Abstract
PhI(OAc)2 serves as a mild and effective oxidant for the synthesis of s-tetrazine derivatives— molecules of emerging significance to the field of bioorthogonal chemistry. This reagent serves as a complementary oxidant to harsher nitrous reagents. Use of PhI(OAc)2 improves the synthesis of 5-amino-di(pyridin-2-yl)-s-tetrazine, a molecule that has been broadly used for cellular imaging and nuclear medicine. The generality of PhI(OAc)2 as the oxidant for tetrazine synthesis is demonstrated for nine tetrazines in 75–98% yield.
Keywords: s-tetrazine, phenyliodonium diacetate, oxidation, bioorthogonal
Bioorthogonal reactions based on the cycloadditions of strained alkenes and cycloalkynes have emerged as powerful tools for chemical biology.1,2 In recent years, the bioorthogonal reactions of strained alkenes with s-tetrazines have served as tools for rapid bioorthogonal labeling, with applications that extend to cell biology,3–5 nuclear medicine6–8 and materials science.9–12 In particular, the bioorthogonal reactions of s-tetrazines with trans-cyclooctene (TCO) derivatives have been shown to be exceptionally rapid, with rate constants in excess of k2 = 106 M−1s−1.13,14
The synthesis of s-tetrazines generally involves the preparation of 1,4-dihydro-s-tetrazine precursors, which are subsequently oxidized to provide s-tetrazine products. Commonly, nitrous reagents (e.g. HONO, NaNO2, isoamylnitrite) are used to oxidize 1,4-dihydro-s-tetrazines to s-tetrazines. This method has good scope and proceeds with yields that are often excellent. However, moderate yields have been reported in several cases, 15–18 and nitrous reagents can fail for substrates with sensitive functionality.19 While oxidants such as chromium trioxide,20 hydrogen peroxide21 and DDQ19,22 have been used for s-tetrazine synthesis, there remains a need to develop general, reliable and efficient methods for preparing s-tetrazines.23
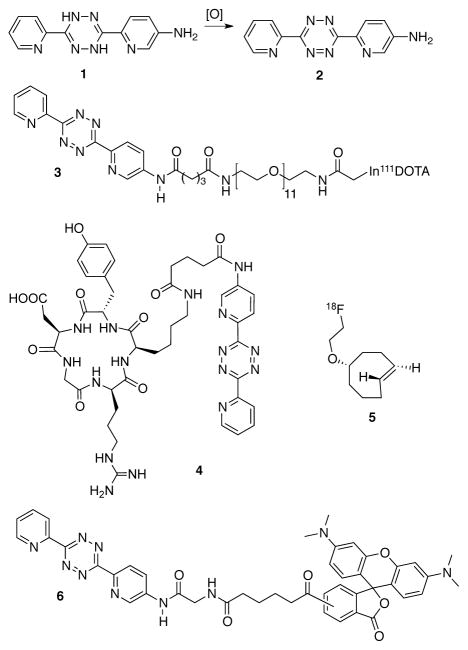
One example of an s-tetrazine that cannot be directly prepared via oxidation with nitrous reagents is the amino substituted di(pyridin-2-yl)-s-tetrazine (2) (Figure 1). Compound 2 was prepared through a statistical combination of 2-cyanopyridine and 5-amino-2-cyanopyridine to give 1,4-dihydro-s-tetrazine 1, which was subsequently oxidized to 2. Attempts to use nitrous reagents were unsuccessful in this synthesis, possibly due to oxidation of the amino functionality. While we found that DDQ served to oxidize 1 in good yield, the hydroquinone byproducts are difficult to remove, and the synthesis could not be readily scaled due to difficult chromatographic steps.19 Developing an improved synthesis of 2 was necessary, as acyl derivatives of 2 have been used in a number of applications (Figure 1). An In(III)-DOTA derivative 3 was used by Robillard and coworkers24 in pretargeted tumor imaging in live mice. Cyclic RGD analog 4 has been used in combination with 18F-labelled TCO 5 for PET imaging in live mice.6 Recently, a 11C-labeled derivative of 2 for PET imaging applications has also been described.25 Fluorescently labeled s-tetrazines such as 6 have been used for site specific labeling in live mammalian cells with proteins that contain either norbornene–15 or TCO–containing26 amino acids. For the preparation of 6, NaNO2 mediated oxidation of a BOC-protected glycine derivative proceeded in moderate yield.26
Figure 1.
Amino-substituted di(pyridin-2-yl)-s-tetrazine (2) cannot be directly prepared from 1 by oxidation with nitrous reagents. Derivatives 3, 4 and 6 have been applied to radiochemical and cellular imaging.
