Abstract
Objective
To estimate whether moderate/severe stress urinary incontinence (SUI) in middle-aged women is associated with overall lifetime physical activity (including leisure, household, outdoor, and occupational), as well as lifetime leisure (recreational), lifetime strenuous and strenuous activity during the teen years.
Study design
Recruitment for this case-control study was conducted in primary care level family medicine and gynecology clinics. 1538 enrolled women ages 39–65 years underwent a Pelvic Organ Prolapse Quantification examination to assess vaginal support. Based on Incontinence Severity Index scores, cases had moderate/severe and controls no/mild SUI. We excluded 349 with vaginal descent at/below the hymen (pelvic organ prolapse), 194 who did not return questionnaires, and 110 with insufficient activity data for analysis. 213 cases were frequency-matched 1:1 by age group to controls. Physical activity was measured using the Lifetime Physical Activity Questionnaire, in which women recall activity from menarche to present. We created separate multivariable logistic regression models for activity measures.
Results
SUI odds increased slightly with overall lifetime activity (OR 1.20 per 70 additional MET-hrs/wk; 95% CI 1.02, 1.41), and were not associated with lifetime strenuous activity (OR 1.11; 95% CI 0.99, 1.25). In quintile analysis of lifetime leisure activity, which demonstrated a non-linear pattern, all quintiles incurred about half the odds of SUI compared to reference (2nd quintile; p=0.009). Greater strenuous activity in teen years modestly increased SUI odds (OR 1.37 per 7 additional hours/week; 95% CI 1.09, 1.71); OR 1.75; 95% CI 1.15, 2.66 in sensitivity analysis adjusting for measurement error. The predicted probability of SUI rose linearly in women exceeding 7.5 hours of strenuous activity/week during teen years. Teen strenuous activity had a similar effect on SUI odds when adjusted for subsequent strenuous activity during ages 21–65 years.
Conclusion
In middle-aged women, a slight increased odds of SUI was noted only after substantially increased overall lifetime physical activity. Increased lifetime leisure activity decreased and lifetime strenuous activity appeared unrelated to SUI odds. Greater strenuous activity during teen years modestly increased SUI odds.
INTRODUCTION
More than one-quarter of nulliparous athletes report stress urinary incontinence (SUI) while doing their activity.1–4 Even active teenagers leak urine: 80% of trampoline jumpers reported leakage while jumping.5 Young women exercising at higher intensities are more likely to report SUI during sports than are those whose exercise does not include repetitive impact.1,2 It is clear that young women notice SUI during strenuous activities, but whether such activity increases the odds of future SUI is not known. Indeed, in middle-aged women, regular low intensity activity is associated with lower odds of new and persistent SUI.6–9
The teenage years may constitute a particularly vulnerable time during which strenuous activity may have a greater deleterious effect because of the musculoskeletal, hormonal and reproductive changes occurring in young women during that time.
Understanding how lifetime physical activity impacts SUI is important: roughly 10–20% of women between ages 40 and 80 report moderate or severe incontinence, and over half have symptoms of primarily SUI.10,11 Physical activity is a modifiable risk factor with the potential for both positive and negative effects on SUI. To date, studies examining this association have not assessed lifetime physical activity or included activities other than leisure.
The aims of this study were to estimate, in a population of middle-aged women without pelvic organ prolapse recruited from non-tertiary care settings, whether moderate to severe SUI is associated with overall lifetime activity (including leisure, household, outdoor, and occupational), lifetime leisure activity, lifetime strenuous activity and strenuous activity during the teen years.
METHODS
Local institutional review boards approved this study. All participants completed an informed consent process.
Research nurses recruited women for this study, as well as a separate case-control study exploring physical activity and pelvic organ prolapse (POP)12, from 17 primary care level gynecologic and family medicine clinics located across the Salt Lake Valley between 10/3/09 and 1/14/13. Women were also initially recruited from community advertising. Complete methods have been published.13
We excluded women that were pregnant or within six months postpartum, < 39 or > 65 years, had a body mass index (BMI) < 18.5 kg/m2 or ≥ 40 kg/m2, had prior surgical treatment for POP or urinary incontinence (UI), were not able to walk independently, had medical conditions associated with UI or low physical activity, those currently undergoing cancer treatment and those with moderate to severe urgency incontinence (score of ≥ 3 on the validated Incontinence Severity Index14 and either pure urgency incontinence, or mixed urgency predominant incontinence, based on the “3IQ” validated tool15). We chose our inclusion age range because the physical activity questionnaire used in this study (described below) was validated in women 39 to 65 years and because women in this range are both likely to have developed SUI and to maintain physical activity.
Trained research nurses performed the Pelvic Organ Prolapse Quantification (POP-Q) examination to assess vaginal support.16–18 Participants completed study instruments at home. An exercise science graduate student reviewed missing and improbable response with participants using an established protocol.
To assess lifetime physical activity, we used the self-administered and reliable Lifetime Physical Activity Questionnaire (LPAQ) which is designed for use in women, includes leisure (i.e., recreational) activity, outdoor work and housework, and assesses physical activity over four age periods, menarche to age 21 (the “teen” epoch), 22–34, 35–50, and 51–65 years.19,20 The LPAQ is scored using METs (metabolic equivalents) obtained from the Compendium of Physical Activities21 to calculate MET hours per week. As the LPAQ does not include occupational activity, we added the Occupation Questionnaire (OQ), a component of the Lifetime Overall Physical Activity Questionnaire.22
To calculate overall lifetime physical activity, we multiplied each activity’s MET score by the reported number of hours/week, fraction of months/year, and fraction of years lived in each age epoch, and added the average MET hours/week calculated on the Occupation Questionnaire. Overall leisure activity included only activities related to traditional exercise and recreation. Strenuous activities included those associated with repetitive impact and/or relatively higher intra-abdominal pressures.23 Vigorous activities included those with > 6 METs.
