Abstract
Matrix metalloproteinase-3 (MMP3) plays a key role in tissue degradation in periodontitis. The relationship between the MMP3 -1171 5A/6A polymorphism (rs35068180) and periodontitis has been widely studied. However, existing studies have yielded contradictory results. We therefore conducted a meta-analysis to comprehensively investigate these inconclusive findings. Several electronic databases were searched for eligible articles. Seven case-control studies from 6 articles were searched without any language restrictions. Pooled estimates indicated that MMP3 -1171 5A/6A polymorphism is associated with a decreased risk of periodontitis (allelic genetic model: OR = 0.70, 95% CI: 0.62–0.80, Pheterogeneity = 0.315; heterozygous model: OR = 0.50, 95% CI: 0.39–0.65, Pheterogeneity = 0.221; homozygous model: OR = 0.42, 95% CI: 0.25–0.69, Pheterogeneity = 0.265; dominant model: OR = 0.49, 95% CI: 0.38–0.62, Pheterogeneity = 0.238, respectively). Similar results were also found in chronic periodontitis (CP), Asian, Asian&CP, and non-smokers subgroups. Moreover, MMP3 rs35068180 polymorphism might be associated with a lower risk of aggressive periodontitis (AgP) in Asians (allelic genetic model: OR = 0.66, 95% CI: 0.48–0.91, Pheterogeneity = 0.945), and CP in Caucasians and Brazilians. In conclusion, this meta-analysis demonstrates that MMP3 -1171 5A/6A polymorphism may be associated with decreased risk of both CP and AgP in Asians. Large independent studies to replicate these results are necessary to validate these associations in other populations.
Periodontitis is a globally prevalent inflammatory disease that affects the tooth-supporting apparatus1. Periodontitis is extremely common; chronic periodontitis (CP) is the most common form of the disease and affects between 5.0% and 79.6% of the world’s population2. CP is also the major cause of tooth loss, particularly in the elderly. Aggressive periodontitis (AgP), which generally presents in early adulthood, is characterised by a rapid, severe destruction of alveolar bone and periodontal attachment loss3. Although microorganisms are thought to be the prerequisite factor4, environmental (e.g., stress and smoking)5,6 and genetic factors (e.g., IL-1, IL-8 and TGF-β1)7,8,9 are also involved in the pathogenesis of periodontitis.
Matrix metalloproteinases (MMPs) are the most important pathway in periodontitis-associated tissue breakdown, owing to their role in the pathological destruction of extracellular matrix and immune responses related to periodontal inflammation10. MMP3 (stromelysin-1) can degrade collagen in the basal membrane and can induce the synthesis of other MMPs, such as MMP1 and MMP9. The MMP3 gene, located on chromosome 11q22.2–22.3 (adjacent to the MMP1 and MMP12 genes), is an endopeptidase produced by smooth muscle cells, macrophages and synovial cells11. The insertion/deletion of a single adenosine (5A/6A) at position -1171 of the MMP3 promoter region (rs35068180 or rs3025058) can alter its transcription levels12. This may affect its ability to degrade connective tissue and lead to the progression of periodontitis. A prioritisation analysis of candidate genes for periodontitis using multiple computational tools identified MMP3 as one of the most promising genes to be involved in periodontitis13.
Over the past decade, many genetic association studies have been conducted to evaluate the association between MMP3 -1171 5A/6A polymorphism and the risk of periodontitis. However, the results of these studies were inconsistent. The discrepancies among studies may be due to the relatively small sample size in each investigation, as well as varying population characteristics. Therefore, we performed a meta-analysis to investigate the potential association of MMP3 -1171 5A/6A polymorphism with the risk of periodontitis.
Results
Characteristics of eligible studies
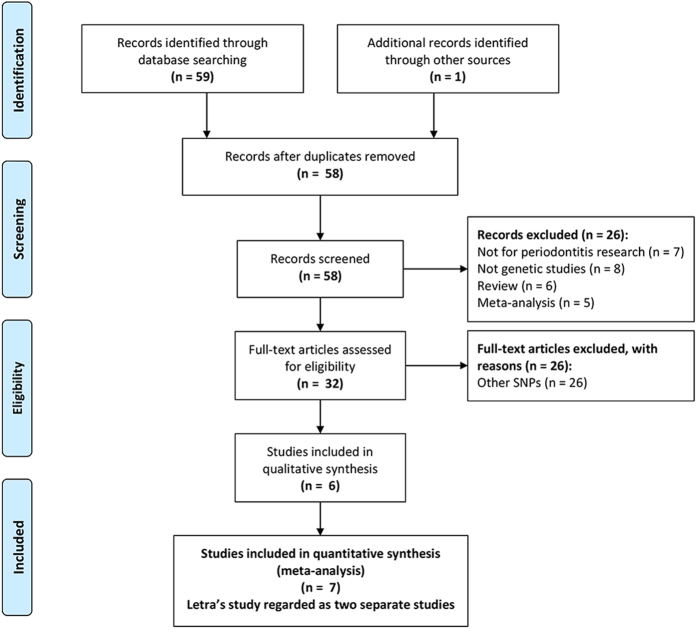
Seven case-control studies from six publications were identified in the meta-analysis (a flow diagram is shown in Fig. 1). Basic characteristics and the quality assessment scores of the selected articles are all listed in Table 1. Overall, all of the included studies were moderate- to high-quality, with a mean quality score of 29.1/40 (range from 27 to 31). Of these case-control studies, four studies including 963 cases and 1227 controls were undertaken in Asians14,15,16,17, two studies containing 183 cases and 402 controls were conducted in a mixed Brazilian population18,19, and one study with 67 cases and 202 controls was performed in a Caucasian population19. Three studies enrolled non-smokers, which permitted smoking to be analysed as a risk factor14,16,18. Five eligible studies mentioned quality controls of the genetic analyses, such as blind genotyping, validation of genotyping accuracy, and random replications15,16,17,19. Two studies supplied only the allele frequency to compute OR in the allelic genetic model15,19. The control populations of three studies were not consistent with Hardy–Weinberg equilibrium (HWE)16,17,18. The minor allele frequency (MAF) for controls and genotype distributions of analysed polymorphism in different ethnicities are all listed in Table 2.
