Abstract
Background: Food additives attract consumers, improve foods quality, control weight, and replace sugar in foods, while it may affect seriously children and adults health. Aim: To investigate the adverse effects of saccharin and methylsalicyltaes on lipid profile, blood glucose, renal, hepatic function, and oxidative stress/antioxidant (lipid peroxidation, Catalase and reduced glutathione (GSH) in liver tissues). Methods: 46 young male albino rats were used. Saccharin and methylsalicylate were giving orally as low and high dose for 30 days. Rats were divided into 5 groups, 1st control group, 2nd and 3rd low and high saccharin-treated groups and 4th and 5th low and high methylsalicylate-treated group. Results: Serum total cholesterol, triglyceride, glucose levels and body weight gain were decreased in saccharin high dose compared to control. Rats ingested high dose of saccharin presented a significant reduction in serum triglycerides, cholesterol and LDL levels. Low and high doses of saccharin exhibited a significant increase in liver function marker of ALT, AST, ALP activity, total proteins and albumin levels and renal function test (urea and creatinine levels) in comparison with control group. Saccharin high dose induce a significant decline in hepatic GSH levels, catalase and SOD activities while increased in hepatic MDA level. Conclusion: It could be concluded that, saccharin affects harmfully and alters biochemical markers in hepatic and renal tissues not only at greater doses but also at low doses. Whereas uses of metylsalicylates would not pose a risk for renal function and hepatic oxidative markers.
Keywords: Food additives, liver, oxidative biomarkers, saccharin, methyl-salicylates
Introduction
In the last decades, the rising concern about health and life quality have encouraged societies to exercise, eat healthy food and reduce the consumption of food rich in sugar, salt and fat. With increased consumer interest in reducing sugar intake, food products made with sweeteners rather than sugar have become more common [1]. The low-calorie artificial sweeteners, such as aspartame, saccharin, acesulfame-K and cyclamate, have become sugar alternatives to replace sucrose [2], and have been widely used in dairy products, energy control diet and diabetes in Africa, Asia, Europe and in the USA.
Saccharin is the non-nutritive, non-caloric intense artificial sweeteners, as it has 300-500 times the sweetness of sucrose, but it has a slightly bitter aftertaste [3]. It still of the most important and widely used sweeteners [4] as it is a very stable compound with respect to heat and time so that it can be used in hot beverages, canned vegetables, bakery products and reduced sugar jams [5]. Saccharin is an important sweetener, especially for diabetics, as it goes directly through the human digestive system without being digested. Although saccharin thus has no food energy, it can trigger the release of insulin in rats, apparently as a result of its taste [6]. There are different forms of saccharin including sodium saccharin, calcium saccharin, potassium and acid saccharin. Sodium saccharin is more often used as it is more palatable. Saccharin has the chemical formula C7H5NO3S, can be produced in various ways and can be used to prepare exclusively disubstituted amines from alkyl halides via a Gabriel synthesis. In the European Union, saccharin is also known under the E number (additive code) E954. The accepted daily intake of saccharin is 2.5 mg/kg body weight [7].
The degree of saccharin absorption occurs rapidly and dependent on food intake, after its removal from the diet, almost complete tissue clearance resulted in 3 days. Saccharine of renal, bladder, liver and muscle tissues and plasma levels increased in diets containing 7.5% or 10% saccharin for 22 days. This was attributed to the animals’ reduced ability to eliminate saccharin [8]. So cause its accumulation in the tissue when it consumed 2-3 times a week.
The renal and hepatic effects of in vivo treatment with saccharin have been determined as increased DNA elutability in the range 130-210% compared with controls [9]. Consumption of large amounts of saccharin might produce hypoglycemia [10], reduce hyperinsulinemia, decrease the insulin resistance and improve glycemic control during saccharin intake in hyperglycemic obese mice [11]. In contrast intake of foods or fluids containing non-nutritive sweeteners was accompanied by increased food intake, body weight gain, accumulation of body fat, and weaker caloric compensation [12]. In the US, food containing saccharin must be labeled with a warning that use of this product may be hazardous to health and has caused cancer in laboratory animals. Some studies showing a correlation between saccharin consumption and increased frequency of cancer [especially bladder cancer] and others finding no such correlation, some studies have shown a correlation between consumption and cancer incidence [13]. There are shortages in data about saccharine and its oxidative stress action that may correlate to carcinogenic effects.
Salicylate esters, a chemicals extensively used as flavor and fragrance additives in foods, beverages and a wide variety of consumer products, are suspected to have estrogenic activity [14]. Dermal exposures to methyl salicylate, oral exposures to salicylic acid and sodium salicylate, are all associated with reproductive and developmental toxicity. An exposure to cosmetic product would be only 20 % of the level seen with ingestion of a “baby” aspirin [81 mg] on a daily basis. The oral bioavailability of salicylates is supposed to be 100% and well absorbed by mouth. The salicylates are undergoing extensive hydrolysis, primarily in the hepatic tissues, to salicylic acid that conjugated with either glucuronide or glycine and is excreted via urine as salicyluric acid and acyl and phenolic glucuronides. The probable metabolism of the salicylates does not provide toxicological concerns [15]. Moderate oral toxicity of the salicylates found in acute case, with toxicity commonly reducing with increasing size of the ester R-group. The aromatic salicylates are of low to moderate acute oral toxicity [1300 to > 5000 mg/kg body weight). Differences in acute oral toxicity are correlated to the virtual amount of the molecular weight produced as salicylic acid after hydrolysis [16]. From the genetic toxicity data (2 years investigation of oral methyl salicylate) and the metabolism of the salicylates (simple alcohols and acids metabolites), it seems that the salicylates are improbably to be oncogenic [17].
Uses of salicylates and salicylic acid in cosmetic products would not produce a risk for reproductive or developmental effects in human being according to the Cosmetic Ingredient Review Board. Synthetic sweetener and flavoring materials are routinely widely used due to their taste and sensory pleasure fragrance properties to food and beverage [18], consistency, stability and low price, however many of them become toxic after extended use, initiating health complications such as indigestion, anemia and allergic reactions as urticarial, asthma and, pathological injuries in the brain, kidney, spleen and liver, tumors, paralysis, mental retardation, nervous manifestations, abnormalities in offsprings, growth retardation and eye faults [19,20]. From the previous literature there are contradictory suggestion on the role of saccharin and methylsalicyltaes on health.
