Abstract
Objective
Trimethoprim-sulfamethoxazole (TS) prophylaxis is recommended for HIV-exposed infants until breastfeeding ends and HIV infection has been excluded. Extending prophylaxis with a focus on preventing malaria may be beneficial in high transmission areas. We investigated three regimens for the prevention of malaria in young HIV-exposed children.
Design
Open-label, randomized controlled trial.
Setting
Tororo, Uganda, a rural area with intense, year-round, malaria transmission.
Participants
200 infants aged 4-5 months enrolled and 186 randomized after cessation of breastfeeding and confirmed to be HIV uninfected (median 10 months of age).
Intervention
No chemoprevention, monthly sulfadoxine-pyrimethamine (SP), daily TS, or monthly dihydroartemisinin-piperaquine (DP) given from randomization to 24 months of age.
Main outcome measures
The primary outcome was the incidence of malaria during the intervention period. Secondary outcomes included the incidence of hospitalization, diarrheal illness, or respiratory tract infection; prevalence of anemia and asymptomatic parasitemia; measures of safety; and incidence of malaria over 1 year after the intervention was stopped.
Results
During the intervention, the incidence of malaria in the no chemoprevention group was 6.28 episodes per person-year at risk. Protective efficacy was 69% (95% CI, 53-80%, p<0.001) for DP, 49% (95% CI, 23-66%, p=0.001) for TS, and 9% for SP (95% CI, −35 to 38%, p=0.65). There were no significant differences in any secondary outcomes, with the exception of a lower prevalence of asymptomatic parasitemia in the DP arm.
Conclusions
Monthly chemoprevention with DP was safe and associated with a significant reduction in malaria in young HIV-exposed children.
Keywords: HIV-exposed uninfected infants, malaria chemoprevention, dihydroartemsinin piperaquine, trimethoprim sulfamethoxazole prophylaxis, sulfadoxine pyrimethamine
INTRODUCTION
Due to the success of prevention of mother-to-child transmission (PMTCT) interventions [1], HIV-exposed uninfected (HEU) children (HIV negative children born to HIV-infected mothers) are a growing population in Africa. Importantly, these children have been reported to have an increased risk of morbidity and mortality compared with children born to HIV-uninfected mothers [2-6]. This association is thought to be mediated through several factors, including impaired immunity[7-10], premature cessation of breastfeeding [11], and indirect factors such as increased parental mortality, exposure to maternal infections, and poverty.
Given the increased morbidity and mortality in HEU children and high rates of maternal to child transmission of HIV in the absence of PMTCT interventions, the World Health Organization (WHO) recommends placing HEU children on trimethoprim-sulfamethoxazole (TS) prophylaxis starting at 6 weeks of age [12]. This recommendation was initially made following studies in HIV-infected adults showing that TS effectively reduces morbidity by preventing opportunistic infections [13]. Subsequent studies showed that TS prophylaxis reduces morbidity in HIV-infected [14-16] and HEU children [11, 17, 18]. Current guidelines recommend discontinuation of TS prophylaxis in HEU children after the period of HIV exposure (i.e. after breastfeeding ends and HIV infection has been excluded) [12].
One of the principal benefits of TS prophylaxis in individuals living in Sub-Saharan Africa is the prevention of malaria. Several studies have shown a benefit of TS in preventing malaria in HIV-infected and uninfected children and adults [15, 19, 20]. Further, there is evidence of continued benefit of TS prophylaxis in immune reconstituted HIV-infected children and adults on antiretroviral therapy when administered beyond current guideline recommendations, largely through prevention of malaria [21, 22]. We previously showed that extending TS prophylaxis beyond breastfeeding cessation to 24 months of age was safe and resulted in 39% protective efficacy against malaria among HEU children living in a high malaria transmission setting [23]. In that study, TS did not have significant benefit against diarrhea or respiratory tract infections, two common infectious syndromes associated with HIV infection. We therefore asked whether we could improve upon the efficacy of daily TS in preventing malaria in HEU children beyond the period of HIV exposure by comparing it with two strategies of intermittent preventative therapy (IPT): monthly sulfadoxine pyrimethamine (SP), another antifolate medication used for prevention of malaria during pregnancy and in childhood [24-26], or monthly dihydroartemisinin-piperaquine (DP), a regimen with excellent treatment efficacy against malaria and a prolonged post-treatment prophylactic effect [27]. Children were randomized after the cessation of breastfeeding to one of these three chemoprevention strategies (daily TS, monthly SP, or monthly DP) or to no chemoprevention (discontinue TS prophylaxis) through 24 months of age, and then followed for an additional year after the intervention.
METHODS
Study design, site and population
We performed a randomized, controlled, open-label trial comparing the efficacy and safety of 3 regimens versus no therapy for the prevention of malaria in HEU children living in Tororo District, eastern Uganda, an area with intense year-round malaria transmission and an entomological inoculation rate estimated at 125 infectious bites per person-year in 2011-12[28].