Figure 1. PRISMA flowchart for inclusion of studies in the meta-analysis.
Table 1. Characteristics of eligible studies included in the meta-analysis.
| First author | Year | Country | Ethnicity | Form of Disease | Sample size (Case/Control) | Distribution of genotypes and alleles |
Smoking status | Genotyping method | Quality scores | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case |
Control |
|||||||||||||
| 5A/5A/5A/6A/6A/6A | 5A | 6A | 5A/5A/5A/6A/6A/6A | 5A | 6A | |||||||||
| Itagaki | 2004 | Japan | Asian | CP | 205/142 | 5/58/142 | 68 | 342 | 4/38/100 | 46 | 238 | Non-smokers | TaqMan | 31 |
| Itagaki | 2004 | Japan | Asian | AgP | 37/142* | 0/17/20 | 17 | 57 | 4/38/100 | 46 | 238 | Non-smokers | TaqMan | 31 |
| Astolfi | 2006 | Brazil | Mixed | CP | 90/103 | 19/52/19 | 90 | 90 | 8/70/25 | 86 | 120 | Non-smokers | PCR-RFLP | 29 |
| Kobayashi | 2009 | Japan | Asian | CP | 147/303 | — | 37 | 257 | — | 72 | 534 | S/N | DNA chip & PCR | 29 |
| Kobayashi | 2009 | Japan | Asian | AgP | 172/303* | — | 58 | 286 | — | 72 | 534 | S/N | DNA chip & PCR | 29 |
| Loo | 2011 | China | Asian | CP | 280/250 | 154/115/11 | 423 | 137 | 100/135/15 | 335 | 165 | Non-smokers | PCR-RFLP | 28 |
| Letra | 2012 | Brazil | Mixed | CP | 93/299 | — | 117 | 69 | — | 356 | 242 | S/N | TaqMan | 30 |
| Letra | 2012 | USA | Caucasian | CP | 67/202 | — | 78 | 56 | — | 193 | 211 | S/N | TaqMan | 30 |
| Li | 2012 | China | Asian | CP | 122/532 | 75/44/3 | 194 | 50 | 213/283/36 | 709 | 355 | S/N | PCR-RFLP | 27 |
CP, chronic periodontitis; AgP, aggressive periodontitis; S/N, smokers and nonsmokers.
*The control group for AgP used the same subjects as the control group for CP. In the pooled analysis, the CP and AgP cases were combined into one group and the control group was counted only one time.
Table 2. Minor allele frequency and genotype distribution in different sources of ethnicities.
| Source of Ethnicity | Numbers of comparisons | Minor Allele Frequency | 5A/5A/5A/6A/6A/6A |
|---|---|---|---|
| Asian | 4 | 0.53 | 0.34/0.50/0.16 |
| Caucasian | 1 | 0.52 | — |
| Mixed (Brazilian) | 2 | 0.45 | 0.08/0.68/0.24 |
Quantitative data synthesis
Between-study heterogeneity was not evident in all five comparisons (Table 3). Overall, we found that with the exception of a marginal association in the recessive model (6A/6A vs. 5A/5A + 5A/6A: OR = 0.74, 95% CI = 0.53–1.02, Pheterogeneity = 0.539, p = 0.06), the other four genetic models contributed to decreased susceptibility to periodontitis (6A allele vs. 5A allele: OR = 0.70, 95% CI = 0.62–0.80, Pheterogeneity = 0.315; 5A/6A vs. 5A/5A: OR = 0.50, 95% CI = 0.39–0.65, Pheterogeneity = 0.221; 6A/6A vs. 5A/5A: OR = 0.42, 95% CI = 0.25–0.69, Pheterogeneity = 0.265; 5A/6A + 6A/6A vs. 5A/5A: OR = 0.49, 95% CI = 0.38–0.62, Pheterogeneity = 0.238, respectively). After the HWE-violating studies were excluded, a significant association was found in the allele contrast (6A allele vs. 5A allele: OR = 0.80, 95% CI = 0.67–0.96, Pheterogeneity = 0.655).
