Abstract
Components of the innate immune system such as macrophages and dendritic cells are instrumental in determining the fate of immune responses and are, also, among the most sensitive targets of early life environmental alterations including developmental immunotoxicity (DIT). DIT can impede innate immune cell maturation, disrupt tissue microenvironment, alter immune responses to infectious challenges, and disrupt regulatory responses. Dysregulation of inflammation, such as that observed with DIT, has been linked with an increased risk of chronic inflammatory diseases in both children and adults. In this review, we discuss the relationship between early-life risk factors for innate immune modulation and promotion of dysregulated inflammation associated with chronic inflammatory disease. The health risks from DIT-associated inflammation may extend beyond primary immune dysfunction to include an elevated risk of several later-life, inflammatory-mediated diseases that target a wide range of physiological systems and organs. For this reason, determination of innate immune status should be an integral part of drug and chemical safety evaluation.
Keywords: developmental immunotoxicity, inflammation, TLRs, risk factors, macrophages, dendritic cells, innate immunity
Introduction
The developing immune system is extremely sensitive to alteration through exposure to environmental toxicants (Bunn et al. 2001a, 2001b; Luebke et al. 2006). These alterations lead to inappropriate inflammatory responses, and since there are critical windows of immune development during which key, often one-time, maturational processes occur, the alterations can persist throughout life. Although environmental risk for disease is usually compartmentalized into prenatal versus neonatal exposure and/or by the different categories of environmental factors (environmental chemicals, drugs, maternal and childhood diet, infections, and stress), these risks do not occur in isolation and often act in combination (Chen et al. 2004; Wigle et al. 2008). For example, certain prenatal environmental exposures including maternal dietary factors can either enhance (Fujiwara et al. 2010) or alternatively, reduce (Grandjean et al. 2010) the normally beneficial impact of breastfeeding on neonatal immune regulation. Interactions between genetic and environmental factors during these critical immune windows also determine later-life, immune-based health risks. As a result, timing, genetics, and combinations of exposure to environmental risk factors, all determine the eventual immunological outcome and later-life disease risk (Bunn et al. 2001a, 2001b). Such later-life sequelae include allergy, autoimmune disease, metabolic syndrome, inflammatory diseases, and cancer (Dietert 2011).
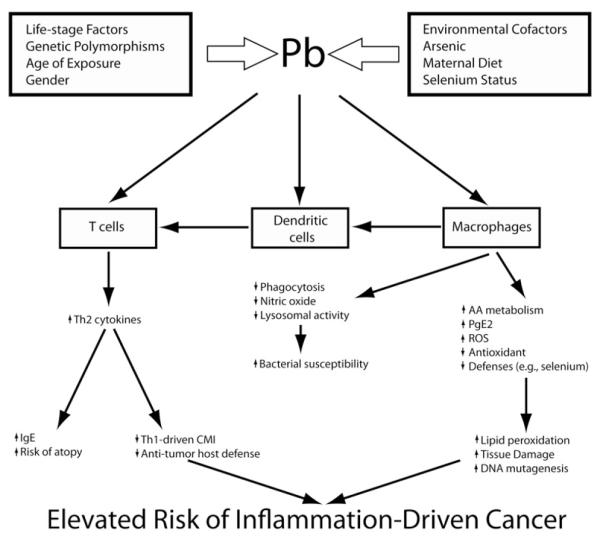
Environmental factors can affect almost any aspect of the immune system. But when environmentally-induced immune insult occurs developmentally, it has the potential to result in a dysfunctional immune response pattern that may persist across a lifetime. Concern for the ramifications of developmental immunotoxicity (DIT) extends beyond humans (Dietert 2009) to include both agricultural (Bunn, Marsh, and Dietert 2000; Lavoie and Grasman 2007; Peden-Adams et al. 2009) and wildlife species (Grasman and Fox 2001). In a prior review, the ramifications of developmental immunotoxicity (DIT) on the risk of pediatric asthma and allergy were discussed (Dietert and Zelikoff 2008). In this review, we discuss another major outcome associated with early life environment and DIT: misregulated innate immune responses, inflammatory dysfunction, and the risk of chronic inflammatory disease. We discuss examples of how environmental exposures change the phenotype and function of innate immune cells, and describe in detail one example, lead (Pb), and its effect on a critical innate immune cell, the macrophage. We illustrate an integrative model for metal-induced immunotoxicity of dendritic cells and macrophages using lead (shown in Figure 1) and suggest that changes in the early immune system through environmental exposures can alter both the repertoire of immune responses and the capacity for effective inflammatory regulation. Lastly, we suggest that in response to the recent findings on the sensitivity of the innate immune system during development and the importance of appropriate innate immune function for health of humans. DIT testing of environmental toxicants must incorporate measures of: (1) innate immune cell function, (2) the expression and function of the innate immune cell receptors, and (3) newly discovered regulatory mechanisms that control inflammation.
Figure 1.
Lead as a specific example of effects on innate immune function. During early development, a single immunotoxicant, the heavy metal, Pb, can target macrophages, dendritic cells and T cells producing innate immune dysfunction, hyperinflammation and Th2 bias. The elevated health risks from developmental exposure to Pb extend beyond the risk of allergic disease to include a variety of innate immune cell-influenced conditions.
Innate immunity and the developing immune system
Environmental interference during development of the innate immune system has the potential to limit the normal expansion of immune function and result in Th2-biased immunity or inappropriate/chronic inflammatory responses (Gao, Mondal, and Lawrence 2007; Kasten-Jolly, Heo, and Lawrence, 2010) and is, therefore, an important consideration for childhood vaccine development (Philbin and Levy 2009). Newborns have an inherently immature immune system (Zhao et al. 2008), and the default profile of immune responses during pregnancy and at birth is Th2-biased (Kollmann et al. 2009; Lee et al. 2008; White et al. 2010). Early life microbial exposures facilitate the maturation of dendritic cells (DCs) (Hoeman, Dhakal, and Zaghouani 2010; Troy and Kasper 2010) and expand the repertoire of immune functions to include Th1, Th17, and other immune response patterns. Prenatal and/or early postnatal exposure to toxicants (e.g., alcohol, heavy metals, tobacco smoke), certain infectious agents (e.g., gram-negative bacteria), and dietary factors (e.g., prenatal overnutrition, postnatal formula feeding) can alter the normal trajectory of innate immune maturation contributing to tissue pathology rather than adequate host resistance and tissue homeostasis (Auten et al. 2009; Beloosesky et al. 2010; Bry, Hogmalm, and Backstrom 2010; Caicedo et al. 2008; Calderon-Garciduenas et al. 2009; Ding et al. 2010; Fry et al. 2007; Sharkey et al. 2009; Tomat, Costa Mde, and Arranz 2011). These alterations in immune function have the potential to change the trajectory of subsequent pathogen-stimulated innate immune responses throughout life (Perrone et al. 2010; Strunk et al. 2011). Failure to appropriately clear infection or to regulate inflammatory responses can lead to long-lasting inflammation. Chronic inflammation has been correlated with many different diseases and syndromes such as chronic obstructive pulmonary disease (COPD), inflammatory bowel disease, rheumatoid arthritis, psoriasis, and the metabolic syndrome-related conditions of childhood obesity, hepatic steatosis, and atherosclerotic vascular diseases (Konner and Bruning 2011; Monteiro and Azevedo 2010; Sacheck 2008). We suggest that environmental insults during critical windows of innate immune cell maturation are likely to elevate the risk of adult inflammatory-related diseases many of which will arise in non-lymphoid tissues.
Direct effects of DIT on innate immune cells
Innate immune cells such as DCs and macrophages, seed tissues, and organs early in prenatal development, and have an increased likelihood of encountering the full range of environmental factors in the neonate due to their residence in the portals of environmental exposure (e.g., lung, skin, gut) (Maeda et al. 2010; Novak et al. 2010). Furthermore, the function of these innate immune cells is directly impacted by exposure to environmental toxicants (Bahri, Saidane-Mosbahi, and Rouabhia 2010; Gupta et al. 2010; Myrtek et al. 2008). Since these cells participate in homeostasis, respond to infectious challenge, and promote tissue remodeling, alterations in their function can change the outcome of responses in tissues such as the lung, skin, liver, and brain (Kramer et al. 2009; Verney et al. 2010; Zhao et al. 2009). Some of the developmental risk factors that directly impact innate immune cells include maternal smoking and environmental tobacco smoke (ETS), polychlorinated biphenyls (PCBs), heavy metals such as lead (Pb), air and traffic pollution, ozone, prenatal and postnatal dietary fatty acid intake, lack of animal-microbial exposure in early life, inadequate vitamin D intake, lack of breastfeeding, birth delivery mode, and paracetamol use (Tables 1 and 2).
Table 1.
Developmental risk factors for innate immunity and inflammation: environmental chemicals and drugs.
| Developmental risk factors |
Innate immune effects(s) |
Altered inflammatory conditions |
References |
|---|---|---|---|
| Maternal smoking and ETS |
Altered expression of TLRs; increased numbers of lung mast cells and eosinophils; decreased phago- cytosis but ele- vated production of ROS by macrophages |
Dyslipidemia, hyper- inflammation; higher infant oxi- dative stress |
Blacquiere et al. (2009) Ishida et al. (2009) Ng et al. (2009) Noakes et al. (2006, 2007) Pfefferle, Pinkenburg, and Renz (2010) Yu et al. (2009) |
| Air and/or traffic pollution |
Variant TLR-2 and 4 alleles affect pol- lutant impact |
Increased eosinophil- lic inflammation and airway remodeling |
Clark et al. (2010) Herr et al. (2010, 2011) Kerkhof et al. (2010) Morgenstern et al. (2008) |
| Ozone | Disrupted epithelial barrier; altered macrophage function |
Interaction with maternal PM exposure to pro- duce childhood airway hyperreactivity |
Auten et al. (2009)
Hollingsworth, Kleeberger, and Foster (2007) Lin et al. (2008) |
| Pb and synergistic effects with other metals |
Altered dendritic cell maturation (pro- motion of Th2); excessive TNF- alpha and ROS by macrophages |
Inflammatory dys- function; promo- tion of tissue damage, apoptosis and DNA mutagenesis |
Bishayi and Sengupta (2006)
Kasten-Jolly, Heo, and Lawrence (2010) Snyder et al. (2000) |
| Polychlorinated biphenyls |
Macrophage apopto- sis; disrupted epi- thelial barrier function |
Upregulation of ICAM-1, MCP-1; Notch3, CCLs and IL-8; enhanced vascular adhesion and inflammation |
Choi et al. (2010)
Eum et al. (2009) Felty, Yoo, and Kennedy (2010) Grandjean et al. (2010) Majkova et al. (2009) Shin et al. (2000 |
| Maternal and early childhood paracetamol use |
Decreased gluthathione, TNF-alpha and IL-6 production by pulmonary macrophages |
Glutathione deple- tion; increased oxidant-induced inflammation |
Bakkeheim et al. (2010)
Beasley et al. (2008) Dimova et al. (2005) Etminan et al. (2009) Farquhar et al. (2010) Gonzalez-Diaz et al. (2010) Shaheen, Newson, Ring, et al. (2010) |
Table 2.
