Abstract
Increased p21 activated kinase (PAK) signaling and expression has been identified in the invasive fronts of aggressive papillary thyroid cancers (PTCs), including those with RET/PTC, BRAF V600E, and mutant RAS expression. Functionally, thyroid cancer cell motility in vitro is dependent on Group 1 PAKs particularly PAK1. In the present study, we hypothesize that BRAF, a central kinase in PTC tumorigenesis and invasion, regulates thyroid cancer cell motility in part through PAK activation. Using three well-characterized human thyroid cancer cell lines, we demonstrated in all cell lines that BRAF knockdown reduced PAK phosphorylation of direct downstream targets. In contrast, inhibition of MEK activity either pharmacologically or with siRNA did not reduce PAK activity indicating MEK is dispensable for PAK activity. Inhibition of cell migration through BRAF loss is rescued by overexpression of either constitutive active (CA) MEK1 or PAK1, demonstrating that both signaling pathways are involved in BRAF-regulated cell motility. To further characterize BRAF-PAK signaling, immunofluorescence and immunoprecipitation demonstrated that both exogenously overexpressed and endogenous PAK1 and BRAF co-localize and physically interact, and that this interaction was enhanced in mitosis. Finally, we demonstrated that acute induction of BRAFV600E expression in vivo in murine thyroid glands results in increased PAK expression and activity confirming a positive signaling relationship in vivo. In conclusion, we have identified a signaling pathway in thyroid cancer cells which BRAF activates and physically interacts with PAK and regulates cell motility.
Keywords: Thyroid cancer, Cell motility, PAK1, BRAF, Cancer Biology
INTRODUCTION
Over the past three decades, the incidence of thyroid cancer, particularly papillary thyroid cancer (PTC) has been increasing. While this rise is in large part due to improved diagnostics and early detection, the occurrence of larger tumors and the annual number of individuals who die from thyroid cancer are rising (Pellegriti, et al. 2013). Most thyroid cancers are curable if detected at an early stage; however, for patients with aggressive forms of thyroid cancer treatment options are limited and prognosis is poor (Ringel 2009). Predictors of death include the presence of gross local invasion and the presence of larger distant metastases (Wong, et al. 2013). Defining the mechanisms underlying thyroid cancer development, invasion, and metastases is important in order to develop effective targeted therapies.
PTC tumorigenesis is driven largely by activation of the MAP kinase pathway through several genetic events, the most common of which are activating mutations in BRAF (most frequently BRAF V600E), RAS isoforms, or gene rearrangements involving the RET tyrosine kinase (Fagin and Mitsiades 2008; Nucera, et al. 2011). Whether activated by wild-type (WT) BRAF signaling through RAF homodimers or heterodimers, or by monomeric signaling from constitutively activated BRAFV600E, activated MEK leads to a downstream cascade of signaling events resulting in increased proliferation, invasiveness, reduced apoptosis, and reduced tissue-specific function (Knauf, et al. 2005; Melillo, et al. 2005; Riesco-Eizaguirre, et al. 2009). BRAFV600E has been particularly well studied in PTC. Expression of BRAFV600E in the mouse causes thyroid cancer and thyroid cell dedifferentiation in vivo (Knauf et al. 2005; Knauf, et al. 2011) and occurs in ~40% of all human PTC samples, although this prevalence varies depending on geographic location and ethnicity. BRAFV600E in PTC is associated with more aggressive clinical behavior (Xing 2007; Xing M 2013) and recent data suggests that other genetic abnormalities, such as mutations in the hTERT promoter, may cooperate with BRAFV600E resulting in more aggressive tumor behavior (Landa, et al. 2013E; Liu, et al. 2014). The frequency of BRAFV600E also is reported to be high in tumors from patients with progressive PTC enrolled in clinical trials (Kloos, et al. 2009). For these reasons, there have been major efforts to evaluate the efficacy of inhibiting BRAF, and/or MEK in thyroid and other BRAF-mediated cancers. This has resulted in FDA-approval of compounds targeting either all RAF isoforms or BRAFV600E specifically. However, these treatments are not curative and acquired resistance is nearly universal. Strategies to increase the effectiveness of BRAF-targeted compounds and to alleviate mechanisms of acquired resistance are being studied.
Because gross tumor invasion predicts poor prognosis in thyroid cancer, we evaluated expression profiles of the invasive fronts of large invasive PTCs to identify potential therapeutic targets. This work demonstrated that PTC invasion was associated with signaling leading to epithelial-to-mesenchymal transition (EMT) (Vasko, et al. 2007). The studies implicated known thyroid cancer pathways such as PI3K and TGFβ signaling cascades in the invasive fronts, but also suggested a previously undefined role for p21-activated kinases (PAKs). Subsequently we confirmed that PAK expression and phosphorylation were increased in the invasive fronts of aggressive PTCs and occurred in tumors with MAPK activating genetic alterations. We further demonstrated that inhibition of group I PAKs (PAK1 in particular) reduced motility in six different human thyroid cancer cell lines (McCarty, et al. 2010).