Convenience sampling was used to enroll a cohort of 200 infants 4-5 months of age from the Tororo District Hospital Maternal and Child Health clinic between June 2010 and July 2011. Eligibility criteria included: 1) confirmed HIV positive status of biological mother; 2) confirmed negative HIV DNA PCR test of infant at time of enrollment; 3) infant actively breastfeeding at time of enrollment; 4) residency within 30 km of the study clinic with no intention of moving outside the study area; 5) agreement to come to the study clinic for any illness and to avoid medications outside the study protocol; 6) provision of informed consent by parent/guardian; 7) no history of allergy or sensitivity to any study drugs; 8) absence of active medical problem requiring in-patient evaluation or chronic medical conditions requiring frequent attention; 9) absence of clinically significant electrocardiogram abnormalities, family history of long QT syndrome, or current use of drugs that prolong the QT interval. Only one eligible child was enrolled per household, and at enrollment, all children were prescribed daily TS prophylaxis for the duration of breastfeeding as standard of care and each household was given 2 long lasting insecticide treated bednets (ITNs).
Study drugs and treatment allocation
Six weeks following cessation of breastfeeding, a DNA PCR test was done for each participant and those who remained HIV uninfected were randomized. A randomization list using permuted variable sized blocks of 4, 8, and 12 was computer generated by a member of the study not directly involved in patient care. Randomization was done using pre-made, consecutively numbered, sealed envelopes. Treatment allocation was performed by nurses not involved with patient care. Study drugs were dosed as: TS (Co-trimoxazole, Kampala Pharmaceutical Industries, Uganda) single dose once daily, SP (Kamsidar, Kampala Pharmaceutical Industries, Uganda) single dose each month, and DP (Duo-Cotexin, Holley-Cotec, Beijing, China) once daily for 3 consecutive days each month; each provided for administration at home according to weight-based guidelines. At the time of treatment allocation parents/guardians were given a 2 month supply of drugs and a diary with dates for dosing and check-offs to indicate administration. Parents/guardians were instructed to re-administer drugs if children vomited within 30 minutes of administration and to bring children to the clinic if they vomited again. During each visit to the study clinic parent/guardians were questioned about study drug use.
Study Procedures
Participants received all of their medical care at a designated study clinic open every day. Children who presented with a documented fever (tympanic temperature ≥ 38.0°C) or history of fever in the previous 24 hours had blood obtained by finger prick for a thick blood smear. If the smear was positive, the patient was diagnosed with malaria and a complete blood count (CBC) and thin blood smear for parasite speciation were performed. Episodes of uncomplicated malaria were treated with artemether-lumefantrine (AL). AL was administered twice a day for 3 days, with the first dose each day directly observed in the clinic and the second administered at home. Episodes of complicated malaria (severe malaria or danger signs) [29] or treatment failures occurring within 14 days of prior therapy were treated with quinine. Routine evaluations, including thick blood smears and assessment of adherence with ITNs and study drugs, were done monthly. CBC, glucose, and alanine aminotransferase (ALT) levels were assessed every 4 months. HIV DNA PCR tests were done every 60 days during breastfeeding, at 6 weeks following cessation of breastfeeding and at 18 months of age. Adverse events were assessed and graded according to severity (mild, moderate, severe, life-threatening) using standardized criteria at every clinic visit. Diagnosis of incident episodes of non-malarial illnesses, including diarrheal illnesses and respiratory tract infections, were based on a pre-specified list of diagnostic criteria developed by the study team. Medications with antimalarial activity were avoided for the treatment of non-malarial illnesses when possible. Antihelminthics, iron sulfate, and vitamin A were prescribed following Integrated Management of Childhood Illness guidelines.
Chemoprevention was stopped at 24 months of age and study participants were followed-up 1 additional year until they reached 36 months of age. Study participants were prematurely withdrawn from the study for: 1) documented HIV infection by DNA PCR (these children were referred to an appropriate HIV care center), 2) movement out of the study area, 3) failure to be seen in the study clinic for > 60 consecutive days, 4) withdrawal of informed consent, or 5) inability to comply with the study schedule and procedures.
Laboratory procedures
Thick and thin blood smears were stained with 2% Giemsa for 30 minutes. Parasite density was estimated by counting the number of asexual parasites per 200 white blood cells and assuming a white blood cell count of 8,000 per μL. A smear was deemed negative if no parasites were seen in 100 high powered fields. Microscopy quality control included re-reading all blood smears and resolution of any discrepancies by a third microscopist. Piperaquine (PQ) drug levels were measured from capillary blood collected on filter paper on the day malaria was diagnosed among study participants randomized to monthly DP, as previously described [30].
Statistical analysis
The study was designed to test the hypotheses that chemoprevention will lower the incidence of malaria compared to no chemoprevention, and that the optimal chemoprevention regimen will be DP. We assumed that the incidence of malaria would be 1.85 episodes per person year with TS based on a prior cohort study in the same area [23], and thus that we would need to enroll 50 participants in each arm to detect a reduction in the incidence of malaria in either the monthly SP or DP arms (two-sided significance level = 0.05) compared to the daily TS arm of 48% or greater with 80% power at 95% significance (two-sided), allowing for 10% loss to follow-up.