Table 3. Meta-analysis of the MMP3 gene -1171 5A/6A polymorphism (rs35068180) on periodontitis risk.
| Variables | N (Case/Control) | 6A allele vs. 5A allele |
5A/6A vs. 5A/5A |
6A/6A vs.5A/5A |
5A/6A + 6A/6A vs.5A/5A |
6A/6A vs. 5A/5A + 5A/6A |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Phet, I2 (%) | OR (95% CI) | Phet, I2 (%) | OR (95% CI) | Phet, I2 (%) | OR (95% CI) | Phet, I2 (%) | OR (95% CI) | Phet, I2 (%) | ||
| All | 7 (1213/1831) | 0.70 (0.62, 0.80) | 0.315, 15.0 | 0.50 (0.39, 0.65) | 0.221, 31.9 | 0.42 (0.25, 0.69) | 0.265, 24.4 | 0.49 (0.38, 0.62) | 0.238, 29.1 | 0.74 (0.53, 1.02) | 0.539, 0.0 |
| HWE | 4 (721/946) | 0.80 (0.67, 0.96) | 0.655, 0.0 | 1.58 (0.40, 6.22) | — | 1.30 (0.34, 4.94) | — | 1.37 (0.36, 5.20) | — | 0.85 (0.54, 1.33) | — |
| Type of periodontitis | |||||||||||
| CP | 7 (1004/1831) | 0.72 (0.63, 0.82) | 0.163, 34.8 | 0.50 (0.39, 0.64) | 0.345, 9.6 | 0.41 (0.25, 0.68) | 0.336, 11.3 | 0.48 (0.38, 0.62) | 0.317, 14.9 | 0.77 (0.56, 1.07) | 0.438, 0.0 |
| AgP (Asian) | 2 (209/445) | 0.66 (0.48, 0.91) | 0.945, 0.0 | 4.09 (0.21, 80.21) | — | 1.84 (0.10, 35.43) | — | 2.44 (0.13, 46.28) | — | 0.49 (0.24, 1.04) | — |
| Ethnicity | |||||||||||
| Asian | 4 (963/1227) | 0.68 (0.58, 0.80) | 0.149, 43.7 | 0.52 (0.40, 0.68) | 0.198, 38.2 | 0.45 (0.25, 0.81) | 0.173, 42.9 | 0.51 (0.39, 0.66) | 0.202, 37.4 | 0.71 (0.49, 1.03) | 0.357, 2.9 |
| Asian&CP | 4 (754/1227) | 0.73 (0.55, 0.96) | 0.052, 61.2 | 0.52 (0.40, 0.68) | 0.333, 9.0 | 0.44 (0.24, 0.79) | 0.226, 32.8 | 0.50 (0.39, 0.65) | 0.284, 20.6 | 0.75 (0.52, 1.10) | 0.260, 25.7 |
| Caucasian (CP) | 1 (67/202) | 0.66 (0.44, 0.97) | — | — | — | — | — | — | — | ||
| Brazilian Mixed (CP) | 2 (183/402) | 0.80 (0.62, 1.04) | 0.477, 0.0 | 0.31 (0.13, 0.77) | — | 0.32 (0.12, 0.89) | — | 0.31 (0.13, 0.76) | — | 0.83 (0.42, 1.64) | — |
| Smoke | |||||||||||
| Non-smokers | 3 (612/495) | 0.73 (0.60, 0.88) | 0.414, 0.0 | 0.54 (0.39, 0.75) | 0.151, 47.1 | 0.50 (0.28, 0.89) | 0.260, 25.8 | 0.53 (0.39, 0.73) | 0.189, 40.0 | 0.80 (0.57, 1.13) | 0.825, 0.0 |
N, Number of comparisons; OR, odds ratio; 95% CI, 95% confidence interval; CP, chronic periodontitis; AgP, aggressive periodontitis.
