Abstract
Objective
To determine the balance of metabolism of free bisphenol A (BPA) to the inactive conjugate, BPA glucuronide, in neonates.
Study design
Free BPA and BPA glucuronide concentrations were measured in 78 urine samples collected between December 2012 and August 2013 from a cohort of 44 healthy full term (≥ 37 weeks’ gestation) neonates at two intervals (3 - 6 days and 7 - 27 days of age). A questionnaire was administered at the time of sample collection. Neonates recruited into the study were born in an urban, tertiary care hospital.
Results
Only BPA glucuronide was detected in the urine samples; concentrations ranged from <0.1 μg/L to 11.21 μg/L (median: 0.27 μg/L). Free BPA concentrations were below the limit of quantification of 0.1 μg/L. Age, but not sex or type of diet was significantly associated with urinary BPA glucuronide concentration (p=0.002).
Conclusions
Our results illustrate widespread BPA exposure in healthy full-term neonates and efficient conjugation of BPA to its readily excretable and biologically inactive form (BPA glucuronide) as early as 3 days of age. Factors other than type of diet may be important contributors to BPA exposure in neonates.
Keywords: endocrine disrupting chemical, environmental exposure, early life metabolism, postnatal development, biomarkers
Bisphenol A (BPA) is a high-production volume chemical present in food and drink can liners, dental sealants, medical equipment and cash register receipts.1 Detection of BPA in urine of 90% of the general population in countries around the world demonstrates widespread exposure,2-5 primarily through dietary consumption. BPA leaches into food from packaging, especially the linings of cans.6 Through its estrogenic properties, BPA may induce a variety of adverse outcomes, including developmental effects (on the brain, lung and reproductive organs), diabetes, obesity, cancer, and cardiovascular disease.7-15
Upon ingestion, BPA is metabolized into BPA glucuronide, a biologically inert form that is rapidly cleared in the urine (half-life < 6 hours).16 Glucuronidation of some compounds is limited in the newborn (eg, bilirubin), and this impairment has been hypothesized to slow clearance of BPA and increase serum and urine concentrations of free BPA in neonates.17-20
Sensitive and specific methods are available to separately quantify free BPA and BPA conjugates in human biological samples, but contamination of samples with BPA from background sources in the laboratory and in the field has hampered the use of urinary biomarkers to study BPA metabolism in humans.21
In a previous study, we measured urinary free BPA and BPA glucuronide concentrations in a small cohort of young infants using methods that reduce sample contamination.22 The data demonstrated universal BPA exposure in those infants, with free BPA concentrations below the limit of quantification (0.1 μg/L). Given the rapid increase in hepatic metabolism in the neonatal period,17, 18 we sought to assess changes in BPA glucuronidation through the first month of life. We hypothesized that less efficient BPA metabolism in the first week of the neonatal period would result in higher urine free BPA concentrations in neonates during the first week of life compared with the later part of the neonatal period.
Methods
Postpartum mothers and their healthy, full-term neonates (≥ 37 0/7 weeks gestation) were recruited during their hospitalization in the Full Term Nursery at the Johns Hopkins Hospital between December, 2012 and August, 2013. Newborns receiving pediatric primary care at the Johns Hopkins Harriet Lane Primary Care Clinic were eligible to participate. Babies were excluded if they were either large or small size for gestational age, were diagnosed with intrauterine growth restriction, had an APGAR score of less than 5 at 5 minutes of age, had delayed voiding or stooling (greater than 24 hours after birth), were admitted to the Neonatal Intensive Care Unit (NICU) for management of hyperbilirubinemia, or had certain specific risk factors for hyperbilirubinemia (eg, cephalohematoma, polycythemia). Infants born to mothers with documented tobacco use during pregnancy, a positive urine toxicology screen for cocaine, marijuana, heroin, or methadone at delivery, and/or anti-epileptic drug use in pregnancy were also excluded. Infants with hyperbilirubinemia not requiring a NICU admission or with blood type incompatibility with the mother (ABO or Rh) were included. Recruitment and follow up protocols were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, and participant mothers provided informed consent.