Data were double-entered and verified in Microsoft Access and statistical analyses performed using Stata, version 12 (Stata-Corp). All analyses used an intention-to-treat approach for participants who were randomized to therapy. The primary outcome was the incidence of malaria, defined as the number of incident episodes per time at risk, during the period the intervention was given. Treatments within 14 days of a prior episode were not considered incident events. Time at risk was from the day following the initiation of study drugs to the last day of observation, minus 14 days after each treatment for malaria. Secondary outcomes included the incidence of complicated malaria, hospitalizations, diarrheal illnesses, respiratory tract infections, and serious adverse events or adverse events of moderate or greater severity (grade 3-4); the prevalence of moderate-severe anemia (hemoglobin < 8 gm/dL) at the time of each episode of malaria and upon routine testing every 4 months and the prevalence of parasitemia and gametocytemia seen in monthly routine blood smears. To assess the impact of chemoprevention on the development of naturally acquired immunity, the incidence of malaria, complicated malaria, and hospitalizations were compared after the intervention was stopped, between 24-36 months of age.
Incidence outcomes were compared using a negative binomial regression model, and prevalence outcomes and measure of compliance with ITNs at the time of monthly assessments were compared using generalized estimating equations with adjustment for repeated measures. For all analyses, only the assigned treatment arm was included as a covariate, with the exception of a multivariate analysis performed for the primary outcome of malaria incidence, which also included age at randomization and incidence of malaria prior to randomization, covariates thought to be potential independent risk factors for malaria. Measures of association were expressed as protective efficacy (PE = 1 minus the incident rate ratio or prevalence ratio) during the intervention and incident rate ratios after the intervention was stopped. A p value < 0.05 was considered statistically significant.
Ethics statement
Ethical approval was obtained from the Uganda National Council for Science and Technology, the Makerere University School of Medicine Research and Ethics Committee, and the University of California, San Francisco Committee on Human Research.
RESULTS
Trial profile and baseline characteristics
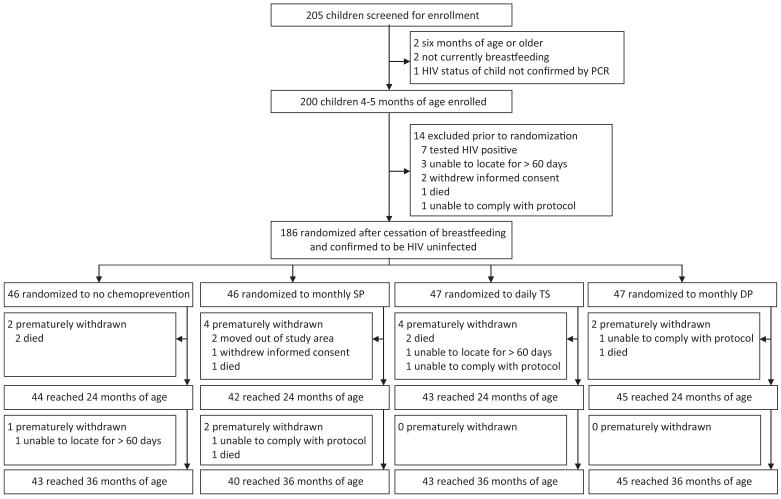
We screened 205 children and 200 were enrolled (Figure). Of enrolled children, 14 (7.0%) were excluded before randomization, including 7 who became infected with HIV. The 186 enrolled children were randomized to one of the four treatment arms at a median age of 10 months: 46 to no chemoprevention, 46 to monthly SP, 47 to daily TS, and 47 to monthly DP. Baseline characteristics were similar across treatment arms (Table 1). Prior to randomization, the incidence of malaria was lower in children subsequently randomized to all three treatment arms compared to children randomized to no chemoprevention, although these differences were not statistically significant (Table 1). Among the 186 infants who were randomized, 174 (95.5%) and 171 (91.9%) were followed-up to 24 months and 36 months of age, respectively (Figure). At monthly assessments 96.8% reported sleeping under an ITN the prior evening, without significant differences between the study arms (p=0.31).
Figure.
Trial Profile.
Table 1. Baseline characteristics of study participants randomized to the intervention.
| Characteristic | Intervention arm |
|||
|---|---|---|---|---|
| Control (n=46) |
Monthly SP (n=46) |
Daily TS (n=47) |
Monthly DP (n=47) |
|
| At the time of enrollment | ||||
|
| ||||
| Age in months, mean (SD) | 4.8 (0.7) | 4.7 (0.7) | 4.8 (0.8) | 4.7 (0.8) |
| Female gender, n (%) | 22 (47.8%) | 21 (45.7%) | 27 (57.5%) | 25 (53.2%) |
| Slept under any bednet prior night, n (%) | 27 (58.7%) | 27 (58.7%) | 26 (55.3%) | 29 (61.7%) |
| Slept under ITN prior night, n (%) | 22 (47.8%) | 24 (52.2%) | 19 (40.4%) | 22 (46.8%) |
| Taking TS prophylaxis, n (%) | 5 (10.9%) | 7 (15.2%) | 5 (10.6%) | 9 (19.2%) |
| Stunted1, n (%) | 6 (13.0%) | 7 (15.2%) | 6 (12.8%) | 7 (14.9%) |
| Wasted2, n (%) | 0 | 4 (8.7%) | 1 (2.1%) | 4 (8.5%) |
| Hemoglobin (gm/dL), mean (SD) | 10.1 (1.2) | 10.0 (1.4) | 10.1 (1.1) | 10.0 (1.5) |