The LPAQ+OQ was considered insufficient for analysis if women recorded no physical activity of any type for an entire age epoch, no leisure or household activity over the entire LPAQ, overall physical activity exceeding 168 hours/week, or exceeded 671 MET hours/week in any age epoch.24
From the initial participant pool, we excluded women with POP (vaginal descent at or below the hymen) 25,26 and those whose activity questionnaires were either unreturned or of insufficient quality.
Cases had moderate/severe urinary incontinence defined as a score of ≥ 3 on the Incontinence Severity Index (which correlates well with incontinence severity according to pad weight, bladder diary and quality of life instruments) 14,27 and pure or predominantly SUI according to responses on the 3-IQ15; controls had no or mild UI defined as a score of ≤ 2 on the Incontinence Severity Index and also had no POP. Research nurses obtaining outcome measures were masked to LPAQ + OQ results, and exercise science researchers were masked to group assignment.
The sample size of at least 175 cases and 175 controls was calculated a priori to provide 80% power at the 2-sided 5% significance level to detect an odds ratio of 0.295 for a 1 SD increase in actual physical activity, taking measurement error into account.28 This sample size also had 80% power at the 2-sided 5% significance level to detect the scenario in which the odds ratio is nonlinear in quintiles of physical activity based on the control group, with an inverted U-shape represented by a distribution of cases from lowest to highest quintile of 10 to 30%, or more extreme.28 Computations used nQuery v 6.0 software.
We planned a priori to frequency match controls and cases for age, BMI and recruitment source. However, because we recruited only 13 cases from community advertising, but 213 from primary care clinics, we excluded community participants from our final models, as low cell sizes would preclude analysis. Additionally, before beginning data analysis, we elected not to frequency match or adjust for BMI, as two prospective cohort studies published after the start of our study reasoned that lifetime PA ‘causes’ BMI.29,30 Thus, BMI is on the direct pathway between lifetime activity and SUI and is an effect of lifetime PA; including BMI could eliminate the association of activity with SUI by overadjustment.31
We frequency matched controls to cases 1:1 by age (39–49, 50–60, 61–65 years), and selected controls using a computerized random number generator when more than 1 was eligible.
We grouped physical activity variables into quintiles based on their distribution in the selected control group, assigning the 2nd quintile as the reference such that we could investigate the potential deleterious effect of low activity32. We performed logistic regression with variable selection guided by an updated directed acyclic graph (DAG), in which BMI was depicted as an intermediate variable, developed using DAGitty version 2.0.33,34 We adjusted for education and age and further adjusted for number of vaginal deliveries and hysterectomy status, based on past literature, which was permissible per the DAG. Regression diagnostics were checked for multicollinearity and influential observations. We examined the functional form of the relationship between each physical activity variable and SUI by inspecting plots of initial regression coefficients and using the Stata multivariable fractional polynomials procedure to identify the best polynomial fit. In the event of a non-linear, non-polynomial pattern, such as a threshold effect, we pooled quintiles with common odds ratios based on the Akaike Information Criterion (AIC). As there were only two missing observations in the dataset, we did not perform multiple imputation. As sensitivity analyses, we re-estimated odds ratios using simulation-extrapolation (SIMEX),35 with bootstrapped standard errors to adjust for measurement error and also re-estimated odds ratios comparing cases to controls with no UI (that is, Incontinence Severity Index score of 0).
We generally used a 5% significance level, but considered tests for individual quintiles versus the reference category to be significant if p<0.01, to adjust for multiple comparisons. All statistical programming calculations were verified by a second independent research team member. Analysis was performed using SAS 9.3 and the multivariable fractional polynomial and simulation extrapolation procedures in Stata 11 and 12.
RESULTS
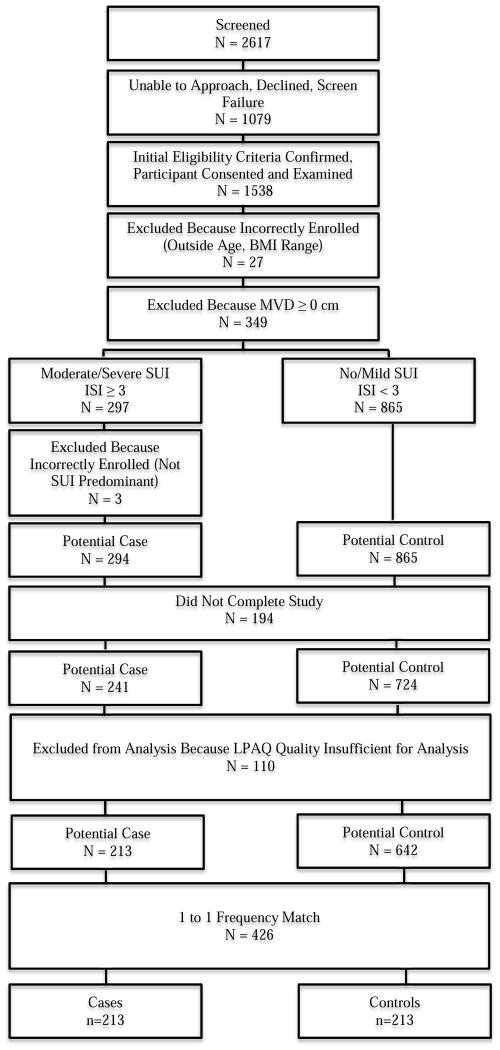
From the primary care clinics, 1538 women met initial screening criteria and were enrolled; an additional 72 were enrolled through community advertising. The participant flow for the primary care clinic population is summarized in Figure 1. After applying exclusion criteria, there were 213 potential cases and 642 potential controls from primary care; and 13 and 31, from the community. All cases were successfully frequency matched by age group, resulting in a primary care sample of 213 cases and 213 controls; and a community sample of 13 cases and 13 controls. There were no differences in demographic characteristics between women with sufficient vs insufficient LPAQ quality (p>0.05, data not shown). Women enrolled through community advertising were of similar age, BMI and race/ethnicity as those enrolled through primary care clinics (p>0.05, data not shown).
