Abstract
To address information gaps that limit informed clinical decisions on medication use in pregnancy, the Centers for Disease Control and Prevention (CDC) solicited expert input on a draft prototype outlining a systematic approach to evaluating the quality and strength of existing evidence for associated risks. The draft prototype outlined a process for the systematic review of available evidence and deliberations by a panel of experts to inform clinical decision making for managing health conditions in pregnancy. At an expert meeting convened by the CDC in January 2013, participants divided into working groups discussed decision points within the prototype. This report summarizes their discussions of best practices for formulating an expert review process, developing evidence summaries and treatment guidance, and disseminating information. There is clear recognition of current knowledge gaps and a strong collaboration of federal partners, academic experts, and professional organizations willing to work together toward safer medication use during pregnancy.
Keywords: Centers for Disease Control and Prevention, expert review, medications, pregnancy, teratogens
The National Center on Birth Defects and Developmental Disabilities (NCBDDD) of the Centers for Disease Control and Prevention (CDC) is working toward safer medication use in pregnancy as a strategy to prevent birth defects with the Treating for Two: Safer Medication Use in Pregnancy initiative.1 Originating in the CDC Birth Defects Branch, this initiative identifies birth defects prevention as a priority outcome, but also aims to optimize maternal health by enhancing informed clinical decisions about management of common conditions during pregnancy and the reproductive years. NCBDDD engaged colleagues in other relevant areas of CDC as well as partner federal agencies, academic institutions, professional societies, and consumer organizations to develop and advance this initiative.
Medication use is common and prevalence of use during pregnancy is increasing.2 However, the vast majority of maternal medications have an undetermined risk for birth defects or other adverse fetal outcomes because they have not been adequately studied in human pregnancy.3 An earlier report from CDC established the urgent need for “a panel of experts to set priorities and standards, interpret data, and make recommendations” regarding medication use during pregnancy.4 The results from an expert review could be used by health care providers to inform prescribing decisions, and this information would also be shared with the Food and Drug Administration (FDA) for their review. In early 2012, in partnership with relevant federal agencies and academic experts, CDC formed a steering committee tasked with planning a meeting of experts to discuss a concrete plan for moving forward with a systematic approach, including an evidence review, for promoting safer medication use in pregnancy.
Prototype
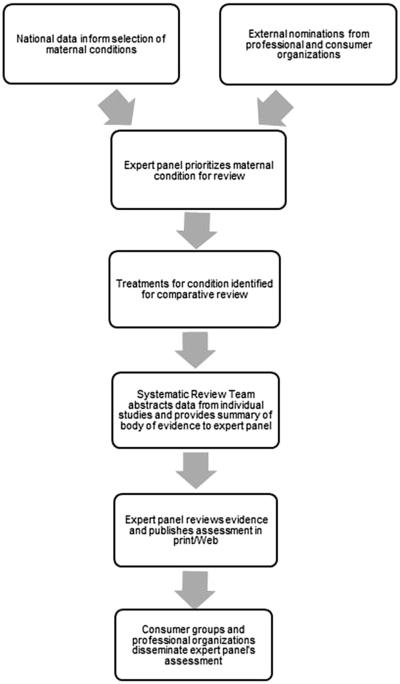
In consultation with steering committee members, CDC scientists drafted a prototype for a formal review process to evaluate the quality and strength of existing evidence for embryonic/fetal and perinatal risks associated with medications used to treat medical conditions among reproductive-age women. This proposed review process includes 2 primary components: an evidence synthesis based on systematic reviews, and evidence review and guidance development via an independent panel of clinical, public health, and prevention experts (Figure).
FIGURE. Proposed flow diagram of expert review panel model.
Broussard. A systematic approach to safer medication use during pregnancy. Am J Obstet Gynecol 2014.
