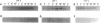Abstract
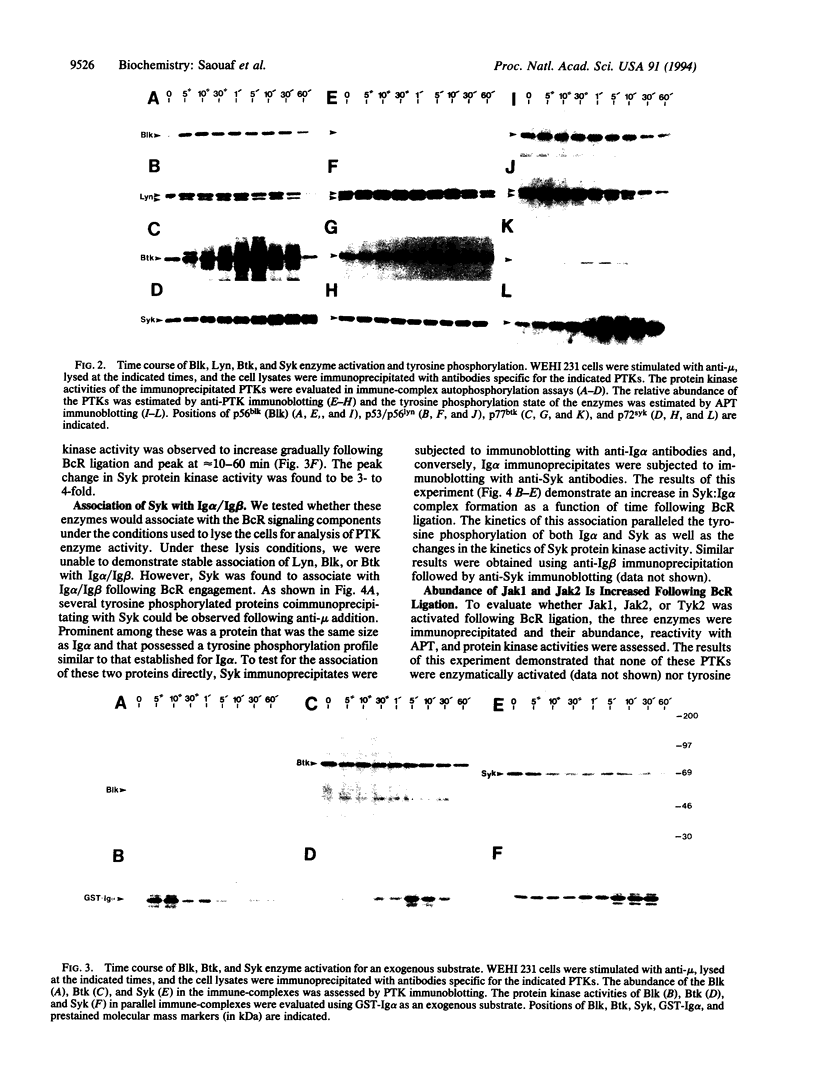
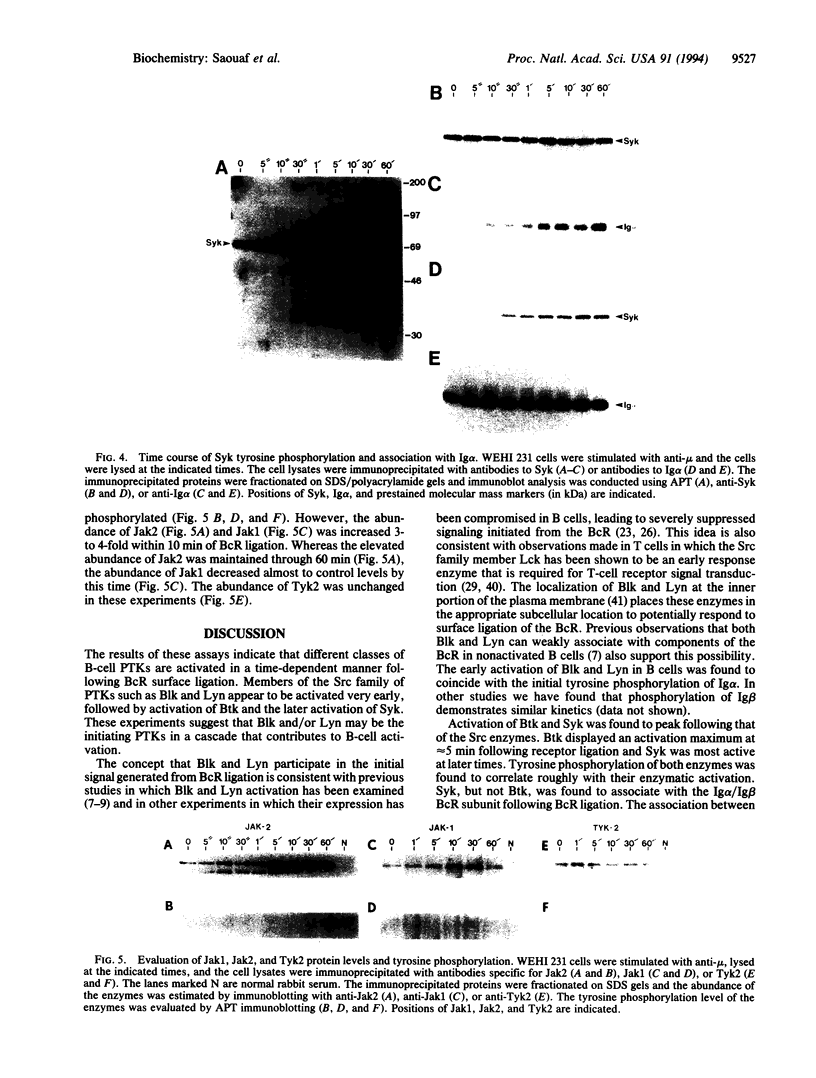
We evaluated in WEHI 231 B cells the time-dependent responses of Lyn, Blk, Btk, Syk, and three members of the Jak family of protein tyrosine kinases following antibody-mediated surface engagement of the B-cell antigen receptor. Our results show that the enzyme activities of Lyn and Blk were stimulated within seconds of antigen receptor engagement and correlated with the initial tyrosine phosphorylation of the Ig alpha and Ig beta subunits of the B-cell antigen receptor. Btk enzyme activity was also transiently stimulated and was maximal at approximately 5 min after B-cell receptor surface binding. Syk activity gradually increased to a maximum at 10-30 min following receptor ligation and was found to parallel the association of Syk with the tyrosine phosphorylated Ig alpha and Ig beta subunits of the receptor. While the specific activities of the Jak1, Jak2, and Tyk2 protein tyrosine kinases were unaltered following B-cell receptor ligation, the abundance of Jak1 and Jak2 were increased 3- to 4-fold within 10 min of receptor engagement. These results demonstrate that multiple families of non-transmembrane protein tyrosine kinases are temporally regulated during the process of B-cell antigen receptor-initiated intracellular signal transduction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A. L., Costa T., Misulovin Z., Stealy B., Bolen J. B., Nussenzweig M. C. Ig alpha and Ig beta are functionally homologous to the signaling proteins of the T-cell receptor. Mol Cell Biol. 1994 Feb;14(2):1095–1103. doi: 10.1128/mcb.14.2.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C., Campbell K. S. Membrane immunoglobulin and its accomplices: new lessons from an old receptor. FASEB J. 1992 Oct;6(13):3207–3217. doi: 10.1096/fasebj.6.13.1397843. [DOI] [PubMed] [Google Scholar]
- Campbell K. S., Hager E. J., Friedrich R. J., Cambier J. C. IgM antigen receptor complex contains phosphoprotein products of B29 and mb-1 genes. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3982–3986. doi: 10.1073/pnas.88.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Association between B-lymphocyte membrane immunoglobulin and multiple members of the Src family of protein tyrosine kinases. Mol Cell Biol. 1992 May;12(5):2315–2321. doi: 10.1128/mcb.12.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with anti-immunoglobulin. EMBO J. 1990 Jul;9(7):2125–2131. doi: 10.1002/j.1460-2075.1990.tb07381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Campbell K. S., Kazlauskas A., Johnson S. A., Hertz M., Potter T. A., Pleiman C., Cambier J. C. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science. 1992 Oct 2;258(5079):123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- Costa T. E., Franke R. R., Sanchez M., Misulovin Z., Nussenzweig M. C. Functional reconstitution of an immunoglobulin antigen receptor in T cells. J Exp Med. 1992 Jun 1;175(6):1669–1676. doi: 10.1084/jem.175.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco A. L. Structure and function of the B cell antigen receptor. Annu Rev Cell Biol. 1993;9:377–410. doi: 10.1146/annurev.cb.09.110193.002113. [DOI] [PubMed] [Google Scholar]
- Desiderio S. Human genetics. Becoming B cells. Nature. 1993 Jan 21;361(6409):202–203. doi: 10.1038/361202a0. [DOI] [PubMed] [Google Scholar]