Developmental risk factors for innate immunity and inflammation: physical, microbial and dietary factors.
| Developmental risk factors |
Innate immune effects (s) |
Altered inflammatory conditions |
References |
|---|---|---|---|
| Caesarian delivery | Increased levels of IL-13; reduced IFN-gamma |
Restricted diversity of gut microbiota |
Biasucci et al. (2008)
Ly et al. (2006) Pistiner et al. (2008) Protonotariou et al. (2010) Tollanes et al. (2008) |
| Low maternal infant dietary n – 3 fatty acid and high n – 6 fatty acid-trans fat |
Receptor-dependent functional alter- ations in macrophages |
Increased risk of inflammatory dis- orders; elevated neonatal oxidative damage and LTB4 production |
Dunstan et al. (2007)
Furuhjelm et al. (2009) Innis (2007) Oh da et al. (2010) Prescott et al. (2007) Thijs et al. (2011) |
| Low prenatal- neonatal animal bacterial exposures |
Depressed MyD88 (myeloid) driven recruitment of T regs to the airways |
Shifts in the types of inflammatory response observed |
Cao et al. (2010)
Pfefferle, Pinkenburg, and Renz (2010) von Mutius and Radon (2008) |
| Absence of pro- longed breast feeding (e.g., formula feeding) |
Delayed development of innate immune cells |
Increased later life risk of inflamma- tory disease (e.g., metabolic syndrome) |
Andersson et al. (2009)
Codispoti et al. (2010) Nobili et al. (2009) |
| Vitamin D deficiency |
Neonatal innate immune misregula- tion; suppressed autophagy in human macro- phages; reduced IL-10 by dendritic cells and Tregs; suppressed catheli- cidins; reduced metabolism of 25-OHD |
Chronic low grade inflammation and increased risk of tissue-localized inflammatory damage |
Erkkola et al. (2009)
Hewison (2010) Kamen and Tangpricha (2010) Lee et al. (2008) Litonjua (2009) Misawa et al. (2009) Rochat et al. (2010) Sandhu and Casale (2010) Yuk et al. (2009) |
Exposure of DCs to environmental insults during their maturation in vitro or in vivo changes their functional properties in many different ways. Bone-marrow DCs differentiated in the presence of nicotine are functional but express increased surface costimulatory molecules and major histocompatibility antigens (Nouri-Shirazi, Tinajero, and Guinet 2007). Therefore, these cells will have a lower threshold for inducing T cell responses. However, these cells are deficient in Th1 promoting cytokines and, therefore, promote a Th2-biased T cell repertoire characterized by increased interleukin (IL) 4 and reduced interferon gamma (IFN-γ). Similar effects were observed with exposure to organic dust associated with agriculture (Poole et al. 2009). Urban aerosols also enhanced costimulatory molecule expression on dendritic cells, but unlike nicotine and organic dust, proinflammatory cytokines such as IL-1 and IL-6 were increased. This suggests that these DCs will induce a dysregulated and more robust inflammatory response. Increased production of proinflammatory cytokines correlated with higher antigen specific immunoglobulin (Ig) G and IgE following intranasal ovalbumin challenge in a mouse model (Yoshida et al. 2010). Together, these studies demonstrate that environmental exposures directly alter DC function in a way that promotes excessive and/or Th2-biased chronic inflammatory responses.
Exposure to environmental toxins also alters the function of specialized tissue resident macrophages such as alveolar macrophages in the lung and microglial cells in the brain (Campbell 2004; Moreno et al. 2009a, 2009b). Manganese exposure in juvenille mice led to increased activation of microglia cells and upregulation of nitric oxide (NO) synthase, an enzyme critical for the production of reactive nitrogen intermediates (Campbell 2004; Moreno et al. 2009a, 2009b). These early effects resulted in alterations in glial cell function in adult mice. Pesticides such as lindane and dieldrin accumulate in the brain and enhance production of reactive oxygen by microglial cells (Mao and Liu 2008). Microglial-produced reactive oxygen has been linked to neurodegeneration (Lull and Block 2010), suggesting that pesticide exposure may increase the risk for neuroinflammatory diseases such as Parkinson’s disease (Mao and Liu 2008).
Alveolar macrophages are located at the interface between the host and the environment and are, therefore, constantly bombarded with a myriad of different environmental insults that can alter their function (Bateson and Schwartz 2008; Bhalla et al. 2009). Ambient particulate matter and diesel exhaust act directly on human alveolar macrophages to suppress inflammatory cytokine production (Sawyer et al. 2010). While this seems counterintuitive to the initiation of chronic inflammation, these responses are critical for host defense and may lead to failure to eliminate pathogens and result in chronic inflammation. This study also showed that some particulates reduced inflammatory cytokines, but actually increased the neutrophil chemotactic factor IL-8. Since neutrophils are a major mediator of tissue damage in response to inflammatory and infectious stimuli, these changes could lead to increased immune-mediated inflammatory tissue damage. Human blood-derived macrophages treated with arsenic displayed marked alterations in morphology, surface marker expression, phagocytic uptake, and cytokine secretion (Lemarie et al. 2006). In a mouse model, cigarette smoke reduced macrophage-mediated bacterial phagocytosis and clearance (Phipps et al. 2010). Lastly, exposure of a mouse macrophage cell line to synthetic pyrethroid insecticides resulted in dramatically increased reactive oxygen response (Zhang et al. 2010) reinforcing the observations that a broad array of chemically-driven effects on inflammatory cell function are possible depending upon the specific toxicants and concentration involved.
A specific example: lead exposure, macrophage function, and disease susceptibility
To emphasize the linkage of DIT to innate immune dysfunction, a more detailed example involving the heavy metal lead (Pb) is shown in Figure 1. Multiple genetic, life-stage, gender, nutritional, and environmental factors influence the sensitivity to Pb exposure (Bishayi and Sengupta 2006; Bunn et al. 2001a, 2001b; Miller et al. 1998; Snyder et al. 2000). Following Pb exposure, multiple cell types exhibit altered function including macrophages and DCs (Gao, Mondal, and Lawrence 2007; Guo, Mudzinski, and Lawrence 1996; Sengupta and Bishayi 2002). Pb exposure of dendritic cells reduces phagocytosis and limits bactericidal activities of lysosomes necessary for bacterial killing (Bishayi, Sengupta, and Ghosh 2004). This leads to increased sensitivity to bacterial infection. Similarly, macrophages are primary targets for Pb-induced immunotoxicity resulting in the overproduction of inflammatory mediators such as TNF-α (Cheng, Yang, and Liu 2006), prostaglandin-E2 (Grizzo and Cordellini 2008), and reactive oxygen species (ROS) (Pineda-Zavaleta et al. 2004; Shabani and Rabbani 2000). The elevated risk of innate immune-mediated oxidative damage in tissues is further exacerbated by a Pb-induced reduction in cellular glutathione and defenses against oxidative damage (Chetty et al. 2005). Alterations in innate immune cell function lead to a significant bias toward Th2 responses by effector T cells, which can also have their function directly affected by Pb exposure (Gao and Lawrence 2010; Gao, Mondal, and Lawrence 2007; Heo, Lee, and Lawrence 1998). Together, these alterations largely define the hallmarks of Pb-induced immunotoxicity (Dietert and Piepenbrink 2006).
Interactive immune effects among environmental risk factors
Although the vast majority of studies have examined the impact of single environmental risk factors on the developing immune system, it is important to recognize that synergistic or antagonistic interactions between categories of environmental risk factors (both positive and negative) can alter the relative likelihood for DIT and inflammation-promoted disease. Exposure to more than one environmental risk factor for DIT can arise either through exposure to mixtures (e.g., metal mixtures in water, air or soil) or via the exposure to multiple sources of immunomodulatory factors (e.g., environmental tobacco smoke and breastfeeding). In the latter case of multiple sources, the exposures could occur simultaneously during development or during different life stages.
For example, prenatal exposure of rats to Pb when the dams were fed a 20% protein diet resulted in a significant elevation of TNF-α production ex vivo in the offspring. However, if instead the dams were fed a 10% protein isocaloric diet, the effect of Pb exposure on the production of this proinflammatory cytokine was not significantly different from controls (Chen et al. 2004). This suggests the possibility that maternal diet might affect the risk of Pb-induced inflammatory dysfunction. But there is evidence suggesting that the reverse is also likely where exposure to an immunotoxicant can overcome the beneficial effects of other immunomodulatory factors. Early-life farming environments protect against the risk of childhood asthma and atopy, which may be associated with changes among innate immune cell receptors and innate immune responses (Ege et al. 2006; von Mutius 2010). But other agriculturally-related exposures appear to undermine the benefit of a microbial-rich early-life environment. Hoppin et al. (2008) reported that pesticide use among women growing up on a farm elevated their risk of asthma versus women raised on a farm who were not involved with pesticides (Hoppin et al. 2008). So, direct exposure to some pesticides during childhood may blunt the benefits of immune maturation in a diverse microbial environment.