Collection of urine samples and administration of a questionnaire took place at the Harriet Lane Clinic by trained research staff during scheduled pediatric well visits at 3-6 days of age (Visit 1) and at 7-27 days of age (Visit 2). At each of the two appointments, urine samples were collected from each infant using BPA-free pediatric urine collection bags (U-Bag, Hollister, Inc., Libertyville, IL). All urine collection bags were purchased at the outset of the study and were from the same lot. The bags and other equipment used to handle and store samples were tested for the presence of BPA using a BPA-free synthetic urine mixture to generate quality control samples.23 All free BPA and BPA glucuronide concentrations in the quality control samples fell below the limit of quantification (0.1 μg/L). Urine samples were transported to the laboratory on ice within 3 hours of sample collection and were stored at −80°C until laboratory analysis.
Urine samples were analyzed using high performance liquid chromatography with tandem mass spectrometry (HPLC-MS/MS) following derivatization with dansyl chloride according to a previously modified published method.24 Modifications included direct measurement of BPA glucuronide, which eliminated the need for enzymatic hydrolysis or solvent extraction of the samples. D6-BPA glucuronide was added as an internal standard. In addition, the HPLC column was changed from a 1 mm ID C8 to a 2 mm ID C18, which also required a flow increase from 80 to 250 μl/min. The limit of quantitation (LOQ) was 0.1 μg/L for both free BPA and BPA glucuronide. Urine specific gravity was measured by handheld refractometer (Model: PAL 10-S, Atago, Bellview, WA).
Statistical Analyses
Statistical analysis was performed using STATA 10 (StataCorp, College Station, TX) and Excel 2010 (Microsoft, Redmond, WA). The contribution of potential sources of BPA to exposure in the study population was evaluated by multiple linear regression, performed using a generalized estimating equation with urinary BPA glucuronide as the dependent variable.25
Covariates in the model were age (dichotomous) and sex of the neonate, and a dietary variable for formula and/or breast milk intake. A uniform correlation structure was specified to account for correlation between measurements from the same individual (Spearman ρ=0.46). Prior to regression analysis, BPA glucuronide concentrations were normalized based on specific gravity (reference value: 1.003) and log-transformed.26 A Wald test was performed to determine the significance of the effect of dietary type. Sample concentrations below the LOQ were replaced by the LOQ divided by the square root of 2.
Results
Out of 66 eligible mothers and infants, 51 were enrolled into the study. At least one sample was collected from each of the 44 neonates who participated. Samples at Visit 1 and Visit 2 were collected from 34 participants. For five participants, a sample was collected at Visit 1, but not Visit 2, and for five others, a sample was collected at Visit 2, but not Visit 1. At the time of the first sample collection, 51% of the neonates in the study were fed exclusively formula, 28% were exclusively breast fed, and 21% were fed a combination of breast milk and formula (Table I). These diets remained relatively stable between the two visits, with only 3 infants who were exclusively breast fed at Visit 1 switching to either formula or a combination of breast milk and formula by Visit 2.
Table 1.
Population Characteristics
| Mother-Neonate Pairs1 | 44 |
| Sex of Neonate | |
| Male | 25 (57%) |
| Female | 19 (43%) |
| Maternal Race | |
| African-American | 43 (98%) |
| Hispanic | 1 (2%) |
| Age (days) | |
| Sample 1 | 4.3 (±1.0) |
| Sample 2 | 12.1 (±3.8) |
| Feeding Type (Visit 1; Visit2) | |
| Breast milk only | 11 (28%); 8 (21%) |
| Formula only | 20 (51%); 21 (54%) |
| Both | 8 (21%); 10 (25%) |
A total of 78 samples were collected from 44 neonates: 39 at the first time point (Visit 1) and 39 at the second time point (Visit 2).