On Jan. 28-29, 2013, the Division of Birth Defects and Developmental Disabilities of NCBDDD convened the Treating for Two: Safer Medication Use in Pregnancy meeting to seek expert advice on the draft prototype toward its initiative. The meeting brought together 66 experts from academic institutions, professional organizations, and federal agencies (Appendix). The group included persons with backgrounds representing diverse expertise in the fields of teratology, maternal-fetal medicine, developmental toxicology, pharmacoepidemiology, perinatal psychiatry, systematic review methodology, bioethics, and others, including developers of existing teratogen information resources, such as REPROTOX, TERIS, and Briggs Drugs in Pregnancy and Lactation.5–9 In this report, we have synthesized the meeting discussions around the components of the proposed review process.
Conference summary
Priority setting
Experts within the priority setting workgroup were tasked with developing criteria by which a maternal condition could be selected for expert review to develop treatment guidance. Guiding prioritization of conditions for expert review would be an emphasis on the clinical utility and anticipated public health impact of resulting guidance. Assessment of the potential public health impact includes consideration of the prevalence of the condition among reproductive-age women, the frequency of medication use for the condition among pregnant and reproductive-age women, the severity of the condition being treated, and the potential consequences of no pharmacotherapy.
Suspicion of adverse fetal effects based on animal studies or clinical data should also be a factor in assessing the likely public health impact of prioritizing a particular condition. Some maternal conditions likely to be prioritized based on these principles include maternal infections, asthma, depression/anxiety, allergies, thyroid disorders, nausea and vomiting of pregnancy, diabetes, seizure disorders, and migraines.10–19 While all are important, the expert review panel could consider the criteria above in determining how to best prioritize these conditions for review. It would be important for the expert review panel to prioritize maternal conditions for which guidelines for treatment in pregnancy are lacking or for which current guidelines fail to provide adequate direction.
To provide information that is most useful to clinicians, it would be important to prioritize maternal conditions for which there are at least 2 treatment options (one comparator could be no medication treatment if that is a plausible option) that can be compared in terms of relative safety or risk for use during pregnancy. In addition, when possible, the expert review panel would need to consider both the current use and projected future use of specific medications, such that some guidance can be provided in a timely manner for medications with rapidly increasing use.
The expert review panel could meet an important need by prioritizing the evidence review for medications used to treat those acute or chronic maternal conditions that pose the most challenging treatment decisions to clinicians providing health care to women just before and during pregnancy. Surveys of obstetrician–gynecologists and other health care providers could be used to evaluate the greatest perceived needs by clinicians either for conditions lacking guidance or for those which clinicians indicate the available guidance is inadequate. This information should be factored into prioritization decisions. In addition, professional groups representing both health care providers and consumers could be afforded the opportunity to nominate specific maternal conditions for consideration for review to ensure that there is a specific mechanism for input from these key stakeholders.
The primary outcomes of interest to be evaluated by the expert review panel would be perinatal outcomes including preterm birth, fetal death, structural birth defects, poor fetal growth, and severe adverse maternal events. In addition, the review would consider the potential role of medication exposures in pregnancy on developmental disabilities and neurocognitive and behavioral effects if there are published studies on which to base such an evaluation. While many studies will be outcome specific, the endpoint of interest remains the overall relative safety or risk of specific treatment options during pregnancy, and casting a broad net for outcomes would result in an evidence summary that more closely addresses this key question.
Systematic review
Under the proposed approach, once a condition has been selected for review, a formal review process to assess the maternal and fetal effects of exposure to medications used to treat the condition would be conducted. The review would encompass evaluations of the quality and strength of existing evidence. Reviews could be conducted by an independent systematic review team with oversight from designated expert panel members.
The systematic review of evidence would include all medications used to treat the selected maternal condition and potentially a review of delayed treatment or no treatment if these are viable options for a specific condition. For each medication identified, all available evidence would be reviewed. In addition, all medications commonly used, including those used off-label, for the selected maternal condition should be considered for review. Some reviews are likely to include an entire class of medications, but whenever possible, individual medications should be evaluated separately because risks to the fetus can vary within a given class of medications.20
We expect that the adequacy and amount of information for each medication will vary widely; information will be occasionally limited and frequently incomplete. However, the adequacy of data would not modify the established priorities because the assessment of the quantity, quality, and consistency of available information would be a valuable contribution of the review process. A brief report, such as a State of Science review concluding that data are lacking, would be informative in itself and would identify knowledge gaps and future lines of research. Once the review process for all medications for a given condition is completed, a surveillance system could be maintained to systematically monitor publications and prompt an update of the initial review after an appropriate number of years. Length of time until rereview would vary depending on such factors as emerging public health concerns, new publications, or newly marketed medications to treat the condition.