Similar interactive effects among risk factors were seen in a recent study from the Faroe Islands. Evidence suggests that prolonged breastfeeding can benefit innate immunity and effective immune balance when compared versus formula feeding (Andersson et al. 2009). It also helps to reduce the risk of childhood asthma possibly via the innate immune modulating, soluble CD14 molecule (Rothenbacher et al. 2005). But exposure to environmental toxicants such as PCBs in the Faroe Island diet can undermine the normal benefit of breastfeeding. Grandjean et al. (2010) reported that PCB contamination of breast milk above a certain threshold concentration increased the risk of allergic sensitization in children (Grandjean et al. 2010). Therefore, it is important to consider the interactive effects of multiple or mixed exposures across all of innate immune development and not only the effects of a single environmental risk factor during a narrow period of immune development (e.g., neonatal microbial environment).
Lifetime health risks associated with DIT mediated innate immune dysfunction
Defects in fetal-neonatal innate immune development can lead to imbalanced and inappropriate responses that persist throughout a lifetime (Bellinger, Lubahn, and Lorton 2008; Selgrade et al. 2008; Thornton 2010). These uncontrolled or inappropriate responses increase risk for later-life inflammatory disease. However, it is important to note that such later-life adverse outcomes are components of much broader immune-based disease patterns, which include depression, sleep disorders, sensory impairment, and cancer (Dietert, Dietert, and Gavalchin 2010).
Psychiatric disturbances such as depression and mood disorders correlate with developmentally-associated inflammatory dysfunction and resulting inflammatory disease (Ahola et al. 2010; Fang et al. 2010; Gershon et al. 2010; Leonard 2010; Wilson et al. 2010). For example, chronic overproduction of proinflammatory cytokines and disruption of the pro- and anti-inflammatory cytokine balance can contribute to an increased risk of depression, mood disturbances, suicidal behavior, and sleep disturbances (Ferini-Strambi 2011; Janelidze et al. 2011; Reeves et al. 2007; Song and Wang 2010). Beside the chronic nature of these conditions, it is clear that the underlying innate immune dysfunction and the dysregulated inflammation pattern act beyond the lung or gut to affect other major health parameters that negatively impact the quality of life and may require medical intervention. Table 3 illustrates the extent to which inflammation-associated chronic diseases with putative early life risk factors affect most systems and organs of the body. Many of these diseases (e.g., asthma, coronary heart disease, COPD, diabetes, Alzhemier’s disease) have a significant prevalence among present adult populations, represent significant public health concerns, and are a focus of risk reduction strategies.
Table 3.
Inflammatory diseases and reported developmental risk factors.
| Inflammatory disease or condition |
Target system or organ |
Putative environmental risk factor(s) |
Reference(s) |
|---|---|---|---|
| Atherosclerosis | Cardiovascular | Air pollution | Brook and Rajagopolan (2010) |
| Coronary heart disease |
Cardiovascular | Traffic-associated pollution |
Gan et al. (2010) |
| Psoriasis | Dermal | Environmental tobacco smoke (women) |
Jankovic et al. (2009) |
| Dental caries/ Periodontal disease |
Dental | Maternal smoking; Environmental tobacco smoke; Dietary fatty acid intake |
Erdemir et al. (2010), Julihn, Ekbom, and Modeer (2009) and Naqvi et al. (2010) |
| Celiac disease | Gastrointestinal | Neonatal infections | Sandberg-Bennich, Dahlquist, and Kallen (2002) |
| Colon cancer | Gastrointestinal | Microbial depletion in early environment; Early life diet |
Davis and Milner (2009), Roberfroid et al. (2010) and Xiao et al. (2007) |
| Inflammatory bowel disease |
Gastrointestinal | Early antibiotic use |
Hviid, Svanstrom, and Frisch (2011) and Shaw, Blanchard, and Bernstein (2010) |
| Nonalcohol fatty liver disease |
Hepatic | Heavy metals | Cave et al. (2010) |
| Chronic kidney disease |
Nephrotic | Heavy metals |
Fadrowski et al. (2010), Gobe and Crane (2010) and Soderland et al. (2010) |
| Alzheimer’s disease | Neurological | Metals | Duce and Bush (2010) |
| Multiple sclerosis | Neurological | Vitamin D and/or ultraviolet radiation deficiency; smoking exposure |
Handel et al. (2011), Sloka et al. (2011) and Staples, Ponsonby, and Lim (2010) |
| Parkinson’s disease | Neurological | Certain pesticide exposures |
Elbaz et al. (2009) and Gatto et al. (2009) |
| Otitis media | Otological | PCB/PCDF exposure |
Dallaire et al. (2006) and Guo et al. (2004) |
| Type 1 diabetes | Pancreatic | Prenatal nutrient over- load and/or Reduced neonatal microbial environment |
D’Angeli et al. (2010) |
| Type 2 diabetes | Pancreatic | Prenatal nutrient energy overload or Prenatal nutrient- insufficiency |
Kanaka-Gantenbein (2010) |
| Childhood asthma | Respiratory | Traffic pollution; Environmental tobacco smoke; Paracetamol |
Baena-Cagnani et al. (2009), Carlsten et al. (2011) and McConnell et al. (2010) |
|
Shaheen, Newson, Smith, et al. (2010)
Sly (2011) |
|||
| COPD | Respiratory | Tobacco smoke; Biomass smoke exposure |
Beyer, Mitfessel, and Gillissen (2009), Grigg (2009) and Taylor (2010) |
| Juvenile idiopathic arthritis |
Skeletal | Maternal smoking | Berkun and Padeh (2010) and Jaakkola and Gissler (2005) |
| Osteoporosis/Risk of bone fractures |
Skeletal | Cadmium exposure; Vitamin D deficiency |
Nawrot et al. (2010), Papandreou et al. (2010) and Shin, Paek, and Yoon (2011) |
| Systemic sclerosis | Systemic: connec- tive tissue disease |
Vitamin D deficiency | Caramaschi et al. (2010) and Vacca et al. (2009) |
Chronic disease outcomes of inflammatory dysfunction are not restricted to non-cancerous conditions. When innate immune dysfunction and chronic inflammation persist across decades of life, two types of cancers are commonly observed: tissue-specific cancers and leukemic cancers (Dietert 2011). Although chronic inflammatory diseases are often negatively correlated with the risk of cancer, the risk is often elevated for the tissue that is a target of persistent inflammatory insult (Dietert, DeWitt, et al. 2010). This can include both the risk of tissue-specific cancer itself (Ji et al. 2009) as well as the association of inflammation with the recruitment of circulating tumor cells to the target tissue (Taranova et al. 2008). Additionally, leukemic cancers are commonly associated with some forms of immune dysfunction-based patterns (e.g., celiac disease, psoriasis) suggesting that dysfunctional immunoregulation established early in life can promote both childhood- and adult-onset cancer (Dietert 2011).
The mechanistic connection between chronic inflammation and cancer is linked to the high production of growth factors needed to repair tissue damage and an abundance of inflammatory cytokines (Coussens and Werb 2002; Karin and Greten 2005; Terzic et al. 2010). Although low levels of inflammation promote tissue homeostasis, tumors take advantage of proinflammatory cytokines to promote their own survival and growth (e.g. TNF, IL-1, IL-6, IL-8) (Chen et al. 2007; Rakoff-Nahoum, Hao, and Medzhitov 2006; Rakoff-Nahoum and Medzhitov 2007).
Given the importance of inflammatory-based diseases across a lifetime (Dietert and Zelikoff 2010), increased attention to other health risks linked with innate immune dysfunction is needed. Effective management of innate immune maturation requires both avoidance of hazardous environmental chemicals, drugs, and physical factors, as well as promotion of positive factors such as prolonged breastfeeding, adequate vitamin D levels, and useful balances of dietary fatty acids (Dietert, DeWitt, et al. 2010). DIT-induced dysfunctional innate immunity can impact the risk of adult leukemic and/or tissue-specific cancer that is linked to several chronic inflammation-associated diseases (Dietert 2011).
Identification of innate immune and inflammatory dysfunction in safety evaluation
This review highlights one of the major problems in the preclinical safety screening of drugs and in the safety screening of environmental chemicals for immunotoxicity. To date, the primary focus of immune safety assessment has been on histopathology combined with a limited number of adaptive immune responses usually directed against protein or xenogeneic cell antigens (Dietert 2009, 2011). Comprehensive innate immune analysis and assessment of inflammatory regulation in response to infectious agent challenge is rarely required in adult safety assessment (FDA S8 guidelines), although such protocols are available (Burleson and Burleson 2007, 2008; Neff-LaFord et al. 2007). This is a significant problem if protocols utilized for immune assessment lack the capacity for evaluating the regulation of inflammation. Adult immunotoxicity assessment is not routine (FDA S8 guidelines) and, when performed, usually lacks measures of inflammatory responses. Relevant innate immune assessment of the developing immune system is even less common.
The list of chronic inflammatory diseases shown in Table 3 and their relative importance to public health underscores the need for such immune safety information. Given the immature status of the innate immune system in the neonate and the risk for drug and chemical exposures to cause inflammatory dysfunction in later life, a priority should be given for ensuring that drug and chemical safety screening is designed to detect developmentally-induced inflammatory dysfunction.
Focus on pattern recognition receptors
A promising focus for the evaluation of innate immune-directed immunotoxicity in neonates and adults is the family of pattern recognition receptors (PRRs) including the Toll-like receptors (TLRs). The TLRs are expressed on innate immune cells and bind directly to microbial structures such as lipopolysaccharide and components of Gram+ bacterial cell walls (Takeda, Kaisho, and Akira 2003). TLR stimulation initiates inflammatory cytokine secretion and leads to maturation of dendritic cells (Medzhitov, Preston-Hurlburt, and Janeway 1997), which is critical to induce effector T cell responses. Depending on the cues that dendritic cells receive during this maturational process, different types of effector T cell responses can occur (Mazzoni et al. 2001, 2003; Mazzoni and Segal 2004, 2006). The importance of appropriate regulation of these responses initiated through TLRs is highlighted in inflammation-mediated autoimmune disease, such as systemic lupus erythematosus. In mouse models of lupus, inappropriate and uncontrolled responses through TLRs result in overproduction of cytokines, and type I interferons, which results in inflammatory damage (Leadbetter et al. 2002). Therefore, it is critical to understand the molecular mechanisms regulating these receptors and consider the effects of environmental exposures on their expression and regulation.