PAKs are a family of serine/ threonine kinases that phosphorylate downstream targets that alter cell motility by regulating cytoskeletal proteins involved in promoting lamellopod extension, enhancing proliferation, and inhibiting apoptosis (Radu M 2014). PAKs play important roles in breast cancer development and progression (Rider, et al. 2013; Shrestha, et al. 2012), in schwannoma development as effectors of NF2 (Flaiz, et al. 2009), and in neurological syndromes (Ma QL 2012). The six isoforms of PAK are divided into group I (PAKs 1-3) and group II (PAKs 4-6) based on structural and functional similarities (Radu M 2014). RAC1 and CDC42 are the primary activators of Group I PAKs (Radu M 2014) that normally exist as inactive homodimers through the binding of the auto inhibitory domain (AID) of one kinase to the kinase domain of another (Whale, et al. 2011). When RAC1 and CDC42 bind to PAK, the homodimer relaxes allowing for activation (Lei, et al. 2005). Once activated, PAKs phosphorylate downstream effectors including vimentin, cRAF, ROCK, and many others (Radu M 2014).
The relationship between PAK and RAF/MEK signaling is complex. PAK is known to phosphorylate CRAF and MEK enhancing activation, suggesting a potentiating role for PAK in RAF and MEK signaling (Radu M 2014; Slack-Davis, et al. 2003; Wang, et al. 2013). In addition to their kinase activity, group 1 PAKs have a kinase-independent scaffold function that sequesters CRAF and MEK1 at the plasma membrane enhancing signaling (Wang et al. 2013). Similar scaffolding functions allow PAK1 to promote AKT signaling (Higuchi, et al. 2008). PAK1 coordinately activates MET and MAPK signaling in breast cancer cells and PAK1 amplification has been proposed to be an alternative pathway for MAPK activation in this tissue type (Shrestha et al. 2012). It is of interest that in melanoma tissue microarrays (presumably from central tumor cores), PAK1 expression levels are lower in BRAFV600E-positive tumors vs wild-type tumors (Ong, et al. 2011). Concordantly, PAK1 mediates MAPK activation in melanoma cells with WT BRAF more so than in those homozygous for BRAFV600E through phosphorylation of CRAF and MEK (Ong et al. 2011). However, a role for BRAF beyond kinase inhibition (i.e. through scaffolding or other mechanisms) or the mechanism for its regulation of PAK function has not been reported. In addition, the regulation of PAK by MAPK signaling proteins has not yet been reported in thyroid cancer cells that have unique resistance mechanisms to BRAF inhibitors (Montero-Conde, et al. 2013).
In the present study, we demonstrate in thyroid cancer cells that PAK activity is dependent on BRAF expression. We also demonstrate that MEK does not regulate PAK signaling and report the new observation that BRAF and PAK physically interact. Interestingly, the BRAF-PAK1 interaction appears to be enhanced in mitosis. Finally, in vivo acute overexpression of BRAFV600E results in increased PAK expression and activity in addition to ERK phosphorylation. These data together are consistent with the conclusion that PAK is a MEK-independent functional downstream effector of BRAF that may play a role in thyroid tumorigenesis and progression.
MATERIALS AND METHODS
Cell Culture
Human thyroid carcinoma BCPAP, TPC1, and FTC133 cell lines (heterozygous for BRAF V600E; express RET/PTC1, or are BRAF wild type, respectively) were the generous gifts of Drs. R. Schweppe (University of Colorado Denver) (Schweppe, et al. 2008) with permission from the researchers who originally established cell lines: FTC133--P. Goretzki, University of Leipzig, Germany (Goretzki, et al. 1990); BCPAP--D. N. Fabien, Centre Hospitalier Lyon-Sud, France (Fabien, et al. 1994); TPC1--H. Sato, Kanazawa University, Japan (Kurebayashi, et al. 2000). The obtained cell lines were independently confirmed to be of thyroid origin by DNA fingerprinting using methods as previously described (Schweppe et al. 2008). Cells were cultured as described (McCarty et al. 2010). Human embryonic kidney (HEK) 293 cells were purchased from the American Type Culture Collection (ATCC) and grown in conditions described (Ding, et al. 2013).
Cell Synchronization
TPC1 cells were treated with 2mM of Thymidine in DMEM with 10% FBS for 24 hours, released for 2 hours, and treated with 100ng/mL of nocodazole in DMEM with 10% FBS for 24hrs. TPC1 cells were released for 1 hour and protein was isolated using the method described below.
cDNA Constructs
Vectors containing the cDNAs encoding constitutively active (CA) PAK1 and GFP-tagged PAK inhibitory domain (PID) were the generous gifts of J. Chernoff (Fox Chase Cancer Center) (Beeser and Chernoff 2005). The vector containing the cDNA for murine CA MEK1 was a gift from. M. Ostrowski (The Ohio State University, Columbus OH) (Huang W 1994). The vector containing the cDNA for MYC-tagged BRAF was a gift from Dr. James Fagin (Memorial Sloan-Kettering Cancer Center, New York, NY). The FLAG-PAK1 vector was constructed by excising cDNA from a wild-type PAK1 vector using BamH1 and Hind III. The fragment underwent PCR to add a FLAG-tag and was then inserted into the pCMV-Tag2B vector.