Systematic reviews should utilize all publicly available information and not be limited to peer-reviewed publications. However, unpublished or gray literature should be considered very carefully. In all cases, publication bias should be considered.21
It is crucial to consider in vitro, in vivo, and animal studies in addition to human data. This is especially important in the early postmarketing period for a drug when data from nonclinical studies might be all that is available. These data can be obtained from publications, drug labeling, FDA reports, background documents for advisory committees, and direct cooperation with pharmaceutical companies. Moreover, given that the number of pregnant women evaluated in preapproval randomized trials is often insufficient to evaluate the fetal risk, most human data will likely come from the postmarketing setting.
While data from case reports and case series can offer clues, except for select major teratogens (eg, thalidomide), valid information on human fetal safety will most likely come from epidemiologic studies such as cohort (eg, pregnancy registries) and case-control designs. Although these observational studies lack randomization and blinding, they can be informative if adequately designed and carefully conducted, particularly when evaluating unintended medication effects or identifying safety signals.
The systematic review team would abstract and summarize data for different study types (animal vs human), individual study designs (eg, controlled vs observational), and different outcomes for each study. Standardized abstraction forms would be developed to define a set of quality standards for information to be systematically abstracted for each study reviewed. Data abstracted should include a set of critical elements that would allow for the evaluation of level of risk of exposure and quality of information, with or without quality scores quantification. When possible, absolute risks and risk differences should also be abstracted or estimated.
All sources of information could be combined qualitatively by separately summarizing animal and human data. In addition, evidence could be synthesized quantitatively. Metaanalyses would allow the estimation of pooled relative risk estimates when the study designs and outcomes among individual studies are relatively homogeneous.
The collective body of evidence would be appraised. Established grading and weighting systems for the body of evidence (eg, GRADE approach22,23) consider multiple domains: direction (strength of association), quantity/quality of data to inform it (precision/biases), and consistency (studies pointing in each direction).
While integration of all the domains on a final scale to inform recommendations seems appealing, assigning weights might be challenging, particularly in the presence of conflicting results. In addition, mechanical evaluation and oversimplification might result in incorrect conclusions. As we have learned from existing systems (eg, pregnancy categories for FDA labeling), simple scores cannot adequately capture or convey the level of complexity inherent in these summaries.3,24–27
Comparative safety approaches may address an important clinical question, namely, “which medication has the best risk vs benefit profile to treat a given condition?” However, assessment of comparative safety is often difficult, and reliance on evaluation of uncontrolled or nonrandomized data may lead to incorrect guidance.
Guidance development
Meeting participants considered whether the expert review panel should develop guidelines, guidance, or treatment recommendations for the conditions reviewed, or if it should restrict its scope to summarizing and grading the body of evidence. In the latter scenario, a professional society or societies could build on the evidence summary to develop specific treatment guidelines. There are examples of the CDC developing guidelines or recommendations in collaboration with external partners for several key issues of public health importance such as the Recommendations of the Advisory Committee on Immunization Practices,28,29 and guidelines for diagnosis and treatment of sexually transmitted infections,30 and prevention of intrapartum vertical transmission of group B streptococcus.31
No recommendations should be made in the absence of clear evidence. However, if there is clear evidence to support a recommendation, directive guidelines with action statements such as “clinicians should do this” might be warranted. Meeting participants considered how the expert panel could formally evaluate available evidence to develop guidelines. Alternative options discussed for data review included a “Delphi” method32 similar to that used for the TERIS online database6,7 and a more formalized system such as that used by the US Preventive Services Task Force.33 A rigid grading system probably would not provide adequate flexibility for individual clinical decision making during pregnancy.