Seven participants who enrolled in the study were lost to follow up, due mostly to late or early clinic arrival (when research staff was not present) or infant hospitalization. One participant elected to withdraw from the study for unspecified reasons.
Urinary Free BPA and BPA Glucuronide Concentrations
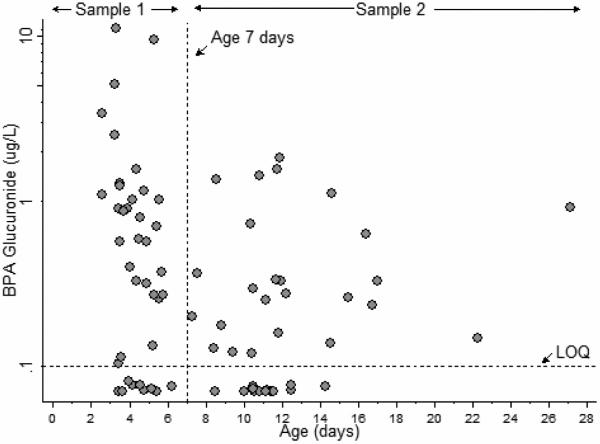
Free BPA concentrations were below the LOQ in all of the 78 urine samples collected (Table II and Figure 1). BPA glucuronide was quantifiable in 71% of the samples (77% at Visit 1, 64% at Visit 2) (Figure 2). Median BPA glucuronide concentrations at age 3-6 days were higher compared with those at age 7-27 days. Four outliers, the highest observed BPA glucuronide concentrations, 3.67, 4.41, 8.49 and 11.21 μg/L were all measured in samples collected at ages 3-6 days. BPA glucuronide concentrations in urine collected from these four individuals at the second visit (at ages 7-27 days) were < LOQ, 0.13, 0.80 and 0.61 μg/L, respectively.
Table 2.
Concentrations of Free BPA and BPA Glucuronide (BPAG) in the Urine of 44 Neonates (μg/L)
| Analyte | Age Group | N1 | Mean | SD | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|
| BPAG | 3-6 days | 39 | 1.2 | 2.3 | 0.10 | 0.49 | 1.09 | 11.21 |
| 7-27 days | 39 | 0.37 | 0.48 | <0.1 | 0.16 | 0.33 | 2.02 | |
| All Ages | 78 | 0.79 | 1.7 | <0.1 | 0.27 | 0.91 | 11.21 | |
| Free BPA | 3-6 days | 39 | <0.1 | - | <0.1 | <0.1 | <0.1 | <0.1 |
| 7-27 days | 39 | <0.1 | - | <0.1 | <0.1 | <0.1 | <0.1 | |
| All Ages | 78 | <0.1 | - | <0.1 | <0.1 | <0.1 | <0.1 | |
The populations at ages 3-6 (N=39) and at ages 7-27 (N=39) differ slightly; each includes 5 participants who are not in the other age group.
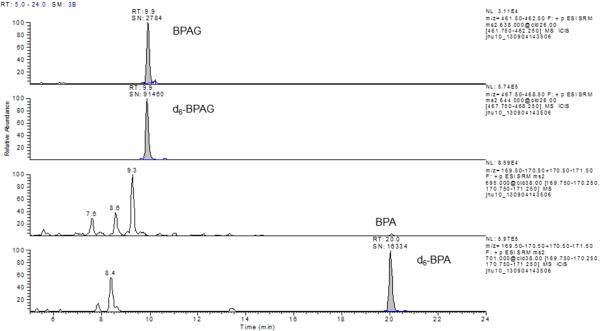
Figure 1.
Representative chromatogram for urine sample with a BPA glucuronide concentration of 0.8 μg/L (a) and 8.5 μg/L (b)
Figure 2.