| Positive thick blood smear, n (%) | 8 (17.4%) | 4 (8.7%) | 8 (17.0%) | 6 (12.8%) |
|
| ||||
| At the time of randomization | ||||
|
| ||||
| Age in months, median (range) | 10.0 (6.0-18.0) | 9.3 (6.5-18.8) | 11.6 (6.2-18.7) | 10.3 (6.5-20.0) |
| Duration of follow-up in months, median (range) | 5.7 (1.9-13.4) | 5.1 (2.0-14.8) | 6.1 (1.8-14.7) | 5.2 (1.8-15.9) |
| Incidence of malaria per PYAR3 (95% CI) | 2.12 (1.57-2.80) | 1.51 (1.05-2.10) | 1.63 (1.18-2.20) | 1.82 (1.32-2.44) |
Length-for-age Z-score < −2
Weight-for-age Z-score < −2
Person-year at risk from date of enrollment to date of randomization
Protective efficacy of chemoprevention regimens against malaria
From the time of randomization to 24 months of age, the incidence of malaria in the no chemoprevention group was 6.28 episodes per person year (Table 2). After adjusting for age at randomization and incidence of malaria prior to randomization, monthly DP had the greatest protective efficacy (PE) against malaria (PE=69%, 95% CI, 53-80% p<0.001), followed by daily TS (PE=49%, 95% CI, 23-66%, p=0.001). Monthly SP did not have significant PE compared to the no chemoprevention group (PE=9%, 95% CI, −35-38%, p=0.65). In comparison to daily TS, monthly DP was associated with a marginally significant protective efficacy (PE = 39%, 95% CI 0-62%, P=0.05). In all 4 arms the incidence of malaria increased with age and the protective efficacies in the daily TS and monthly DP arms were greater for children 16 months or younger compared to those 17-24 months of age (Table 2, Supplemental Figure).
Table 2. Protective efficacy against incident episodes of malaria stratified by age.
| Treatment arm |
Randomization to 24 months of age |
Randomization to 16 months of age |
17 to 24 months of age |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of cases |
PYAR1 | Incidenc e per PYAR1 |
PE2 (95% CI) | p value | Incidence per PYAR1 |
PE2 (95% CI) | p value | Incidence per PYAR1 |
PE2 (95% CI) | p value | |
| Control | 240 | 38.2 | 6.28 | reference | - | 5.42 | reference | - | 7.04 | reference | - |
| Monthly SP | 182 | 40.4 | 4.50 | 9% (−35 to 38) | 0.65 | 3.72 | 2% (−64 to 42) | 0.93 | 5.22 | 14% (−29 to 43) | 0.47 |
| Daily TS | 116 | 40.6 | 2.86 | 49% (23 to 66) | 0.001 | 1.70 | 63% (34 to 79) | 0.001 | 3.79 | 42% (12 to 62) | 0.01 |
| Monthly DP | 82 | 44.7 | 1.83 | 69% (53 to 80) | <0.001 | 0.90 | 84% (69 to 92) | <0.001 | 2.67 | 61% (39 to 75) | <0.001 |
PYAR = person-year at risk
PE = protective efficacy adjusted for age at randomization and incidence of malaria prior to randomization
The proportion of assigned doses of study medications administered at home based on diaries completed by the primary care givers were 93% for SP, 99% for TS, and 96% for DP. To further explore adherence among study participants randomized to monthly DP, PQ blood concentrations were measured at the time of each episode of malaria. Among 82 episodes, 81 samples were available for analysis. For 3 episodes PQ levels were > 100 ng/mL and the primary caregivers reported giving the DP within the prior 24 hours, suggesting the coincidental onset of malaria just before the scheduled time for monthly DP dosing. For the remaining 78 samples PQ levels were below the level of detection (< 10 ng/mL) on the day malaria was diagnosed in 53% of episodes, suggesting that a complete dose of DP was not administered in the previous month [31, 32]. Thus, despite caregiver reports to the contrary, our results suggest frequent non-adherence with study dosing schedules.
Protective efficacy of chemoprevention regimens against secondary outcomes
Overall, complicated malaria was uncommon. Of a total of 620 treatments for malaria during the intervention period, 18 (2.9%) were for complicated malaria; 10 for severe malaria (9 severe anemia and 1 respiratory distress) and 8 for danger signs (6 single convulsions and 2 persistent vomiting). There were no significant differences in the incidence of complicated malaria, hospitalization, diarrheal illnesses, respiratory tract infections or the prevalence of moderate to severe anemia or gametocytemia between the treatment arms (Table 3). Monthly DP was significantly protective against asymptomatic parasitemia compared to the no chemoprevention arm (PE= 88%, 95% CI, 48-91%, p=0.001). Daily TS and monthly SP were not significantly protective against asymptomatic parasitemia (Table 3).