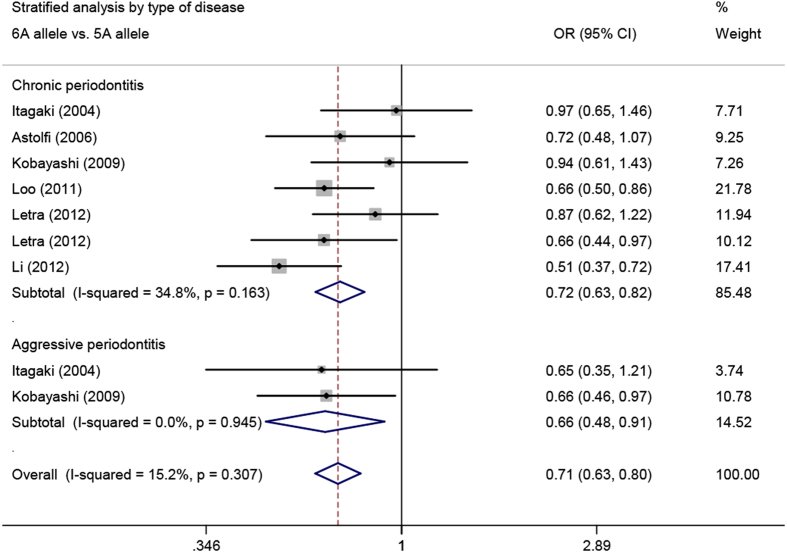
When stratified by the type of periodontitis, we found a significant association of the -1171 5A/6A polymorphism in the MMP3 promoter region with both CP (6A allele vs. 5A allele: OR = 0.72, 95% CI = 0.63–0.82, Pheterogeneity = 0.163; 5A/6A vs. 5A/5A: OR = 0.50, 95% CI = 0.39–0.64, Pheterogeneity = 0.345; 6A/6A vs. 5A/5A: OR = 0.41, 95% CI = 0.25–0.68, Pheterogeneity = 0.336; 5A/6A + 6A/6A vs. 5A/5A: OR = 0.48, 95% CI = 0.38–0.62, Pheterogeneity = 0.317, respectively) and AgP (6A allele vs. 5A allele: OR = 0.66, 95% CI = 0.48–0.91, Pheterogeneity = 0.945) (Fig. 2).
Figure 2. Meta-analysis of MMP3 -1171 5A/6A polymorphism with chronic and aggressive periodontitis.
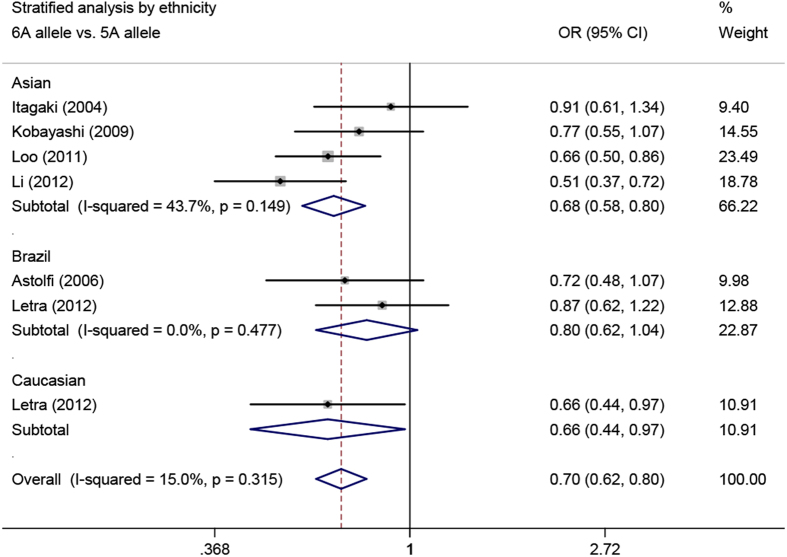
In the ethnicity-stratified analysis, the associations were significant in Asians (6A allele vs. 5A allele: OR = 0.68, 95% CI = 0.58–0.80, Pheterogeneity = 0.149; 5A/6A vs. 5A/5A: OR = 0.52, 95% CI = 0.40–0.68, Pheterogeneity = 0.198; 6A/6A vs. 5A/5A: OR = 0.45, 95% CI = 0.25–0.81, Pheterogeneity = 0.173; 5A/6A + 6A/6A vs. 5A/5A: OR = 0.51, 95% CI = 0.39–0.66, Pheterogeneity = 0.202, respectively), Caucasians (all CP cases) (6A allele vs. 5A allele: OR = 0.66, 95% CI = 0.44–0.97), and Brazilian mixed populations (all CP cases) (5A/6A vs. 5A/5A: OR = 0.31, 95% CI = 0.13–0.77; 6A/6A vs. 5A/5A: OR = 0.32, 95% CI = 0.12–0.89; 5A/6A + 6A/6A vs. 5A/5A: OR = 0.31, 95% CI = 0.13–0.76, respectively) (Fig. 3). Furthermore, after excluding the 209 AgP cases in Asians, the magnitude of the effect on CP was similar in Asians (Table 3).
Figure 3. Meta-analysis of MMP3 -1171 5A/6A polymorphism with periodontitis in different ethnicities.