Figure 1.
Participant flow diagram for primary care recruitment population
Participant characteristics are summarized in Table 1. Participants had mean (SD) age of 50(7) years. Compared to controls, SUI cases were more likely to have delivered vaginally, be overweight or obese, and to report lower health status. Among controls, the Incontinence Severity Index scores were 0 in 111 (49.1%), 1 in 58 (25.7%) and 2 in 57 (25.2%) women.
Table 1.
Characteristics of Cases and Controls
| Control (N=226) | SUI Case (N=226) | Univariate OR (95% CI) | ||
|---|---|---|---|---|
| Age, years | Mean (SD) | 49.76 (7.03) | 49.69 (7.09) | NA |
| 39 to 49 | N | 121 | 121 | |
| 50 to 60 | N | 83 | 83 | |
| 61 to 65 | N | 22 | 22 | |
|
| ||||
| Body Mass Index, kg/m2 | Mean (SD) | 25.61 (5.11) | 27.09 (4.92) | 1.1 (1.0, 1.1) |
| 18.5 to 24.9 | N (%) | 130 (57.52) | 95 (42.04) | 1.0 |
| 25 to 29.9 | N (%) | 52 (23.01) | 64 (28.32) | 1.7 (1.1, 2.6) |
| 30 to 39.9 | N (%) | 44 (19.47) | 67 (29.65) | 2.1 (1.3, 3.3) |
|
| ||||
| Parity | Median (range) | 2 (0, 8) | 2 (0,12) | 1.2 (1.0, 1.3) |
| 0 | N (%) | 63 (28.00) | 39 (17.26) | 1.0 |
| 1 | N (%) | 33 (14.67) | 39 (17.26) | 1.9 (1.0, 3.5) |
| 2 | N (%) | 67 (29.78) | 74 (32.74) | 1.8 (1.1, 3.0) |
| 3+ | N (%) | 62 (27.56) | 74 (32.74) | 1.9 (1.1, 3.3) |
| Missing | N | 1 | 0 | |
|
| ||||
| No. of Vaginal Deliveries | Median (range) | 1 (0, 8) | 2 (0, 12) | 1.2 (1.1, 1.3) |
| 0 | N (%) | 97 (43.11) | 62 (27.43) | 1.0 |
| 1 | N (%) | 30 (13.33) | 38 (16.81) | 2.0 (1.1, 3.5) |
| 2 | N (%) | 49 (21.78) | 59 (26.11) | 1.9 (1.1, 3.1) |
| 3+ | N (%) | 49 (21.78) | 67 (29.65) | 2.1 (1.3, 3.5) |
| Missing | N | 1 | 0 | |
|
| ||||
| No. of Cesarean Deliveries | Median (range) | 0 (0, 6) | 0 (0, 6) | 0.8 (0.6, 1.1) |
| 0 | N (%) | 174 (77.33) | 189 (83.63) | 1.0 |
| 1 | N (%) | 28 (12.44) | 22 (9.73) | 0.7 (0.4, 1.3) |
| 2 | N (%) | 16 (7.11) | 11 (4.87) | 0.6 (0.3, 1.4) |
| 3+ | N (%) | 7 (3.11) | 4 (1.77) | 0.5 (0.2, 1.8) |
| Missing | N | 1 | 0 | |
|
| ||||
| Race | NA | |||
| Asian | N (%) | 7 (3.14) | 2 (0.90) | |
| Black/African American | N (%) | 0 (0) | 3 (1.35) | |
| Hawaiian/Pacific Islander | N (%) | 1 (0.45) | 0 (0) | |
| Am. Indian/Alaskan Native | N (%) | 2 (0.90) | 5 (2.25) | |
| Caucasian | N (%) | 213 (95.52) | 212 (95.50) | |
| Missing | N | 3 | 4 | |
|
| ||||
| Ethnicity | ||||
| Non-Hispanic | N (%) | 219 (97.77) | 210 (93.33) | 1.0 |
| Hispanic | N (%) | 5 (2.23) | 15 (6.67) | 3.1 (1.1, 8.8) |
| Missing | N | 2 | 1 | |
|
| ||||
| Education | ||||
| High School or Less | N (%) | 24 (10.62) | 23 (10.18) | 1.0 |
| Some College or College Grad. | N (%) | 127 (56.19) | 135 (59.73) | 1.1 (0.6, 2.1) |
| Grad./Professional Degree | N (%) | 75 (33.19) | 68 (30.09) | 0.9 (0.5, 1.8) |
|
| ||||
| Current Smoker | N (%) | 9 (3.98) | 12 (5.31) | 1.4 (0.6, 3.3) |
|
| ||||
| Caffeine consumption | ||||
| < once per month | N (%) | 37 (16.37) | 35 (15.56) | 1.0 |
| Between monthly and daily | N (%) | 31 (13.72) | 33 (14.67) | 1.1 (0.6, 2.2) |
| 1–3 times per day | N (%) | 138 (61.06) | 118 (52.44) | 0.9 (0.5, 1.5) |
| > 3 times per day | N (%) | 20 (8.85) | 39 (17.33) | 2.1 (1.0, 4.2) |
| Missing | N | 0 | 1 | |
|
| ||||
| Prior hysterectomy | N (%) | 25 (11.06) | 31 (13.78) | 1.3 (0.7, 2.3) |
|
| ||||
| Menopausal Status | ||||
| Premenopausal | N (%) | 134 (61.75) | 140 (63.06) | 1.0 |
| Postmenopausal | N (%) | 83 (38.25) | 82 (36.94) | 0.9 (0.6, 1.4) |