Transient Transfections
CA PAK1 cDNA, CA MEK1 cDNA, PID cDNA and BRAF siRNA combinations were transfected into BCPAP, TPC1 and FTC133 cells using Optifect Reagent (Life Technologies Co., Carlsbad, CA) (McCarty et al. 2010). HEK293 cells were transfected with MYC-tagged BRAF, FLAG-tagged PAK1 and HA control using Lipofectamine Plus (Life Technologies Co.) as previously described (Porchia, et al. 2007).
siRNA Transfections
BCPAP, TPC1 and FTC133 cells were grown to 30-40% confluence and transfected with scrambled siRNA control (cat. sc-37007 Santa Cruz, Biotechnology, Inc., Santa Cruz, CA), BRAF specific siRNAs (cat. sc-36368 Santa Cruz, Biotechnology, Inc.), PAK1 specific siRNAs (cat. sc-29700 Santa Cruz, Biotechnology, Inc.), MEK1 specific siRNAs (cat. sc-29396 Santa Cruz, Biotechnology, Inc.) or MEK2 (cat. Sc-35905 Santa Cruz, Biotechnology, Inc.) using Lipofectamine 2000 (Life Technologies Co.) as previously described (McCarty et al. 2010).
U0126 Treatment
BCPAP, TPC1 and FTC133 cells were grown to 50% confluence and treated with U0126 (Cell Signaling Technology) at various concentrations for 24 hours (0-10 µM). For migration experiments involving U0126, the cells were seeded, allowed to settle for 1 hour, and incubated with U0126 or DMSO (2µM) for 2 hours before being exposed to a serum gradient.
Protein Isolation and Western Blotting
Protein was isolated using either M-PER or lysis buffer and then WB was performed as previously described (Ringel, et al. 2001; Vasko et al. 2007). Primary antibodies against PAK1, phospho-Thr423 PAK1/Thr402 PAK2, cRAF, phospho-Ser338 cRAF, MEK1/2, phospho-Ser217 MEK1/Ser221MEK2, phospho-Ser298 MEK1, LIMK1, LIMK2, phospho-Thr508 LIMK1/Thr505LIMK2, ERK1/2, phospho-Thr202 ERK1/Thr204 ERK2, MYC-TAG and GAPDH were from Cell Signaling Technology (Danvers, MA), Antibodies against BRAF and GFP were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The antibody against vimentin and phospho-Ser55 vimentin were from Sigma-Aldrich, Inc. (St. Louis, MO) and MBL Co. (Nagoya, Japan), respectively.
Migration Assay
Migration assays were performed on BCPAP, FTC133 and TPC1 cells as previously described (McCarty et al. 2010).
Cell Viability Analysis
The effect of transfection and U0126 treatment on cell viability was assessed using the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) assay as previously described (Porchia et al. 2007).
Immunofluorescence and Confocal Microscopy
HEK293, cells were grown on glass cover slips in 6-well dishes, transfected, fixed with 4% paraformaldehyde (Affymetric, Cleveland, OH), permeabilized with 0.1% Triton X-100, and incubated for 15 minutes in Vectastain (Vector Laboratories, Burlingame, CA) blocking solution. Primary rabbit FLAG-Tag and mouse BRAF antibodies were applied for overnight at 4°C. After washing twice with PBS, cells were incubated with Alexa Fluor 488 and Alexa Fluor 594 secondary antibodies in PBS for 1 hour in the dark. Cover slips were mounted onto the slides with Slow Fade Antifade reagent with DAPI (Life Technology Co.), dried in the dark for 2 hour, and stored in the dark at 4°C. Images were collected with a single-photon Olympus Flowview 1000 Laser Scanning Confocal microscope. TPC1 and FTC133 cells underwent the same process except they were not transfected and were seeded in a Labteck Chamber slide (Sigma). Primary antibodies against PAK1 and phospho-Thr423 PAK1/Thr402 PAK2 (#2601) were used.