- Dymecki S. M., Niederhuber J. E., Desiderio S. V. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990 Jan 19;247(4940):332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- Dymecki S. M., Zwollo P., Zeller K., Kuhajda F. P., Desiderio S. V. Structure and developmental regulation of the B-lymphoid tyrosine kinase gene blk. J Biol Chem. 1992 Mar 5;267(7):4815–4823. [PubMed] [Google Scholar]
- Gold M. R., Law D. A., DeFranco A. L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990 Jun 28;345(6278):810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Matsuuchi L., Kelly R. B., DeFranco A. L. Tyrosine phosphorylation of components of the B-cell antigen receptors following receptor crosslinking. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3436–3440. doi: 10.1073/pnas.88.8.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992 Apr 25;267(12):8613–8619. [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Iwashima M., Irving B. A., van Oers N. S., Chan A. C., Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994 Feb 25;263(5150):1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- Keegan A. D., Paul W. E. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol Today. 1992 Feb;13(2):63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- Kiener P. A., Rankin B. M., Burkhardt A. L., Schieven G. L., Gilliland L. K., Rowley R. B., Bolen J. B., Ledbetter J. A. Cross-linking of Fc gamma receptor I (Fc gamma RI) and receptor II (Fc gamma RII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993 Nov 15;268(32):24442–24448. [PubMed] [Google Scholar]
- Li Z. H., Mahajan S., Prendergast M. M., Fargnoli J., Zhu X., Klages S., Adam D., Schieven G. L., Blake J., Bolen J. B. Cross-linking of surface immunoglobulin activates src-related tyrosine kinases in WEHI 231 cells. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1536–1544. doi: 10.1016/0006-291x(92)90477-3. [DOI] [PubMed] [Google Scholar]
- Page D. M., Gold M. R., Fahey K. A., Matsuuchi L., DeFranco A. L. Mutational analysis of antigen receptor regulation of B lymphocyte growth. Evidence for involvement of the phosphoinositide signaling pathway. J Biol Chem. 1991 Mar 25;266(9):5563–5574. [PubMed] [Google Scholar]
- Pleiman C. M., Chien N. C., Cambier J. C. Point mutations define a mIgM transmembrane region motif that determines intersubunit signal transduction in the antigen receptor. J Immunol. 1994 Mar 15;152(6):2837–2844. [PubMed] [Google Scholar]
- Pleiman C. M., Hertz W. M., Cambier J. C. Activation of phosphatidylinositol-3' kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994 Mar 18;263(5153):1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- Rawlings D. J., Saffran D. C., Tsukada S., Largaespada D. A., Grimaldi J. C., Cohen L., Mohr R. N., Bazan J. F., Howard M., Copeland N. G. Mutation of unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993 Jul 16;261(5119):358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Interaction of tyrosine kinase oncoproteins with cellular membranes. Biochim Biophys Acta. 1993 Dec 23;1155(3):307–322. doi: 10.1016/0304-419x(93)90012-2. [DOI] [PubMed] [Google Scholar]
- Samelson L. E., Klausner R. D. Tyrosine kinases and tyrosine-based activation motifs. Current research on activation via the T cell antigen receptor. J Biol Chem. 1992 Dec 15;267(35):24913–24916. [PubMed] [Google Scholar]