| Missing | N | 9 | 4 | |
|
| ||||
| Medical Conditions | ||||
| Seasonal allergy | N (%) | 78 (34.51) | 95 (42.04) | 1.4 (0.9, 2.0) |
| Arthritis | N (%) | 36(15.93) | 39 (17.26) | 1.1 (0.7, 1.8) |
| Hypertension | N (%) | 25 (11.06) | 34 (15.04) | 1.4 (0.8, 2.5) |
| Major depression | N (%) | 17 (7.52) | 26 (11.50) | 1.6 (0.8, 3.0) |
| Cancer history | N (%) | 24 (10.62) | 18 (7.96) | 0.7 (0.4, 1.4) |
| Sleep apnea | N (%) | 7 (3.10) | 9 (3.98) | 1.3 (0.5, 3.5) |
| Diabetes | N (%) | 7 (3.10) | 6 (2.65) | 0.9 (0.3, 2.6) |
| Chronic cough | N (%) | 2 (o.88) | 4 (1.77) | 2.0 (0.4, 11.1) |
| Myocardial Ischemia | N (%) | 1 (0.44) | 1 (0.44) | 1.0 (0.1, 16.1) |
|
| ||||
| Current # of Prescription Medications | Median (range) | 1 (0, 9) | 1 (0, 12) | 1.1 (1.0, 1.2) |
| 0 | N (%) | 76 (33.93) | 77 (34.22) | 1.0 |
| 1 | N (%) | 65 (29.02) | 50 (22.22) | 0.9 (0.7, 1.3) |
| 2 | N (%) | 31 (13.8) | 46 (20.4) | 0.9 (0.6, 1.3) |
| 3 | N (%) | 20 (8.93) | 13 (5.78) | 0.9 (0.6, 1.5) |
| 4 | N (%) | 12 (5.36) | 16 (7.11) | 0.7 (0.4, 1.2) |
| 5+ | N (%) | 20 (8.93) | 23 (10.22) | 0.6 (0.4, 0.9) |
| Missing | N | 2 | 1 | |
|
| ||||
| Self-reported health | ||||
| Excellent | N (%) | 72 (31.86) | 49 (21.68) | 1.0 |
| Very good | N (%) | 105 (46.46) | 113 (50.00) | 1.6 (1.0, 2.5) |
| Good | N (%) | 47 (20.80) | 51 (22.57) | 1.6 (0.9, 2.7) |
| Fair | N (%) | 2 (0.88) | 13 (5.75) | 9.5 (2.0, 44.1) |
|
| ||||
| Sites | NA | |||
| Primary Care | N (%) | 213 (94.25) | 213 (94.25) | |
| Community | N (%) | 13 (5.75) | 13 (5.75) | |
Results for Primary Exposures
Table 2 summarizes our results. Overall lifetime activity was associated with slightly increased SUI odds SUI (OR 1.20 (1.02, 1.41), per 70 additional MET-hours/week, p=0.03) in multivariable analysis adjusted for frequency matched age categories, education, number of vaginal deliveries, and hysterectomy. As lifetime leisure activity demonstrated a non-linear, non-polynomial pattern, we compared quintiles of activity between cases and controls and found that compared to the 2nd quintile, the lowest (OR 0.53; 95% CI 0.29, 0.98) and third (OR 0.3; 95% CI 0.2, 0.6), but not fourth, quintiles incurred about half the odds of SUI in adjusted analyses (p-value across all quintiles, p=0.0092).
Table 2.
Odds of SUI by physical activity measure
| Physical activity measure | Age adjusted crude OR(95% CI) | Multivariable Adjusted OR (95% CI)* | Multivariable OR (95% CI) adjusted for measurement error |
|---|---|---|---|
|
| |||
| Primary exposures | |||
|
| |||
| Overall lifetime activity (units = 70 MET-hrs/wk1) | 1.206 (1.029, 1.413) P=0.0205 |
1.196 (1.017, 1.407) P=0.0307 |
1.321 (1.017, 1.714) P=0.037 |
|
| |||
| Lifetime leisure activity** | P=0.0070 | P=0.0092 | N/A |
| Quintile 1 | 0.508 (0.282, 0.916) | 0.534 (0.291, 0.980) | |
| Quintile 2 | 1 | 1 | |
| Quintile 3 | 0.302 (0.158, 0.578) | 0.300 (0.155, 0.583) | |
| Quintile 4 | 0.621 (0.351, 1.097) | 0.706 (0.392, 1.271) | |
| Quintile 5 | 0.592 (0.334, 1.046) | 0.653 (0.363, 1.175) | |
|
| |||
| Lifetime strenuous activity (units=7 hrs/wk2) | 1.113 (0.998, 1.241) P=0.0545 |
1.112 (0.994, 1.244) P=0.0628 |
1.161 (0.946, 1.424) P=0.152 |
|
| |||
| Teen epoch*** strenuous activity (units=7 hrs/wk2) | 1.356 (1.091, 1.685) P=0.0061 |
1.367 (1.094, 1.709) P=0.0061 |
1.750 (1.153, 2.657) P=0.009 |
|
| |||
| Secondary exposures | |||
|
| |||