Natural food free from pesticide residue and pollution or additives is the best choice for human. Our goal is to maintain the people healthy during their life. Drinking juice, meat food products, some milk products etc, all contain synthetic additives, to let us eat much. We choose and ingest food to get its benefit, what about taking food with additives that may increase its consumption. We face environmental pollution, many diseases, daily stresses, metabolic changes during life and aging, so we need to have natural, functional food to keep us withstand, these stressors not another stress from food additives specially when the consumer pay for that.
Therefore, the aim of the present work was to evaluate the toxic harmful effect of some synthetic food additives of sweeteners and odorant or flavors on liver, renal and metabolic biomarkers in rats.
Material and methods
Animals and diet
A total of 46 young male Rattus Norvegicus albino rats weighting about 70-80 g, of about 5 weeks old, were used in the current study. They were obtained from National Research Center, Cairo, Egypt. Animals were retained under observation for 7 days before the start of the investigation to eliminate any intercurrent infection. They were preserved in stainless steel cages at ordinary atmospheric temperature of 27 ± 5°C and in good ventilation. This study was carried out in agreement with the rules of Beni Suef University for animal uses and these animals were used for the studied food additives.
Rats were nourished on the regular basal diet and supplied with tap water and the contents of experimental diets were according to Kim et al., [21], it composed of: lipid 5%, carbohydrates 6%, protein 20.3%, fiber 5%, salts 3.7%, and vitamins 1%.
Compounds and chemicals
Chemicals utilized in the experiment included saccharin (food sweeteners) that is found in its sodium salt, white crystalline powder, and sodium saccharin content is 99.62%, arsenic less than 2 ppm, foreign substance less than 10 ppm, from El-Goumhoria Company (Egypt) purchased from Taianjin North Food Co. LTD (China).
Methylsalicylates (food odorant) is a clear colorless liquid, its concentration is 99.8% purchased from El-Goumhoria Company (Egypt). Saccharine and methylsalicylate were in a solid condition and both prepared as solutions of 2 doses low and high through dissolving in distilled water. The low and high doses of Saccharin were 10 and 500 mg/kg bw, while doses Methylsalicylates were 80 and 250 mg/kg bw respectively.
Normal ingestion of these compound may be safe at ADI, however abnormal much continuous, heavy using of these additive threatening the life of many peoples, some countries has regulation to avoid using children and patient from using those additive and have law for the company, other have nothing to do with the market and companies.
The dose that we used is high compared with ADI to show the safety margin of these compound, the substance with higher safety border consider safer than those with low. I mean if the chosen saccharine is safe, have non-significant effect on the body markers (liver function, kidney function and oxidative stress) in double or triple or 4 times than ADI, it consider more safe.
We choose higher dose to determine the safety border, you will say the children will not consume that much amount. The kids or consumer use too much of synthetic sweetener because many additives have been provided here. If you take in the morning biscuit which contain sweeteners, with tea having saccharin and in the work you got cookie having sweetener and flavor, in your car taste some fried.
We like to say most food in the supermarket contain flavor, sweetener, and coloring to attract the consumer. Even Fanta have tartrazine that have serious effect on the body organs.
We choose saccharine because many people use it especially diabetic who having metabolic disturbances include changes in oxidative stress so saccharine exaggerates the condition. We like to aware the consumer about the effectiveness of saccharine on the oxidative stress and its toxicity limit.
Manufacturer aimed to attract the consumer for each product and this would accumulate through days, so we need to aware the consumer. Food additive is not a medication to give ADI or dose, it take with food and when we eat food we did not look at its dose ADI or small, we (people) just eat until satisfy, the condition is serious.
Experimental design and animal grouping
Rats were classified into the following: Group 1), Control (10 rats) didn’t administrate any chemicals. Group 2), Saccharin low dose (8 rats) were administered 10 mg/kg bw per day for 30 days per os using stomach tube. Group 3), Saccharin high doses (10 rats) were administered 500 mg/kg bw per day for 30 days orally using stomach tube. Group 4), Methylsalicylates low doses (8 rats) were administered 80 mg/kg bw/day for thirty days per os using stomach tube. Group 5), Methylsalicylates high dose (10 rats) were administered 250 mg/kg bw per day for 30 days per os using stomach tube.
Sampling and tissue preparation
At the completion of the experimental time, venous blood samples were harvested from the orbital sinus of control and tested rats by glass capillaries after fasting overnight. The blood samples were collected in clean dry centrifuge tubes and permitted to coagulate at room temperature then centrifuged at 3500 rpm for 15 min. for getting the serum. Hepatic tissues homogenates were set by dissolving 0.25 g of hepatic tissue in 5 ml saline (0.9% NaCl) then minced by homogenizer for 15 min followed by centrifugation using centrifuge for 10 min at 3000 rpm, the formed supernatants were collected for measuring the oxidative stress biomarkers.
Serum and tissue biochemical analysis
Serum ALT and AST activities were determined according to Reitman and Frankel [22] using kits obtained from Randox Company UK. Total protein was measured in agreement with the method of Henry [23], serum urea according to method of Patton and Crouch [24], serum total cholesterol, triglycerides and HDL-cholesterol were estimated by enzymatic colorimetric methods. Serum level of glucose was analysed using enzymatic colorimetric method consistent with Trinder [25]. Catalase activity was assessed in hepatic homogenate according to Cohen et al. [26]. Hepatic GSH and lipid peroxidation levels was evaluated by using the procedure of Beutler et al. [27] and Presuss [28] respectively.
Statistical analysis
The statistical analysis performed by GraphPad Instat software [version 3, ISS-Rome, Italy]. Data were analysed using one-way analysis of variance (ANOVA) followed by Tukey-Kramer (TK) multiple comparisons post-test. Values of P < 0.05 were considered as significant. The data recorded in tables as mean ± standard error (SE).