- Sanchez M., Misulovin Z., Burkhardt A. L., Mahajan S., Costa T., Franke R., Bolen J. B., Nussenzweig M. Signal transduction by immunoglobulin is mediated through Ig alpha and Ig beta. J Exp Med. 1993 Sep 1;178(3):1049–1055. doi: 10.1084/jem.178.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992 Aug 21;70(4):585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Takata M., Sabe H., Hata A., Inazu T., Homma Y., Nukada T., Yamamura H., Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994 Mar 15;13(6):1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Kobayashi T., Kondo J., Takahashi K., Nakamura H., Suzuki J., Nagai K., Yamada T., Nakamura S., Yamamura H. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991 Aug 25;266(24):15790–15796. [PubMed] [Google Scholar]
- Thomas J. D., Sideras P., Smith C. I., Vorechovský I., Chapman V., Paul W. E. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993 Jul 16;261(5119):355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- Tsukada S., Saffran D. C., Rawlings D. J., Parolini O., Allen R. C., Klisak I., Sparkes R. S., Kubagawa H., Mohandas T., Quan S. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993 Jan 29;72(2):279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Veillette A., Horak I. D., Horak E. M., Bookman M. A., Bolen J. B. Alterations of the lymphocyte-specific protein tyrosine kinase (p56lck) during T-cell activation. Mol Cell Biol. 1988 Oct;8(10):4353–4361. doi: 10.1128/mcb.8.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetrie D., Vorechovský I., Sideras P., Holland J., Davies A., Flinter F., Hammarström L., Kinnon C., Levinsky R., Bobrow M. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993 Jan 21;361(6409):226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Peaker C. J., Patel K. J., Neuberger M. S. The alpha/beta sheath and its cytoplasmic tyrosines are required for signaling by the B-cell antigen receptor but not for capping or for serine/threonine-kinase recruitment. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):474–478. doi: 10.1073/pnas.91.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Taniguchi T., Yang C., Yasue S., Saito H., Yamamura H. Association with B-cell-antigen receptor with protein-tyrosine kinase p72syk and activation by engagement of membrane IgM. Eur J Biochem. 1993 Apr 1;213(1):455–459. doi: 10.1111/j.1432-1033.1993.tb17781.x. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Fukui Y., Wongsasant B., Kinoshita Y., Ichimori Y., Toyoshima K., Yamamoto T. Activation of Src-like protein-tyrosine kinase Lyn and its association with phosphatidylinositol 3-kinase upon B-cell antigen receptor-mediated signaling. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1118–1122. doi: 10.1073/pnas.89.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Kakiuchi T., Mizuguchi J., Yamamoto T., Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991 Jan 11;251(4990):192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- Yao X. R., Scott D. W. Antisense oligodeoxynucleotides to the blk tyrosine kinase prevent anti-mu-chain-mediated growth inhibition and apoptosis in a B-cell lymphoma. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7946–7950. doi: 10.1073/pnas.90.17.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]