Any assessment of safety must take into account the severity of the maternal condition, the potential severity and frequency of adverse outcomes, and how potential adverse events are likely to be influenced by dose and timing of exposure (relative to gestational age). If the available data were not compelling, a less directive summary could be written to address the data that are available and could emphasize current gaps in knowledge that prevent a more prescriptive or definitive guideline. If the quantity or quality of data are inadequate to direct what medication should be used, but there are adequate data to state what should not be used, based on clearly defined safety findings, that definitive statement would be helpful to clinicians. Absent adequate data, there should not be an obligatory ranking of medications; individual clinicians should make these specific treatment decisions.
Statements written in collaboration with clinical and professional societies would promote harmonized treatment guidelines among partner groups for each condition examined. The CDC could partner with specific professional societies to develop guidelines based on the evidence summaries adopting a model similar to the group B streptococcus guidelines published jointly by CDC, the American Congress of Obstetricians and Gynecologists, and the American Academy of Pediatrics.31,34,35
Meeting participants generally agreed that the expert review process would focus on fetal and maternal safety; however, final treatment guidelines would also need to consider pharmacokinetics/pharmacodynamics and efficacy in pregnant women. Assessments of effectiveness of a product used during pregnancy as a routine part of the expert review process will likely be beyond the scope of this project. Decisions regarding the final assessment will have to balance completeness with feasibility of reviewing this additional body of literature.
The expert review panel might work best as an independent entity that could consult externally for technical and organizational support as needed. A chairperson or co-chairs could preside over the expert review panel. It is important that members of the expert review panel sign disclosure statements to certify that they have no potential conflicts of interest that could bias conclusions or even give the appearance of bias. There would probably need to be a full-time staff to manage the work of drafting evidence summaries or guidance as well as rotating volunteers assigned as liaison representatives of their agencies or professional societies.
Details of the composition of the expert review panel to address different conditions and duration of service for individuals to provide adequate continuity and institutional memory would need further discussion. Appropriate attention to potential legal implications of guidance must include the fact that there will inevitably be parties who try to establish the guidance as the standard of care in medicolegal liability cases, and others who sue to have them overturned if they disagree with the contents of the guidance.
Communication and collaboration between the expert review panel and the FDA would be needed. FDA may be able to share safety data with the expert review panel, and findings from the panel would help to inform FDA’s regulatory actions, such as product labeling revisions, drug safety communications, and risk evaluation and mitigation strategies.
Dissemination
A crucial step toward safer medication use in pregnancy is the dissemination of the final evidence summaries or guidance to targeted audiences, namely health care providers and women of reproductive age. The main objective guiding dissemination efforts would be to provide the very best evidence possible in a way that supports empowerment through shared decision making. We anticipate that health care providers would serve in an implementation role largely for prescription medications but also for over-the-counter medications.
The primary information to be disseminated to all audiences would be the guidance developed by the expert review panel. Dissemination and implementation efforts should be directed toward a wide range of health care providers as well as patients and consumers, but the content and approach should differ. This information should convey credibility and should be tailored to each specific audience to increase accessibility to reliable information about treatment options for maternal conditions during pregnancy.
For instance, evidence summaries quantitatively comparing different treatment options would be important for health care providers, whereas this technical information could be translated more simply for patients by suggesting questions women should ask a health care provider, such as “What will happen if I take/do not take this medication during pregnancy?” or “Is there a safer medication that I should take instead or another alternative to manage my condition?”
Key messages should be nuanced in such a way that they are informative and factual, while not alarmist. The target audience should expand beyond women to include individuals close to them, including their parents and partners. Irrespective of the audience, the message should be kept simple, and audience testing will be important. For today’s audience, dissemination through electronic media is key; therefore, any guidance developed would be most helpful if posted and readily available to health care providers and the public electronically.
Because of its leadership and coordination of the broader Treating for Two: Safer Medication Use in Pregnancy initiative, and given its staff with technical expertise in health communication, CDC could lead dissemination efforts of the expert review panel’s guidance and could offer technical support for communication and dissemination activities. A comprehensive multistage communication plan devised by CDC in collaboration with relevant partner groups would be important. Ongoing collaboration with professional societies on dissemination efforts would help to extend the reach of the expert review panel’s guidance. It is anticipated that each relevant partner group would actively participate in the dissemination of panel guidance, in whichever format is most appropriate for their respective constituents. In particular, this information is expected to be especially useful to FDA in its role as the regulatory agency for medications.