Results
Effect of saccharin and methyl-salicylate on body weight gain and growth rat
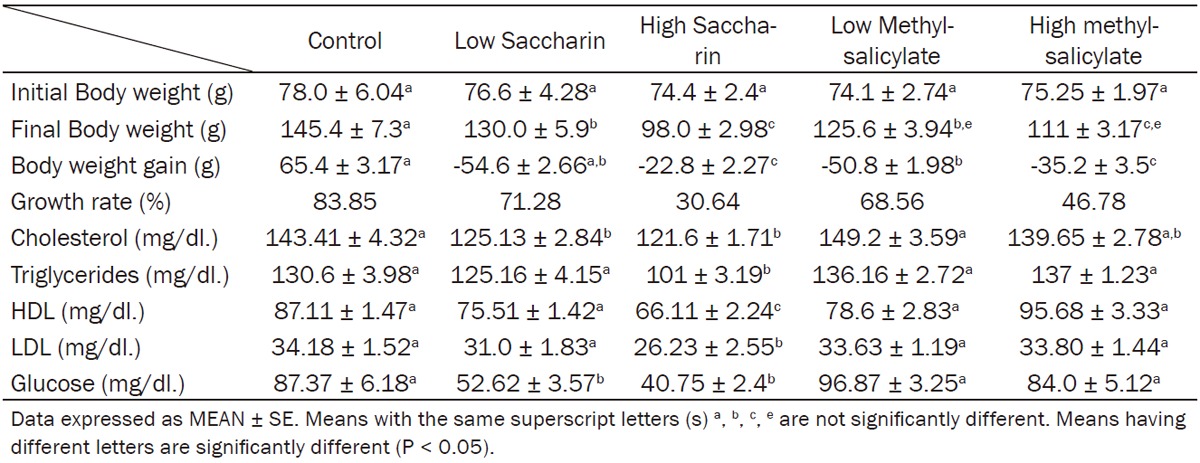
Data showing the effect of low and high dosages of food sweeteners and odorant on final body weight and body weight gain were expressed at Table 1.
Table 1.
Effect of saccharin (10 and 500 mg/kg bw) and methyl-salicylate (80 and 250 mg/kg bw) on body weight, serum lipid profile and glucose
|
Rats consumed saccharin high dose, exhibited a significant reduction, while low dose indicated a non-significant changes in final body weight in comparison with normal control group.
Rats consumed low dose of methylsalicylates and that consumed high dose of the same food flavor, produced a significant decline in final body weight comparing with normal rats, and extraordinary methylsalicylates exhibited a significant reduction than low dose of methylsalicylates on final body weight. Little and high doses of methylsalicylates induced a significant decrease in body weight gain in comparison with control group.
Effect of saccharin and methyl-salicylate on lipid profile and serum glucose
Data showing the effect of food sweeteners (saccharin) both low and high doses on lipid profile (serum total cholesterol, triglycerides, HDL and LDL-Cholesterol) and fasting serum glucose were expressed in Table 1.
Rats consumed saccharin in low and high doses revealed a significant decrease in serum total cholesterol and glucose level when compared to control rats (Table 1).
Rats consumed high dose of saccharin showed a significant decrease in serum triglycerides, cholesterol and LDL levels when compared to control rats.
High dose saccharin showed the maximum reduction in serum fasting glucose in all groups.
Methylsalicyaltes food odors both low and high doses showed a non significant changes in lipid profile (serum total cholesterol, triglycerides, HDL and LDL-Cholesterol) and fasting serum glucose when consumed orally by young rats daily for 30 days (Table 1).
Effect of saccharin and methyl-salicylate on hepatic function markers
Low and High dose of both Saccharin and Methyl-salicylate exhibited a significant increase in serum ALT, ALP activity when compared to control rats and high dose of Saccharin showed the highest level among the groups (Table 2).
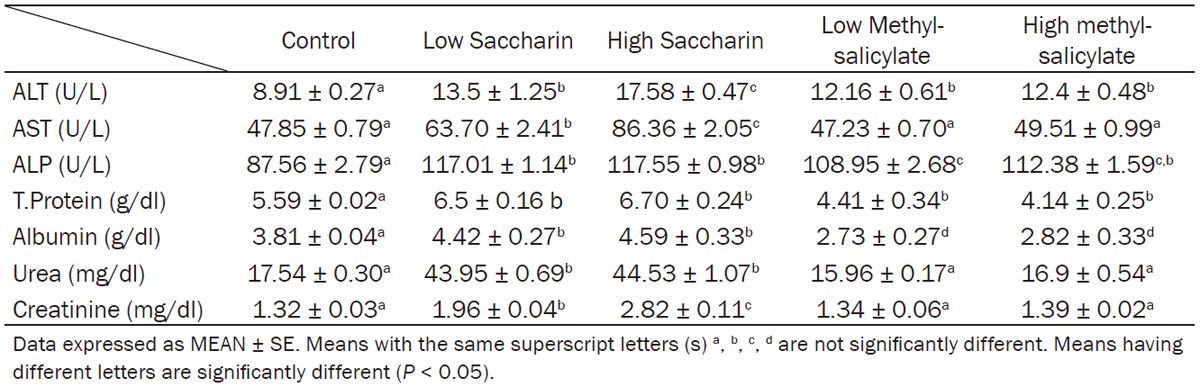
Table 2.
Effect of saccharin (10 and 500 mg/kg bw) and methyl-salicylate (80 and 250 mg/kg bw) on serum liver function and renal function markers
|
Rats ingested low and high doses of saccharin exhibited a significant increase in liver function marker of serum ALT, AST, ALP activity, total proteins and albumin concentration in comparison with control group (Table 2).
Rats consumed low dose or high dose of methylsalicylate presented a significant decrease in total protein level of serum comparing to normal control group, while serum albumin was not significantly changed (Table 2).
Effect of saccharin and methyl-salicylate on renal function tests
Saccharin low and high dose produced a significant elevation in blood urea and creatinine levels and the higher concentration recorded with high dose, whereas low and high methylsalicyltes not induce any significant changes with urea and creatinine when compared with control rats (Table 2).