Table 3. Comparative effectiveness against secondary outcomes.
| Treatment arm | Number of cases |
PYAR | Incidence per PYAR |
PE (95% CI) | p value |
|---|---|---|---|---|---|
| Complicated malaria | |||||
|
| |||||
| Control | 5 | 47.3 | 0.106 | reference | - |
| Monthly SP | 8 | 47.2 | 0.170 | −57% (−417 to 52) | 0.46 |
| Daily TS | 3 | 44.9 | 0.067 | 37% (−181 to 86) | 0.55 |
| Monthly DP | 2 | 47.8 | 0.042 | 61% (−110 to 93) | 0.27 |
|
| |||||
| All-cause hospital admissions | |||||
|
| |||||
| Control | 10 | 47.3 | 0.211 | reference | - |
| Monthly SP | 17 | 47.2 | 0.360 | −60% (−342 to 42) | 0.36 |
| Daily TS | 8 | 44.9 | 0.178 | 21% (−147 to 75) | 0.69 |
| Monthly DP | 6 | 47.8 | 0.126 | 46% (−81 to 84) | 0.32 |
|
| |||||
| Diarrheal illnesses | |||||
|
| |||||
| Control | 101 | 47.3 | 2.13 | reference | - |
| Monthly SP | 82 | 47.2 | 1.74 | 21% (−20 to 47) | 0.27 |
| Daily TS | 98 | 44.9 | 2.18 | −2% (−53 to 32) | 0.91 |
| Monthly DP | 77 | 47.8 | 1.61 | 25% (−13 to 51) | 0.17 |
|
| |||||
| Respiratory tract infections | |||||
|
| |||||
| Control | 146 | 47.3 | 3.08 | reference | - |
| Monthly SP | 144 | 47.2 | 3.05 | 6% (−33 to 33) | 0.73 |
| Daily TS | 158 | 44.9 | 3.52 | −8% (−52 to 23) | 0.65 |
| Monthly DP | 170 | 47.8 | 3.56 | −11 (−57 to 21) | 0.53 |
|
| |||||
| Incident episodes of malaria after intervention stopped (24 to 36 months of age) | |||||
|
| |||||
| Control | 294 | 32.4 | 9.08 | reference | - |
| Monthly SP | 219 | 32.5 | 6.75 | 0.83 (0.55-1.24) | 0.36 |
| Daily TS | 266 | 32.7 | 8.13 | 1.06 (0.71-1.59) | 0.77 |
| Monthly DP | 243 | 35.8 | 6.78 | 0.78 (0.52-1.16) | 0.22 |
|
| |||||
| Complicated malaria after intervention stopped (24 to 36 months of age) | |||||
|
| |||||
| Control | 7 | 43.6 | 0.161 | reference | - |
| Monthly SP | 6 | 40.9 | 0.147 | 1.00 (0.16-6.26) | 0.99 |
| Daily TS | 5 | 43.0 | 0.116 | 0.73 (0.12-4.66) | 0.74 |
| Monthly DP | 2 | 45.1 | 0.044 | 0.28 (0.03-2.35) | 0.24 |
|
| |||||
| All cause hospital admissions after intervention stopped (24 to 36 months of age) | |||||
|
| |||||
| Control | 20 | 43.6 | 0.459 | reference | - |
| Monthly SP | 13 | 40.9 | 0.318 | 0.99 (0.18-5.58) | 0.99 |
| Daily TS | 8 | 43.0 | 0.186 | 0.41 (0.07-2.40) | 0.32 |
| Monthly DP | 4 | 45.1 | 0.089 | 0.20 (0.03-1.29) | 0.09 |
|
| |||||
| Prevalence of moderate-severe anemia (Hb < 8 gm/dL) | |||||
|
| |||||
| Control | 42/362 (11.6%) | reference | - | ||
| Monthly SP | 46/347 (13.3%) | −11% (−128 to 46) | 0.77 | ||
| Daily TS | 22/285 (7.7%) | 7% (−100 to 57) | 0.86 | ||
| Monthly DP | 25/282 (8.9%) | 27% (−62 to 67) | 0.44 | ||
|
| |||||
| Prevalence of asymptomatic parasitemia | |||||
|
| |||||
| Control | 37/228 (16.2%) | reference | - | ||
| Monthly SP | 23/228 (10.1%) | 41% (−17 to 70) | 0.13 | ||
| Daily TS | 22/282 (7.8%) | 46% (−8 to 72) | 0.08 | ||
| Monthly DP | 12/369 (3.3%) | 88% (48 to 91) | 0.001 | ||
|
| |||||
| Prevalence of gametocytemia | |||||
|
| |||||
| Control | 10/228 (4.4%) | reference | - | ||
| Monthly SP | 3/228 (1.3%) | 67% (−84 to 94) | 0.21 | ||
| Daily TS | 4/282 (1.4%) | 61% (−44 to 89) | 0.16 | ||
| Monthly DP | 6/369 (1.6%) | 61% (−41 to 89) | 0.15 | ||
PYAR = person-year at risk. PE = protective efficacy. SP = sulfadoxine-pyrimethamine. TS = trimethoprim-sulfamethoxazole. DP = dihydroartemisinin-piperaquine.
The incidence of malaria was 9.08 episodes per person year at risk among children 24-36 months of age who had not received chemoprevention (Table 3). Following the cessation of the interventions, the incidence of malaria was 6.75, 8.13, and 6.78 episodes per person year for children who had been on monthly SP, daily TS and monthly DP, respectively. There were no significant differences in these incidences, although there were trends towards a lower incidence in children randomized to SP or DP. Similarly, there were no significant differences in the incidence of complicated malaria or hospitalizations between the treatment arms in the year after the interventions were stopped, although there were trends towards a lower incidence in children randomized to DP (Table 3).