Several potential dissemination strategies by intended audience were discussed (Table). Formative research with both health care providers and patients will allow us to assess the knowledge, attitudes, practices, and access to information about medication use during pregnancy and help to inform the translation of the expert review panel’s guidance into messaging appropriate to all audiences.
TABLE.
Suggested dissemination products by target audience
| Variable | Health care providersa | Patients/consumers | Both audiences |
|---|---|---|---|
| Products | Brief abstract with focused “top line” message reflecting critical information |
CDC podcast or video | 1-page overview document or At-a-Glance piece |
|
|
|||
| Joint letter from CDC and professional organizations |
Guest blog or expert commentary on pregnancy- related websites such as WhatToExpect.com, MarchOfDimes.com, MotherToBaby.org |
||
|
|
|||
| Key messages and FAQs | Case studies/stories | ||
|
|
|||
| Announcement via listservs | Article included in pregnancy-related week-by-week newsletter |
||
|
|
|||
| Provider toolkits | |||
|
|
|||
| Medscape Expert Commentary available online |
|||
|
|
|||
| Clinical Decision Support Tools | |||
|
| |||
| Potential channels |
Online or mobile applications36,37 | Popular internet search engines (eg, Google, YouTube) |
CDC website |
|
|
|||
| Continuing education credit modules | Pregnancy-related week-by-week newsletter | Email blast to key partners | |
|
|
|||
| Electronic health/medical records (for Clinical Decision Support Tools) Presentations at annual professional conferences |
Brief message to be included in mobile or social media initiatives targeting expectant parents (eg, Text-4-Baby, Facebook, Twitter) |
||
CDC, Centers for Disease Control and Prevention; FAQ, frequently asked question.
Health care providers include primary care physicians, obstetrician-gynecologists, and physician specialists such as maternal fetal medicine or psychiatrists, as well as certified nurse midwives, nurse practitioners, and pharmacists.
Broussard. A systematic approach to safer medication use during pregnancy. Am J Obstet Gynecol 2014.
Conclusion
A multidisciplinary panel of experts proposes the involvement of all stakeholders in the development of a strategy to prioritize, synthesize, evaluate, and disseminate the body of evidence on the comparative safety of different therapeutic strategies for the treatment of conditions in pregnant women of the highest clinical and public health relevance.
ACKNOWLEDGMENTS
We are especially grateful to speakers and moderators who presented at premeeting webinars and the panel discussion during the meeting: Gerald Briggs, BPharm, Memorial Care Center for Women, Miller Children’s Hospital, Long Beach Memorial Medical Center, Hun tington Beach, CA; Jan Friedman, MD, PhD, University of British Columbia, Vancouver, British Columbia, Canada; Allen Mitchell, MD, Boston University Schools of Public Health and Medicine, Boston, MA; and Anthony Scialli, MD, Reproductive Toxicology Center, Washington, DC. We also thank all meeting participants who contributed to this work. A full list of individuals and organizations participating in the meeting is available at ajog.org.
This work was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention (CDC) administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and the CDC (M.T.F.).
Dr Hernandez-Diaz has been an adviser for pregnancy registries sponsored by Novartis and GSK Biologicals and has received training grants from Pfizer, Millennium, and PhRMA. Dr Chambers received research funding support from Amgen, Ab-Vie, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Sanofi-Genzyme, Roche-Genentech, Pfizer, Janssen, Teva, Sandoz, Kali, and Apotex.
Appendix to: Developing a systematic approach to safer medication use during pregnancy
Special Report by Broussard et al.