Recent studies suggest that traditional mRNA profiles of innate immune genes, such as TLRs, are insufficient to correlate receptor expression with inflammatory responses. Instead, modifications of TLRs after protein synthesis determine the magnitude of cellular responses. For example, mRNA levels do not necessarily correlate with protein function, since intracellular localization and proteolytic processing control cellular response to TLR9 ligands (Barton, Kagan, and Medzhitov 2006; Chockalingam et al. 2009, 2011; Leifer et al. 2004, 2006). Therefore, to determine the effects of environmental exposure on innate immune function, new targets and assays are desperately needed. We propose that new targets to evaluate innate immune function are (1) to examine the effect of prenatal exposure to toxicants on TLR signaling in neonatal and adult macrophages and dendritic cells and (2) to determine whether processes such as bacterial killing by adult macrophages, which depends in part on recognition of the bacterium through TLRs, is reduced by prenatal exposure to toxicants. Since these types of evaluations are lacking in current safety testing, we may be underestimating the effect that prenatal and early life exposures have on immune cell function.
Conclusions
The newborn emerges with an immune system that favors Th2 adaptive responses and lacks the innate immune maturity that will be needed to effectively protect the child. Prenatal or postnatal environmental conditions that impair the continued and effective maturation of the innate immune system create a hyperinflammatory state. Dysfunctional innate immunity alters host responses to infections. This in turn causes tissue damage and promotes chronic inflammation. DIT has the potential to affect the ability of resident innate immune cells in their seeding of organs, expansion-repopulation of cells in the organ, acquisition of specialized phenotypes, interaction with other resident cells in the tissue, response to environmental stimuli, and recruitment of additional immune cells to the tissue. Yet, to date, the functional status of these cells following early-life exposure to drugs or environmental chemicals is usually only known retrospectively and is rarely, if ever, evaluated in required safety testing (Dietert 2009, 2011). We argue that it is not possible to evaluate the risk that a chemical or drug poses without determining its effect on the functional status of resident innate immune cells and its potential to elevate the risk of later-life inflammatory disease.
Acknowledgments
The authors thank Janice Dietert for her editorial suggestions. The research of CAL on innate immune cells and toll-like receptors is supported by NIH (AI076588, AI076588-S1).
References
- Ahola AJ, Thorn LM, Saraheimo M, Forsblom C, Groop PH. Depression is associated with the metabolic syndrome among patients with type 1 diabetes. Annals of Medicine. 2010;42:495–501. doi: 10.3109/07853890.2010.503660. [DOI] [PubMed] [Google Scholar]
- Andersson Y, Hammarstrom ML, Lonnerdal B, Graverholt G, Falt H, Hernell O. Formula feeding skews immune cell composition toward adaptive immunity compared to breastfeeding. Journal of Immunology. 2009;183:4322–8. doi: 10.4049/jimmunol.0900829. [DOI] [PubMed] [Google Scholar]
- Auten RL, Potts EN, Mason SN, Fischer B, Huang Y, Foster WM. Maternal exposure to particulate matter increases postnatal ozone-induced airway hyperreactivity in juvenile mice. American Journal of Respiratory and Critical Care Medicine. 2009;180:1218–26. doi: 10.1164/rccm.200901-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Baena-Cagnani CE, Gomez RM, Baena-Cagnani R, Canonica GW. Impact of environmental tobacco smoke and active tobacco smoking on the development and outcomes of asthma and rhinitis. Current Opinion in Allergy & Clinical Immunology. 2009;9:136–40. doi: 10.1097/ACI.0b013e3283294038. [DOI] [PubMed] [Google Scholar]
- Bahri R, Saidane-Mosbahi D, Rouabhia M. Cytokine release and cytotoxicity in human keratinocytes induced by polycyclic aromatic hydrocarbons (1-methylpyrene and perylene) Journal of Toxicology and Environmental Health A. 2010;73:552–64. doi: 10.1080/15287390903566617. [DOI] [PubMed] [Google Scholar]
- Bakkeheim E, Mowinckel P, Carlsen KH, Haland G, Lodrup Carlsen KC. Paracetamol in early infancy; the risk of childhood allergy and asthma. Acta Paediatrica. 2010;100:90–6. doi: 10.1111/j.1651-2227.2010.01942.x. [DOI] [PubMed] [Google Scholar]
- Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nature Immunology. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Children’s response to air pollutants. Journal of Toxicology and Environmental Health A. 2008;71:238–43. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- Beasley R, Clayton T, Crane J, von Mutius E, Lai CK, Montefort S, Stewart A. Association between paracetamol use in infancy and childhood, and risk of asthma, rhinoconjunctivitis, and eczema in children aged 6–7 years: Analysis from phase three of the ISAAC programme. The Lancet. 2008;372:1039–48. doi: 10.1016/S0140-6736(08)61445-2. [DOI] [PubMed] [Google Scholar]
- Bellinger DL, Lubahn C, Lorton D. Maternal and early life stress effects on immune function: Relevance to immunotoxicology. Journal of Immunotoxicology. 2008;5:419–44. doi: 10.1080/15476910802483415. [DOI] [PubMed] [Google Scholar]
- Beloosesky R, Maravi N, Weiner Z, Khatib N, Awad N, Boles J, Ross MG, Itskovitz-Eldor J. Maternal lipopolysaccharide-induced inflammation during pregnancy programs impaired offspring innate immune responses. American Journal of Obstetrics and Gynecology. 2010;203:185, e181–4. doi: 10.1016/j.ajog.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Berkun Y, Padeh S. Environmental factors and the geoepidemiology of juvenile idiopathic arthritis. Autoimmunity Reviews. 2010;9:A319–24. doi: 10.1016/j.autrev.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Beyer D, Mitfessel H, Gillissen A. Maternal smoking promotes chronic obstructive lung disease in the offspring as adults. European Journal of Medical Research. 2009;4(Suppl 14):27–31. doi: 10.1186/2047-783X-14-S4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla DK, Hirata F, Rishi AK, Gairola CG. Cigarette smoke, inflammation, and lung injury: A mechanistic perspective. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2009;12:45–64. doi: 10.1080/10937400802545094. [DOI] [PubMed] [Google Scholar]
- Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. Journal of Nutrition. 2008;138:1796S–1800S. doi: 10.1093/jn/138.9.1796S. [DOI] [PubMed] [Google Scholar]
- Bishayi B, Sengupta M. Synergism in immunotoxicological effects due to repeated combined administration of arsenic and lead in mice. International Immunopharmacology. 2006;6:454–64. doi: 10.1016/j.intimp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Bishayi B, Sengupta M, Ghosh S. Lead induced modulation of splenic macrophage responses on humoral and cell mediated immunity. Acta Microbiologica et Immunologica Hungarica. 2004;51:31–45. doi: 10.1556/AMicr.51.2004.1-2.2. [DOI] [PubMed] [Google Scholar]
- Blacquiere MJ, Timens W, Melgert BN, Geerlings M, Postma DS, Hylkema MN. Maternal smoking during pregnancy induces airway remodelling in mice offspring. European Respiratory Journal. 2009;33:1133–40. doi: 10.1183/09031936.00129608. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Current Atherosclerosis Reports. 2010;12:291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- Bry K, Hogmalm A, Backstrom E. Mechanisms of inflammatory lung injury in the neonate: Lessons from a transgenic mouse model of bronchopulmonary dysplasia. Seminars in Perinatology. 2010;34:211–21. doi: 10.1053/j.semperi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Bunn TL, Marsh JA, Dietert RR. Gender differences in developmental immunotoxicity to lead in the chicken: Analysis following a single early low-level exposure in vivo. Journal of Toxicology and Environmental Health A. 2000;61:677–93. doi: 10.1080/00984100050195152. [DOI] [PubMed] [Google Scholar]
- Bunn TL, Parsons PJ, Kao E, Dietert RR. Gender-based profiles of developmental immunotoxicity to lead in the rat: Assessment in juveniles and adults. Journal of Toxicology and Environmental Health A. 2001a;64:223–40. doi: 10.1080/15287390152543708. [DOI] [PubMed] [Google Scholar]
- Bunn TL, Parsons PJ, Kao E, Dietert RR. Exposure to lead during critical windows of embryonic development: Differential immunotoxic outcome based on stage of exposure and gender. Toxicological Sciences. 2001b;64:57–66. doi: 10.1093/toxsci/64.1.57. [DOI] [PubMed] [Google Scholar]
- Burleson GR, Burleson FG. Influenza virus host resistance model. Methods. 2007;41:31–37. doi: 10.1016/j.ymeth.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Burleson GR, Burleson FG. Testing human biologicals in animal host resistance models. Journal of Immunotoxicology. 2008;5:23–31. doi: 10.1080/15476910801897557. [DOI] [PubMed] [Google Scholar]
- Caicedo RA, Li N, Des Robert C, Scumpia PO, Hubsher CP, Wasserfall CH, Schatz DA, Atkinson MA, Neu J. Neonatal formula feeding leads to immunological alterations in an animal model of type 1 diabetes. Pediatric Research. 2008;63:303–7. doi: 10.1203/PDR.0b013e31815ed662. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Macias-Parra M, Hoffmann HJ, Valencia-Salazar G, Henriquez-Roldan C, Osnaya N, Monte OC, et al. Immunotoxicity and environment: Immunodysregulation and systemic inflammation in children. Toxicologic Pathology. 2009;37:161–9. doi: 10.1177/0192623308329340. [DOI] [PubMed] [Google Scholar]
- Campbell A. Inflammation, neurodegenerative diseases, and environmental exposures. Annals of the New York Academy of Sciences. 2004;1035:117–32. doi: 10.1196/annals.1332.008. [DOI] [PubMed] [Google Scholar]
- Cao L, Wang J, Zhu Y, Tseu I, Post M. Maternal endotoxin exposure attenuate allergic airway disease in infant rats. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2010;298:L670–7. doi: 10.1152/ajplung.00399.2009. [DOI] [PubMed] [Google Scholar]
- Caramaschi P, Dalla Gassa A, Ruzzenente O, Volpe A, Ravagnani V, Tinazzi I, Barausse G, Bambara LM, Biasi D. Very low levels of vitamin D in systemic sclerosis patients. Clinical Rheumatology. 2010;29:1419–25. doi: 10.1007/s10067-010-1478-3. [DOI] [PubMed] [Google Scholar]
