Abstract
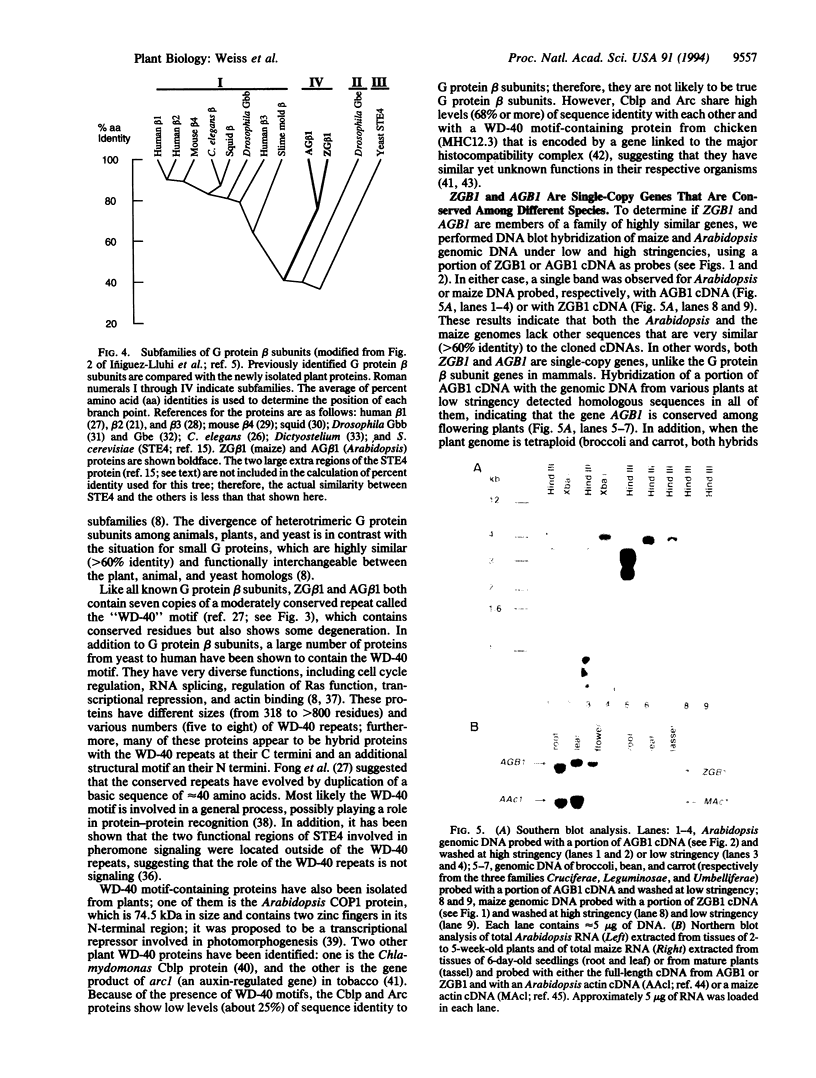
We have isolated cDNAs from maize (ZGB1) and Arabidopsis (AGB1) encoding proteins homologous to beta subunits of guanine nucleotide-binding protein (G protein). The predicted ZGB1 and AGB1 gene products are 76% identical to each other and 41% or more identical to animal G protein beta subunits. Both predicted proteins contain seven repeats of the so-called "WD-40" motif, where WD is Trp-Asp. RNA blot analysis indicates that ZGB1 mRNA is present in the root, leaf, and tassel and that AGB1 mRNA is expressed in the root, leaf, and flower. DNA blot hybridizations indicate that maize and Arabidopsis genomes contain no other genes that are highly similar to ZGB1 and AGB1, respectively, suggesting that the newly isolated G protein beta-subunit homologues are likely to have unique functions. Furthermore, these G protein beta-subunit homologues are conserved among other plant species and may play important role(s) in plant signaling.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L. Receptor-to-effector signaling through G proteins: roles for beta gamma dimers as well as alpha subunits. Cell. 1992 Dec 24;71(7):1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bourne H. R. G-protein subunits. Who carries what message? Nature. 1989 Feb 9;337(6207):504–504. doi: 10.1038/337504a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Neer E. J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature. 1993 Sep 30;365(6445):403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- Deng X. W., Matsui M., Wei N., Wagner D., Chu A. M., Feldmann K. A., Quail P. H. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell. 1992 Nov 27;71(5):791–801. doi: 10.1016/0092-8674(92)90555-q. [DOI] [PubMed] [Google Scholar]
- Duronio R. J., Gordon J. I., Boguski M. S. Comparative analysis of the beta transducin family with identification of several new members including PWP1, a nonessential gene of Saccharomyces cerevisiae that is divergently transcribed from NMT1. Proteins. 1992 May;13(1):41–56. doi: 10.1002/prot.340130105. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Mulligan J. T., Ramer S. W., Spottswood M., Davis R. W. Lambda YES: a multifunctional cDNA expression vector for the isolation of genes by complementation of yeast and Escherichia coli mutations. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1731–1735. doi: 10.1073/pnas.88.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairley-Grenot K., Assmann S. M. Evidence for G-Protein Regulation of Inward K+ Channel Current in Guard Cells of Fava Bean. Plant Cell. 1991 Sep;3(9):1037–1044. doi: 10.1105/tpc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Amatruda T. T., 3rd, Birren B. W., Simon M. I. Distinct forms of the beta subunit of GTP-binding regulatory proteins identified by molecular cloning. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebl M., Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991 May;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Guillemot F., Billault A., Auffray C. Physical linkage of a guanine nucleotide-binding protein-related gene to the chicken major histocompatibility complex. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4594–4598. doi: 10.1073/pnas.86.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Novel mechanisms of translational control in Saccharomyces cerevisiae. Trends Genet. 1988 Jun;4(6):169–174. doi: 10.1016/0168-9525(88)90023-6. [DOI] [PubMed] [Google Scholar]