Additional individuals and organizations participating in the Treating for Two: Safer Medication Use in Pregnancy meeting. Convened by the Division of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Jan. 28-29, 2013
Elizabeth C. Ailes, PhD, MPH, Angela Colson, MA, Janet Cragan, MD, MPH, Andreea Creanga, MD, PhD, Kathryn Curtis, PhD, Nicole Dowling, PhD, Randy Elder, PhD, MEd, J. David Erickson, DDS, PhD, MPH, Suzanne M. Gilboa, PhD, Yvonne Green, RN, CNM, MSN, Denise J. Jamieson, MD, MPH, James E. Kucik, PhD, MPH, Dana Meaney-Delman, MD, MPH, Elizabeth W. Mitchell, PhD, MA, Cynthia Moore, MD, PhD, Kara N. D. Polen, MPH, Jennita Reefhuis, PhD, Teresa Schnorr, PhD, Joe Sniezek, MD, MPH, Vasavi Thomas, RPh, MPH, Phoebe Thorpe, MD, MPH, Sarah Tinker, PhD, Centers for Disease Control and Prevention, Atlanta, GA; Sandra Kweder, MD, Pamela Scott, PhD, MA, Melissa Tassinari, PhD, Mary Willy, PhD, Food and Drug Administration, Silver Spring, MD; Merle Paule, PhD, Food and Drug Administration, Jefferson, AR; Indira Jevaji, MD, MSL, Zhaoxia Ren, MD, PhD, National Institutes of Health, Bethesda, MD; Kembra L. Howdeshell, PhD, National Toxicology Program, National Institute of Environmental Health Sciences, Research Triangle Park, NC; Philip Anderson, PharmD, University of California San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences, La Jolla, CA; Hani Atrash, MD, MPH, Health Resources and Services Administration, Rockville, MD; Steven R. Brown, MD, American Academy of Family Physicians, Banner Good Samaritan Family Medicine Residency, Phoenix, AZ; Elizabeth Conover, MS, Nebraska Teratogen Information Service, University of Nebraska Medical Center, Omaha, NE; William Cooper, MD, MPH, Vanderbilt University School of Medicine, Nashville, TN; Robert L. Davis, MD, MPH, University of Tennessee Health Science Center, Center in Biomedical Informatics, and Department of Pediatrics, College of Medicine, Memphis, TN; Siobhan M. Dolan, MD, MPH, March of Dimes, Albert Einstein College of Medicine, Montefiore Medical Center, New York, NY; Sara Ephross, PhD, GlaxoSmithKline, Research Triangle Park, NC; Michael Fraser, PhD, CAE, Association of Maternal and Child Health Programs, Washington, DC; Craig Hansen, PhD, Kaiser Permanente Center for Health Research, Atlanta, GA; Lewis Holmes, MD, MassGeneral Hospital for Children, Harvard Medical School, Boston, MA; Tekoa King, CNM, MPH, American College of Nurse-Midwives, University of California San Francisco Medical Center, San Francisco, CA; Zita Lazzarini, JD, MPH, University of Connecticut School of Medicine, Farmington, CT; Lisa Longo, PharmD, Department of Veterans Affairs, Washington, DC; Anne Drapkin Lyerly, MD, MA, University of North Carolina at Chapel Hill, Chapel Hill, NC; Kimford Meador, MD, Emory University School of Medicine, Atlanta, GA (currently with Stanford University, Stanford, CA); Janine Polifka, PhD, University of Washington, Seattle, WA; Kenneth Rothman, DrPH, RTI Health Solutions, Research Triangle Park, NC; Michael Schatz, MD, MS, Kaiser Permanente, University of California San Diego School of Medicine, La Jolla, CA; Kim Schofield, DMIN, MLC, Georgia Lupus Registry, Emory University School of Medicine, Atlanta, GA; Dixie E. Snider, MD, MPH, Atlanta, GA; Rosenie Thelus, PhD, Department of the Army, Silver Spring, MD; John van den Anker, MD, PhD, American Academy of Pediatrics, Children’s National Medical Center, Washington, DC; Richard Wild, MD, JD, MBA, Centers for Medicare and Medicaid Services, Atlanta, GA; Katherine L. Wisner, MD, MS, Northwestern University Feinberg School of Medicine, Chicago, IL; Kimberly A. Yonkers, MD, Yale School of Medicine, New Haven, CT.
Footnotes
The remaining authors report no conflict of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or other agencies.