Safety outcomes
Among those assigned to study drugs, there were 144 grade 3 or 4 adverse events, but only 8 (5.6%) were classified as possibly related to study drugs (3 for TS and 5 for DP, Table 4). The incidence of grade 3 or 4 adverse events, anemia and elevated temperature were significantly lower in the DP arm compared to the control arm. The incidence of grade 3 or 4 anemia was significantly lower in the TS arm compared to the control arm (p=0.009). There were no significant differences in the incidence of serious adverse events between the treatment arms (Table 4).
Table 4. Comparative safety outcomes.
| Characteristic | Number of events (incidence per PYAR1) by treatment arm |
|||
|---|---|---|---|---|
| Control | Monthly SP | Daily TS | Monthly DP | |
| All grade 3-4 adverse events | 62 (1.214) | 77 (1.478) | 32 (0.659) | 35 (0.678)3 |
| Individual grade 3-4 adverse events2 | ||||
| Anemia | 36 (0.705) | 32 (0.631) | 10 (0.206) 4 | 15 (0.291) 3 |
| Elevated temperature | 22 (0.431) | 27 (0.532) | 10 (0.206) | 9 (0.174) 3 |
| Thrombocytopenia | 0 | 6 (0.118) | 0 | 3 (0.058) |
| Elevated aspartate aminotransferase | 2 (0.039) | 1 (0.020) | 2 (0.041) | 2 (0.039) |
| Malnutrition | 1 (0.020) | 1 (0.020) | 1 (0.021) | 2 (0.039) |
| Grade 3-4 events possibly related to study drugs | N/A | 0 | 3 (0.062) | 5 (0.097) |
| All serious adverse events | 21 (0.411) | 23 (0.453) | 16 (0.330) | 10 (0.194) |
PYAR = person-year at risk
Only includes those with at least 5 total events
p-value< 0.05 compared to control group
p-value< 0.01 compared to control group
DISCUSSION
We evaluated the efficacy and safety of 3 different malaria chemoprevention strategies given to HEU children beyond the period of HIV exposure when TS prophylaxis is normally discontinued: continuation of daily TS; monthly SP; or monthly DP. Despite the use of ITNs, the incidence of malaria in those discontinuing TS was remarkably high, at more than 6 episodes per person-year at risk from randomization to 24 months of age, and more than 9 episodes per person-year at risk from 24-36 months of age. Monthly SP provided no significant protection against malaria. Continuation of daily TS prophylaxis reduced the incidence of malaria by 49%. Monthly DP was the most effective regimen, reducing the incidence of malaria by 69% and the prevalence of asymptomatic parasitemia by 88%. None of the regimens provided significant protection against diarrheal illnesses or respiratory tract infections. All three regimens were safe and there were no differences in the incidence of malaria between the treatment arms after the intervention was stopped.
Current WHO guidelines recommend discontinuation of TS prophylaxis in HEU children after breastfeeding cessation, assuming that the main benefit of prophylaxis is prevention of opportunistic infections, such as Pneumocystis pneumonia, if a child is infected with HIV [12]. Several studies have shown that a major benefit of TS prophylaxis in sub-Saharan Africa is prevention of malaria [15, 19, 20], and that this benefit extends beyond immune reconstitution in HIV-infected children and adults on antiretroviral therapy [21, 22]. We previously showed that extending TS prophylaxis in HEU children beyond the period of HIV exposure was safe and modestly efficacious at preventing malaria [23]. In the present study, daily TS again provided modest benefit against malaria, without significant benefit against diarrheal or respiratory tract infections, suggesting that the main benefit in extending TS prophylaxis in HEU children in this setting is the prevention of uncomplicated malaria. The other intensive antifolate regimen studied, monthly SP, offered no significant protection against malaria, which may not be surprising given the high prevalence of mutations in P. falciparum target genes associated with antifolate resistance in Uganda [23, 33]. Monthly DP was the most protective against malaria and asymptomatic parasitemia. Importantly, the true protective efficacies of all 3 studied regimens may have been underestimated, as treatments were not directly observed, and measurements of PQ blood levels suggested frequent noncompliance with chemoprevention regimens. Overall, our results were not surprising given recent studies showing excellent protective efficacy of monthly DP in African children [34, 35], including a parallel study showing 58% protective efficacy of DP compared to no chemoprevention in HIV-unexposed children in Tororo [30].
We were also interested in the subsequent impact of chemoprevention after cessation of study drugs, given concerns that chemoprevention might delay the acquisition of antimalarial immunity, leading to a rebound in the incidence of malaria after the intervention is stopped [36, 37]. Interestingly, recent studies of IPT have not been associated with a rebound in the incidence of malaria [25], with one study showing a sustained reduction in the incidence of malaria in the year after the intervention was stopped, suggesting that IPT could actually enhance the development of naturally acquired immunity [38]. In the present study, in the year following the discontinuation of study drugs, the incidence of malaria was similar in children randomized to the 3 chemopreventive regimens and in children who had not received prior chemoprevention, suggesting that, in this high transmission setting, the use of chemopreventive regimens with a range of protective efficacies did not negatively impact the development of antimalarial immunity. Further, there were trends towards lower incidences of malaria, complicated malaria, and all cause hospital admissions after cessation of chemoprevention in children randomized to monthly DP, suggesting a more rapid acquisition of immunity in those children.