Immunoprecipitation
200-400 µg of total protein was incubated with pre-conjugated EZview Red anti-FLAG M2 affinty gel (Sigma), EZview Red anti-c-MYC affinity gel (Sigma), EZview Red anti-HA affinity gel (Sigma), non-conjugated anti-PAK1 antibody from Cell Signaling Technology (Danvers, MA), or non-conjugated anti-BRAF antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) overnight at 4°C. For the PAK1 and BRAF immunoprecipitation (IP) 1/10 volume of protein G agarose beads was added for 2 hours to eliminate non-specific binding. The beads were centrifuged and the supernatant was collected for IP. The same amount of protein G agarose beads was used as for the IP. Both mixtures were centrifuged and the pellets were washed with M-PER buffer three times. Loading buffer for WB (1 volume of dye and 3 volume of distilled water) was added and the mixtures were boiled to release the bound proteins and Western blot was performed. For experiments using preconjugated beads, control experiments in which preconjugated anti-hemaglutinin antibody beads was used for IP were included; for non-preconjugated bead experiments, IgG precipitation was the negative control.
Immunohistochemical Staining and Western Blot of Mouse Thyroid Tissue
Mice with thyroid-specific, doxycycline-inducible BRAFV600E (iBRAFV600E) and paraffin blocks of thyroids from these mice following one week of induction, and wild type control littermates were the gifts of J. Fagin (Memorial Sloan Kettering Cancer Center, NY) (Chakravarty, et al. 2011). The blocks were cut into sections, which were placed on slides and soaked in Xylene followed by 100% and 95% ethanol. Endogenous peroxidase was quenched using 3% Peroxidase. The slides were prepared by heating in Antigen Unmasking Solution for 7 minutes (Vector Laboratories Inc., Burlingame CA). For blocking and primary antibody staining, immunostaining racks and cover plates (Thermo Scientific Inc., Pittsburg, PA) were used. Primary antibodies against Thr 423 pPAK, PAK1, PAK2, and PAK3 were diluted, 1:100, in PBS and incubated on the slides overnight at 4°C. After the primary antibody, the slides were treated with a secondary antibody followed by streptavidin/peroxidase preformed complex solution and a DAB Substrate Mixture (Vector Laboratories). They were then immersed in hematoxylin for 30 seconds and coverslips were mounted using 30% glycerol. Negative controls were slides that underwent the same process minus the primary antibody. IHC was scored based on the number of positive cells and the intensity of the immune-activity independently by three investigators (SAM, MS, and VV) on a 0-2 scale. Scoring was compared and averages were calculated.
For Western blot analysis, we also isolated mouse thyroid tissues from iBRAFV600E mice as well as age matched wild type and rtTA-expressing control mice all after 1 week treatment with doxcycline-impregnated food pellets (2,500 ppm) as previously described (Chakravarty et al. 2011). Thyroid tissue was homogenized in M-PER buffer and protein was isolated from individual treated iBRAFV600E mice. For the treated wild-type and rTTA control mice, pooled thyroid gland tissue from each genotype group (due to the small thyroid size) was homogenized M-PER buffer and protein was isolated. Proteins were concentrated using Amicon Ultra-0.5 Centrifugal Filter Devices (EMD Millipore Co, Billerica, MA), concentrations were measured as above, and Western blots were performed. The mouse studies were approved by the Institutional Animal Care and Use Committee.
Statistical Analysis
For cell migration studies, to investigate whether inhibiting BRAF and MEK1/2 (compared to control) would affect cell migration, linear mixed models were used to account for the correlation among observations from the same experiment. For the MTT assays, the data were first log transformed to reduce variance. For the migration rescue experiments, linear mixed models with an interaction effect were used to analyze whether introducing either CA PAK1 or CA MEK1 into BRAF siRNA transfected cells rescued the effect of decreased migration caused by the BRAF siRNA. As a secondary analysis, an interaction between the interaction term and cell lines was used to test whether the rescue effect differed between cell lines. All analyses were performed using SAS/STAT software version 9.2 (SAS Institute Inc., Cary, NC). To quantify the co-localization of BRAF with PAK1 and pPAK, the confocal images were analyzed using Pearson’s Coefficient of Correlation (r). A coefficient of 1 or -1 implies perfect positive or negative correlation, while 0 implies none. The Pearson’s coefficient can then be converted into a percentage that represents the amount of overlap or co-localization. Complete details of the methods and mathematics are as reported previously (French, et al. 2008; Zinchuk, et al. 2007).
