markers of inflammation in obesity. We were specifically interested in changes in TFM and VAT during the study period because such longitudinal estimates are less likely to be confounded and reflect modifiable changes that occur during the period of interest i.e. adolescence. Given that MCP-1 and IL-8 were independently related to changes in VAT, but not TFM, our data is consistent with the notion that visceral adiposity is more detrimental than total adiposity. Analyzing the adiposity variables separately to minimize decreased power due to multicollinearity identified additional positive relationships between adiposity and adipocytokines, particularly with TFM. Larger studies, which would be less affected by multicollinearity, will be required to determine whether VAT is a greater independent determinant of circulating inflammatory markers than TFM in children with obesity. The sparse and weak associations with adiposity detected in our analysis were unexpected, as several reports have found relationships between BMI or WC and cytokines in children, including Hispanics (14, 18, 21, 33). However, other studies have shown no associations between obesity and these same adipocytokines, including: IL-6 (7), TNF-α (7, 19), IL-8 (17), and MCP-1 (15, 17, 19). In contrast, increases in either TFM or VAT were associated with worse dyslipidemia over the same time period. These latter findings indicate that we observed biologically relevant changes in adiposity during the study period, but it is possible that greater changes in adiposity or maintenance of these changes for a longer period is required to detect changes in circulating inflammatory markers (34). Furthermore, longitudinal estimates frequently differ from crosssectional estimates (27).
The final aim of this study was to examine the independent contribution of circulating adipocytokines to changes in metabolic parameters across puberty. Higher baseline MCP-1 and IL-6 were each associated with a further decline in SI during puberty that fully recovered by post-puberty, suggesting that higher levels of these cytokines did not have a lasting influence on SI. The exaggerated decreases in SI with higher MCP-1 and IL-6 were accompanied by compensatory increases in AIR, explaining why did not observe any relationships between adipocytokines and DI. In addition, MCP-1 (i.e. ΔMCP-1) was the only adipocytokine measured that was independently and inversely associated with SI, AIR, and fasting insulin across time. The independent contribution of inflammatory markers to insulin resistance in obese pediatric populations is often not tested (34), or the associations between inflammatory biomarkers and insulin resistance are nearly entirely explained by adiposity (19, 23). Thus, a major strength of our findings with MCP-1 is that they are independent of total and visceral adiposity. Interestingly, Herder et al. similarly reported that MCP-1 was only one of seven inflammatory markers to be independently associated with HOMA-IR (19). The potential importance of MCP- 1 may be attributable to its potent biological actions. MCP-1 is primarily produced by adipose tissue and binds to chemokine receptor-2 on circulating monocytes to induce macrophage recruitment and activation in adipose tissue (35). Furthermore, in differentiated human muscle cells, MCP-1 was capable of blunting insulin signaling via ERK1/2 activation at physiologically-relevant concentrations as low as 20pg/ml (13). Other non-inflammatory mechanisms that could explain decreased insulin sensitivity in this cohort could be the accumulation of ectopic fat in the liver, which unfortunately, could not be assessed in this current cohort (36, 37). Furthermore, we have recently shown that fasting free fatty acids are elevated in pre-diabetic adolescents compared to their glucose tolerant counterparts, suggesting that a progressive decline of BCF in our cohort could be attributed to lipotoxicity of the pancreas (38). Of course, our results must be interpreted in the context of several limitations.
By design, the SOLAR cohort is restricted to overweight and obese adolescents. Including lean participants may have allowed us to detect more substantial effects of adiposity on markers of inflammation. On the other hand, not all individuals with obesity demonstrate a strong inflammatory phenotype. A previous study by our group reported 50% of Hispanic young adults with obesity were positive for biopsy confirmed adipose tissue inflammation (39). Thus, our design prevented us from examining these relationships among individuals with a wide range of adiposity, but potentially strengthened our ability to detect relationships between inflammation and metabolic parameters. Another limitation of our study is that we did not perform adipose tissue biopsies to directly measure tissue levels of inflammation, and thus cannot exclude the potential importance of tissue cytokine levels. A very recent report employing functional and histochemical assays in fat biopsies from over 150 children provides strong evidence of early alterations and macrophage infiltration in the adipose tissue of children with obesity (40). Furthermore, many adipocytokines may primarily act through paracrine mechanisms within adipose tissue and liver, and therefore circulating levels may be less relevant than tissue concentrations (11). In light of this, our paper further emphaizes the need to obtain adipose tissue biopsies or identify better circulating markers of tissue inflammation in pediatric patients.