- Ishida S., Takahashi Y., Nagata T. Isolation of cDNA of an auxin-regulated gene encoding a G protein beta subunit-like protein from tobacco BY-2 cells. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11152–11156. doi: 10.1073/pnas.90.23.11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez-Lluhi J., Kleuss C., Gilman A. G. The importance of G-protein beta lambda subunits. Trends Cell Biol. 1993 Jul;3(7):230–236. doi: 10.1016/0962-8924(93)90122-h. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Scherübl H., Hescheler J., Schultz G., Wittig B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992 Jul 30;358(6385):424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- Kleuss C., Scherübl H., Hescheler J., Schultz G., Wittig B. Selectivity in signal transduction determined by gamma subunits of heterotrimeric G proteins. Science. 1993 Feb 5;259(5096):832–834. doi: 10.1126/science.8094261. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Dignard D., Harcus D., Thomas D. Y., Whiteway M. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J. 1992 Dec;11(13):4815–4824. doi: 10.1002/j.1460-2075.1992.tb05587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E., Dignard D., Hougan L., Thomas D. Y., Whiteway M. Dominant-negative mutants of a yeast G-protein beta subunit identify two functional regions involved in pheromone signalling. EMBO J. 1992 Dec;11(13):4805–4813. doi: 10.1002/j.1460-2075.1992.tb05586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. A., Smallwood P. M., Moen P. T., Jr, Helman L. J., Ahn T. G. Molecular cloning of beta 3 subunit, a third form of the G protein beta-subunit polypeptide. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2329–2333. doi: 10.1073/pnas.87.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Assmann S. M. Characterization of a G-protein-regulated outward K+ current in mesophyll cells of vicia faba L. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):262–266. doi: 10.1073/pnas.90.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly P., Wu L., Welker D. L., Devreotes P. N. A G-protein beta-subunit is essential for Dictyostelium development. Genes Dev. 1993 Jun;7(6):986–995. doi: 10.1101/gad.7.6.986. [DOI] [PubMed] [Google Scholar]
- Lohmer S., Maddaloni M., Motto M., Salamini F., Thompson R. D. Translation of the mRNA of the maize transcriptional activator Opaque-2 is inhibited by upstream open reading frames present in the leader sequence. Plant Cell. 1993 Jan;5(1):65–73. doi: 10.1105/tpc.5.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Yanofsky M. F., Huang H. Isolation and sequence analysis of TGA1 cDNAs encoding a tomato G protein alpha subunit. Gene. 1991 Nov 15;107(2):189–195. doi: 10.1016/0378-1119(91)90318-6. [DOI] [PubMed] [Google Scholar]
- Ma H., Yanofsky M. F., Meyerowitz E. M. Molecular cloning and characterization of GPA1, a G protein alpha subunit gene from Arabidopsis thaliana. Proc Natl Acad Sci U S A. 1990 May;87(10):3821–3825. doi: 10.1073/pnas.87.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn C. J., Winesett L., Ferl R. J. Nucleotide sequence of an actin gene from Arabidopsis thaliana. Gene. 1988 May 30;65(2):247–257. doi: 10.1016/0378-1119(88)90461-1. [DOI] [PubMed] [Google Scholar]
- Neuhaus G., Bowler C., Kern R., Chua N. H. Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell. 1993 Jun 4;73(5):937–952. doi: 10.1016/0092-8674(93)90272-r. [DOI] [PubMed] [Google Scholar]
- Ruggieri R., Tanaka K., Nakafuku M., Kaziro Y., Toh-e A., Matsumoto K. MSI1, a negative regulator of the RAS-cAMP pathway in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8778–8782. doi: 10.1073/pnas.86.22.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryba N. J., Pottinger J. D., Keen J. N., Findlay J. B. Sequence of the beta-subunit of the phosphatidylinositol-specific phospholipase C-directed GTP-binding protein from squid (Loligo forbesi) photoreceptors. Biochem J. 1991 Jan 1;273(Pt 1):225–228. doi: 10.1042/bj2730225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. A. A Chlamydomonas gene encodes a G protein beta subunit-like polypeptide. Mol Gen Genet. 1990 May;221(3):443–452. doi: 10.1007/BF00259410. [DOI] [PubMed] [Google Scholar]