Effect of saccharin and methyl-salicylate on hepatic oxidative stress markers
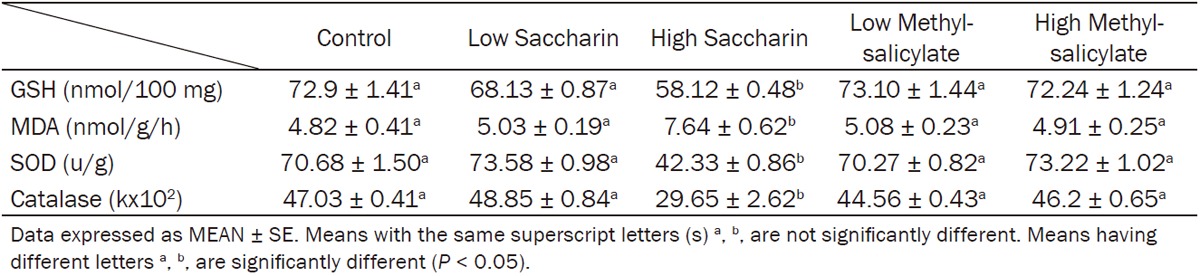
Saccharin high dose induced a significant reduction in hepatic GSH levels, catalase and SOD activities while increased in hepatic MDA level. However biomarkers of oxidative stress in liver homogenate were not significantly changed with low saccharin dose and both low and high methylsalicylates (Table 3).
Table 3.
Effect of Saccharin (10 and 500 mg/kg bw) and Methyl-salicylate (80 and 250 mg/kg bw) on oxidative stress biomarkers (GSH, MDA, SOD and catalase) in liver homogenate
|
Discussion
The synthetic and some of the naturally present food additives, have been reviewed for toxicity. Limit values have been evaluated for dietary intake by humans on the basis of conclusion of data attained in experimental animals.
In our work we tried to examin the side toxic effects and biochemical variations in blood and tissues of experimental rats treated with low and high dose of two compounds saccharine and methylsalicylates that are commonly used in Egyptian and saudian field in different product (grocery, companies and many hyper and supermarket) of food additives.
We considered low dose (double of ADI) because young children in Egypt can consume a double of ADI (or more) daily in several products without control, in addition we used the high dose (a much higher than ADI) to evaluate the toxicity and health hazards of these additives on biochemical assay and oxidative stress.
Food additives is not antibiotic have ADI dose, not exceed that dose but people eat what they taste, smell and look good, this is the issue so higher dose should be examined as it related to uncontrolled food consumption, not drugs. Once again it is food not tablet take it with care and our role is to explore the reality.
The significant body weight losses with high saccharine may be attributed to reduce food consumption per day and may be a consequence to the hypotriglyceredemia and hypocholesterolemic effect as revealed by a decrease in total serum cholesterol especially with the high dose treated groups [29,30].
These results are in agreement with the results obtained by Dib et al., [31] who reported a significant reduction in body weight of rats [50%] after administration of a 14-day sodium saccharin.
Though, the present results are in contrary with that obtained by Polyák et al., [32] who reported that body weight increase in saccharin consumed rats. However, some works suggests that frequent consumers of Saccharin as sugar substitutes may also be at increased risk of excessive weight gain, metabolic syndrome, type 2 diabetes, and cardiovascular disease [33], while this study did not provide sufficient evidence for increased body weight.
The poor body weight gain in case of animals consumed methylsalicylates may attribute to uncoupling oxidative phosphorylation, inhibiting Krebs cycle enzymes (succinate and α-ketoglutarate dehydrogenase) by salicylate, and inhibiting amino acid synthesis; this can lead to decreasing in tissue build and reduce the growth rate of these animals [34].
Body weight loss is considered by some authors to be a good reliable sensitive toxicity indicator [35]. Thus the body weight loss in the present study may represent the primary marker of food additive bad effect. These data indicated that artificial sweeteners saccharin assist in weight management, control of blood glucose of diabetics, and also can be used to replace sugar in foods.
The hypoglycemia observed in saccharin dosed rats are in accordance with Abdallah [30] and Horwitz et al., [10] who reported that feeding of high quantities of saccharin might reduce blood glucose level. Moreover, Osfor & Elias [36] reported that blood glucose levels of rats given saccharin significantly dropped after 12 weeks of oral administration.
Although saccharin has no food energy, it can trigger the release of insulin in rats, apparently as a result of its taste; this may lead to reduction in blood glucose level so it was believed that saccharin is beneficial to diabetic people. Saccharin causes a reflex release of insulin mediated by the gustatory nerves and hypoglycemia is produced as readily after washing the mouth with saccharin as by drinking the saccharin solution. It is the “sweet taste” of saccharin which is responsible for its hypoglycemic effect, a property not common to the sulfonamides as a group [6].
In addition, Bailey et al. [11] reported a reduction of hyperinsulinemia, decrease the insulin resistance and improve glycemic control during saccharin consumption in hyperglycemic obese mice.
Saccharin causes a reflex release of insulin mediated by the gustatory nerves. This theory has been proposed by Kun and Hormath [37] and is supported by their observation that the hypoglycemia is produced as readily after washing the mouth with saccharin as by drinking the saccharin solution. It is the “sweet taste” of saccharin which is responsible for its hypoglycemic effect, a property not common to the sulfonamides as a group.
In contrast Swithers et al., [12] suggested that one mechanism by which exposure to high-intensity sweeteners that interfere with a predictive relation between sweet tastes and calories may impair energy balance which could alter glucose homeostasis and reduce satiety. We concluded that if the satiety reduced how the body weight gain increased. So our study provides evidence that reduced blood glucose level and hypotriglyceridemia induced low body weight gain.
High and low saccharin induced hypocholesterolemia and high saccharin induce hypotriglyceridemia when compared to control groups in agreement with results recorded by Sharma et al., [38] and Ashour & Abdelaziz [39]. Moreover, Osfor & Elias [36] reported the same effect for 12 weeks.
Cholesterol is a soft waxy substance in the blood stream and in the body’s cells. It is used to form cell membranes and to produce certain hormones. Its total body content depends on the balance between body synthesis plus that absorbed from diet. The intestinal cholesterol pool comes from dietary cholesterol and the majority from biliary excretion. Approximately 50% of the intestinal cholesterol pool is reabsorbed by the intestines [40] and recirculated via the enterohepatic circulation, with the remainder excreted in feces. The deviation from normal serum cholesterol levels is considered as symptoms of liver diseases [41]. In the present study the decreased cholesterol level implies liver damage which is in accordance with increased alkaline phosphatase level.