RESULTS
PAK Function is regulated by BRAF
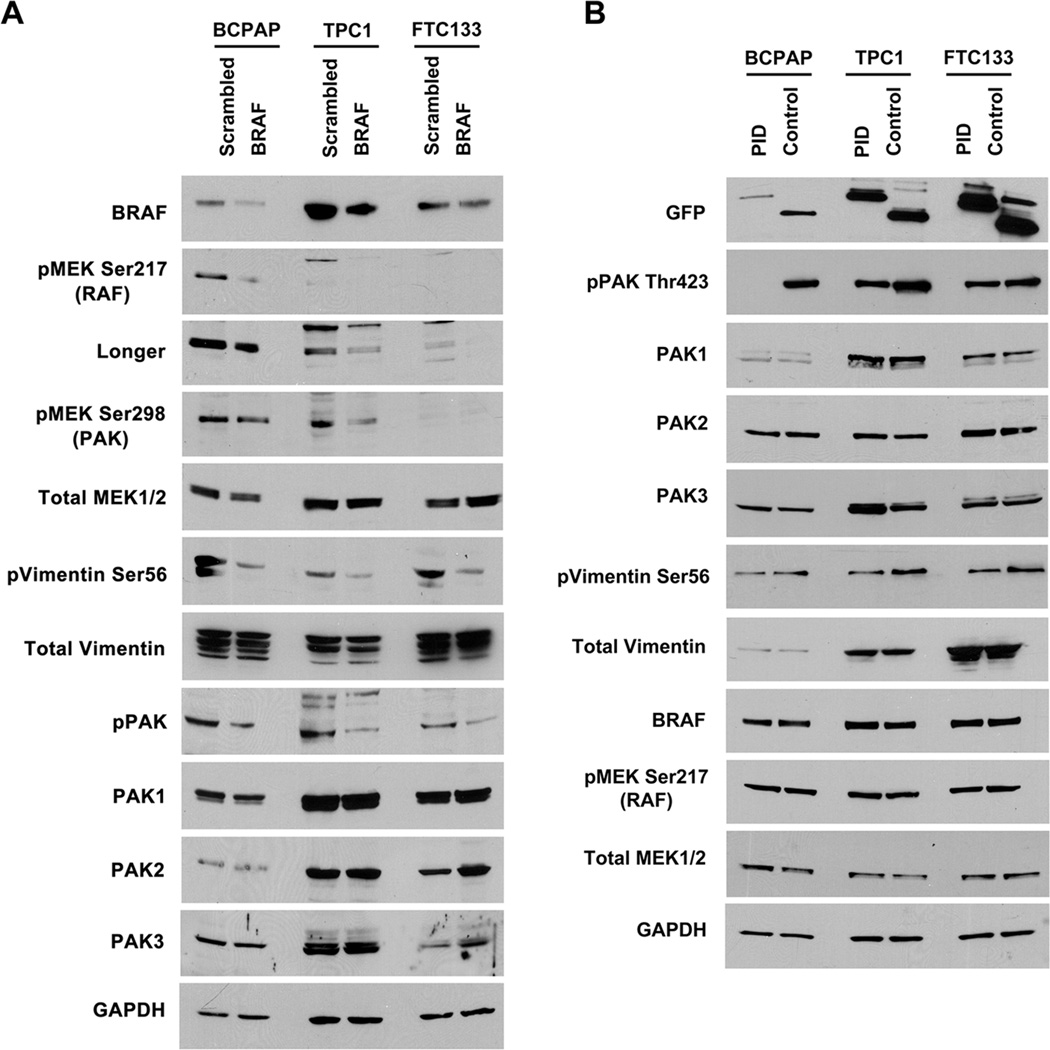
To determine whether PAK function is dependent on BRAF expression, BRAF or control siRNA was transfected into the human thyroid cancer cell lines BCPAP, TPC1 and FTC133 that harbor different thyroid cancer-causing genetic mutations (see materials and methods section). Western blot was performed using specific antibodies for known PAK phosphorylation sites on vimentin (Ser56) and MEK (Ser298). In all cases, partial BRAF suppression was sufficient to reduce PAK-mediated phosphorylation of downstream targets as well as BRAF-mediated MEK phosphorylation (Fig. 1A). In contrast, when group I PAK activity was specifically suppressed using exogenous PAK autoinhibitory domain (PID), no effect was observed on BRAF-mediated MEK phosphorylation despite reductions in PAK-mediated phosphorylation (Fig. 1B).
FIGURE 1.
PAK function is BRAF-dependent. A. BRAF siRNA blocked PAK-mediated phosphorylation of vimentin and MEK in BCPAP, TPC1 and FTC133 cell lines versus scrambled sequence control. Transfection efficiencies were approximately 40-60%. “Longer” refers to a longer exposure of the same blot for adequate visualization of the bands of all cell lines for the pMEK immunoblot. B. Expression of a molecular inhibitor of group 1 PAKs, GFP-tagged PID, did not reduce BRAF-mediated MEK1/2 phosphorylation in all three-thyroid cancer cell lines versus vector transfection control. Representative Western blots are shown for experiments repeated independently on at least two occasions.