Urinary BPA glucuronide concentrations in neonates, by age
Determinants of BPA Exposure
Urinary BPA glucuronide concentrations decreased with age, with the difference between the two age groups being statistically significant (p=0.002). Concentrations were higher in males (geometric mean [GM]: 0.36 μg/L) than females (GM: 0.24 μg/L), but the difference was not significant (p=0.133). Neonates fed exclusively breast milk had higher concentrations of BPA glucuronide in the urine (GM: 0.39 μg/L) than those fed either formula alone (GM: 0.32 μg/L) or a combination of breast milk and formula, (GM: 0.17 μg/L). After normalizing BPA glucuronide concentrations to specific gravity, neonates fed exclusively formula had the highest concentrations, followed by those fed exclusively breast milk and those fed a combination of formula and breast milk. However, both with and without specific gravity normalization, the differences in BPA glucuronide concentrations between the three feeding regimens were not statistically significant based on a Wald test with two degrees of freedom.
Discussion
In this study, we examined whether healthy full-term neonates efficiently form glucuronide conjugates of BPA. The question of the balance of free BPA to BPA glucuronide is critical to efforts to evaluate health risks from BPA exposure, because free BPA mimics estrogen and may have deleterious effects on developing neonates. Because of BPA's short half-life, urinary BPA glucuronide concentrations reflect recent exposure but also demonstrate effective conversion to a biologically inert form. We measured BPA glucuronide concentrations in 71% of urine samples collected at age 3-6 days and age 7-27 days. In every sample, free BPA concentrations fell below the LOQ of 0.1 μg/L. These findings demonstrate both widespread exposure to BPA and efficient glucuronidation during the neonatal period.
Although in vitro studies and animal model experiments have provided some insight into neonatal BPA metabolism, studies of BPA glucuronidation using human biomarkers have been sparse and hindered by analytical technology.27, 28 We previously reported urinary concentrations of BPA glucuronide in samples from 11 neonates and 1 young infant between 7 to 44 days of age; free BPA was below the LOQ in all 12 individuals as in the present study.22 BPA glucuronide concentrations were above the LOQ in 100% of the samples in the previous study but only 71% in this study, which could reflect a downward trend in BPA exposure in the population, possibly attributable to reduced use of BPA in consumer products, including baby bottles and formula packaging.29-31
Several studies have reported measurements of free and total BPA (i.e., the sum of free BPA and BPA glucuronide) in the urine of infants. Volkel et al demonstrated efficient glucuronidation in infants as early as 1 month of age.32 In another study of infants 2-15 months of age, free BPA was detected in 28% of urine samples, but study investigators speculated that free BPA in the urine may have resulted from sample contamination during sample collection and handling.33 In a study of premature infants in a NICU, free BPA was detected in 92% of samples, suggesting that poor glucuronidation capacity may impact free BPA internal dose and clearance in infants who are born preterm.34 Higher BPA levels among the preterm population due to BPA exposure (from NICU medical equipment) and possible developmental differences in glucuronidation activity between preterm and full-term neonates and infants may account for the difference between those data and our results.
Although our findings indicate widespread BPA exposure in our study population, the major sources of this exposure are unclear. BPA glucuronide concentrations were lower in neonates who were exclusively breast fed or drank a combination of breast milk and formula compared with those who drank formula only, but the difference was not significant. This finding is consistent with a report that diet did not contribute to urinary total BPA in infants in a NICU.35 Although BPA has been detected in both breast milk and liquid formula, powder formula is not a likely source of BPA exposure.36, 37 The presence of BPA glucuronide in 20 (80%) of samples from infants who drank exclusively powder formula was surprising and unexplained. Although used and mixed with powder formula by 88% of parents of these infants, commercially sold bottled water is not a known source of BPA exposure.38 Baby bottles were an unlikely source as most baby bottle manufacturers switched from polycarbonate to non-BPA-based materials in 2009.39 Study participants were asked about the age of the bottles and none reported using bottles purchased before 2009. Overall, our findings suggest that nondietary sources may contribute to BPA exposure in neonates.