| Lifetime vigorous activity (activities with >6 METs; units=7 hrs/wk3) | 0.860 (0.517,1.428) P=0.5590 |
0.891 (0.528, 1.504) P = 0.6664 |
0.818 (0.310, 2.163) P=0.686 |
|
| |||
| Past year overall activity | 1.085 (0.864, 1.362) P=0.4821 |
1.072 (0.850, 1.352) P=0.5573 |
1.124 (0.739, 1.709) P=0.583 |
|
| |||
| Past year leisure activity (units=35 MET-hrs/wk4) | 0.806 (0.657, 0.990) P=0.0398 |
0.809 (0.654, 1.001) P=0.0515 |
0.684 (0.444, 1.051) P=0.083 |
|
| |||
| Past year strenuous activity (units=7 hrs/wk2) | 1.235 (0.990, 1.541) P=0.0618 |
1.227 (0.981, 1.534) P=0.0727 |
1.364 (0.940, 1.980) P=0.101 |
adjusted for age, education, hysterectomy, vaginal deliveries
Unable to calculate OR based on continuous measure, as this variable demonstrates non-linear relationship with SUI
menarche to age 21 years
70 units is equivalent to an increase of 10 MET-hrs per day for each day of the week (for example, running at 10 minutes per mile pace for one extra hour per day or doing child care for 3.5 extra hours per day each day of the week)
7 units is equivalent to an increase of 1 strenuous hour per day for each day of the week (for example, doing calisthenics such as push-ups, sit-ups, pull-ups and lunges for one extra hour per day)
7 units is equivalent to an increase of 1 hour per day spent doing activities with >6 METs (for example, riding a road bicycle at 14 – 15.9 mph for one extra hour per day)
35 units is equivalent to an increase of 5 MET-hours per day for each day of the week (for example, playing doubles tennis for one extra hour per day)
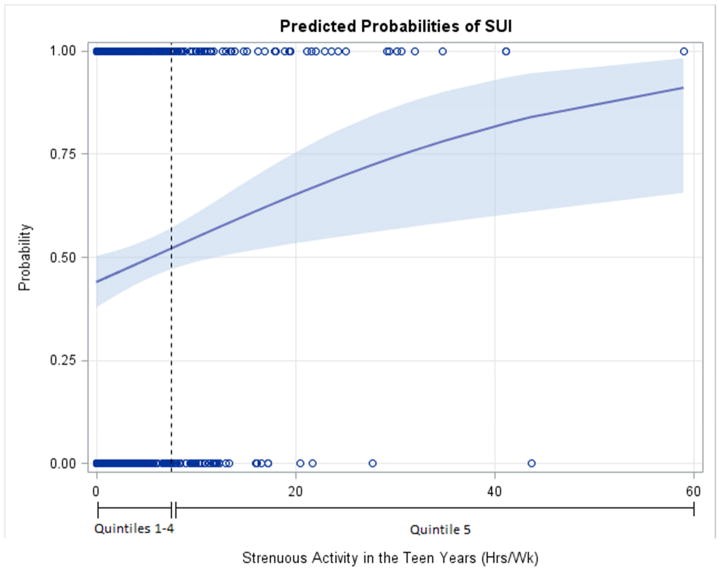
The odds of SUI were not associated with lifetime strenuous hours/week (OR 1.11 per 7 additional hours/week; 95% CI 0.99, 1.25, p=0.06). However, increasing strenuous activity in the teen years was associated with modestly increased odds of SUI (OR 1.37 per 7 additional hours/week (95% CI 1.09, 1.71), p=.006) in multi-variable analysis. The predicted probability of SUI rose linearly in women exceeding 7.5 hours of strenuous activity/week during teen years (the top quintile; Figure 2.) Teen strenuous activity had a similar effect on SUI odds when adjusted for subsequent strenuous activity during ages 21–65 years, while subsequent strenuous activity adjusted for teen strenuous activity was not associated with SUI (OR 1.02; 95% CI 0.90, 1.15).
Figure 2.
Predicted probabilities of stress urinary incontinence by strenuous activity in the teen years. The shaded area indicates 95% CI.
Results for Secondary Exposures
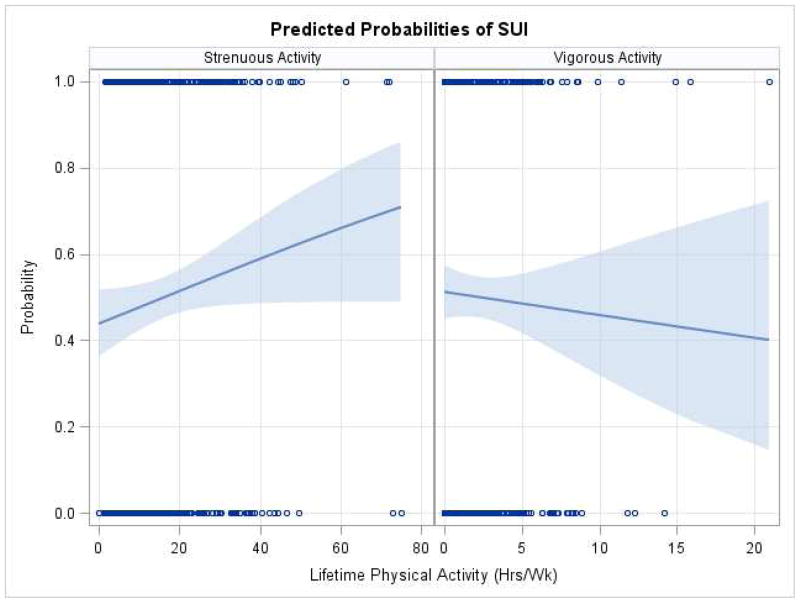
Lifetime vigorous activity showed a threshold effect: women in quintiles 2 through 5 had nearly half the odds of SUI compared to women in the lowest quintile of vigorous activity (adjusted OR=0.55; 95% CI 0.34, 0.88; p=0.01.) Figure 3 demonstrates the contradictory trends observed for the predicted probabilities of SUI for lifetime strenuous and lifetime vigorous activity.
Figure 3.
Predicted probabilities of stress urinary incontinence by lifetime strenuous and lifetime vigorous activity. The shaded area indicates 95% CI.
There were no significant associations between the odds of SUI and total past year activity or strenuous activity during the past year. We found a protective effect of higher levels of leisure activity during the past year on SUI (OR 0.6 for quintiles 4 and 5 vs. quintiles 1–3; 95% CI 0.43 0.97; p=0.04).