BRAF-Regulated Thyroid Cancer Cell Motility is Dependent on MEK and PAK 1
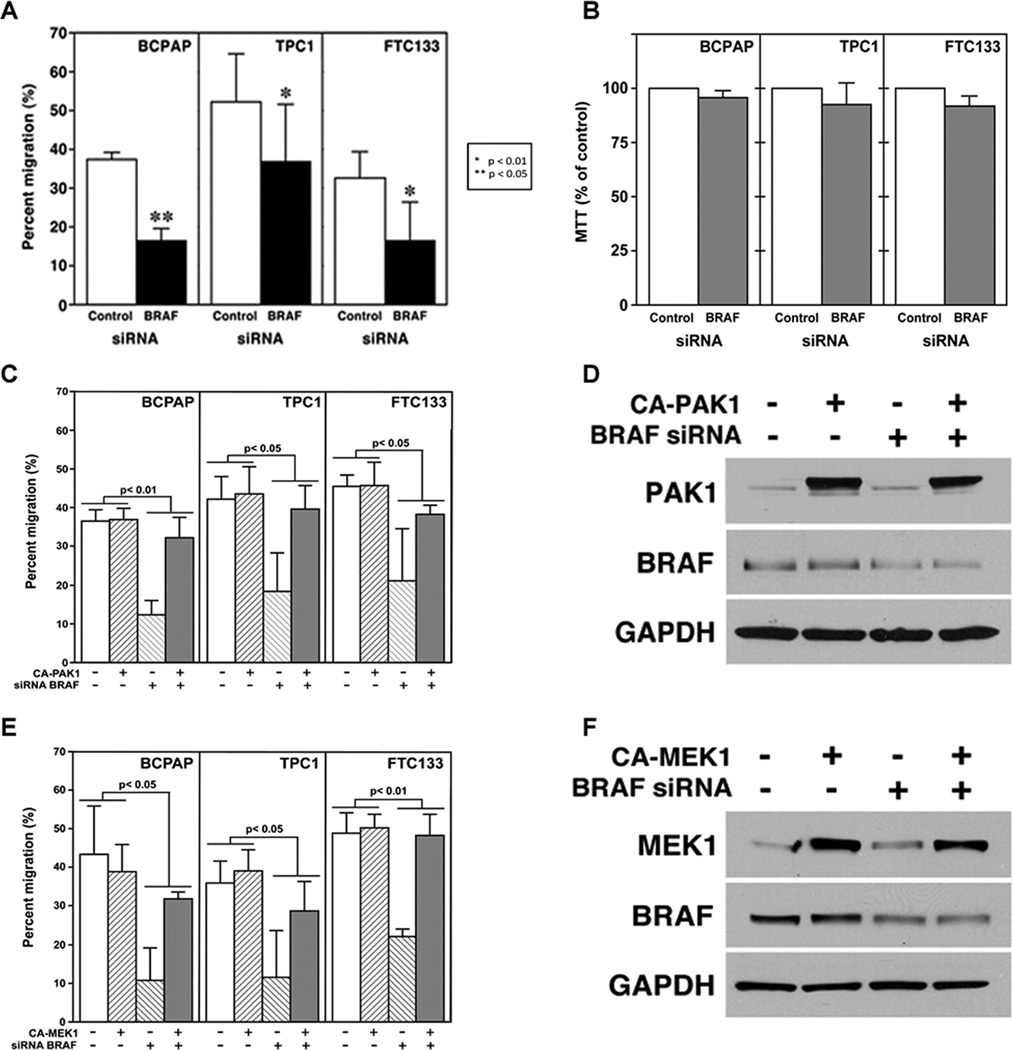
Both BRAF and PAKs regulate cancer cell motility (Arozarena, et al. 2011; Makrodouli E 2011; McCarty et al. 2010). BRAF siRNA blocked serum gradient-induced BCPAP, TPC1, and FTC133 thyroid cancer cell migration compared to the scrambled control in all three-cell lines (Fig. 2A). To ensure that BRAF suppression had a lesser effect on cell viability in the low confluency conditions employed in the migration assays, MTT assays were performed and showed no significant effects (Fig. 2B). To determine whether PAK1 played a role in BRAF-regulated thyroid cell migration induced by a strong serum gradient, rescue experiments were performed in all three-cell lines. Constitutively active (CA) PAK1, when expressed in BRAF siRNA-transfected thyroid cancer cells, resulted in a statistically significant rescue of migration (Fig, 2C). This effect was consistent for all three-cell lines tested when analyzed using a linear mixed statistical model. Western Blot confirmed CA PAK1 expression and BRAF knockdown (Fig. 2D). MTT assays confirmed that the transfections had minimal effects on cell viability in the conditions used for the experiments (data not shown). Similar rescue experiments using CA MEK1 and demonstrated that CA MEK1 also rescued the effect of BRAF siRNA on migration, and that the rescue was consistent for all three-cell lines. A similar mixed model with an interaction effect was used as for the CA PAK1 rescue experiment. (Fig. 2E). Western blot confirmed CA MEK1 expression and a partial BRAF knock-down (Fig. 2F) and MTT assays confirmed that the transfections only minimally altered cell viability in the same experimental conditions (data not shown).
FIGURE 2.