- Carlsten C, Dybuncio A, Becker A, Chan-Yeung M, Brauer M. Traffic-related air pollution and incident asthma in a high-risk birth cohort. Occupational and Environmental Medicine. 2011;68:291–5. doi: 10.1136/oem.2010.055152. [DOI] [PubMed] [Google Scholar]
- Cave M, Falkner KC, Ray M, Joshi-Barve S, Brock G, Khan R, Bon Homme M, McClain CJ. Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology. 2010;51:474–81. doi: 10.1002/hep.23321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Huang J, Gong W, Iribarren P, Dunlop NM, Wang JM. Toll-like receptors in inflammation, infection and cancer. International Immunopharmacology. 2007;7:1271–85. doi: 10.1016/j.intimp.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Chen S, Golemboski K, Piepenbrink M, Dietert R. Developmental immunotoxicity of lead in the rat: Influence of maternal diet. Journal of Toxicology and Environmental Health A. 2004;67:495–511. doi: 10.1080/15287390490276520. [DOI] [PubMed] [Google Scholar]
- Cheng YJ, Yang BC, Liu MY. Lead increases lipopolysaccharide-induced liver-injury through tumor necrosis factor-alpha overexpression by monocytes/macrophages: Role of protein kinase C and P42/44 mitogen-activated protein kinase. Environmental Health Perspectives. 2006;114:507–13. doi: 10.1289/ehp.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetty CS, Vemuri MC, Campbell K, Suresh C. Lead-induced cell death of human neuroblastoma cells involves GSH deprivation. Cellular & Molecular Biology Letters. 2005;10:413–23. [PubMed] [Google Scholar]
- Chockalingam A, Brooks JC, Cameron JL, Blum LK, Leifer CA. TLR9 traffics through the Golgi complex to localize to endolysosomes and respond to CpG DNA. Immunology and Cell Biology. 2009;87:209–17. doi: 10.1038/icb.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chockalingam A, Cameron JL, Brooks JC, Leifer CA. Negative regulation of signaling by a soluble form of Toll-like receptor 9. European Journal of Immunology. 2011 doi: 10.1002/eji.201041034. DOI: 10.1002/eji.201041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YJ, Seelbach MJ, Pu H, Eum SY, Chen L, Zhang B, Hennig B, Toborek M. Polychlorinated biphenyls disrupt intestinal integrity via NADPH oxidase-induced alterations of tight junction protein expression. Environmental Health Perspectives. 2010;118:976–81. doi: 10.1289/ehp.0901751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NA, Demers PA, Karr CJ, Koehoorn M, Lencar C, Tamburic L, Brauer M. Effect of early life exposure to air pollution on development of childhood asthma. Environmental Health Perspectives. 2010;118:284–90. doi: 10.1289/ehp.0900916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codispoti CD, Levin L, LeMasters GK, Ryan P, Reponen T, Villareal M, Burkle J, et al. Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rhinitis. Journal of Allergy and Clinical Immunology. 2010;125:1054–60. doi: 10.1016/j.jaci.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angeli MA, Merzon E, Valbuena LF, Tirschwell D, Paris CA, Mueller BA. Environmental factors associated with childhood-onset type 1 diabetes mellitus: An exploration of the hygiene and overload hypotheses. Archives of Pediatrics & Adolescent Medicine. 2010;164:732–8. doi: 10.1001/archpediatrics.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallaire F, Dewailly E, Vezina C, Muckle G, Weber JP, Bruneau S, Ayotte P. Effect of prenatal exposure to polychlorinated biphenyls on incidence of acute respiratory infections in preschool Inuit children. Environmental Health Perspectives. 2006;114:1301–5. doi: 10.1289/ehp.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CD, Milner JA. Gastrointestinal microflora, food components and colon cancer prevention. Journal of Nutrition Biochemistry. 2009;20:743–52. doi: 10.1016/j.jnutbio.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert RR. Developmental immunotoxicology: Focus on health risks. Chemical Research in Toxicology. 2009;22:17–23. doi: 10.1021/tx800198m. [DOI] [PubMed] [Google Scholar]
- Dietert RR. Role of developmental immunotoxicity and immune dysfunction in chronic disease and cancer. Reproductive Toxicology. 2011;31:319–26. doi: 10.1016/j.reprotox.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Dietert RR, DeWitt JC, Germolec DR, Zelikoff JT. Breaking patterns of environmentally influenced disease for health risk reduction: Immune perspectives. Environmental Health Perspectives. 2010;118:1091–9. doi: 10.1289/ehp.1001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert RR, Dietert JM, Gavalchin J. Risk of autoimmune disease: Challenges for immunotoxicity testing. Methods in Molecular Biology. 2010;598:39–51. doi: 10.1007/978-1-60761-401-2_4. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Piepenbrink MS. Lead and immune function. Critical Reviews in Toxicology. 2006;36:359–85. doi: 10.1080/10408440500534297. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Zelikoff JT. Early-life environment, developmental immunotoxicology, and the risk of pediatric allergic disease including asthma. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2008;83:547–60. doi: 10.1002/bdrb.20170. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Zelikoff JT. Identifying patterns of immune-related disease: Use in disease prevention and management. World Journal of Pediatrics. 2010;6:111–18. doi: 10.1007/s12519-010-0026-1. [DOI] [PubMed] [Google Scholar]
- Dimova S, Hoet PH, Dinsdale D, Nemery B. Acetaminophen decreases intracellular glutathione levels and modulates cytokine production in human alveolar macrophages and type II pneumocytes in vitro. International Journal of Biochemistry & Cell Biology. 2005;37:1727–37. doi: 10.1016/j.biocel.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Ding T, McConaha M, Boyd KL, Osteen KG, Bruner-Tran KL. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reproductive Toxicology. 2010;31:351–8. doi: 10.1016/j.reprotox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duce JA, Bush AI. Biological metals and Alzheimer’s disease: Implications for therapeutics and diagnostics. Progress in Neurobiology. 2010;92:1–18. doi: 10.1016/j.pneurobio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Dunstan JA, Mitoulas LR, Dixon G, Doherty DA, Hartmann PE, Simmer K, Prescott SL. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: A randomized controlled trial. Pediatric Research. 2007;62:689–94. doi: 10.1203/PDR.0b013e318159a93a. [DOI] [PubMed] [Google Scholar]
- Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, Schram-Bijkerk D, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. Journal of Allergy and Clinical Immunology. 2006;117:817–23. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Clavel J, Rathouz PJ, Moisan F, Galanaud JP, Delemotte B, Alperovitch A, Tzourio C. Professional exposure to pesticides and Parkinson disease. Annals of Neurology. 2009;66:494–504. doi: 10.1002/ana.21717. [DOI] [PubMed] [Google Scholar]
- Erdemir EO, Sonmez IS, Oba AA, Bergstrom J, Caglayan O. Periodontal health in children exposed to passive smoking. Journal of Clinical Periodontology. 2010;37:160–4. doi: 10.1111/j.1600-051X.2009.01510.x. [DOI] [PubMed] [Google Scholar]
- Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, Veijola R, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clinical & Experimental Allergy. 2009;39:875–82. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, Fitzgerald JM. Acetaminophen use and the risk of asthma in children and adults: A systematic review and metaanalysis. Chest. 2009;136:1316–23. doi: 10.1378/chest.09-0865. [DOI] [PubMed] [Google Scholar]
- Eum SY, Andras I, Hennig B, Toborek M. NADPH oxidase and lipid raft-associated redox signaling are required for PCB153-induced upregulation of cell adhesion molecules in human brain endothelial cells. Toxicology and Applied Pharmacology. 2009;240:299–305. doi: 10.1016/j.taap.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, Guallar E, Weaver VM, Furth SL. Blood lead level and kidney function in US adolescents: The Third National Health and Nutrition Examination Survey. Archives of Internal Medicine. 2010;170:75–82. doi: 10.1001/archinternmed.2009.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang BJ, Tonelli LH, Soriano J, Postolache TT. Disturbed sleep: Linking allergic rhinitis, mood and suicidal behavior. Frontiers in Bioscience (Scholar Edition) 2010;2:30–46. doi: 10.2741/s44. [DOI] [PubMed] [Google Scholar]
- Farquhar H, Stewart A, Mitchell E, Crane J, Eyers S, Weatherall M, Beasley R. The role of paracetamol in the pathogenesis of asthma. Clinical & Experimental Allergy. 2010;40:32–41. doi: 10.1111/j.1365-2222.2009.03378.x. [DOI] [PubMed] [Google Scholar]
- Felty Q, Yoo C, Kennedy A. Gene expression profile of endothelial cells exposed to estrogenic environmental compounds: Implications to pulmonary vascular lesions. Life Sciences. 2010;86:919–27. doi: 10.1016/j.lfs.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferini-Strambi L. Sleep disorders in multiple sclerosis. Handbook of Clinical Neurology. 2011;99:1139–46. doi: 10.1016/B978-0-444-52007-4.00025-4. [DOI] [PubMed] [Google Scholar]
- Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, et al. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS Genetics. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara R, Takemura N, Watanabe J, Sonoyama K. Maternal consumption of fructo-oligosaccharide diminishes the severity of skin inflammation in offspring of NC/Nga mice. British Journal of Nutrition. 2010;103:530–8. doi: 10.1017/S000711450999198X. [DOI] [PubMed] [Google Scholar]
- Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Bottcher MF, Falth-Magnusson K, Duchen K. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatrica. 2009;98:1461–67. doi: 10.1111/j.1651-2227.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- Gan WQ, Tamburic L, Davies HW, Demers PA, Koehoorn M, Brauer M. Changes in residential proximity to road traffic and the risk of death from coronary heart disease. Epidemiology. 2010;21:642–9. doi: 10.1097/EDE.0b013e3181e89f19. [DOI] [PubMed] [Google Scholar]