The mechanism of hypocholesterolemic and hypolipidemic effect may be attributed to reduced total cholesterol synthesis by the saccharin suppressed in vivo liver enzymatic activity of acetyl-CoA synthetase, citrate lyase, and mitochondrial citrate exchange leading to a reduction of available cytoplasmic acetyl-CoA, which is required for the synthesis of cholesterol and fatty acids [42]. Moreover liver acetyl-CoA carboxylase, phosphatidate phosphohydralase, and glycerol-3-phosphate acyl transferase activities were markedly reduced by the saccharin analogues. Suppression of these enzymes would lead to a reduction of triglyceride synthesis.
Cyclic AMP (cAMP) is formed from ATP by adenylyl cyclase at the inner surface of cell membranes and acts as an intracellular second messenger. The mechanism of action of the non-nutritive sweetener, saccharin, was to stimulate significantly the activity of adenylate cyclase in membranes derived from skeletal muscle of rat; in addition Sodium saccharin enhanced adenylate cyclase activity in a dose related manner [43]. cAMP activates phosphorylase that triggers glycogenolysis, gluconeogenesis so induce hyperglycemia. Also adenylat cyclase, activates hormone-sensitive lipase that produces lipolysis and converting triglyceride into free fatty acid and glycerol.
The saccharin analogues accelerated bile excretion of cholesterol metabolites and increased the fecal excretion of the cholesterol, triglycerides, neutral lipids, and phospholipids thus, the liver and plasma lipoprotein lipid contents including, cholesterol, triglycerides, and neutral lipids were markedly reduced by the saccharin. Thus the studied food additives saccharin can significantly reduce serum total cholesterol, triglycerides and LDL-cholesterol and act as antihyperlipidemic, so it consider of health benefit.
Both oral low and high methylsalicylates (wintergreen odor) reduce serum albumin significantly and as a result the total serum protein was also fall. It has been reported that aspirin and sodium salicylates act as potent inhibitors of protein synthesis in guinea pig gastric mucosal tissue and rat islets [44].
Inhibition of protein synthesis by salicylates through inducing phosphorylation of the subunit of eukaryotic translation initiation factor 2, resulting in the inhibition of mRNA translation in the cells [45]. Thus, the food odor methylsalicylates induced a significant hypoproteinemia when given orally for 30 days. This decrement may be due to loss of protein formation from the alimentary tract, or to decrease formation of protein in the liver (impaired ability of the liver to form albumin) this depression may be due to an alternation in the intracellular protein synthesis mechanism and the oxidative enzyme change were probably secondary in altering proteins [46].
Artificial sweeteners are widely used nowadays, when the sensitivity of the people to the general health increased, the subject of sweeteners as food additives will take dangerous and effective dimension. It is almost difficult to follow a diet which is completely free from sweeteners and other food additives. The toxicity of sweeteners has become increasing and has attracted the concern of many scientists to study their toxic effect. Several previous studies have revealed that the use of artificial sweeteners may entail some hazards to the users [47].
Low and high dose of Saccharin exhibited a significant increase in serum ALT, AST and ALP activities when compared with control rats. Our results correlate well with Abdallah [30]. In addition Negro et al. [48] reported elevated serum activity of liver enzymes after the oral administration of three different drugs, of which saccharin was the only common constituent. Furthermore Osfor & Elias [36] reported that saccharin treated rats showed a significant increase in ALT activity after both 6 and 12 weeks of administration.
AST levels were significantly higher in saccharine treated group and that chronic saccharin intake reflects various metabolic, hormonal and neural responses in males and females [49]. Also, Shakoori et al. [50] reported that both low and high concentrations of saccharin caused hypertrophy of hepatic cell, its nucleus and nucleoli in addition to excessive vacuolation, in male albino rats at a dose of 65 mg/kg body weight/day (weak dose) for a total period of 39 weeks. The elevation in serum aminotransferase activities could be due to drastic effects caused by free radicals interaction with cellular membranes or may be related to breakdown of liver parenchyma [51].
The changes in liver function attributed to hepatocellular impairment which subsequently caused leakage and the release of greater than normal levels of intracellular enzymes into the blood. Elevation in the activities of aminotransferases indicated an early diagnosis of hepatotoxicity and considers as tissue damage biomarkers.
Our results revealed that both low and high doses of methylsalicylates showed a significant elevation in serum ALT and ALP activities when compared to control group. The elevation of ALT, and ALP activities in groups dosed with methylsalicylates are in agreement with Humphreys [52] who reported that methylsalicylates, showed some enlargement of the liver of dogs when given orally. The elevation in serum alkaline phosphatase (ALP) in low and high saccharin and methylsalicylate may be an evidence of obstructive damage in the liver tissue due to saccharin and methylsalicylate administration.
The liver cells play an important role in both synthesis and secretion of ALP into the bile. Therefore, the alterations in ALP activity caused by saccharin may be attributed to early cholestatic liver damage which primarily affects the liver parenchyma, thus making ALP a sensitive index in the diagnosis of infiltrative diseases [53].
It appears that in lower animals the toxicity of methyl salicylate is essentially identical with that of salicylic acid, although it is conceivable that in man the small proportion of unhydrolyzed ester might have a more toxic action [54]. After ingestion of salicylates, salicylic acid is formed, and readily absorbed in the stomach and small bowel. Salicylate poisoning is manifested clinically by disturbances of several organ systems, including the CNS and the cardiovascular, pulmonary, hepatic, renal, and metabolic systems.
When the acetates administered orally, they hydrolyzed rapidly in liver into acetic acid and their corresponding alcohol. The corresponding alcohol in case of isoamylacetates is isopentanol; liberation of that alcohol can affect the liver cells by increasing the cytotoxicity lead to increase release of hepatic enzymes like ALT, and ALP into circulation [55].
Thus, saccharin and methylsalicylates, have a risky effect on liver and alter hepatic function by elevating serum ALT, AST and ALP in both low and high doses and this effect is more appreciable at high doses.
Significant elevated serum creatinine and urea with high and low dose of saccharin, may be attributed to the toxic effects of saccharin on the kidney especially with high dose that can lead to disorders in the renal function and hence reduced glomerular filtration rate followed by retention of urea and creatinine in the blood [36,56].