In conclusion, we found that, in an area of high malaria endemicity, continuing daily TS or starting monthly DP in HEU children from the time of breastfeeding cessation through 24 months of age had significant protective efficacy against malaria in comparison to the current standard of care, discontinuation of TS prophylaxis. Monthly DP had the greatest protective efficacy, was safe, and was not associated with rebound in malaria morbidity in the 1 year following the intervention. Given the rising number of HEU children living in malaria endemic settings, our results argue strongly for continued malaria prophylaxis beyond the period of HIV exposure. Additional studies are needed to evaluate optimal chemoprevention strategies in breastfeeding children in the era of Option B+, as well as strategies to optimize treatment compliance and maintain surveillance for potential selection of drug-resistant parasites.
Supplementary Material
Supplemental Figure: Incidence of malaria over age, stratified by assigned study arm. PYAR, person-years at risk.
ACKNOWLEDGEMENTS
We thank the study participants, parents and guardians of study participants, study staff, Tororo Community Advisory Board, and the Drug Safety and Monitoring Board for their support and participation in the study. The study was funded by the National Institutes of Health (HD059454). PJ was supported by K23 AI100949. Holley-Cotec provided the dihydroartemisinin-piperaquine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
MRK, DVH, PR, TDC, JA, and GD conceived and designed the study. VB, JK, SK, FM, MKM, AK, and BO supervised the enrolment and follow-up of patients. VB, JK, PJ, and GD assisted with verification, analysis, and interpretation of the data. MK drafted the original version of the manuscript. FA and LH were responsible for measurements of piperaquine drug levels. All authors participated in the writing of the manuscript, and read and approved the final manuscript.
Funding: National Institutes of Health HD059454
Footnotes
Trial registration: ClinicalTrials.gov number P01HD05945.
REFERENCES
- 1.UNAIDS [Accessed July 25, 2014];UNAIDS report on the global AIDS epidemic 2013. 2013 Available at http://www.unaids.org/en/dataanalysis/knowyourepidemic/
- 2.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41:504–508. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 3.Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J. 2011;30:45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 4.Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PLoS One. 2012;7:e47337. doi: 10.1371/journal.pone.0047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakiyingi JS, Bracher M, Whitworth JA, Ruberantwari A, Busingye J, Mbulaiteye SM, et al. Child survival in relation to mother’s HIV infection and survival: evidence from a Ugandan cohort study. AIDS. 2003;17:1827–1834. doi: 10.1097/00002030-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 6.Ng’weshemi J, Urassa M, Isingo R, Mwaluko G, Ngalula J, Boerma T, et al. HIV impact on mother and child mortality in rural Tanzania. J Acquir Immune Defic Syndr. 2003;33:393–404. doi: 10.1097/00126334-200307010-00015. [DOI] [PubMed] [Google Scholar]
- 7.Afran L, Garcia Knight M, Nduati E, Urban BC, Heyderman RS, Rowland-Jones SL. HIV-exposed uninfected children: a growing population with a vulnerable immune system? Clin Exp Immunol. 2014;176:11–22. doi: 10.1111/cei.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–3871. [PubMed] [Google Scholar]
- 9.Farquhar C, Nduati R, Haigwood N, Sutton W, Mbori-Ngacha D, Richardson B, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40:494–497. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazzola TN, da Silva MT, Abramczuk BM, Moreno YM, Lima SC, Zorzeto TQ, et al. Impaired Bacillus Calmette-Guerin cellular immune response in HIV-exposed, uninfected infants. AIDS. 2011;25:2079–2087. doi: 10.1097/QAD.0b013e32834bba0a. [DOI] [PubMed] [Google Scholar]
- 11.Taha TE, Hoover DR, Chen S, Kumwenda NI, Mipando L, Nkanaunena K, et al. Effects of cessation of breastfeeding in HIV-1-exposed, uninfected children in Malawi. Clin Infect Dis. 2011;53:388–395. doi: 10.1093/cid/cir413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UNICEF. WHOa [Accessed July 25, 2014];Co-trimoxazole prophylaxis for HIV-exposed and HIV-infected infants and children: Practical approaches to implementation and scale up. 2009 Available at http://www.unicef.org/aids/files/CotrimoxazoleGuide_2009.pdf.