In conclusion, adipocytokines relevant to adipose tissue inflammation and metabolic dysfunction mostly decline during adolescence in Hispanic youth with obesity. Fat mass, either total or visceral, appears to be only weakly associated with circulating inflammatory markers in adolescents who have already developed obesity. Lastly, while higher levels of MCP-1 and IL-6 were associated with lower SI and greater β-cell compensation during puberty (increased AIR), the overall decline in BCF observed in this longitudinal study was unrelated to circulating adipocytokines. These data suggest that adolescence may be a period of relatively reduced inflammatory tone and extends others’ cross-sectional findings that after controlling for adiposity, circulating adipocytokines are relatively poorly associated with diabetes risk in pediatric populations. However, MCP-1 may be a contributor to insulin resistance in children with obesity, and our findings with this adipocytokine warrant further investigation.
What is known about this subject?
-
-
Children and adolescence with obesity already demonstrate insulin resistance and impaired beta-cell function.
-
-
Inflammation is an important contributor to insulin resistance and diabetes risk in obesity.
-
-
Adipocytokines, circulating inflammatory factors derived from adipose tissue, are frequently cited as mediators of diabetes risk in children with obesity yet few studies have directly examined this relationship.
What does this study add?
-
-
Several well-studied adipocytokines decline during adolescence in our cohort, and appear relatively insensitive to changes in adiposity during this period.
-
-
MCP-1 is longitudinally associated with increasing visceral adiposity, and this adipocytokine predicts a greater fall in insulin sensitivity and increased insulin secretion during puberty.
Acknowledgements
BDK was involved in data collection and was responsible for data analysis and manuscript preparation. CT-C, TLA, and MJW performed data collection, researched data, and reviewed and edited the manuscript. MIG (principal investigator) supervised all aspects of the study.
We are grateful to the children and their families for participating in the study. We would like to thank Christina Ayala, MPH, and Quintilia Avila, MPA, and the SOLAR project staff, as well as the staff of the GCRC and CTSI/CTU for their work and dedication to this project.
Support
This project was supported by Award Number R01DK059211 from the National Institute of Diabetes And Digestive And Kidney Diseases and by the GCRC, National Center for Research Resources Grant MO1-RR-00043.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to disclose.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R, Fisch G, Teague B, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. New England Journal of Medicine. 2002;346:802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 3.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2010;91:1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ball GD, Huang TT, Gower BA, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. J Pediatr. 2006;148:16–22. doi: 10.1016/j.jpeds.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 5.Kelly LA, Lane CJ, Weigensberg MJ, Toledo-Corral CM, Goran MI. Pubertal changes of insulin sensitivity, acute insulin response, and beta-cell function in overweight Latino youth. J Pediatr. 2011;158:442–446. doi: 10.1016/j.jpeds.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in highrisk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 7.Wärnberg J, Nova E, Moreno LA, et al. Inflammatory proteins are related to total and abdominal adiposity in a healthy adolescent population: the AVENA Study. Am J Clin Nutr. 2006;84:505–512. doi: 10.1093/ajcn/84.3.505. [DOI] [PubMed] [Google Scholar]
- 8.Goran MI, Lane C, Toledo-Corral C, Weigensberg MJ. Persistence of pre-diabetes in overweight and obese Hispanic children: association with progressive insulin resistance, poor beta-cell function, and increasing visceral fat. Diabetes. 2008;57:3007–3012. doi: 10.2337/db08-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 10.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-Grade Systemic Inflammation in Overweight Children. Pediatrics. 2001;107:e13–e13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 11.Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–684. doi: 10.1016/j.cell.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proceedings of the National Academy of Sciences. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sell H, Dietze-Schroeder D, Kaiser U, Eckel J. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology. 2006;147:2458–2467. doi: 10.1210/en.2005-0969. [DOI] [PubMed] [Google Scholar]