BRAF regulates migration through PAK and MEK. A. BRAF siRNA or scrambled sequence control was transfected into BCPAP, TPC1, and FTC133 cells. BRAF siRNA signficantly reduced thyroid cancer cell migration in all three-cell lines compared with the control scrambled sequence. p values are as noted in the figure. B. MTT demonstrates no statistical difference between cells transfected with BRAF siRNA or the control scrambled sequence. C. CA PAK1 rescued thyroid cancer cell migration in BRAF-siRNA-transfected cells with significant p values as noted in the figure. D. Western Blot confirmed a reduction BRAF protein by the siRNA and the transfection with the CA PAK1. Negative controls were transfection with a scrambled sequence for the siRNA and empty vector for the CA PAK1, respectively. E. Rescue experiments using murine CA MEK1 also rescued migration of the BRAF-siRNA transfected cells in all three-thyroid cancer cell lines. The transfection efficiencies were approximately 30-40%. The p values were statistically significant as noted in the figure. F. Western Blot confirmed a reduction BRAF protein by the siRNA and the transfection with the CA MEK1. Negative controls were transfection with a scrambled sequence for the siRNA and empty vector for the CA MEK1, respectively
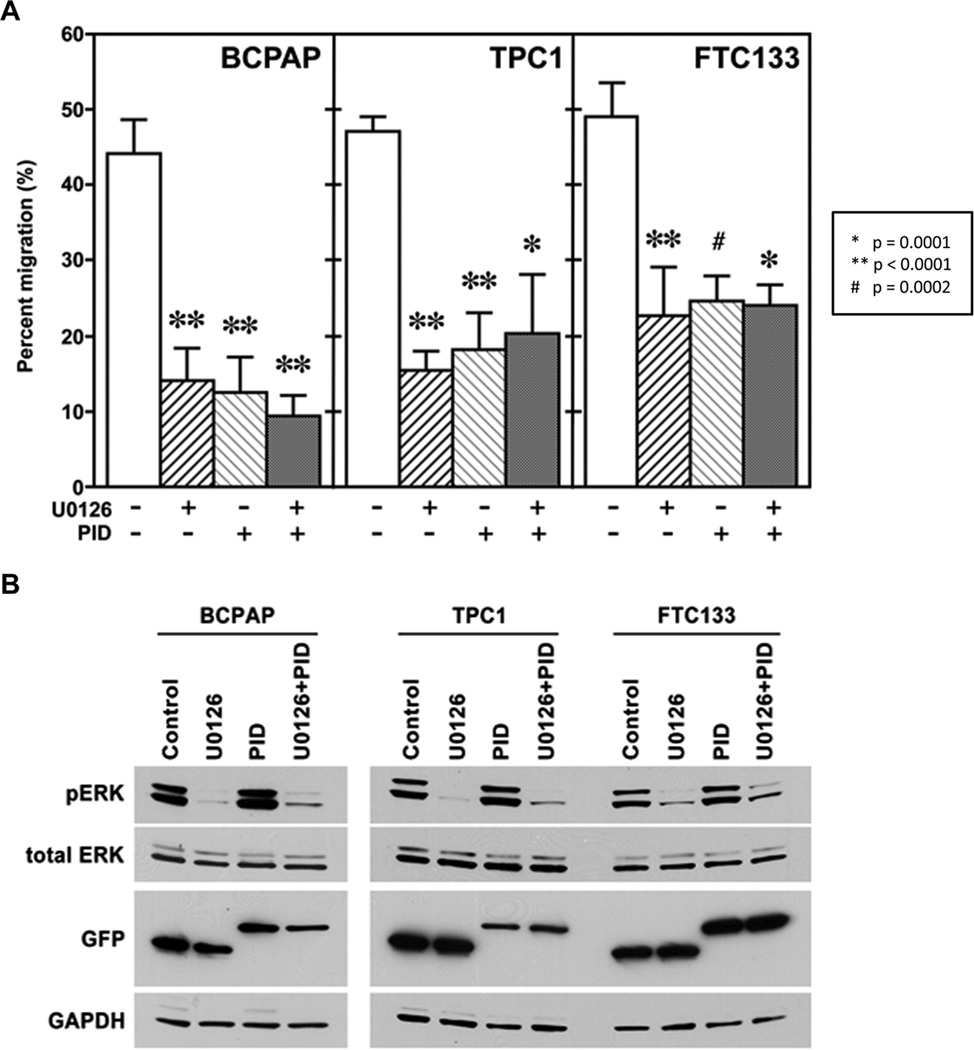
MEK and Group I PAK Pathways do not Synergistically Regulate Migration
MEK and group I PAKs were inhibited using U0126, a specific MEK1/2 inhibitor (2µM), or PID transfection, respectively in each cell line, and migration experiments were performed. The inhibition using either U0126 or PID alone significantly reduced migration compared to the appropriate control. Inhibiting both MEK and group I PAKs simultaneously; however, did not result in a greater decrease in migration. These results suggest either that the two pathways are not synergistic or that they are maximally individually inhibited (Fig. 3A). Western blot confirmed a U0126-induced decrease in MEK activity, indicated by a decrease in pERK1/2, and expression of GFP-tagged PID or GFP control vector (Fig. 3B).
FIGURE 3.