- 13.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, et al. Cotrimo-CI Study Group Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 14.Chintu C, Bhat GJ, Walker AS, Mulenga V, Sinyinza F, Lishimpi K, et al. Cotrimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–1871. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 15.Kamya MR, Gasasira AF, Achan J, Mebrahtu T, Ruel T, Kekitiinwa A, et al. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. Aids. 2007;21:2059–2066. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 16.Mulenga V, Ford D, Walker AS, Mwenya D, Mwansa J, Sinyinza F, et al. Effect of cotrimoxazole on causes of death, hospital admissions and antibiotic use in HIV-infected children. AIDS. 2007;21:77–84. doi: 10.1097/QAD.0b013e3280114ed7. [DOI] [PubMed] [Google Scholar]
- 17.Kourtis AP, Wiener J, Kayira D, Chasela C, Ellington SR, Hyde L, et al. Health outcomes of HIV-exposed uninfected African infants. Aids. 2013;27:749–759. doi: 10.1097/QAD.0b013e32835ca29f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dow A, Kayira D, Hudgens M, Van Rie A, King CC, Ellington S, et al. Effects of cotrimoxazole prophylactic treatment on adverse health outcomes among HIV-exposed, uninfected infants. Pediatr Infect Dis J. 2012;31:842–847. doi: 10.1097/INF.0b013e31825c124a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mermin J, Ekwaru JP, Liechty CA, Were W, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367:1256–1261. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 20.Thera MA, Sehdev PS, Coulibaly D, Traore K, Garba MN, Cissoko Y, et al. Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis. 2005;192:1823–1829. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S, Nahirya-Ntege P, Keishanyu R, Nathoo K, et al. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med. 2014;370:41–53. doi: 10.1056/NEJMoa1214901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell JD, Moore D, Degerman R, Kaharuza F, Were W, Muramuzi E, et al. HIV-infected ugandan adults taking antiretroviral therapy with CD4 counts >200 cells/muL who discontinue cotrimoxazole prophylaxis have increased risk of malaria and diarrhea. Clin Infect Dis. 2012;54:1204–1211. doi: 10.1093/cid/cis013. [DOI] [PubMed] [Google Scholar]
- 23.Sandison TG, Homsy J, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342:d1617. doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . Intermittent Preventive Treatment of malaria in pregnancy using Sulfadoxine-Pyrimethamine (IPTp-SP) Geneva: Oct, 2012. Updated World Health Organization Policy Recommendation. 2012. [Google Scholar]
- 25.Aponte JJ, Schellenberg D, Egan A, Breckenridge A, Carneiro I, Critchley J, et al. Efficacy and safety of intermittent preventive treatment with sulfadoxine-pyrimethamine for malaria in African infants: a pooled analysis of six randomised, placebo-controlled trials. Lancet. 2009;374:1533–1542. doi: 10.1016/S0140-6736(09)61258-7. [DOI] [PubMed] [Google Scholar]
- 26.WHO [accessed October 8, 2013];World Health Organization policy recommendation on intermittent preventive treatment during infancy with sulphadoxine-pyrimethamine (SP-IPTi) for Plasmodium falciparum malaria control in Africa. 2010 http://www.who.int/malaria/news/policy_recommendation_IPTi_032010/en/
- 27.White NJ. Intermittent presumptive treatment for malaria. PLoS Med. 2005;2:e3. doi: 10.1371/journal.pmed.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilama M, Smith DL, Hutchinson R, Kigozi R, Yeka A, Lavoy G, et al. Estimating the annual entomological inoculation rate for Plasmodium falciparum transmitted by Anopheles gambiae s.l. using three sampling methods in three sites in Uganda. Malar J. 2014;13:111. doi: 10.1186/1475-2875-13-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. World Health Organization [Accessed April 10, 2014];WHO guidelines for the treatment of malaria. 2010 Available at http://www.who.int/malaria/publications/atoz/9789241547925/en/ 2010. [PubMed]
- 30.Bigira V, Kapisi J, Clark TD, Kinara S, Mwangwa F, Muhindo MK, et al. Protective efficacy and safety of three antimalarial regimens for the prevention of malaria in young Ugandan children: a randomized controlled trial. PLOS Medicine. 2014 doi: 10.1371/journal.pmed.1001689. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Creek DJ, Bigira V, McCormack S, Arinaitwe E, Wanzira H, Kakuru A, et al. Pharmacokinetic predictors for recurrent malaria after dihydroartemisinin-piperaquine treatment of uncomplicated malaria in Ugandan infants. J Infect Dis. 2013;207:1646–1654. doi: 10.1093/infdis/jit078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarning J, Zongo I, Some FA, Rouamba N, Parikh S, Rosenthal PJ, et al. Population pharmacokinetics and pharmacodynamics of piperaquine in children with uncomplicated falciparum malaria. Clin Pharmacol Ther. 2012;91:497–505. doi: 10.1038/clpt.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malamba S, Sandison T, Lule J, Reingold A, Walker J, Dorsey G, et al. Plasmodium falciparum dihydrofolate reductase and dihyropteroate synthase mutations and the use of trimethoprim-sulfamethoxazole prophylaxis among persons infected with human immunodeficiency virus. Am J Trop Med Hyg. 2010;82:766–771. doi: 10.4269/ajtmh.2010.08-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cisse B, Cairns M, Faye E, ND O, Faye B, Cames C, et al. Randomized trial of piperaquine with sulfadoxine-pyrimethamine or dihydroartemisinin for malaria intermittent preventive treatment in children. PLoS One. 2009;4:e7164. doi: 10.1371/journal.pone.0007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, et al. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis. 2014;58:1404–1412. doi: 10.1093/cid/ciu150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aponte JJ, Menendez C, Schellenberg D, Kahigwa E, Mshinda H, Vountasou P, et al. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med. 2007;4:e242. doi: 10.1371/journal.pmed.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menendez C, Kahigwa E, Hirt R, Vounatsou P, Aponte JJ, Font F, et al. Randomised placebo-controlled trial of iron supplementation and malaria chemoprophylaxis for prevention of severe anaemia and malaria in Tanzanian infants. Lancet. 1997;350:844–850. doi: 10.1016/S0140-6736(97)04229-3. [DOI] [PubMed] [Google Scholar]
- 38.Schellenberg D, Menendez C, Aponte JJ, Kahigwa E, Tanner M, Mshinda H, et al. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet. 2005;365:1481–1483. doi: 10.1016/S0140-6736(05)66418-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Incidence of malaria over age, stratified by assigned study arm. PYAR, person-years at risk.