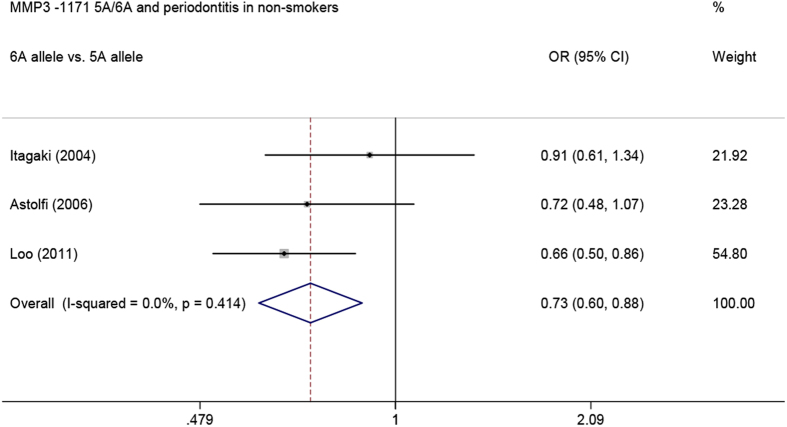
In addition, when analysis was limited to the three studies that recruited non-smokers, we also found that the -1171 5A/6A polymorphism was associated with a decreased risk of periodontitis in nonsmokers (6A allele vs. 5A allele: OR = 0.73, 95% CI = 0.60–0.88, Pheterogeneity = 0.414; 5A/6A vs. 5A/5A: OR = 0.54, 95% CI = 0.39–0.75, Pheterogeneity = 0.151; 6A/6A vs. 5A/5A: OR = 0.50, 95% CI = 0.28–0.89, Pheterogeneity = 0.260; 5A/6A + 6A/6A vs. 5A/5A: OR = 0.53, 95% CI = 0.39–0.73, Pheterogeneity = 0.189, respectively) (Fig. 4).
Figure 4. Meta-analysis of MMP3 -1171 5A/6A polymorphism with periodontitis in nonsmokers.
Sensitivity analysis and publication bias
Sensitivity analysis was performed by omitting individual studies to assess the effect of each publication on the overall results. No single study changed the pooled ORs qualitatively, which suggests that the results of our meta-analysis are accurate.
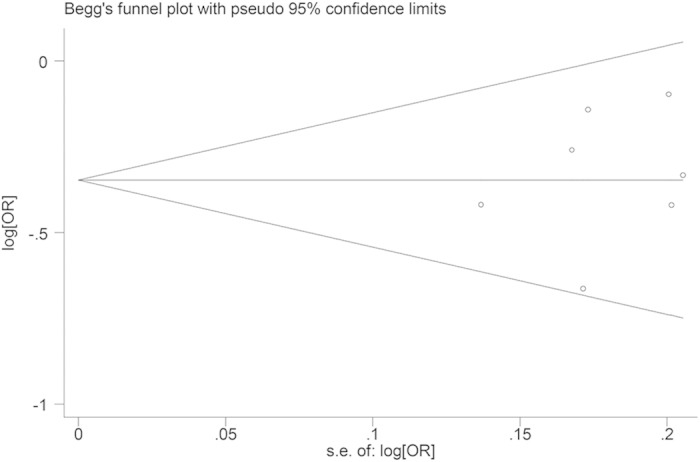
Begger’s funnel plot and Egger’s test were performed to detect the publication bias of included studies. As shown in Fig. 5, the shape of the funnel plot did not reveal any evidence of obvious asymmetry. In addition, the results of Egger’s test did not show any evidence of publication bias.
Figure 5. Funnel plot of MMP3 -1171 5A/6A for the allelic model (6A allele versus 5A allele). SE = standard error.
Discussion
Increasing evidence suggests that MMPs play a pivotal role in periodontitis10. Meta-analyses have demonstrated that both MMP1 -1607 1G/2G and MMP9 -1562C/T polymorphisms may be involved in the development of CP20,21. MMP3 -1171 5A/6A and MMP1 -1607 1G/2G (rs1799750) are in a strong linkage disequilibrium22. The relationship between MMP3 -1171 5A/6A polymorphism and periodontitis has been widely investigated. However, previous studies have yielded contradictory results. We therefore performed this meta-analysis to comprehensively evaluate these inconclusive findings. To the best of our knowledge, this is the first meta-analysis to have investigated the association between MMP3 -1171 5A/6A polymorphism and the susceptibility to periodontitis. Our meta-analysis was based on seven case–control studies that focused on the link between MMP3 -1171 5A/6A polymorphism and periodontitis risk and included a total of 1,213 cases and 1,831 controls. The results of our meta-analysis revealed that -1171 5A/6A polymorphism is associated with a decreased risk of overall periodontitis; this association was consistently significant in all subgroups in analyses that were stratified by periodontitis type, ethnicity and smoking status.