Age was the only statistically significant determinant of BPA exposure, which may indicate a source of BPA exposure unique to the first week of the neonatal period, such as residual in utero exposure. However, correlation between measurements from the same individual also suggests a common postnatal source of exposure at both time points. The impact of age on BPA glucuronide concentration in our study may be attributable to lower fluid intake (i.e., more concentrated urine) among neonates in the younger age group. Of the four individuals with the highest BPA glucuronide concentrations at age 3-6 days of age, three were exclusively breast fed, indicating that maternal lactogenesis, which occurs over a period of days during the first week, likely impacted breast fed neonates’ fluid intake. 40,41 In our data analysis, we controlled for variability in fluid intake by normalizing BPA glucuronide concentrations based on specific gravity. However, specific gravity, like creatinine, is an imperfect measure of urine dilution in neonates.32
To avoid selection of a study population with an underlying predisposition toward efficient glucuronidation, we included neonates with hyperbilirubinemia not requiring admittance to the NICU in the study (though different isoforms of UDP-glucuronosyltransferase are responsible for bilirubin and BPA metabolism).42, 43 Of the neonates participating in this study, two required phototherapy for hyperbilirubinemia. BPA glucuronide was quantifiable in urine samples from both neonates. As in the rest of the study population, no free BPA was detected in the urine these neonates.
A particular strength of this study was the prevention of sample contamination during sample collection and laboratory analysis. In this study, because all free BPA concentrations fell below the LOQ and BPA glucuronide is not expected in the environment, we can confidently report that field contamination did not influence our results or conclusions. The use of repeated measurements in the same neonate further strengthens our data. Because measurements from the same individual were correlated, the use of the generalized estimating equation allowed us to make inferences about the contribution of age, sex and diet, to BPA glucuronide concentration despite a relatively small sample size.25, 44
Limits to the generalizability of this study include the restriction of our cohort to healthy newborns from one center. The prevalence of certain genetic variants for UGT2B15 (and other UGT isoforms that play a role in BPA metabolism) varies by race, and all but one mother in our cohort self-identified as African American.43, 45 Subsets of neonates, such as those born prematurely or with other biological predisposition toward poor glucuronidation (e.g., liver disease), could be less efficient at metabolizing BPA than the neonates in this study. In addition, these results may not apply to neonates who are more highly exposed to BPA, such as those spending time in a NICU.
Acknowledgments
We acknowledge Alison S. Geyh, PhD (Johns Hopkins Bloomberg School of Public Health, deceased), Barry Solomon, MD, MPH (Division of General Pediatrics and Adolescent Medicine), and Pamela Donohue, ScD (Eudowood Neonatal Pulmonary Division, Johns Hopkins University School of Medicine) for their contributions to the design of this study. In addition we thank the Pediatric Nurse Practitioners in the Full Term Nursery at the Johns Hopkins Hospital (Christa Bay, MSN, CPNP; Kristen Byrnes, MSN, CRNP; Krista Kline, CRNP; Carol Long, CRNP IBCLC; Krystina Mints, CPNP; Kimberly Alice Rice, MSN, CPNP; Suzanne Rubin, DNP, MPH, CRNP-P; Patricia H. Smouse, MSN, CPNP; Jo-Ann Swartz, MSN, CRNP) for screening patients for eligibility. We also thank the leadership and nursing staff of the Harriet Lane Clinic for their assistance.
Supported by the Johns Hopkins Center for a Livable Future Lerner Fellowship, the Wendy Klag Memorial Fund, and the National Institutes of Health (P01 ES006052, P30 ES003819, P30 CA006973, N01-CO-12400, and T32 ES007141). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government. The authors declare no conflicts of interest.
List of Abbreviations
- BPA
bisphenol A
- GM
geometric mean
- HPLC
high performance liquid chromatography
- LOQ
limit of quantitation
- NICU
neonatal intensive care unit
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No reprints will be requested by the authors.