- Schmidt R. J., Burr F. A., Aukerman M. J., Burr B. Maize regulatory gene opaque-2 encodes a protein with a "leucine-zipper" motif that binds to zein DNA. Proc Natl Acad Sci U S A. 1990 Jan;87(1):46–50. doi: 10.1073/pnas.87.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinfest C. W., Henderson K. W., Gu J. R., Kottaridis S. D., Besbeas S., Panotopoulou E., Papas T. S. Subtraction hybridization cDNA libraries from colon carcinoma and hepatic cancer. Genet Anal Tech Appl. 1990 May;7(3):64–70. doi: 10.1016/0735-0651(90)90042-e. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Genes encoding actin in higher plants: intron positions are highly conserved but the coding sequences are not. J Mol Appl Genet. 1983;2(1):111–126. [PubMed] [Google Scholar]
- Sharrock R. A., Quail P. H. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989 Nov;3(11):1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Simon M. I., Strathmann M. P., Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Sive H. L., St John T. A simple subtractive hybridization technique employing photoactivatable biotin and phenol extraction. Nucleic Acids Res. 1988 Nov 25;16(22):10937–10937. doi: 10.1093/nar/16.22.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr Signal transduction in yeast mating: receptors, transcription factors, and the kinase connection. Trends Genet. 1991 Nov-Dec;7(11-12):393–398. [PubMed] [Google Scholar]
- Warpeha K. M., Hamm H. E., Rasenick M. M., Kaufman L. S. A blue-light-activated GTP-binding protein in the plasma membranes of etiolated peas. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8925–8929. doi: 10.1073/pnas.88.20.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C. A., Huang H., Ma H. Immunolocalization of the G protein alpha subunit encoded by the GPA1 gene in Arabidopsis. Plant Cell. 1993 Nov;5(11):1513–1528. doi: 10.1105/tpc.5.11.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway M., Hougan L., Dignard D., Thomas D. Y., Bell L., Saari G. C., Grant F. J., O'Hara P., MacKay V. L. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell. 1989 Feb 10;56(3):467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]
- Yarfitz S., Niemi G. A., McConnell J. L., Fitch C. L., Hurley J. B. A G beta protein in the Drosophila compound eye is different from that in the brain. Neuron. 1991 Sep;7(3):429–438. doi: 10.1016/0896-6273(91)90295-b. [DOI] [PubMed] [Google Scholar]
- Yarfitz S., Provost N. M., Hurley J. B. Cloning of a Drosophila melanogaster guanine nucleotide regulatory protein beta-subunit gene and characterization of its expression during development. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7134–7138. doi: 10.1073/pnas.85.19.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Voorn L., Gebbink M., Plasterk R. H., Ploegh H. L. Characterization of a G-protein beta-subunit gene from the nematode Caenorhabditis elegans. J Mol Biol. 1990 May 5;213(1):17–26. doi: 10.1016/s0022-2836(05)80118-4. [DOI] [PubMed] [Google Scholar]
- von Weizsäcker E., Strathmann M. P., Simon M. I. Diversity among the beta subunits of heterotrimeric GTP-binding proteins: characterization of a novel beta-subunit cDNA. Biochem Biophys Res Commun. 1992 Feb 28;183(1):350–356. doi: 10.1016/0006-291x(92)91650-f. [DOI] [PubMed] [Google Scholar]