Saccharin administartion caused a reduction in the renal slice steady-stable accumulation of p-aminohippurate and tetraethylammonium and at 60 days of age, increased urine volume, a decrease in urine osmolality, and increased potassium excretion were also found [57].
A combination of saccharin and aspirin had a high incidence of renal papillary necrosis and calcification. These toxic effects of aspirin and saccharin are independent responses, and administration of both greatly accentuates these responses [58].
The present data revealed that there is no significant change in serum creatinine or urea levels between the groups of rats dosed with methylsalicylates and control groups. From the previous data we postulated that saccharin affect the kidney leading to disturbed renal function and increasing serum creatinine and urea levels.
Oxidative stress is an imbalance between oxidants and antioxidants in favor of the oxidants, potentially causing damage to cells or cellular components [59].
A particularly destructive aspect of oxidative stress is the production of reactive oxygen species (ROS), which include free radicals, H2O2 and peroxides that can cause extensive cellular damage [60]. Most of these oxygen-derived species are produced at a low level by normal aerobic metabolism and the damage they cause to cells is constantly repaired. However, under the severe levels of oxidative stress that cause necrosis, the damage causes ATP depletion, preventing controlled apoptotic death and damage on DNA [61,62].
Our study revealed that high dose of saccharin induced a significant reduction in liver Catalase, SOD, and GSH, while showed a significant increase in liver MDA level when compared to control rats, while methylsalicylate dosed rats didn’t show any significant changes when compared to control rats (Table 3).
We have not been able to find studies targeting the effect of saccharin on hepatic biomarkers of oxidative stress. We attributed the oxidative stress induced by high dose of saccharin to the inflammation initiated to the liver cells; this opinion can be supported by observation of Abdallah [30] who stated that livers of rats administrated saccharin showed a portal infilteration with mononuclear inflammatory cells mainly lymphocytes and macrophages, the same observation was reported by Hassanin [56] who noted that the portal tract in liver sections of rats received saccharin for six weeks showed moderate infiltration by mononuclear inflammatory cells, mainly lymphocytes.
Consequently inflammatory processes induce oxidative or nitrosative stress and lipid peroxidation, thereby generating excess ROS, reactive nitrogen species, and DNA-reactive aldehydes [63]. Stimulated inflammatory cells undergo a respiratory burst and release ROS such as superoxide anion, hydrogen peroxide and numerous secondary oxidants as well as the arachidonic acid cascade [64].
So liberation of ROS by inflammatory cells induced lipid peroxidation by depletion of GSH content and decrease catalase, and SOD activities and increased the formation of malondialdehyde as a product of lipid peroxidation by high dose of saccharin, these free radicals can also lead to damage of the hepatic cells and increase the liberation of AST and ALT enzymes into serum as we observed in saccharin treated rats specially those consumed high dose (500 mg/kg.bw.). Increased oxidative markers in saccharine group may not due to lipid profile elevation but due to liver function disturbances.
Regarding hepatic antioxidant system there was a significant inhibition of the antioxidant defense system during saccharin administration, specifically a decrease of catalase, and SOD activities and equivalent fall in GSH content which prevents the cell death by the toxic radicals so their levels in the tissue homogenate were decreased. on the other hand MDA level was increased as a product of lipid peroxidation arisen by the ROS action on lipids of cellular membrane.
Our study provide novel data and conclusion that saccharin can produce ROS and lipid peroxidation to the lipid of hepatic cell membranes and these ROS lead to depletion of the components of cellular antioxidant system GSH, SOD and catalase because these components are used by the cells to reduce the toxic action of liberated oxygen radicals.
The oxidative stress produced by saccharine provide an explanation of its role in initiation of carcinoma specially when accumulated with large dose in the bladder and other tumors in different tissues as well as reports of induction of hyperplasia.
When we choose higher dose, it means we examine its safety margin and if it have even wide safety limit it should be taken with care as it is food not tablet. Saccharine and methylsalicylates to certain extent are not safe on lipid profile, glucose and hepatic and renal function with our examined dose. So break taking food additives, and back to natural food.
In conclusion, frequent consumption of saccharine have the effect of inducing metabolic derangements that affect body weight, glucose and lipids levels. These results question the effect of saccharine on weight-maintenance or growth rate.
Saccharin induces changes in hepatic and renal function at dose dependent manner and become more risky at higher doses because of its ability to induce oxidative stress by formation of free radicals may be due to liver function disturbances. Food flavoring agent methylsalicylates have a bad effect on liver function and induce an elevation to serum transaminases. Therefore, it is necessary to create consumer awareness regarding the ill effects of these food additives, and mention the type and concentration of each material added to food specially that consumed frequently by children. Finally, it is advisable to limit the uses and consumption of these food odorant or food flavor additives especially by young age.
Disclosure of conflict of interest
None.