We sought an oxidant for 1,4-dihydro-s-tetrazine derivatives that would be mild, general and produce readily separable byproducts. To develop a more scalable one-pot synthesis of 2 from 2-cyanopyridine (8) and 5-amino-2-cyanopyridine (7), a number of oxidants were surveyed as shown in Table 1. Attempts to use NBS, NCS, hydrogen peroxide, peracetic acid and bromine to oxidize 1 were all unsuccessful, and gave only decomposition products. Compound 2 was formed when mCPBA was used as the oxidant in 27% yield over 2 steps, or 55% in the oxidation step. Both benzoquinone and benzoyl peroxide were more successful oxidants, providing 2 in 32% and 35% overall yields, respectively. As was the case for the DDQ-mediated oxidation, the purification of 2 from the benzoquinone mediated oxidation was complicated by difficult chromatography. Of the oxidants that were surveyed, PhI(OAc)2 was the most successful, providing a yield of 42% over 2 steps (86% in the oxidation step). The byproduct of the reaction— iodobenzene— is readily removed at the stage of purification. Importantly, this process could be carried out without purification of the 1,4-dihydro-s-tetrazine intermediate 1, and s-tetrazine 2 could be prepared on 2 gram scale.
Table 1.
Optimization of the oxidant in the one-pot synthesis of tetrazine 2
| ||
|---|---|---|
| oxidant | yield of oxidationa | two-step yield |
| N-chlorosuccinimide | 0% | 0% |
| N-bromoosuccinimide | 0% | 0% |
| hydrogen peroxide | 0% | 0% |
| peracetic acid | 0% | 0% |
| bromine | 0% | 0% |
| m-CPBA | 55% | 27% |
| benzoyl peroxide | 65% | 32% |
| benzoquinone | 71% | 35% |
| PhI(OAc)2 | 86% | 42% |
Yield of the oxidation step was based on the NMR yield of 49% for the first step.
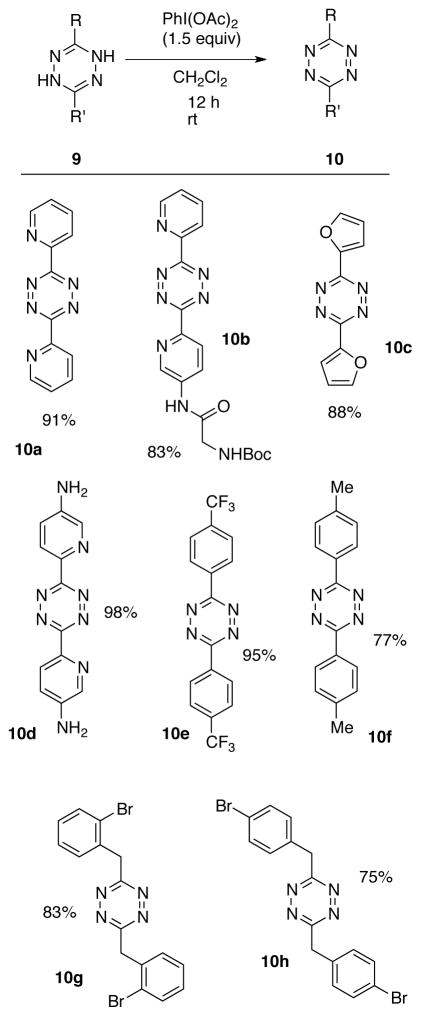
Having identified a suitable oxidant for the preparation of 2, we explored the oxidation of a number of 1,4-dihydro-s-tetrazine derivatives (9) by PhI(OAc)2 as shown in Table 2. Heteroaromatic derivatives 10a-d were prepared in 83–98% yield. Also well tolerated were Diphenyl-s-tetrazines with trifluoromethyl and methyl functionality, as compounds 10e–f could be prepared in 77–95% yield. Dialkyl-s-tetrazines 10g-h could be prepared in 75–83% yield.
Table 2.
Oxidation by PhI(OAc)2 to give s-tetrazines
|
In sum, PhI(OAc)2 has been identified as a mild and effective oxidant for the synthesis of s-tetrazine derivatives, which are of emerging significance to the field of bioorthogonal chemistry. This reagent serves as a complementary method to harsher procedures for the oxidation of 1,4-dihydro-s-tetrazines.
Supplementary Material
Acknowledgments
We gratefully acknowledge support of the National Science Foundation (NSF CHE1112409) and National Institutes of Health (R01 EB014354). For instrumentation support, we thank NIH P20RR017716, NIH S10 RR026962, NSF CHE0840401, CHE- 1229234.