Presented at the 53rd annual meeting of the Teratology Society as part of the TS/OTIS Joint Pregnancy Registry Workshop: Pregnancy Registry Designs that Involve Multiple Drugs for Similar Indications: Advantages, Disadvantages, and Sustainable Funding Models, Tucson, AZ, June 22-26, 2013.
REFERENCES
- 1.Centers for Disease Control and Prevention Treating for two: safer medication use in pregnancy. Available at: http://www.cdc.gov/treatingfortwo. Accessed March 26, 2014.
- 2.Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205:51.e1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet C Semin Med Genet. 2011;157:175–82. doi: 10.1002/ajmg.c.30313. [DOI] [PubMed] [Google Scholar]
- 4.Lagoy CT, Joshi N, Cragan JD, et al. Medication use during pregnancy and lactation: an urgent call for public health action. J Womens Health. 2005;14:104–9. doi: 10.1089/jwh.2005.14.104. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick RB. REPROTOX: an information system on environmental hazards to human reproduction and development. Med Ref Serv Q. 2008;27:73–80. doi: 10.1300/J115v27n01_05. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JM, Polifka JE. TERIS: the teratogen information system. University of Washington; Seattle, WA: 1999. [Google Scholar]
- 7.TERIS: The Teratogen Information System. University of Washington; Seattle, WA: Midland, MI: RightAnswer.com Inc. Available at: http://www.rightanswerknowledge.com. Accessed May 20, 2014. [Google Scholar]
- 8.Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 9th Lippincott Williams and Wilkins; Philadelphia, PA: 2011. [Google Scholar]
- 9.Scialli AR. The REPROTOX system. Georgetown University Medical Center and Reproductive Toxicology Center, Columbia Hospital for Women Medical Center; Washington, DC: Midland, MI: RightAnswer.com Inc. Available at: http://www.rightanswerknowledge.com. Accessed May 20, 2014. [Google Scholar]
- 10.D’Angelo D, Williams L, Morrow B, et al. Centers for Disease Control and Prevention Preconception and interconception health status of women who recently gave birth to a liveborn infant: Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 26 reporting areas, 2004. MMWR Surveill Summ. 2007;56:1–35. [PubMed] [Google Scholar]
- 11.Collier SA, Rasmussen SA, Feldkamp ML, et al. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2009;85:193–201. doi: 10.1002/bdra.20540. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol. 2005;32:523–59. doi: 10.1016/j.clp.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vissenberg R, Van den Boogaard E, Van Wely M, et al. Treatment of thyroid disorders before conception and in early pregnancy: a systematic review. Hum Reprod Update. 2012;18:360–73. doi: 10.1093/humupd/dms007. [DOI] [PubMed] [Google Scholar]
- 14.Lucas S. Medication use in the treatment of migraine during pregnancy and lactation. Curr Pain Headache Rep. 2009;13:392–8. doi: 10.1007/s11916-009-0064-3. [DOI] [PubMed] [Google Scholar]
- 15.Gilboa SM, Strickland MJ, Olshan AF, et al. Use of antihistamine medications during early pregnancy and isolated major malformations. Birth Defects Res A Clin Mol Teratol. 2009;85:137–50. doi: 10.1002/bdra.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ismail SK, Kenny L. Review on hyperemesis gravidarum. Best Pract Res Clin Gastroenterol. 2006;21:755–69. doi: 10.1016/j.bpg.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe PG, Gilboa SM, Hernandez-Diaz S, et al. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf. 2013;22:1013–8. doi: 10.1002/pds.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko JY, Farr SL, Dietz PM, et al. Depression and treatment among US pregnant and nonpregnant women of reproductive age, 2005-2009. J Womens Health. 2012;21:830–6. doi: 10.1089/jwh.2011.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yerby MS. Special considerations for women with epilepsy. J Hum Pharmacol Drug Ther. 2000;20:159–70S. doi: 10.1592/phco.20.12.159s.35249. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell AA. Studies of drug-induced birth defects. In: Strom BL, Kimmel SE, Hennessy S, editors. Pharmacoepidemiology. 5th Wiley-Blackwell; New York, NY: 2012. pp. 487–504. [Google Scholar]