Although phenotypic differences exist between AgP and CP in terms of the genetic predisposition to periodontitis, some scholars believe that both chronic and aggressive periodontitis may share some susceptibility genes23. Furthermore, some genetic polymorphisms (e.g., TNF-α -308G/A and Fcγ receptor IIIB NA1/NA2) have been shown to have similar effects on AgP and CP24,25. In this report, we first analysed the effect of MMP3 -1171 5A/6A polymorphism on two forms of periodontitis. We found that this locus was associated with a decreased risk of periodontitis under four genetic models. Additionally, -1171 5A/6A showed a trend towards protective factor for periodontitis in the recessive model, although the finding was not statistically significant (p = 0.06). When the analysis was limited to the four HWE studies, the association between -1171 5A/6A polymorphism and periodontitis was still significant in the allele model, indicating the robustness of this meta-analysis. Several subgroup analyses were subsequently conducted, and the associations were similar in AgP and CP subgroups in the allele comparison. Additional well-designed studies are required to strengthen our understanding of the association between -1171 5A/6A polymorphism and AgP. Although the minor allele frequency (MAF) and genotype distributions in the included studies for -1171 5A/6A varied by ethnicity (Table 2); the stratified analysis by ethnicity showed a significant association with CP in all three ethnicities. However, all the positive results for the Caucasian and Brazilian mixed populations were derived from a single study. Therefore, future studies with larger samples are warranted to confirm these associations in the Caucasian and Brazilian mixed populations. Tobacco smoking is thought to be an important modifier of the pathogenesis of periodontitis5. In further stratified analyses, only the non-smoker subgroup was included to eliminate the possible confounding effects of smoking, and similarly significant findings were found.
The role of MMP3 in the pathogenesis of periodontitis has been widely investigated. Toyman et al. showed that MMP3 levels in the gingival crevicular fluid (GCF) increased in periodontitis cases and played a role in tissue destruction26. Recent findings have demonstrated that clinical improvements after non-surgical periodontal therapy are accompanied by a reduction of MMP3 in GCF in both CP and AgP patients27,28. Some meta-analyses have investigated the associations between MMP3 -1171 5A/6A polymorphism and systemic diseases29,30,31,32,33 and have demonstrated that this polymorphism is associated with a decreased risk of gastrointestinal cancer30, abdominal aortic aneurysm31, myocardial infarction33 and head and neck cancer32. All of these diseases have been shown to be positively associated with periodontitis in several epidemiological studies34,35,36,37. Our results suggest an association between the transcriptionally more active 5A allele and periodontitis, substantiating previous functional findings regarding the MMP3 rs35068180 variant. We speculate that rs35068180 may influence the development of periodontitis through a similar molecular mechanism. In vitro assays of promoter activity have shown that the 5A allele has a two-fold higher promoter activity than the 6A allele38. Based on these results, our findings are biologically plausible.
The following limitations of the meta-analysis should be recognised. First, we pooled the data based on unadjusted information, while a more precise analysis needs to be conducted if individual data are available. Second, the number of subjects included in this meta-analysis was relatively small for AgP, Caucasian and Brazilian mixed population subgroups. Hence, the results should be interpreted with caution. Third, the genotypic distribution of the controls also deviated from HWE in three of the studies.
In conclusion, this meta-analysis suggests that MMP3 -1171 5A/6A polymorphism contributes to a decreased susceptibility to CP, especially in Asian populations. Furthermore, this polymorphism may decrease the risk of AgP in Asians. Replication studies of our results in large independent populations are necessary to validate this association.
Methods
Search strategy
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed39. A systematic search of studies that addressed the associations between MMP3 -1171 5A/6A polymorphism and periodontitis was performed. The literature was retrieved in electronic bio-medical databases including Google Scholar, PubMed, Embase, Medline and China National Knowledge Infrastructure (the last search update was 19 Apr 2015). A combination of the terms “Matrix Metalloproteinases”, “MMP”, “periodontal diseases”, “periodontitis”, and “polymorphism” were entered both as text words and as Medical Subject Heading components. No language restriction was placed on the search. Manual searches for references cited in published review and original publications were also performed.
Inclusion and exclusion criteria
The following two inclusion criteria were used for all retrieved articles: (1) use of a case–control, cohort, nested case–control, or cross-sectional design; and (2) genotype distribution or allele frequency was available from the published data for both cases and controls. The exclusion criteria were as follows: (1) lack of usable allele frequency or genotype distribution data; and (2) lack of control subjects.
Data extraction
Information was carefully extracted independently from all eligible publications by two investigators. Disagreements were resolved through discussion among the authors to achieve a consensus. The following characteristics were collected for each study: the first author’s family name, publication year, ethnicity (categorised as Caucasian, Asian and mixed), numbers of cases and controls, type of periodontitis (CP or AgP), distribution of genotypes and alleles, smoking status, and genotype identification method.
Study quality assessment
The quality of each study was evaluated independently by two authors according to the modified STROBE quality score systems40. Forty assessment items were used for assessing study quality with scores ranging from 0 to 40. Studies were classified as low- (scores of 0–19), moderate- (scores of 20–29) and high-quality (scores of 30–40). Any disagreement was resolved by discussion among the authors to reach a consensus. The details of the modified STROBE quality score system are listed in Supplementary Table S1.
Statistical analysis
Odds ratios (ORs), along with 95% confidence intervals (CIs), were used to assess the strength of the association between the MMP3 gene -1171 5A/6A polymorphism and periodontitis risk. The Z-test was used to determine the statistical significance of the pooled OR. The pooled ORs were performed for the following genetic models: the mutant allele versus the wild type, homozygote contrast, heterozygote contrast, dominant model and recessive model. In addition, the HWE in the healthy control populations was tested within each study. We applied the chi-squared method to assess if the genotype distribution in the control group was in HWE. Subgroup analyses were also performed for the HWE in controls, type of periodontitis, ethnicity and smoking status.