- 14.Aygun AD, Gungor S, Ustundag B, Gurgoze MK, Sen Y. Proinflammatory cytokines and leptin are increased in serum of prepubertal obese children. Mediators Inflamm. 2005;2005:180–183. doi: 10.1155/MI.2005.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Bhattacharjee R, Kheirandish-Gozal L, et al. Insulin sensitivity, serum lipids, and systemic inflammatory markers in school-aged obese and nonobese children. Int J Pediatr. 2010;2010:846098. doi: 10.1155/2010/846098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauras N, Delgiorno C, Kollman C, et al. Obesity without established comorbidities of the metabolic syndrome is associated with a proinflammatory and prothrombotic state, even before the onset of puberty in children. J Clin Endocrinol Metab. 2010;95:1060–1068. doi: 10.1210/jc.2009-1887. [DOI] [PubMed] [Google Scholar]
- 17.Murdolo G, Nowotny B, Celi F, et al. Inflammatory adipokines, high molecular weight adiponectin, and insulin resistance: a population-based survey in prepubertal schoolchildren. PLoS One. 2011;6:e17264. doi: 10.1371/journal.pone.0017264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breslin WL, Johnston CA, Strohacker K, et al. Obese Mexican American children have elevated MCP-1, TNF-alpha, monocyte concentration, and dyslipidemia. Pediatrics. 2012;129:e1180–e1186. doi: 10.1542/peds.2011-2477. [DOI] [PubMed] [Google Scholar]
- 19.Herder C, Schneitler S, Rathmann W, et al. Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab. 2007;92:4569–4574. doi: 10.1210/jc.2007-0955. [DOI] [PubMed] [Google Scholar]
- 20.Roth CL, Kratz M, Ralston MM, Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism. 2011;60:445–452. doi: 10.1016/j.metabol.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Giordano P, Del Vecchio GC, Cecinati V, et al. Metabolic, inflammatory, endothelial and haemostatic markers in a group of Italian obese children and adolescents. Eur J Pediatr. 2011;170:845–850. doi: 10.1007/s00431-010-1356-7. [DOI] [PubMed] [Google Scholar]
- 22.Stoppa-Vaucher S, Dirlewanger M, Meier CA, et al. Inflammatory and prothrombotic states in obese children of european descent. Obesity. 2012;20:1662–1668. doi: 10.1038/oby.2012.85. [DOI] [PubMed] [Google Scholar]
- 23.Moran A, Steffen LM, Jacobs DR, Jr, et al. Relation of C-reactive protein to insulin resistance and cardiovascular risk factors in youth. Diabetes Care. 2005;28:1763–1768. doi: 10.2337/diacare.28.7.1763. [DOI] [PubMed] [Google Scholar]
- 24.Goran MI. Impaired Glucose Tolerance and Reduced β-Cell Function in Overweight Latino Children with a Positive Family History for Type 2 Diabetes. Journal of Clinical Endocrinology & Metabolism. 2004;89:207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 25.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 26.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. Journal of Clinical Investigation. 1981;68:1456–1467. doi: 10.1172/JCI110398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diggle P, Heagerty P, Liang K-Y, Zeger S, et al. Analysis of longitudinal data. Oxford University Press; 2013. [Google Scholar]
- 28.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 29.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 30.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–e809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klöting N, Fasshauer M, Dietrich A, et al. Insulin sensitive obesity. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 32.Testelmans D, Tamisier R, Barone-Rochette G, et al. Profile of circulating cytokines: impact of OSA, obesity and acute cardiovascular events. Cytokine. 2013;62:210–216. doi: 10.1016/j.cyto.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Tam CS, Garnett SP, Cowell CT, et al. IL-6, IL-8 and IL-10 levels in healthy weight and overweight children. Horm Res Paediatr. 2010;73:128–134. doi: 10.1159/000277632. [DOI] [PubMed] [Google Scholar]
- 34.Tam CS, Clement K, Baur LA, Tordjman J. Obesity and low-grade inflammation: a paediatric perspective. Obes Rev. 2010;11:118–126. doi: 10.1111/j.1467-789X.2009.00674.x. [DOI] [PubMed] [Google Scholar]
- 35.Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toledo-Corral CM, Alderete TL, Hu HH, et al. Ectopic fat deposition in prediabetic overweight and obese minority adolescents. J Clin Endocrinol Metab. 2013;98:1115–1121. doi: 10.1210/jc.2012-3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alderete TL, Toledo-Corral CM, Desai P, Weigensberg MJ, Goran MI. Liver fat has a stronger association with risk factors for type 2 diabetes in African-American compared with Hispanic adolescents. J Clin Endocrinol Metab. 2013;98:3748–3754. doi: 10.1210/jc.2013-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toledo-Corral CM, Alderete TL, Richey J, Sequeira P, Goran MI, Weigensberg MJ. Fasting, post-OGTT challenge, and nocturnal free fatty acids in prediabetic versus normal glucose tolerant overweight and obese Latino adolescents. Acta Diabetol. 2014 doi: 10.1007/s00592-014-0634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lê KA, Mahurkar S, Alderete TL, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes. 2011;60:2802–2809. doi: 10.2337/db10-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Landgraf K, Rockstroh D, Wagner IV, Weise S. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes. 2014 doi: 10.2337/db14-0744. [DOI] [PubMed] [Google Scholar]