References
- 1.Rolls BJ. Effects of intense sweeteners on hunger, food intake, and body weight: a review. Am J Clin Nutr. 1991;53:872–8. doi: 10.1093/ajcn/53.4.872. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay A, Ghoshal S, Mukherjee A. Genotoxicity testing of low-calorie sweeteners: aspartame, acesulfame-K, and saccharin. Drug Chem Toxicol. 2008;31:447–457. doi: 10.1080/01480540802390270. [DOI] [PubMed] [Google Scholar]
- 3.Cook-Fuller CC. Low-calorie Sweeteners. In: Ann , editor. Nutrition. 11th edition. USA: 2000. pp. 15–16. [Google Scholar]
- 4.Spillance WJ. Molecular Structure and Sweet Taste. In: Grenby TH, editor. Advances in Sweeteners. London, Glasgow, New York: 1996. pp. 1–25. [Google Scholar]
- 5.Mitchell ML, Pearson RL. Saccharin. In: Lyn O’Brien Nabors., editor. Alternative Sweeteners. 3rd edition. New York: Basel Hong Kong; 1991. p. 127. [Google Scholar]
- 6.Ionescu E, Rohner F, Proietto J, Rivest RW, Jeanrenaud B. Taste-induced changes in plasma insulin and glucose turnover in lean and genetically obese rats. Diabetes. 1988;37:773–779. doi: 10.2337/diab.37.6.773. [DOI] [PubMed] [Google Scholar]
- 7.Fowlkes KD, Carter G. Atlernative sweeteners. Drug Consults. 1994;86:162–16. [Google Scholar]
- 8.Sweatman TW, Renwick AG. The tissue distribution and pharmacokinetics of saccharin in the rat. Toxicol Appl Pharmacol. 1980;55:18–31. doi: 10.1016/0041-008x(80)90215-x. [DOI] [PubMed] [Google Scholar]
- 9.Cesarone CF, Bolognesi C, Santi L. Renal and hepatic toxicity studies in mice treated with sodium saccharin: breaks in single-stranded DNA. Boll Soc Ital Biol Sper. 1980;56:2486–91. [PubMed] [Google Scholar]
- 10.Horwitz DL, Mclane M, Kobe P. Response to single dose of aspartame or saccharin by NIDDM Patients. Diabetes Care. 1988;11:230–234. doi: 10.2337/diacare.11.3.230. [DOI] [PubMed] [Google Scholar]
- 11.Bailey CJ, Day C, Knapper JM, Turner SL, Flatt PR. Antihyperglycemic effect of saccharin in diabetic ob/ob mice. Br J Pharmacol. 1997;120:74–78. doi: 10.1038/sj.bjp.0700871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swithers SE, Laboy AF, Clark K, Cooper S, Davidson TL. Experience with the high-intensity sweetener saccharin impairs glucose homeostasis and GLP-1 release in rats. Behav Brain Res. 2012;233:1–14. doi: 10.1016/j.bbr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weihrauch MR, Diehl V. Artificial sweeteners do they bear a carcinogenic risk. Ann Oncol. 2004;15:1460–1465. doi: 10.1093/annonc/mdh256. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z, Jia C, Hu Y, Sun L, Jiao J, Zhao L, Zhu D, Li J, Tian Y, Bai H, Li R, Hu J. The estrogenic potential of salicylate esters and their possible risks in foods and cosmetics. Toxicol Lett. 2012;209:146–53. doi: 10.1016/j.toxlet.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 15.EMEA/MRL-The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit. Committee for veterinary medical products salicylic acid, sodium salicylate, aluminium salicylate basic and methyl salicylate basic and methyl salicylate summary report. 1999 [Google Scholar]
- 16.RIFM (Research Institute for Fragrance Materials, Inc.) Acute toxicity studies. RIFM report number 1689 (RIFM, Woodcliff Lake, NJ, USA) 1982 [Google Scholar]
- 17.Belsito D, Bickers M, Bruze P, Calow H, Greim JH, Hanifin AE, Rogers JH, Saurat IG, Sipes H, Tagami A. Toxicologic and dermatologic assessment of salicylates when used as fragrance ingredients. Food Chem Toxicol. 2007;45:S318–S361. doi: 10.1016/j.fct.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 18.Sinki GS, Schlegel WA. Flavouring Agents. In: Branen AL, Davidson PM, Salminen S, editors. Food Additives. New York: Marcell Dekker Inc; 1999. [Google Scholar]
- 19.Amin KA, Abdel Hameid H, Abd Elsttar AH. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food and Chem Toxicol. 2010;48:2994–2999. doi: 10.1016/j.fct.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 20.El-Wahab HM, Moram GS. Toxic effects of some synthetic food colorants and/or flavor additives on male rats. Toxicol Ind Health. 2013;29:224–32. doi: 10.1177/0748233711433935. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Hahm DH, Yang DC, Lee HJ, Shim I. Effect of crude saponin of korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97:124–131. doi: 10.1254/jphs.fp0040184. [DOI] [PubMed] [Google Scholar]
- 22.Reitman S, Frankel S. A colorimetry method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J of Clinic Path. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 23.Henry RJ. Clinical Chemistry. New York: Harper & Row Publishers; 1964. p. 181. [Google Scholar]
- 24.Patton CJ, Crouch SR. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Analytical Chemistry. 1977;49:464–469. [Google Scholar]
- 25.Trinder P. Glucose measurement with enzymatic colorimetric method. Ann Clin Biochem. 1969;6:24. [Google Scholar]
- 26.Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970;34:30–8. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- 27.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clinical Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 28.Presuss HG. The insulin system: influence of antioxidants. J Am Coll Nutr. 1998;17:101–102. doi: 10.1080/07315724.1998.10718732. [DOI] [PubMed] [Google Scholar]
- 29.Aboel-Zahab H, El-Khyat Z, Sidhom G, Awadallah R, Abdel-al W, Mahdy K. Physiological effects of some synthetic food coloring additives on rats. Boll Chim Farm. 1997;136:615–627. [PubMed] [Google Scholar]
- 30.Abdallah IA. Physiological changes induced by long term administration of saccharin compared with aspartame to male albino rats. Egyp J Hospit Med. 2002;8:70–81. [Google Scholar]
- 31.Dib K, Oget I, Wrisez F, El-Jamali A, Aguie-Aguie G, Correze C, Lambert B. Effects of sodium saccharin diet on fat-cell lipolysis: evidence for increased function of the adenylyl cyclase catalyst. Int J Obes Relat Metab Disord. 1996;20:15–20. [PubMed] [Google Scholar]
- 32.Polyák E, Gombos K, Hajnal B, Bonyár-Müller K, Szabó S, Gubicskó-Kisbenedek A, Marton K, Ember I. Effects of artificial sweeteners on body weight, food and drink intake. Acta Physiol Hung. 2010;97:401–7. doi: 10.1556/APhysiol.97.2010.4.9. [DOI] [PubMed] [Google Scholar]