Footnotes
Full experimental details, copies of 1H NMR and 13C NMR spectra are provided.
References and notes
- 1.Sletten EM, Bertozzi CR. Angew Chem Int Ed. 2009;48:6974. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jewett JC, Bertozzi C. Chem Soc Rev. 2010;39:1272. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devaraj NK, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Angew Chem Int Ed. 2009;48:7013. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaraj NK, Hilderbrand S, Upadhyay R, Mazitschek R, Weissleder R. Angew Chem Int Ed. 2010;49:2869. doi: 10.1002/anie.200906120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu DS, Tangpeerachaikul A, Selvaraj R, Taylor MT, Fox JM, Ting AY. J Am Chem Soc. 2011;134:792. doi: 10.1021/ja209325n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvaraj R, Liu S, Hassink M, Huang C-w, Yap L-p, Park R, Fox JM, Li Z, Conti PS. Biorg Med Chem Lett. 2011;21:5011. doi: 10.1016/j.bmcl.2011.04.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Hassink M, Selvaraj R, Yap LP, Park R, Wang H, Chen X, Fox JM, Li Z, Conti PS. Mol Imaging. 2013;12:121. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Liu S, Hassink M, Nair I, Park R, Li L, Todorov I, Fox JM, Li Z, Shively JE, Conti PS, Kandeel F. J Nucl Med. 2013;54:244. doi: 10.2967/jnumed.112.109694. [DOI] [PubMed] [Google Scholar]
- 9.Calahorro AJ, Peñas-Sanjuan A, Melguizo M, Fairen-Jimenez D, Zaragoza G, Fernández B, Salinas-Castillo A, Rodríguez-Diéguez A. Inorg Chem. 2013;52:546. doi: 10.1021/ic302318j. [DOI] [PubMed] [Google Scholar]
- 10.Alge DL, Azagarsamy MA, Donohue DF, Anseth KS. Biomacromolecules. 2013;14:949. doi: 10.1021/bm4000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Šečkutė J, Devaraj NK. Curr Opin Chem Biol. 2013;17:761. doi: 10.1016/j.cbpa.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devaraj NK, Weissleder R. Acc Chem Res. 2011;44:816. doi: 10.1021/ar200037t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossin R, van den Bosch SM, ten Hoeve W, Carvelli M, Versteegen RM, Lub J, Robillard MS. Bioconjugate Chem. 2013;24:1210. doi: 10.1021/bc400153y. [DOI] [PubMed] [Google Scholar]
- 14.Selvaraj R, Fox JM. Curr Opin Chem Biol. 2013;17:753. doi: 10.1016/j.cbpa.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW. Nat Chem. 2012;4:298. doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding J, Li Z, Cui Z, Robertson GP, Song N, Du X, Scoles L. J Poly Sci, Part A (Polym Sci) 2011;49:3374. [Google Scholar]
- 17.Rao GW, Hu WX. Bioorg Med Chem Lett. 2006;16:3702. doi: 10.1016/j.bmcl.2006.04.066. [DOI] [PubMed] [Google Scholar]
- 18.Kaim W, Fees J. Zeitschrift fuer Naturforschung, B: Chemical Sciences. 50:123. [Google Scholar]
- 19.Blackman ML, Royzen M, Fox JM. J Am Chem Soc. 2008;130:13518. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sauer J, Pabst GR, Holland U, Kim H-S, Loebbecke S. Eur J Org Chem. 2001:697. [Google Scholar]
- 21.Coburn MD, Buntain GA, Harris BW, Hiskey MA, Lee KY, Ott DG. J Heterocycl Chem. 1991;28:2049. [Google Scholar]
- 22.Asselin CM, Fraser GC, Hall HK, Jr, Lindsell WE, Padias AB, Preston PN. J Chem Soc, Dalton Trans. 1997;20:3765. [Google Scholar]
- 23.Recently, tetrazines have been synthesized from sequential treatment of tosyl hydrazones with Koser’s reagent, followed by treatment with TBAF. See: Liu H, Wei Y. Tetrahedron Lett. 2013;54:4645.
- 24.Rossin R, Renart Verkerk P, van den Bosch SM, Vulders RCM, Verel I, Lub J, Robillard MS. Angew Chem Int Ed. 2010;49:3375. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- 25.Herth MM, Andersen VL, Lehel S, Madsen J, Knudsen GM, Kristensen JL. Chem Commun. 2013;49:3805. doi: 10.1039/c3cc41027g. [DOI] [PubMed] [Google Scholar]
- 26.Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM, Chin JW. J Am Chem Soc. 2012;134:10317. doi: 10.1021/ja302832g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.