- 21.Balshem H, Stevens A, Ansari M, et al. Agency for Healthcare Research and Quality; Rockville, MD: Nov, 2013. Finding gray literature evidence and assessing for outcome and analysis reporting biases when comparing medical interventions: AHRQ and the effective health care program. Methods guide for comparative effectiveness reviews (prepared by the Oregon Health and Science University and University of Ottawa evidence-based practice centers under contract nos. 290-2007-10057-I and 290-10059-I) Available at: http://www.ncbi.nlm.nih. gov/books/NBK174882/pdf/cerguidegrey.pdf. Accessed May 21, 2014. [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, et al. Rating quality of evidence and strength of recommendations, GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkman ND, Lohr KN, Ansari M, et al. Agency for Healthcare Research and Quality; Rockville, MD: Nov, 2013. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the Agency for Healthcare Research and Quality: an update. Methods guide for comparative effectiveness reviews (prepared by the RTI UNC evidence based practice center under contract no. 290-2007-10056-I). AHRQ publication no. 13(14)-EHC130-EF. Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/457/1752/methods-guidance-grading-evidence-131118.pdf. Accessed May 21, 2014. [PubMed] [Google Scholar]
- 24.Chambers CD, Polifka JE, Friedman JM. Drug safety in pregnant women and their babies: ignorance not bliss. Clin Pharmacol Ther. 2008;83:181–3. doi: 10.1038/sj.clpt.6100448. [DOI] [PubMed] [Google Scholar]
- 25.Teratology Society Public Affairs Committee FDA classification of drugs for teratogenic risk. Teratology. 1994;49:446–7. doi: 10.1002/tera.1420490603. [DOI] [PubMed] [Google Scholar]
- 26.Friedman JM. ABCDXXX: the obscenity of postmarketing surveillance for teratogenic effects. Birth Defects Res A Clin Mol Teratol. 2012;94:670–6. doi: 10.1002/bdra.23043. [DOI] [PubMed] [Google Scholar]
- 27.Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (proposed rule) Fed Regist. 2008 May 29;73:30831–68. [Google Scholar]
- 28.Ahmed F, Temte JL, Campos Outcalt D, Schunemann HJ. Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the US Centers for Disease Control and Prevention (CDC) Vaccine. 2011;29:9171–6. doi: 10.1016/j.vaccine.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45–9. doi: 10.7326/0003-4819-150-1-200901060-00009. [DOI] [PubMed] [Google Scholar]
- 30.Workowski KA, Berman S, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59:1–110. [PubMed] [Google Scholar]
- 31.Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease: revised guidelines from CDC, recommendations and reports. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 32.Adler M, Ziglio E, editors. Gazing into the oracle: the Delphi method and its application to social policy and public health. Jessica Kingsley Publishers; Philadelphia, PA: 1996. [Google Scholar]
- 33.US Preventive Services Task Force. Procedure manual, AHRQ publication no. 08-05118-EF2008. Available at: http://www.uspreventiveservices taskforce.org/uspstf08/methods/procmanual.pdf. Accessed May 21, 2014.
- 34.American College of Obstetricians and Gynecologists Prevention of early-onset group B streptococcal disease in newborns. ACOG Committee opinion no. 485. Obstet Gynecol. 2011;117:1019–27. doi: 10.1097/AOG.0b013e318219229b. [DOI] [PubMed] [Google Scholar]
- 35.Baker CJ, Byington CL, Polin RA. Policy statement: recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics. 2011;128:611–6. doi: 10.1542/peds.2011-1466. [DOI] [PubMed] [Google Scholar]
- 36.Jellinek SP, Cohen V, Stansfield L, Likour ezos A, Sable KN. A survey of drug information references emergency medicine clinicians utilize for prescribing in pregnant patients. Ann Pharmacother. 2010;44:456–61. doi: 10.1345/aph.1M631. [DOI] [PubMed] [Google Scholar]
- 37.Lagan BM, Sinclair M, Kernohan WG. Internet use in pregnancy informs women’s decision making: a web-based survey. Birth. 2010;37:106–15. doi: 10.1111/j.1523-536X.2010.00390.x. [DOI] [PubMed] [Google Scholar]