- Gao D, Lawrence DA. Dendritic cells in immunotoxicity testing. Methods in Molecular Biology. 2010;598:259–81. doi: 10.1007/978-1-60761-401-2_19. [DOI] [PubMed] [Google Scholar]
- Gao D, Mondal TK, Lawrence DA. Lead effects on development and function of bone marrow-derived dendritic cells promote Th2 immune responses. Toxicology and Applied Pharmacology. 2007;222:69–79. doi: 10.1016/j.taap.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson’s disease in rural California. Environmental Health Perspectives. 2009;117:1912–18. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon AS, Wang C, Guan J, To T. Burden of comorbidity in individuals with asthma. Thorax. 2010;65:612–18. doi: 10.1136/thx.2009.131078. [DOI] [PubMed] [Google Scholar]
- Gobe G, Crane D. Mitochondria, reactive oxygen species and cadmium toxicity in the kidney. Toxicology Letters. 2010;198:49–55. doi: 10.1016/j.toxlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Diaz SN, Del Rio-Navarro BE, Pietropaolo-Cienfuegos DR, Escalante-Dominguez AJ, Garcia-Almaraz RG, Merida-Palacio V, Berber A. Factors associated with allergic rhinitis in children and adolescents from northern Mexico: International Study of Asthma and Allergies in Childhood Phase IIIB. Allergy Asthma Proceedings. 2010;31:e53–62. doi: 10.2500/aap.2010.31.3346. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Poulsen LK, Heilmann C, Steuerwald U, Weihe P. Allergy and Sensitization during childhood associated with prenatal and lactational exposure to marine pollutants. Environmental Health Perspectives. 2010;118:1429–33. doi: 10.1289/ehp.1002289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasman KA, Fox GA. Associations between altered immune function and organochlorine contamination in young Caspian terns (Sterna caspia) from Lake Huron, 1997–1999. Ecotoxicology. 2001;10:101–14. doi: 10.1023/a:1008950025622. [DOI] [PubMed] [Google Scholar]
- Grigg J. Particulate matter exposure in children: Relevance to chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society. 2009;6:564–69. doi: 10.1513/pats.200905-026RM. [DOI] [PubMed] [Google Scholar]
- Grizzo LT, Cordellini S. Perinatal lead exposure affects nitric oxide and cyclooxygenase pathways in aorta of weaned rats. Toxicological Sciences. 2008;103:207–14. doi: 10.1093/toxsci/kfn018. [DOI] [PubMed] [Google Scholar]
- Guo TL, Mudzinski SP, Lawrence DA. The heavy metal lead modulates the expression of both TNF-alpha and TNF-alpha receptors in lipopolysaccharide-activated human peripheral blood mononuclear cells. Journal of Leukocyte Biology. 1996;59:932–9. doi: 10.1002/jlb.59.6.932. [DOI] [PubMed] [Google Scholar]
- Guo YL, Lambert GH, Hsu CC, Hsu MM. Yucheng: Health effects of prenatal exposure to polychlorinated biphenyls and dibenzofurans. International Archives of Occupational and Environmental Health. 2004;77:153–8. doi: 10.1007/s00420-003-0487-9. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Arany Z, Seale P, Mepani RJ, Ye L, Conroe HM, Roby YA, Kulaga H, Reed RR, Spiegelman BM. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–23. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handel AE, Williamson AJ, Disanto G, Dobson R, Giovannoni G, Ramagopalan SV. Smoking and multiple sclerosis: An updated meta-analysis. PLoS One. 2011;6:e16149. doi: 10.1371/journal.pone.0016149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo Y, Lee WT, Lawrence DA. Differential effects of lead and cAMP on development and activities of Th1- and Th2-lymphocytes. Toxicological Sciences. 1998;43:172–85. doi: 10.1006/toxs.1998.2457. [DOI] [PubMed] [Google Scholar]
- Herr CE, Dostal M, Ghosh R, Ashwood P, Lipsett M, Pinkerton KE, Sram R, Hertz-Picciotto I. Air pollution exposure during critical time periods in gestation and alterations in cord blood lymphocyte distribution: A cohort of livebirths. Environmental Health. 2010;9:46. doi: 10.1186/1476-069X-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr CE, Ghosh R, Dostal M, Skokanova V, Ashwood P, Lipsett M, Joad JP, et al. Exposure to air pollution in critical prenatal time windows and IgE levels in newborns. Pediatric Allergy and Immunology. 2011;22:75–84. doi: 10.1111/j.1399-3038.2010.01074.x. [DOI] [PubMed] [Google Scholar]
- Hewison M. Vitamin D and the intracrinology of innate immunity. Molecular and Cellular Endocrinology. 2010;321:103–11. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeman C, Dhakal M, Zaghouani H. Overcoming dendritic cell tardiness to triumph over IL-13 receptor: A strategy for the development of effective pediatric vaccines. Discovery Medicine. 2010;9:554–9. [PubMed] [Google Scholar]
- Hollingsworth JW, Kleeberger SR, Foster WM. Ozone and pulmonary innate immunity. Proceedings of the American Thoracic Society. 2007;4:240–6. doi: 10.1513/pats.200701-023AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MC, Sandler DP. Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study. American Journal of Respiratory and Critical Care Medicine. 2008;177:11–18. doi: 10.1164/rccm.200706-821OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid A, Svanstrom H, Frisch M. Antibiotic use and inflammatory bowel diseases in childhood. Gut. 2011;60:49–54. doi: 10.1136/gut.2010.219683. [DOI] [PubMed] [Google Scholar]
- Innis SM. Dietary lipids in early development: Relevance to obesity, immune and inflammatory disorders. Current Opinion in Endocrinology, Diabetes and Obesity. 2007;14:359–64. doi: 10.1097/MED.0b013e3282be90b9. [DOI] [PubMed] [Google Scholar]
- Ishida T, Hirono Y, Yoshikawa K, Hutei Y, Miyagawa M, Sakaguchi I, Pinkerton KE, Takeuchi M. Inhibition of immunological function mediated DNA damage of alveolar macrophages caused by cigarette smoke in mice. Inhalation Toxicology. 2009;21:1229–35. doi: 10.3109/08958370903176727. [DOI] [PubMed] [Google Scholar]
- Jaakkola JJ, Gissler M. Maternal smoking in pregnancy as a determinant of rheumatoid arthritis and other inflammatory polyarthropathies during the first 7 years of life. International Journal of Epidemiology. 2005;34:664–71. doi: 10.1093/ije/dyi006. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin A, Traskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain, Behavior, and Immunity. 2011;25:335–9. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Jankovic S, Raznatovic M, Marinkovic J, Jankovic J, Maksimovic N. Risk factors for psoriasis: A case-control study. Journal of Dermatology. 2009;36:328–34. doi: 10.1111/j.1346-8138.2009.00648.x. [DOI] [PubMed] [Google Scholar]
- Ji J, Shu X, Li X, Sundquist K, Sundquist J, Hemminki K. Cancer risk in hospitalised asthma patients. British Journal of Cancer. 2009;100:829–33. doi: 10.1038/sj.bjc.6604890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julihn A, Ekbom A, Modeer T. Maternal overweight and smoking: Prenatal risk factors for caries development in offspring during the teenage period. European Journal of Epidemiology. 2009;24:753–62. doi: 10.1007/s10654-009-9399-7. [DOI] [PubMed] [Google Scholar]
- Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: Modulation of innate and autoimmunity. Journal of Molecular Medicine. 2010;88:441–50. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaka-Gantenbein C. Fetal origins of adult diabetes. Annals of the New York Academy of Sciences. 2010;1205:99–105. doi: 10.1111/j.1749-6632.2010.05683.x. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nature Reviews Immunology. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kasten-Jolly J, Heo Y, Lawrence DA. Impact of developmental lead exposure on splenic factors. Toxicology and Applied Pharmacology. 2010;247:105–15. doi: 10.1016/j.taap.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkhof M, Postma DS, Brunekreef B, Reijmerink NE, Wijga AH, de Jongste JC, Gehring U, Koppelman GH. Toll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthma. Thorax. 2010;65:690–7. doi: 10.1136/thx.2009.119636. [DOI] [PubMed] [Google Scholar]
- Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. Journal of Immunology. 2009;183:7150–60. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner AC, Bruning JC. Toll-like receptors: Linking inflammation to metabolism. Trends in Endocrinology & Metabolism. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Seminars in Fetal and Neonatal Medicine. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie ET, Grasman KA. Effects of in ovo exposure to PCBs 126 and 77 on mortality, deformities and post-hatch immune function in chickens. Journal of Toxicology and Environmental Health A. 2007;70:547–58. doi: 10.1080/15287390600882226. [DOI] [PubMed] [Google Scholar]
- Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, Divekar R, Tartar DM, Haymaker CL, Zaghouani H. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. Journal of Experimental Medicine. 2008;205:2269–80. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer CA, Brooks JC, Hoelzer K, Lopez J, Kennedy MN, Mazzoni A, Segal DM. Cytoplasmic targeting motifs control localization of toll-like receptor 9. Journal of Biological Chemistry. 2006;281:35585–92. doi: 10.1074/jbc.M607511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. TLR9 is localized in the endoplasmic reticulum prior to stimulation. Journal of Immunology. 2004;173:1179–83. doi: 10.4049/jimmunol.173.2.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarie A, Morzadec C, Bourdonnay E, Fardel O, Vernhet L. Human macrophages constitute targets for immunotoxic inorganic arsenic. Journal of Immunology. 2006;177:3019–27. doi: 10.4049/jimmunol.177.5.3019. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The concept of depression as a dysfunction of the immune system. Current Immunology Reviews. 2010;6:205–12. doi: 10.2174/157339510791823835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Liu X, Le LH, Hwang SA. Chronic exposure to ambient ozone and asthma hospital admissions among children. Environmental Health Perspectives. 2008;116:1725–30. doi: 10.1289/ehp.11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litonjua AA. Childhood asthma may be a consequence of vitamin D deficiency. Current Opinion in Allergy & Clinical Immunology. 2009;9:202–7. doi: 10.1097/ACI.0b013e32832b36cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke RW, Chen DH, Dietert R, Yang Y, King M, Luster MI. The comparative immunotoxicity of five selected compounds following developmental or adult exposure. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2006;9:1–26. doi: 10.1080/15287390500194326. [DOI] [PubMed] [Google Scholar]
- Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7:354–65. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly NP, Ruiz-Perez B, Onderdonk AB, Tzianabos AO, Litonjua AA, Liang C, Laskey D, et al. Mode of delivery and cord blood cytokines: A birth cohort study. Clinical and Molecular Allergy. 2006;4:13. doi: 10.1186/1476-7961-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Nishimura Y, Kumagai N, Hayashi H, Hatayama T, Katoh M, Miyahara N, Yamamoto S, Hirastuka J, Otsuki T. Dysregulation of the immune system caused by silica and asbestos. Journal of Immunotoxicology. 2010;7:268–78. doi: 10.3109/1547691X.2010.512579. [DOI] [PubMed] [Google Scholar]
- Majkova Z, Smart E, Toborek M, Hennig B. Up-regulation of endothelial monocyte chemoattractant protein-1 by coplanar PCB77 is caveolin-1-dependent. Toxicology and Applied Pharmacology. 2009;237:1–7. doi: 10.1016/j.taap.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Liu B. Synergistic microglial reactive oxygen species generation induced by pesticides lindane and dieldrin. Neuroreport. 2008;19:1317–20. doi: 10.1097/WNR.0b013e32830b3677. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Leifer CA, Mullen GE, Kennedy MN, Klinman DM, Segal DM. Cutting edge: Histamine inhibits IFN-alpha release from plasmacytoid dendritic cells. Journal of Immunology. 2003;170:2269–73. doi: 10.4049/jimmunol.170.5.2269. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Segal DM. Controlling the Toll road to dendritic cell polarization. Journal of Leukocyte Biology. 2004;75:721–30. doi: 10.1189/jlb.1003482. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Siraganian RP, Leifer CA, Segal DM. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. Journal of Immunology. 2006;177:3577–81. doi: 10.4049/jimmunol.177.6.3577. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. Journal of Clinical Investigation. 2001;108:1865–73. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, Gauderman J, et al. Childhood incident asthma and traffic-related air pollution at home and school. Environmental Health Perspectives. 2010;118:1021–6. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Miller TE, Golemboski KA, Ha RS, Bunn T, Sanders FS, Dietert RR. Developmental exposure to lead causes persistent immunotoxicity in Fischer 344 rats. Toxicological Sciences. 1998;42:129–35. doi: 10.1006/toxs.1998.2424. [DOI] [PubMed] [Google Scholar]
- Misawa Y, Baba A, Ito S, Tanaka M, Shiohara M. Vitamin D(3) induces expression of human cathelicidin antimicrobial peptide 18 in newborns. International Journal of Hematology. 2009;90:561–70. doi: 10.1007/s12185-009-0452-9. [DOI] [PubMed] [Google Scholar]
- Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators of Inflammation. 2010;2010:289645. doi: 10.1155/2010/289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Legare ME, Tjalkens RB. Developmental exposure to manganese increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicological Sciences. 2009a;112:405–15. doi: 10.1093/toxsci/kfp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicological Sciences. 2009b;112:394–404. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Kramer U, Behrendt H, Herbarth O, von Berg A, Bauer CP, Wichmann HE, Heinrich J. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. American Journal of Respiratory and Critical Care Medicine. 2008;177:1331–7. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- Myrtek D, Muller T, Geyer V, Derr N, Ferrari D, Zissel G, Durk T, et al. Activation of human alveolar macrophages via P2 receptors: Coupling to intracellular Ca2 increases and cytokine secretion. Journal of Immunology. 2008;181:2181–8. doi: 10.4049/jimmunol.181.3.2181. [DOI] [PubMed] [Google Scholar]
- Naqvi AZ, Buettner C, Phillips RS, Davis RB, Mukamal KJ. n-3 Fatty acids and periodontitis in US adults. Journal of the American Dietetic Association. 2010;110:1669–75. doi: 10.1016/j.jada.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, Roels HA, Munters E, Cuypers A, Richart T, Ruttens A, Smeets K, Clijsters H, Vangronsveld J. Cadmium exposure in the population: From health risks to strategies of prevention. Biometals. 2010;23:769–82. doi: 10.1007/s10534-010-9343-z. [DOI] [PubMed] [Google Scholar]
- Neff-LaFord H, Teske S, Bushnell TP, Lawrence BP. Aryl hydrocarbon receptor activation during influenza virus infection unveils a novel pathway of IFN-gamma production by phagocytic cells. Journal of Immunology. 2007;179:247–55. doi: 10.4049/jimmunol.179.1.247. [DOI] [PubMed] [Google Scholar]
- Ng SP, Conklin DJ, Bhatnagar A, Bolanowski DD, Lyon J, Zelikoff JT. Prenatal exposure to cigarette smoke induces diet- and sex-dependent dyslipidemia and weight gain in adult murine offspring. Environmental Health Perspectives. 2009;117:1042–8. doi: 10.1289/ehp.0800193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noakes PS, Hale J, Thomas R, Lane C, Devadason SG, Prescott SL. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. European Respiratory Journal. 2006;28:721–9. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- Noakes PS, Thomas R, Lane C, Mori TA, Barden AE, Devadason SG, Prescott SL. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax. 2007;62:714–17. doi: 10.1136/thx.2006.061630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili V, Bedogni G, Alisi A, Pietrobattista A, Alterio A, Tiribelli C, Agostoni C. A protective effect of breastfeeding on the progression of non-alcoholic fatty liver disease. Archives of Disease in Childhood. 2009;94:801–5. doi: 10.1136/adc.2009.159566. [DOI] [PubMed] [Google Scholar]
- Nouri-Shirazi M, Tinajero R, Guinet E. Nicotine alters the biological activities of developing mouse bone marrow-derived dendritic cells (DCs) Immunology Letters. 2007;109:155–64. doi: 10.1016/j.imlet.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Novak N, Gros E, Bieber T, Allam JP. Human skin and oral mucosal dendritic cells as ‘good guys’ and ’bad guys’ in allergic immune responses. Clinical & Experimental Immunology. 2010;161:28–33. doi: 10.1111/j.1365-2249.2010.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh da Y, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–98. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou D, Malindretos P, Karabouta Z, Rousso I. Possible health implications and low vitamin d status during childhood and adolescence: An updated mini review. International Journal of Endocrinology. 2010;2010:472173. doi: 10.1155/2010/472173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden-Adams MM, Stuckey JE, Gaworecki KM, Berger-Ritchie J, Bryant K, Jodice PG, Scott TR, et al. Developmental toxicity in white leghorn chickens following in ovo exposure to perfluorooctane sulfonate (PFOS) Reproductive Toxicology. 2009;27:307–18. doi: 10.1016/j.reprotox.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Perrone S, Tataranno ML, Negro S, Longini M, Marzocchi B, Proietti F, Iacoponi F, Capitani S, Buonocore G. Early identification of the risk for free radical-related diseases in preterm newborns. Early Human Development. 2010;86:241–4. doi: 10.1016/j.earlhumdev.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Pfefferle PI, Pinkenburg O, Renz H. Fetal epigenetic mechanisms and innate immunity in asthma. Current Allergy and Asthma Reports. 2010;10:434–43. doi: 10.1007/s11882-010-0147-6. [DOI] [PubMed] [Google Scholar]
- Philbin VJ, Levy O. Developmental biology of the innate immune response: Implications for neonatal and infant vaccine development. Pediatric Research. 2009;65:98R–105R. doi: 10.1203/PDR.0b013e31819f195d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps JC, Aronoff DM, Curtis JL, Goel D, O’Brien E, Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infection and Immunity. 2010;78:1214–20. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-Zavaleta AP, Garcia-Vargas G, Borja-Aburto VH, Acosta-Saavedra LC, Vera Aguilar E, Gomez-Munoz A, Cebrian ME, Calderon-Aranda ES. Nitric oxide and superoxide anion production in monocytes from children exposed to arsenic and lead in region Lagunera, Mexico. Toxicology and Applied Pharmacology. 2004;198:283–90. doi: 10.1016/j.taap.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedon JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. Journal of Allergy and Clinical Immunology. 2008;122:274–9. doi: 10.1016/j.jaci.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Thiele GM, Alexis NE, Burrell AM, Parks C, Romberger DJ. Organic dust exposure alters monocyte-derived dendritic cell differentiation and maturation. American Journal of Physiology – Lung Cellular and Molecular Physiology. 2009;297:L767–76. doi: 10.1152/ajplung.00107.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott SL, Dunstan JA. Prenatal fatty acid status and immune development: The pathways and the evidence. Lipids. 2007;42:801–10. doi: 10.1007/s11745-007-3030-z. [DOI] [PubMed] [Google Scholar]
- Protonotariou E, Chrelias C, Kassanos D, Kapsambeli H, Trakakis E, Sarandakou A. Immune response parameters during labor and early neonatal life. In Vivo. 2010;24:117–23. [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–29. doi: 10.1016/j.immuni.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–7. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- Reeves GM, Tonelli LH, Anthony BJ, Postolache TT. Precipitants of adolescent suicide: Possible interaction between allergic inflammation and alcohol intake. International Journal of Adolescent Medicine and Health. 2007;19:37–43. doi: 10.1515/ijamh.2007.19.1.37. [DOI] [PubMed] [Google Scholar]
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, et al. Prebiotic effects: Metabolic and health benefits. British Journal of Nutrition. 2010;104(Suppl 2):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- Rochat MK, Ege MJ, Plabst D, Steinle J, Bitter S, Braun-Fahrlander C, Dalphin JC, et al. Maternal vitamin D intake during pregnancy increases gene expression of ILT3 and ILT4 in cord blood. Clinical & Experimental Allergy. 2010;40:786–94. doi: 10.1111/j.1365-2222.2009.03428.x. [DOI] [PubMed] [Google Scholar]