- 33.Swithers SE. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol Metab. 2013;24:431–41. doi: 10.1016/j.tem.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mogilevskaya E, Demin O, Goryanin I. Kinetic model of mitochondrial krebs cycle: unraveling the mechanism of salicylate hepatotoxic effects. J Biol Phys. 2006;32:245–71. doi: 10.1007/s10867-006-9015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yokogawa K, Watannabe M, Takeshia H, Nomura M, Mano Y, Miyamoto K. Serum aminotransferase activity as a predictor of clearance of drugs metabolized by CYP isoforms in rats with acute hepatic failure induced by carbon tetrachloride. Int J Pharm. 2004;269:479–489. doi: 10.1016/j.ijpharm.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 36.Osfor MM, Elias TR. Nutritional and biochemical studies on some artificial sweerteners administrated to male albino rats. Bulletin of the National Research Center. 2003;28:377–401. [Google Scholar]
- 37.Kun E, Hormath I. Influence of oral saccharm on blood sugar. Proc Soc Exp Biol Med. 1947;66:175. doi: 10.3181/00379727-66-16025. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S, Goyal RP, Chakravarty G, Sharma A. Tomato red toxicity: haematological and serological changes in the blood of Swiss albino mice, mus musculus. Ind J Environ Sci. 2006;10:145–148. [Google Scholar]
- 39.Ashour AA, Abdelaziz I. Role of fast green on the blood of rats and the therapeutic action of vitamins C or E. International Journal Integrative Biology. 2009;6:6. [Google Scholar]
- 40.Turley SD, Dietschy JM. Sterol absorption by the small intestine. Curr Opin Lipidol. 2003;14:233–40. doi: 10.1097/00041433-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Singh RL, Khanna SK, Singh GB. Acute and short term toxicity of a popular blend of yellow and orange II in albino rats. Indian J Exp Biol. 1988;26:105–111. [PubMed] [Google Scholar]
- 42.Hall IH, Voorstad PJ, Cocolas GH. Antihyperlipidemic activity of saccharin analogues in rodents. J Pharm Sci. 1983;72:1192–8. doi: 10.1002/jps.2600721022. [DOI] [PubMed] [Google Scholar]
- 43.Striem JB, Naim M, Zehavi U, Ronen T. Saccharin induce changes in adenylate cyclase activity in liver and muscle membranes in rats. Life Sci. 1990;46:803–810. doi: 10.1016/0024-3205(90)90068-3. [DOI] [PubMed] [Google Scholar]
- 44.Kwon G, Hill JR, Corbett JA, Mcdaniel ML. Effects of aspirin on nitric oxide formation and de novo protein synthesis by RINm5F cells and rat islets. Mol Pharmacol. 1997;52:398–405. doi: 10.1124/mol.52.3.398. [DOI] [PubMed] [Google Scholar]
- 45.Silva AM, Wang D, Komar AA, Castilho BA, Williams BR. Salicylates trigger protein synthesis inhibition in a protein kinase r-like endoplasmic reticulum kinase-dependent manner. J Biol Chem. 2007;282:10164–10174. doi: 10.1074/jbc.M609996200. [DOI] [PubMed] [Google Scholar]
- 46.Ganong WF. Review of Medical Physiology. 25th edition. Lange Med Public; 2005. pp. 267–302. [Google Scholar]
- 47.Mukhopadhyay M, Mukherjee A Chakrabarti J. In vivo cytogenetic studies on blends of aspartame and acesulfame-K. Food Chem Toxicol. 2000;38:75–7. doi: 10.1016/s0278-6915(99)00115-5. [DOI] [PubMed] [Google Scholar]
- 48.Negro F, Mondardini A, Palmas F. Hepatotoxicity of saccharin. Engl J Med. 1994;331:134–135. doi: 10.1056/NEJM199407143310220. [DOI] [PubMed] [Google Scholar]
- 49.Andrejić BM, Mijatović VM, Samojlik IN, Horvat OJ, Ćalasan JD, Dolai MA. The influence of chronic intake of saccharin on rat hepatic and pancreatic function and morphology: gender differences. Bosn J Basic Med Sci. 2013;13:94–9. doi: 10.17305/bjbms.2013.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shakoori AR, Iqbal MZ, Ali SS. Biochemical and histopathological effects of saccharin on mouse liver. Pak J Zool. 1995;27:1–13. [Google Scholar]
- 51.Gray CH, Howorth PJ, Rinsler MG. English Language Book Society. 10th edition. In Edward Arnold; 1985. Clinical Chemical Pathology; p. 73. [Google Scholar]
- 52.Humphreys DJ. Veterinary Toxicology. 3rd edition. London, England: Bailliere Tindell; 1988. p. 93. [Google Scholar]
- 53.El-Elaimy A, El-Nabi SE. Influence of thiola on pesticide induced intoxication. II-preventive effect of liver damage. J Environ Sci. 1990;1:67–82. [Google Scholar]
- 54.Clarke DK, Ernest FZ. On the metabolism and toxicity of methyl salicylates. J Pharmacol Exp Ther. 1961;132:207–211. [PubMed] [Google Scholar]
- 55.Bisesi MS. Esters of Mono- and Alkenyl Carboxylic Acids and Mono- and Polyalcohols Amyl Acetates. In: Bingham E, editor. Patty’s Toxicology. 5th edition. 2001. pp. 543–546. [Google Scholar]
- 56.Hassanin NI. A study on the natural nutritive and non-nutritive sweenteners in rats. Vete Med J. 1998;46:133–153. [Google Scholar]
- 57.Berndt WO, Reddy RV, Hayes AW. Evaluation of renal function in saccharin treated rats. Toxicology. 1981;21:305–316. doi: 10.1016/0300-483x(81)90145-1. [DOI] [PubMed] [Google Scholar]
- 58.Johansson SL, Sakata T, Hasegawa R, Zenser TV, Davis BB, Cohen SM. The effect of long-term administration of aspirin and sodium saccharin on the rat kidney. Toxicol Appl Pharmacol. 1986;86:80–92. doi: 10.1016/0041-008x(86)90401-1. [DOI] [PubMed] [Google Scholar]
- 59.Sies H. Oxidative stress; oxidants and antioxidants. Exp Physiol. 1997;82:291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 60.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 61.Lelli JL, Becks LL, Dabrowska MI, Hinshaw DB. ATP converts necrosis to apoptosis in oxidant-injured endothelial cells. Free Radic Biol Med. 1998;25:694–702. doi: 10.1016/s0891-5849(98)00107-5. [DOI] [PubMed] [Google Scholar]
- 62.Evans MD, Cooke MS. Factors contributing to the outcome of oxidative damage to nucleic acids. Bioessays. 2004;26:533–42. doi: 10.1002/bies.20027. [DOI] [PubMed] [Google Scholar]
- 63.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 64.Frenkel K. Carcinogen mediated oxidant formation and oxidative DNA damage. Pharmacol Ther. 1992;53:127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]