The direction of associations did not change following simulation extrapolation to adjust for measurement error, though the odds of SUI associated with teen strenuous activity were greater (OR 1.75 (1.15, 2.66), p=0.009 for a 7-unit increase). We found no interaction effect of vaginal delivery (0 vs 1+) on these associations. Adjusting for BMI did not affect results. Finally, the direction of associations was similar when comparing cases to controls with no UI (Incontinence Severity Index score of 0): adjusted ORs were 1.19 (0.97, 1.49) for overall lifetime activity, 1.13 (1.00, 1.30) for lifetime strenuous activity, 1.31 (1.00, 1.72) for teen strenuous activity and again non-linear with similar odds ratios for lifetime leisure activity.
DISCUSSION
In this study, overall lifetime physical activity was associated with modest increases in SUI odds, while lifetime leisure activity decreased and lifetime strenuous activity appeared unrelated to SUI odds. However, the modest increased odds of SUI was noted only after a substantial increase in lifetime physical activity: an increased odds of SUI of 1.20 per 70 additional MET-hours/week means that a woman would need to do the equivalent of 10 MET-hours per day for each day of the week (e.g., running at 10 minutes/mile pace for one extra hour/day or doing child care for 3.5 extra hours/day) to reach even this modest increase.
Greater strenuous activity during the teen years, even accounting for subsequent strenuous activity, was associated with modest increases in SUI odds in later life. Lifetime strenuous activity tended to increase, and lifetime vigorous activity decrease, SUI odds, suggesting that these two variables measure different entities.
Similar to others, we found that current leisure activity decreased the odds of SUI7,36 While a cross-sectional study is unable to differentiate whether women exercise because they are not incontinent or whether regular exercise prevents incontinence, Danforth et al., in a prospective 12-year analysis of the Nurse’s Health Study confirmed that moderate activity, mainly walking, protected women from developing UI and from having persistent UI.8,9 This may in part be mitigated by weight. In a Finnish twin study, persistent physical activity participation was associated with decreased rate of weight gain over a lifetime; this in turn decreases the odds of SUI.29,37
In contrast to a large body of literature demonstrating that SUI amongst young women during high-impact activity is common1–4, there are very few studies investigating whether strenuous activity while young increases the odds of SUI later in life. In one, former Norwegian athletes were not more likely to report SUI in mid-life compared to controls, while in another, former U.S. Olympians participating in high-impact sports had similar prevalence rates of SUI 25 years later as those participating in swimming s. 38,39 However, these studies also differed in their methods. In the first, cases were between 13–39 years while representing the senior and junior national teams from 38 different sports, while in the second, all women were performing at much higher levels than most athletes and there was no less active control group.
Theoretically, strenuous exercise at younger ages may impact the pelvic floor differently. For example, women engaged in competitive trampoline jumping between mean ages of 11 and 15 years were queried 5–10 years after they stopped the sport; both duration and frequency of trampoline jumping independently increased the odds of current urinary leakage by about 3-fold.40
We previously reported a marginally significant nonlinear relationship between teen strenuous activity and POP with an increase in the log-odds of POP for women reporting ≥ 21 hours/week of strenuous activity.12 The teen years may represent a particularly vulnerable time period, given the dramatic changes in hormones, muscle and bone structure and weight. Given increased risk for connective tissue injury during adolescence in girls,41 it is biologically plausible that high strenuous activity during this period may affect future pelvic floor function.
Similar to others, we found a modest association between parity, or vaginal deliveries, and UI with little additional risk conferred after the first delivery.42–44
Strengths of this study include the recruitment of participants from primary care clinics, rather than from women seeking care for SUI in tertiary care clinics, assessing lifetime activity using a tool validated in middle-aged women, and considering all aspects of physical activity, rather than leisure alone. Further, given the overlap between SUI and POP but the potential for differences in risk factors, we excluded women with vaginal descent at or below the hymen. Our study is most limited by its case-control design---however, a randomized trial assigning girls and women to long-term low versus high levels of activity is impossible, and indeed, unethical. A prospective cohort study in women followed over a lifetime would be difficult to execute. Therefore, we resorted to a case-control design to study the effect of lifetime physical activity on SUI in women currently middle-aged. Our population is largely Caucasian and relatively healthy and may not be generalizable to other populations and our findings may not apply to women with both SUI and POP. Because of the inaccuracy of recall of obstetric events, other than type of delivery, we did not ask more focused questions about childbirth history. 45 While middle-aged women demonstrate good reproducibility in recalling activity during the teen years19, the accuracy of this recall cannot be confirmed. However, by including women not seeking care for SUI who were also masked to the primary goals of the study, it is likely that potential bias was non-differential. In categorizing controls, we grouped Incontinence Severity Index scores of 0–2 together because sporadic, mild incontinence that waxes and wanes is common in women and generally not bothersome. There is large variation in annual incidence rates when UI is defined broadly (‘any’ or ‘monthly’) with ranges from 0.9% to 18.8% and remission rates of 1.2%–42% but these rates are more stable for more frequent UI (1.2–4% annual incidence rates), supporting our case designation. 46,47 Sensitivity analysis comparing cases with only controls with no UI yielded similar directions of associations. Finally, it is possible that excluding women with prior SUI surgery may have biased our results towards the null hypothesis.
The relationship between physical activity and SUI is complicated. Sports that are associated with repetitive jumping or bouncing are associated with exercise-provoked SUI. As UI becomes more severe, women are more likely to curtail physical activity to avoid this leakage trigger. However, even in women with severe UI, only one-third reduced or stopped exercise because of UI.48 It is unlikely that a woman who leaks during strenuous activity could voluntarily stop all domains, including household, occupation, outdoor work and leisure. While stopping all strenuous activity because of UI would bias our conclusion towards the null hypothesis, stopping some strenuous activity might attenuate the strength of the association but would be unlikely to cancel it out.
Given the many beneficial health effects of physical activity, it is reassuring that our results suggest that overall, lifetime physical activity whether leisure or strenuous, increases odds of SUI either modestly or not at all. However, our finding that the highest levels of strenuous activity during the teen years increase the odds of SUI is concerning. However, this finding needs to be replicated using other populations and study designs before using this information in clinical decision making. Finally, our results should not be used to counsel women at potentially higher risk for SUI, such as those immediately following childbirth or pelvic floor surgery.