- Rothenbacher D, Weyermann M, Beermann C, Brenner H. Breastfeeding, soluble CD14 concentration in breast milk and risk of atopic dermatitis and asthma in early childhood: Birth cohort study. Clinical & Experimental Allergy. 2005;35:1014–21. doi: 10.1111/j.1365-2222.2005.02298.x. [DOI] [PubMed] [Google Scholar]
- Sacheck J. Pediatric obesity: An inflammatory condition? Journal of Parenteral and Enteral Nutrition. 2008;32:633–7. doi: 10.1177/0148607108324876. [DOI] [PubMed] [Google Scholar]
- Sandberg-Bennich S, Dahlquist G, Kallen B. Coeliac disease is associated with intrauterine growth and neonatal infections. Acta Paediatrica. 2002;91:30–3. doi: 10.1080/080352502753457905. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Casale TB. The role of vitamin D in asthma. Annals of Allergy, Asthma & Immunology. 2010;105:191–9. doi: 10.1016/j.anai.2010.01.013. quiz 200-192, 217. [DOI] [PubMed] [Google Scholar]
- Sawyer K, Mundandhara S, Ghio AJ, Madden MC. The effects of ambient particulate matter on human alveolar macrophage oxidative and inflammatory responses. Journal of Toxicology and Environmental Health A. 2010;73:41–57. doi: 10.1080/15287390903248901. [DOI] [PubMed] [Google Scholar]
- Selgrade MK, Plopper CG, Gilmour MI, Conolly RB, Foos BS. Assessing the health effects and risks associated with children’s inhalation exposures – Asthma and allergy. Journal of Toxicology and Environmental Health A. 2008;71:196–207. doi: 10.1080/15287390701597897. [DOI] [PubMed] [Google Scholar]
- Sengupta M, Bishayi B. Effect of lead and arsenic on murine macrophage response. Drug and Chemical Toxicology. 2002;25:459–72. doi: 10.1081/dct-120014796. [DOI] [PubMed] [Google Scholar]
- Shabani A, Rabbani A. Lead nitrate induced apoptosis in alveolar macrophages from rat lung. Toxicology. 2000;149:109–14. doi: 10.1016/s0300-483x(00)00232-8. [DOI] [PubMed] [Google Scholar]
- Shaheen SO, Newson RB, Ring SM, Rose-Zerilli MJ, Holloway JW, Henderson AJ. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. Journal of Allergy and Clinical Immunology. 2010;126:1141–8. doi: 10.1016/j.jaci.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen SO, Newson RB, Smith GD, Henderson AJ. Prenatal paracetamol exposure and asthma: Further evidence against confounding. International Journal of Epidemiology. 2010;39:790–4. doi: 10.1093/ije/dyq049. [DOI] [PubMed] [Google Scholar]
- Sharkey D, Gardner DS, Symonds ME, Budge H. Maternal nutrient restriction during early fetal kidney development attenuates the renal innate inflammatory response in obese young adult offspring. American Journal of Physiology – Renal Physiology. 2009;297:F1199–207. doi: 10.1152/ajprenal.00303.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SY, Blanchard JF, Bernstein CN. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. American Journal of Gastroenterology. 2010;105:2687–92. doi: 10.1038/ajg.2010.398. [DOI] [PubMed] [Google Scholar]
- Shin KJ, Bae SS, Hwang YA, Seo JK, Ryu SH, Suh PG. 2,2′,4,6,6′-Pentachlorobiphenyl induces apoptosis in human monocytic cells. Toxicology and Applied Pharmacology. 2000;169:1–7. doi: 10.1006/taap.2000.9034. [DOI] [PubMed] [Google Scholar]
- Shin M, Paek D, Yoon C. The relationship between the bone mineral density and urinary cadmium concentration of residents in an industrial complex. Environmental Research. 2011;111:101–9. doi: 10.1016/j.envres.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Sloka S, Silva C, Pryse-Phillips W, Patten S, Metz L, Yong VW. A quantitative analysis of suspected environmental causes of MS. Canadian Journal of Neurological Sciences. 2011;38:98–105. doi: 10.1017/s0317167100011124. [DOI] [PubMed] [Google Scholar]
- Sly PD. The early origins of asthma: Who is really at risk? Current Opinion in Allergy & Clinical Immunology. 2011;11:24–28. doi: 10.1097/ACI.0b013e328342309d. [DOI] [PubMed] [Google Scholar]
- Snyder JE, Filipov NM, Parsons PJ, Lawrence DA. The efficiency of maternal transfer of lead and its influence on plasma IgE and splenic cellularity of mice. Toxicological Sciences. 2000;57:87–94. doi: 10.1093/toxsci/57.1.87. [DOI] [PubMed] [Google Scholar]
- Soderland P, Lovekar S, Weiner DE, Brooks DR, Kaufman JS. Chronic kidney disease associated with environmental toxins and exposures. Advances in Chronic Kidney Disease. 2010;17:254–64. doi: 10.1053/j.ackd.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Progress in Neuropsychopharmacology & Biological Psychiatry. 2010;35:760–8. doi: 10.1016/j.pnpbp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Staples J, Ponsonby AL, Lim L. Low maternal exposure to ultraviolet radiation in pregnancy, month of birth, and risk of multiple sclerosis in offspring: Longitudinal analysis. British Medical Journal. 2010;340:c1640. doi: 10.1136/bmj.c1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunk T, Currie A, Richmond P, Simmer K, Burgner D. Innate immunity in human newborn infants: Prematurity means more than immaturity. Journal of Maternal-Fetal and Neonatal Medicine. 2011;24:25–31. doi: 10.3109/14767058.2010.482605. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual Review of Immunology. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Taranova AG, Maldonado D, 3rd, Vachon CM, Jacobsen EA, Abdala-Valencia H, McGarry MP, Ochkur SI, et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Research. 2008;68:8582–9. doi: 10.1158/0008-5472.CAN-08-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JD. COPD and the response of the lung to tobacco smoke exposure. Pulmonary Pharmacology and Therapeutics. 2010;23:376–83. doi: 10.1016/j.pupt.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Thijs C, Muller A, Rist L, Kummeling I, Snijders BE, Huber M, van Ree R, Simoes-Wust AP, Dagnelie PC, van den Brandt PA. Fatty acids in breast milk and development of atopic eczema and allergic sensitisation in infancy. Allergy. 2011;66:58–67. doi: 10.1111/j.1398-9995.2010.02445.x. [DOI] [PubMed] [Google Scholar]
- Thornton CA. Immunology of pregnancy. Proceedings of the Nutrition Society. 2010;69:357–65. doi: 10.1017/S0029665110001886. [DOI] [PubMed] [Google Scholar]
- Tollanes MC, Moster D, Daltveit AK, Irgens LM. Cesarean section and risk of severe childhood asthma: A population-based cohort study. Journal of Pediatrics. 2008;153:112–16. doi: 10.1016/j.jpeds.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Tomat AL, Costa Mde L, Arranz CT. Zinc restriction during different periods of life: Influence in renal and cardiovascular diseases. Nutrition. 2011;27:392–8. doi: 10.1016/j.nut.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Troy EB, Kasper DL. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Frontiers in Bioscience. 2010;15:25–34. doi: 10.2741/3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacca A, Cormier C, Piras M, Mathieu A, Kahan A, Allanore Y. Vitamin D deficiency and insufficiency in 2 independent cohorts of patients with systemic sclerosis. Journal of Rheumatology. 2009;36:1924–9. doi: 10.3899/jrheum.081287. [DOI] [PubMed] [Google Scholar]
- Verney C, Monier A, Fallet-Bianco C, Gressens P. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. Journal of Anatomy. 2010;217:436–48. doi: 10.1111/j.1469-7580.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mutius E. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Farm lifestyles and the hygiene hypothesis. Clinical & Experimental Immunology. 2010;160:130–5. doi: 10.1111/j.1365-2249.2010.04138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mutius E, Radon K. Living on a farm: Impact on asthma induction and clinical course. Immunology and Allergy Clinics of North America. 2008;28:631–647. IX–X. doi: 10.1016/j.iac.2008.03.010. [DOI] [PubMed] [Google Scholar]
- White OJ, Rowe J, Richmond P, Marshall H, McIntyre P, Wood N, Holt PG. Th2-polarisation of cellular immune memory to neonatal pertussis vaccination. Vaccine. 2010;28:2648–52. doi: 10.1016/j.vaccine.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Wigle DT, Arbuckle TE, Turner MC, Berube A, Yang Q, Liu S, Krewski D. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2008;11:373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Wilson DH, Appleton SL, Taylor AW, Tucker G, Ruffin RE, Wittert G, Hugo G, Goldney RD, Findlay C, Adams RJ. Depression and obesity in adults with asthma: Multiple comorbidities and management issues. Medical Journal of Australia. 2010;192:381–3. doi: 10.5694/j.1326-5377.2010.tb03559.x. [DOI] [PubMed] [Google Scholar]
- Xiao R, Hennings LJ, Badger TM, Simmen FA. Fetal programming of colon cancer in adult rats: Correlations with altered neonatal growth trajectory, circulating IGF-I and IGF binding proteins, and testosterone. Journal of Endocrinology. 2007;195:79–87. doi: 10.1677/JOE-07-0256. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Yoshioka Y, Fujimura M, Yamashita K, Higashisaka K, Nakanishi R, Morishita Y, et al. Potential adjuvant effect of intranasal urban aerosols in mice through induction of dendritic cell maturation. Toxicology Letters. 2010;199:383–8. doi: 10.1016/j.toxlet.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Yu Y, Tsai HJ, Liu X, Mestan K, Zhang S, Pearson C, Ortiz K, Xu X, Zuckerman B, Wang X. The joint association between F5 gene polymorphisms and maternal smoking during pregnancy on preterm delivery. Human Genetics. 2009;124:659–68. doi: 10.1007/s00439-008-0589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host & Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang C, Sun L, Li L, Zhao M. Cytotoxicity of lambda-cyhalothrin on the macrophage cell line RAW 264. Journal of Environmental Sciences (China) 2010;22:428–32. doi: 10.1016/s1001-0742(09)60125-x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Ma JK, Barger MW, Mercer RR, Millecchia L, Schwegler-Berry D, Castranova V, Ma JY. Reactive oxygen species- and nitric oxide-mediated lung inflammation and mitochondrial dysfunction in wild-type and iNOS-deficient mice exposed to diesel exhaust particles. Journal of Toxicology and Environmental Health A. 2009;72:560–70. doi: 10.1080/15287390802706330. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7528–33. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]