Both the fixed-effects model (the Mantel–Haenszel method)41 and the random-effects model (the DerSimonian and Laird method)42 were used to calculate the pooled ORs. For each genetic comparison, the heterogeneity between studies was assessed using the chi-square based Q-test and was quantified by the I2 statistic. The fixed-effects model was used when heterogeneity was not significant (Pheterogeneity > 0.10 or I2 < 50%); otherwise, the random-effects model was applied. Sensitivity analysis was performed to assess the stability of the association. Begg’s funnel plot43 and Egger’s weighted regression method44 were applied to evaluate the underlying publication bias. All statistical analyses were conducted with STATA software, version 11.0 (StataCorp, College Station, TX, USA), using two-sided p-values.
Additional Information
How to cite this article: Ding, C. et al. Matrix Metalloproteinase-3 -1171 5A/6A Polymorphism (rs35068180) is Associated with Risk of Periodontitis. Sci. Rep. 5, 11667; doi: 10.1038/srep11667 (2015).
Supplementary Material
Acknowledgments
This study was supported by grants from Project of Health and Science in Hangzhou (Grant No. 2013A23 & 2014A18).
Footnotes
Author Contributions N.C. and L.-J.Z. designed the study. C.D. and X.C. collected, analyzed the data and drafted the paper. P.-T.Z., J.-P.H. and Y. X. summarized data and revised this article. All authors reviewed the manuscript and approved the final draft.
References
- Pihlstrom B. L., Michalowicz B. S. & Johnson N. W. Periodontal diseases. Lancet 366, 1809–1820 (2005). [DOI] [PubMed] [Google Scholar]
- Dye B. A. Global periodontal disease epidemiology. Periodontol 2000 58, 10–25 (2012). [DOI] [PubMed] [Google Scholar]
- Armitage G. C. Periodontal diagnoses and classification of periodontal diseases. Periodontol 2000 34, 9–21 (2004). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Metagenomic sequencing reveals microbiota and its functional potential associated with periodontal disease. Sci Rep 3, 1843; 10.1038/srep01843 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom J. Cigarette smoking as risk factor in chronic periodontal disease. Community Dent Oral Epidemiol 17, 245–247 (1989). [DOI] [PubMed] [Google Scholar]
- Genco R. J., Ho A. W., Grossi S. G., Dunford R. G. & Tedesco L. A. Relationship of stress, distress and inadequate coping behaviors to periodontal disease. J Periodontol 70, 711–723 (1999). [DOI] [PubMed] [Google Scholar]
- Chen X., Huang J., Zhong L. & Ding C. Quantitative Assessment of the Associations Between Interleukin-8 Polymorphisms and Periodontitis Susceptibility. J Periodontol 86, 292–300 (2015). [DOI] [PubMed] [Google Scholar]
- Ding C., Zhao L., Sun Y., Li L. & Xu Y. Interleukin-1 receptor antagonist polymorphism (rs2234663) and periodontitis susceptibility: a meta-analysis. Arch Oral Biol 57, 585–593 (2012). [DOI] [PubMed] [Google Scholar]
- Huang J., Ding C., Chen X., He R. & Chen N. Association of TGF-beta1 -509C/T, +869T/C and +915G/C polymorphisms with periodontitis susceptibility. Oral Dis. 10.1111/odi.12297 (2014). [DOI] [PubMed] [Google Scholar]
- Sorsa T. et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 38, 306–321 (2006). [DOI] [PubMed] [Google Scholar]
- Zitka O. et al. Matrix metalloproteinases. Curr Med Chem 17, 3751–3768 (2010). [DOI] [PubMed] [Google Scholar]
- Ye S. Polymorphism in matrix metalloproteinase gene promoters: implication in regulation of gene expression and susceptibility of various diseases. Matrix Biol 19, 623–629 (2000). [DOI] [PubMed] [Google Scholar]
- Zhan Y. et al. Prioritization of candidate genes for periodontitis using multiple computational tools. J Periodontol 85, 1059–1069 (2014). [DOI] [PubMed] [Google Scholar]
- Itagaki M. et al. Matrix metalloproteinase-1 and -3 gene promoter polymorphisms in Japanese patients with periodontitis. J Clin Periodontol 31, 764–769 (2004). [DOI] [PubMed] [Google Scholar]
- Kobayashi T. et al. Genetic risk factors for periodontitis in a Japanese population. J Dent Res 88, 1137–1141 (2009). [DOI] [PubMed] [Google Scholar]
- Loo W. T., Wang M., Jin L. J., Cheung M. N. & Li G. R. Association of matrix metalloproteinase (MMP-1, MMP-3 and MMP-9) and cyclooxygenase-2 gene polymorphisms and their proteins with chronic periodontitis. Arch Oral Biol 56, 1081–1090 (2011). [DOI] [PubMed] [Google Scholar]
- Li G. et al. Association of matrix metalloproteinase (MMP)-1, 3, 9, interleukin (IL)-2, 8 and cyclooxygenase (COX)-2 gene polymorphisms with chronic periodontitis in a Chinese population. Cytokine 60, 552–560 (2012). [DOI] [PubMed] [Google Scholar]
- Astolfi C. M. et al. Genetic polymorphisms in the MMP-1 and MMP-3 gene may contribute to chronic periodontitis in a Brazilian population. J Clin Periodontol 33, 699–703 (2006). [DOI] [PubMed] [Google Scholar]