References
- 1.European Commission Joint Research Center (Institute for Health and Consumer Protection) European union risk assessment report: Bisphenol-A. 2003 EUR 20843 EN. [Google Scholar]
- 2.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003-2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bushnik T, Haines D, Levallois P, Levesque J, Van Oostdam J, Viau C. Lead and bisphenol A concentrations in the canadian population. Health Rep. 2010;21:7–18. [PubMed] [Google Scholar]
- 4.Koch HM, Kolossa-Gehring M, Schroter-Kermani C, Angerer J, Bruning T. Bisphenol A in 24 h urine and plasma samples of the German environmental specimen bank from 1995 to 2009: A retrospective exposure evaluation. J Expo Sci Environ Epidemiol. 2012;22:610–616. doi: 10.1038/jes.2012.39. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Alomirah H, Cho HS, Li YF, Liao C, Minh TB, et al. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ Sci Technol. 2011;45:7044–7050. doi: 10.1021/es200976k. [DOI] [PubMed] [Google Scholar]
- 6.Carwile JL, Ye X, Zhou X, Calafat AM, Michels KB. Canned soup consumption and urinary bisphenol A: A randomized crossover trial. JAMA. 2011;306:2218–2220. doi: 10.1001/jama.2011.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 8.Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- 10.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 11.Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155:805–817. doi: 10.1210/en.2013-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinhouse C, Anderson OS, Bergin IL, Vandenbergh DJ, Gyekis JP, Dingman MA, et al. Dose-dependent incidence of hepatic tumors in adult mice following perinatal exposure to bisphenol A. Environ Health Perspect. 2014;122:485–491. doi: 10.1289/ehp.1307449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acevedo N, Davis B, Schaeberle CM, Sonnenschein C, Soto AM. Perinatally administered bisphenol A acts as a mammary gland carcinogen in rats. Environ Health Perspect. 2013;121:1040–1046. doi: 10.1289/ehp.1306734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spanier AJ, Fiorino EK, Trasande L. Bisphenol A exposure is associated with decreased lung function. J Pediatr. 2014;164:1403–8.e1.. doi: 10.1016/j.jpeds.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donohue KM, Miller RL, Perzanowski MS, Just AC, Hoepner LA, Arunajadai S, et al. Prenatal and postnatal bisphenol A exposure and asthma development among inner-city children. J Allergy Clin Immunol. 2013;131:736–742. doi: 10.1016/j.jaci.2012.12.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkel W, Colnot T, Csanady GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem Res Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 17.McCarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: Phase II conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002;300:361–366. doi: 10.1124/jpet.300.2.361. [DOI] [PubMed] [Google Scholar]
- 18.Divakaran K, Hines RN, McCarver DG. Human hepatic UGT2B15 developmental expression. Toxicol Sci. 2014;141:292–299. doi: 10.1093/toxsci/kfu126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edginton AN, Ritter L. Predicting plasma concentrations of bisphenol A in children younger than 2 years of age after typical feeding schedules, using a physiologically based toxicokinetic model. Environ Health Perspect. 2009;117:645–652. doi: 10.1289/ehp.0800073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mielke H, Gundert-Remy U. Bisphenol A levels in blood depend on age and exposure. Toxicol Lett. 2009;190:32–40. doi: 10.1016/j.toxlet.2009.06.861. [DOI] [PubMed] [Google Scholar]
- 21.Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environ Health Perspect. 2013;121:283–286. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachman RM, Fox SD, Golden WC, Sibinga E, Veenstra TD, Groopman JD, et al. Urinary free bisphenol A and bisphenol A-glucuronide concentrations in newborns. J Pediatr. 2013;162:870–872. doi: 10.1016/j.jpeds.2012.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Laboratory procedure manual (bisphenol A, urine): On line SPE-HPLC-MS/MS. 2005 [Google Scholar]
- 24.Fox SD, Falk RT, Veenstra TD, Issaq HJ. Quantitation of free and total bisphenol A in human urine using liquid chromatography-tandem mass spectrometry. J Sep Sci. 2011;34:1268–1274. doi: 10.1002/jssc.201100087. [DOI] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 26.Cone EJ, Caplan YH, Moser F, Robert T, Shelby MK, Black DL. Normalization of urinary drug concentrations with specific gravity and creatinine. J Anal Toxicol. 2009;33:1–7. doi: 10.1093/jat/33.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Nachman RM, Hartle JC, Lees PSJ, Groopman JD. Early life metabolism of bisphenol a: A systematic review of the literature. Curr Envir Health Rpt. 2014;1:90–100. doi: 10.1007/s40572-013-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Toxicology Program (Center for the Evaluation of Risks to Human Reproduction) NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. 2008;NIH Publication No. 08 – 5994. [Google Scholar]
- 29.77 FR 41899 (July 17, 2012) (abandonment of BPA use in baby bottles).