Acknowledgments
Grant support acknowledgement: The project described was supported by Grant Number R01HD057895-01 from the Eunice Kennedy Schriver National Institute of Child Health and Human Development and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764).
We gratefully acknowledge the assistance of Wei Chen, PhD, MStat, Chang-Chung Chou, MStat, and Khanh K. Thai, MStat for data analysis.
Footnotes
Reprints will not be available
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Disclosures: The authors report no financial disclosures.
Condensation
In middle-aged women, overall lifetime physical activity was associated with slight increases in stress urinary incontinence (SUI) odds, while lifetime recreational activity decreased SUI odds.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ingrid E. NYGAARD, Email: Ingrid.nygaard@hsc.utah.edu, Professor, Department of Obstetrics and Gynecology, University of Utah.
Janet M. SHAW, Email: Janet.Shaw@health.utah.edu, Associate Professor, Department of Exercise and Sport Science, University of Utah.
Tyler BARDSLEY, Email: Tyler.bardsley@hsc.utah.edu, Department of Obstetrics and Gynecology, University of Utah.
Marlene J. EGGER, Email: Marlene.egger@hsc.utah.edu, Professor, Department of Family and Preventive Medicine, University of Utah.
References
- 1.Nygaard IE, Thompson FL, Svengalis SL, Albright JP. Urinary incontinence in elite nulliparous athletes. Obstet Gynecol. 1994;84(2):183–187. [PubMed] [Google Scholar]
- 2.Bo K, Borgen JS. Prevalence of stress and urge urinary incontinence in elite athletes and controls. Med Sci Sports Exerc. 2001;33(11):1797–1802. doi: 10.1097/00005768-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Thyssen HH, Clevin L, Olesen S, Lose G. Urinary incontinence in elite female athletes and dancers. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13(1):15–17. doi: 10.1007/s001920200003. [DOI] [PubMed] [Google Scholar]
- 4.Fozzatti C, Riccetto C, Herrmann V, et al. Prevalence study of stress urinary incontinence in women who perform high-impact exercises. Int Urogynecol J. 2012;23(12):1687–1691. doi: 10.1007/s00192-012-1786-z. [DOI] [PubMed] [Google Scholar]
- 5.Eliasson K, Larsson T, Mattsson E. Prevalence of stress incontinence in nulliparous elite trampolinists. Scand J Med Sci Sports. 2002;12(2):106–110. doi: 10.1034/j.1600-0838.2002.120207.x. [DOI] [PubMed] [Google Scholar]
- 6.Townsend MK, Danforth KN, Lifford KL, et al. Incidence and remission of urinary incontinence in middle-aged women. Am J Obstet Gynecol. 2007;197(2):167 e161–165. doi: 10.1016/j.ajog.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu L, Lang J, Wang H, Han S, Huang J. The prevalence of and potential risk factors for female urinary incontinence in Beijing, China. Menopause. 2008;15(3):566–569. doi: 10.1097/gme.0b013e31816054ac. [DOI] [PubMed] [Google Scholar]
- 8.Danforth KN, Shah AD, Townsend MK, et al. Physical activity and urinary incontinence among healthy, older women. Obstet Gynecol. 2007;109(3):721–727. doi: 10.1097/01.AOG.0000255973.92450.24. [DOI] [PubMed] [Google Scholar]
- 9.Devore EE, Minassian VA, Grodstein F. Factors associated with persistent urinary incontinence. Am J Obstet Gynecol. 2013;209(2):145, e141–146. doi: 10.1016/j.ajog.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunskaar S, Burgio K, Diokno A, Herzog AR, Hjälmås K, Lapitan MC. Epidemiology and natural history of urinary incontinence in women. Urology. 2003;62(4 Suppl 1):16–23. doi: 10.1016/s0090-4295(03)00755-6. [DOI] [PubMed] [Google Scholar]
- 12.Nygaard IE, Shaw JM, Bardsley T, Egger MJ. Lifetime physical activity and pelvic organ prolapse in middle-aged women. Am J Obstet Gynecol. 2014;210(5):477.e471–477.e412. doi: 10.1016/j.ajog.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nygaard I, Shaw J, Egger MJ. Exploring the association between lifetime physical activity and pelvic floor disorders: study and design challenges. Contemp Clin Trials. 2012;33(4):819–827. doi: 10.1016/j.cct.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandvik H, Seim A, Vanvik A, Hunskaar S. A severity index for epidemiological surveys of female urinary incontinence: comparison with 48-hour pad-weighing tests. Neurourol Urodyn. 2000;19(2):137–145. doi: 10.1002/(sici)1520-6777(2000)19:2<137::aid-nau4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Brown JS, Bradley CS, Subak LL, et al. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144(10):715–723. doi: 10.7326/0003-4819-144-10-200605160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobak WH, Rosenberger K, Walters MD. Interobserver variation in the assessment of pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7(3):121–124. doi: 10.1007/BF01894199. [DOI] [PubMed] [Google Scholar]
- 17.Hall AF, Theofrastous JP, Cundiff GW, et al. Interobserver and intraobserver reliability of the proposed International Continence Society, Society of Gynecologic Surgeons, and American Urogynecologic Society pelvic organ prolapse classification system. Am J Obstet Gynecol. 1996;175(6):1467–1470. doi: 10.1016/s0002-9378(96)70091-1. discussion 1470-1461. [DOI] [PubMed] [Google Scholar]
- 18.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 19.Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155(3):282–289. doi: 10.1093/aje/155.3.282. [DOI] [PubMed] [Google Scholar]