- Letra A. et al. MMP3 and TIMP1 variants contribute to chronic periodontitis and may be implicated in disease progression. J Clin Periodontol 39, 707–716 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D. et al. Association between MMP-1 g.-1607dupG polymorphism and periodontitis susceptibility: a meta-analysis. PloS one 8, e59513; 10.1371/journal.pone.0059513 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. et al. MMP-9 -1562C > T contributes to periodontitis susceptibility. J Clin Periodontol 40, 125–130 (2013). [DOI] [PubMed] [Google Scholar]
- Dorr S. et al. Association of a specific haplotype across the genes MMP1 and MMP3 with radiographic joint destruction in rheumatoid arthritis. Arthritis Res Ther 6, R199–R207 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie H., Kobayashi T., Tai H. & Galicia J. C. The role of genetic polymorphisms in periodontitis. Periodontol 2000 43, 102–132 (2007). [DOI] [PubMed] [Google Scholar]
- Ding C., Ji X., Chen X., Xu Y. & Zhong L. TNF-alpha gene promoter polymorphisms contribute to periodontitis susceptibility: evidence from 46 studies. J Clin Periodontol 41, 748–759 (2014). [DOI] [PubMed] [Google Scholar]
- Dimou N. L., Nikolopoulos G. K., Hamodrakas S. J. & Bagos P. G. Fcgamma receptor polymorphisms and their association with periodontal disease: a meta-analysis. J Clin Periodontol 37, 255–265 (2010). [DOI] [PubMed] [Google Scholar]
- Toyman U. et al. Evaluation of gingival crevicular fluid levels of tissue plasminogen activator, plasminogen activator inhibitor 2, matrix metalloproteinase-3 and interleukin 1-beta in patients with different periodontal diseases. J Periodontal Res 50, 44–51 (2015). [DOI] [PubMed] [Google Scholar]
- Goncalves P. F. et al. Periodontal treatment reduces matrix metalloproteinase levels in localized aggressive periodontitis. J Periodontol 84, 1801–1808 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuter G. et al. Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1. J Clin Periodontol 32, 1011–1015 (2005). [DOI] [PubMed] [Google Scholar]
- Zhou H. et al. Genetic polymorphism of matrix metalloproteinase family and chronic obstructive pulmonary disease susceptibility: a meta-analysis. Sci Rep 3, 2818; 10.1038/srep02818 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. et al. Association of matrix metalloproteinase-3 -1171(5A > 6A) polymorphism with cancer risk: a meta-analysis of 41 studies. PloS one 9, e87562; 10.1371/journal.pone.0087562 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Biros E., Cronin O., Kuivaniemi H. & Golledge J. The association of genetic variants of matrix metalloproteinases with abdominal aortic aneurysm: a systematic review and meta-analysis. Heart 100, 295–302 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang C. et al. Meta-analysis of MMP2, MMP3, and MMP9 promoter polymorphisms and head and neck cancer risk. PloS one 8, e62023; 10.1371/journal.pone.0062023 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. et al. Polymorphisms of matrix metalloproteinases in myocardial infarction: a meta-analysis. Heart 97, 1542–1546 (2011). [DOI] [PubMed] [Google Scholar]
- Zeng X. T. et al. Periodontal disease and risk of head and neck cancer: a meta-analysis of observational studies. PloS one 8, e79017; 10.1371/journal.pone.0079017 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud D. S., Liu Y., Meyer M., Giovannucci E. & Joshipura K. Periodontal disease, tooth loss, and cancer risk in male health professionals: a prospective cohort study. Lancet Oncol 9, 550–558 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J. et al. High incidence of periodontitis in Japanese patients with abdominal aortic aneurysm. Int Heart J 55, 268–270 (2014). [DOI] [PubMed] [Google Scholar]
- Yu Y. H., Chasman D. I., Buring J. E., Rose L. & Ridker P. M. Cardiovascular Risks Associated with Incident and Prevalent Periodontal Disease. J Clin Periodontol 42, 21–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Watts G. F., Mandalia S., Humphries S. E. & Henney A. M. Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J 73, 209–215 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G., Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6, e1000097; 10.1371/journal.pmed.1000097 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa B. R., Cevallos M., Altman D. G., Rutjes A. W. & Egger M. Uses and misuses of the STROBE statement: bibliographic study. BMJ open 1, e000048; 10.1136/bmjopen-2010-000048 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. & Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22, 719–748 (1959). [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.