- 30.78 FR 41840 (July 12, 2013) (abandonment of BPA in formula packaging).
- 31.Wells J, Koontz Decline in urinary bisphenol A concentrations in the U.S. Epidemiology. 2013;24(1):167–8. doi: 10.1097/EDE.0b013e31827849b4. [DOI] [PubMed] [Google Scholar]
- 32.Volkel W, Kiranoglu M, Fromme H. Determination of free and total bisphenol A in urine of infants. Environ Res. 2011;111:143–148. doi: 10.1016/j.envres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Mendonca K, Hauser R, Calafat AM, Arbuckle TE, Duty SM. Bisphenol A concentrations in maternal breast milk and infant urine. Int Arch Occup Environ Health. 2012 doi: 10.1007/s00420-012-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, et al. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–644. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duty SM, Mendonca K, Hauser R, Calafat AM, Ye X, Meeker JD, et al. Potential sources of bisphenol A in the neonatal intensive care unit. Pediatrics. 2013;131:483–489. doi: 10.1542/peds.2012-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ackerman LK, Noonan GO, Heiserman WM, Roach JA, Limm W, Begley TH. Determination of bisphenol A in U.S. infant formulas: Updated methods and concentrations. J Agric Food Chem. 2010;58:2307–2313. doi: 10.1021/jf903959u. [DOI] [PubMed] [Google Scholar]
- 37.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:110–115. doi: 10.1016/j.jchromb.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 38.Health Canada Survey of bisphenol A in bottled water products. 2009 doi: 10.1080/02652030802563290. [DOI] [PubMed] [Google Scholar]
- 39.Layton L. No BPA for baby bottles in US: 6 makers announce decision on chemical. Washington Post; Mar 6, 2009. 2009 http://www.washingtonpost.com/wp-dyn/content/article/2009/03/05/AR2009030503285.html. [Google Scholar]
- 40.Neville MC, Morton J, Umemura S. Lactogenesis: The transition from pregnancy to lactation. Pediatr Clin North Am. 2001;48:35–52. doi: 10.1016/s0031-3955(05)70284-4. [DOI] [PubMed] [Google Scholar]
- 41.Benitez OA, Benitez M, Stijnen T, Boot W, Berger HM. Inaccuracy in neonatal measurement of urine concentration with a refractometer. J Pediatr. 1986;108:613–616. doi: 10.1016/s0022-3476(86)80850-2. [DOI] [PubMed] [Google Scholar]
- 42.Hanioka N, Naito T, Narimatsu S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere. 2008;74:33–36. doi: 10.1016/j.chemosphere.2008.09.053. doi: 10.1016/j.chemosphere.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 43.Beutler E, Gelbart T, Demina A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: A balanced polymorphism for regulation of bilirubin metabolism?. Proc Natl Acad Sci U S A. 1998;95:8170–8174. doi: 10.1073/pnas.95.14.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of Longitudinal Data. 2nd ed. Oxford University Press; USA: 2002. [Google Scholar]
- 45.Hanioka N, Oka H, Nagaoka K, Ikushiro S, Narimatsu S. Effect of UDP-glucuronosyltransferase 2B15 polymorphism on bisphenol A glucuronidation. Arch Toxicol. 2011;85:1373–81. doi: 10.1007/s00204-011-0690-5. [DOI] [PubMed] [Google Scholar]