- 20.Chasan-Taber L, Erickson JB, Nasca PC, Chasan-Taber S, Freedson PS. Validity and reproducibility of a physical activity questionnaire in women. Med Sci Sports Exerc. 2002;34(6):987–992. doi: 10.1097/00005768-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 22.Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. 1998;30(2):266–274. doi: 10.1097/00005768-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Nygaard IE, Shaw JM, Bardsley T, Egger MJ. Lifetime Physical Activity and Pelvic Organ Prolapse in Middle-Aged Women. Am J Obstet Gynecol. 2014 doi: 10.1016/j.ajog.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nygaard IE. Exploring the association between lifetime physical activity and pelvic floor disorders: study and design challenges. 2012 doi: 10.1016/j.cct.2012.04.001. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeLancey JO, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 26.Woodman PJ, Swift SE, O’Boyle AL, et al. Prevalence of severe pelvic organ prolapse in relation to job description and socioeconomic status: a multicenter cross-sectional study. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(4):340–345. doi: 10.1007/s00192-005-0009-2. [DOI] [PubMed] [Google Scholar]
- 27.Sandvik H, Espuna M, Hunskaar S. Validity of the incontinence severity index: comparison with pad-weighing tests. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(5):520–524. doi: 10.1007/s00192-005-0060-z. [DOI] [PubMed] [Google Scholar]
- 28.Tosteson TD, Buzas JS, Demidenko E, Karagas M. Power and sample size calculations for generalized regression models with covariate measurement error. Stat Med. 2003;22(7):1069–1082. doi: 10.1002/sim.1388. [DOI] [PubMed] [Google Scholar]
- 29.Waller K, Kaprio J, Kujala UM. Associations between long-term physical activity, waist circumference and weight gain: a 30-year longitudinal twin study. Int J Obes (Lond) 2008;32(2):353–361. doi: 10.1038/sj.ijo.0803692. [DOI] [PubMed] [Google Scholar]
- 30.Hankinson AL, Daviglus ML, Bouchard C, et al. Maintaining a high physical activity level over 20 years and weight gain. JAMA. 2010;304(23):2603–2610. doi: 10.1001/jama.2010.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner A, Dallongeville J, Haas B, et al. Sedentary behaviour, physical activity and dietary patterns are independently associated with the metabolic syndrome. Diabetes Metab. 2012;38(5):428–435. doi: 10.1016/j.diabet.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Sung VW. Reducing bias in pelvic floor disorders research: using directed acyclic graphs as an aid. Neurourol Urodyn. 2012;31(1):115–120. doi: 10.1002/nau.21183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 35.Cook J, Stefanski LA. A simulation extrapolation method for parametric measurement error models. J Amer Statistical Assoc. 1995;89:1314–1328. [Google Scholar]
- 36.Hannestad YS, Rortveit G, Daltveit AK, Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG. 2003;110(3):247–254. [PubMed] [Google Scholar]
- 37.Mishra GD, Hardy R, Cardozo L, Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women: results from a British prospective cohort. Int J Obes (Lond) 2008;32(9):1415–1422. doi: 10.1038/ijo.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bo K, Sundgot-Borgen J. Are former female elite athletes more likely to experience urinary incontinence later in life than non-athletes? Scand J Med Sci Sports. 2010;20(1):100–104. doi: 10.1111/j.1600-0838.2008.00871.x. [DOI] [PubMed] [Google Scholar]
- 39.Nygaard IE. Does prolonged high-impact activity contribute to later urinary incontinence? A retrospective cohort study of female Olympians. Obstet Gynecol. 1997;90(5):718–722. doi: 10.1016/S0029-7844(97)00436-5. [DOI] [PubMed] [Google Scholar]
- 40.Eliasson K, Edner A, Mattsson E. Urinary incontinence in very young and mostly nulliparous women with a history of regular organised high-impact trampoline training: occurrence and risk factors. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(5):687–696. doi: 10.1007/s00192-007-0508-4. [DOI] [PubMed] [Google Scholar]
- 41.Wild CY, Steele JR, Munro BJ. Why do girls sustain more anterior cruciate ligament injuries than boys?: a review of the changes in estrogen and musculoskeletal structure and function during puberty. Sports Med. 2012;42(9):733–749. doi: 10.1007/BF03262292. [DOI] [PubMed] [Google Scholar]
- 42.Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194(2):339–345. doi: 10.1016/j.ajog.2005.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sampselle CM, Harlow SD, Skurnick J, Brubaker L, Bondarenko I. Urinary incontinence predictors and life impact in ethnically diverse perimenopausal women. Obstet Gynecol. 2002;100(6):1230–1238. doi: 10.1016/s0029-7844(02)02241-x. [DOI] [PubMed] [Google Scholar]
- 44.Rortveit G, Hannestad YS, Daltveit AK, Hunskaar S. Age- and type-dependent effects of parity on urinary incontinence: the Norwegian EPINCONT study. Obstet Gynecol. 2001;98(6):1004–1010. doi: 10.1016/s0029-7844(01)01566-6. [DOI] [PubMed] [Google Scholar]
- 45.Elkadry E, Kenton K, White P, Creech S, Brubaker L. Do mothers remember key events during labor? Am J Obstet Gynecol. 2003;189(1):195–200. doi: 10.1067/mob.2003.371. [DOI] [PubMed] [Google Scholar]
- 46.Ebbesen MH, Hunskaar S, Rortveit G, Hannestad YS. Prevalence, incidence and remission of urinary incontinence in women: longitudinal data from the Norwegian HUNT study (EPINCONT) BMC Urol. 2013;13(1):27. doi: 10.1186/1471-2490-13-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Møller LA, Lose G, Jørgensen T. Incidence and remission rates of lower urinary tract symptoms at one year in women aged 40–60: longitudinal study. BMJ. 2000;320(7247):1429–1432. doi: 10.1136/bmj.320.7247.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nygaard I, Girts T, Fultz NH, Kinchen K, Pohl G, Sternfeld B. Is urinary incontinence a barrier to exercise in women? Obstet Gynecol. 2005;106(2):307–314. doi: 10.1097/01.AOG.0000168455.39156.0f. [DOI] [PubMed] [Google Scholar]