MEK and group I PAKs regulate thyroid cancer cell motility. A. MEK1/2 and group I PAK activity were inhibited using U0126 (2µM) or DMSO and GFP-PID or GFP-control, respectively. Migration experiments showed that inhibiting both MEK and group I PAKs independently and simultaneously significantly inhibited migration to a similar degree (p-values as shown in figure). B. Western Blot confirmed inhibition of MEK activity by U0126 and successful transfection of the PID and control vector by immunoblot for phosphorylated ERK and GFP, respectively. The transfection efficiency was approximately 30-40% based on imaging of GFP.
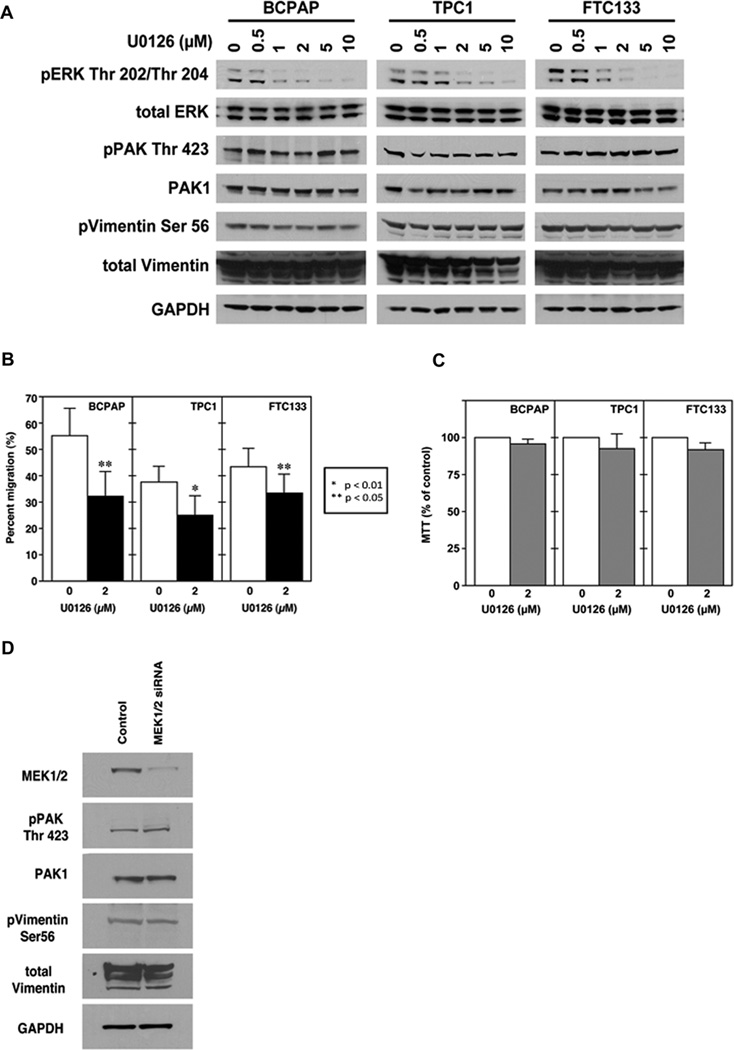
MEK Inhibition Does not Alter PAK Activity
Because MEK is a downstream target of BRAF that is functionally interactive with PAK (Slack-Davis et al. 2003), we performed experiments to determine whether MEK regulated PAK activity in thyroid cancer cells. MEK kinase activity was first inhibited using U0126 at increasing concentrations in BCPAP, TPC1, and FTC133 cells. U0126 did not reduce levels of phosphorylated PAK or PAK-mediated phosphorylated vimentin (Fig. 4A). Treatment with U0126 at a dose (2.0 µM) in which MEK inhibition is near-complete (Fig. 4A) results in significantly reduced thyroid cancer cell motility (Fig. 4B) without reducing cell viability (Fig. 4C). This result is consistent with PAK-independent motility inhibition. We also knocked down MEK1 and 2 using siRNA and confirmed that loss of MEK expression does not alter PAK activity (Fig 4D).
FIGURE 4.
PAK activity is not MEK-dependent. A. Western Blot of protein isolated from BCPAP, TPC1 and FTC133 cell lines treated with U0126 (or control DMSO) at doses sufficient to inhibit MEK kinase function did not alter PAK-mediated phosphorylation of vimentin at serine 56. B. 2.0 µM of U0126 reduced thyroid cancer cell migration compared to DSMO control. Statistical significance (p values) as noted in the figure. C. MTT assays confirmed that 2.0 µM of U0126 in the conditions used for migration did not reduce cell viability. WB demonstrates that a reduction of MEK1/2 levels with siRNA also did not affect pPAK or PAK1 protein levels versus cells transfected scrambled sequence control. Western blots are representative of experiments performed on at least three independent occasions.
Exogenous BRAF and PAK 1 Co-localize and Co-immunoprecipitate
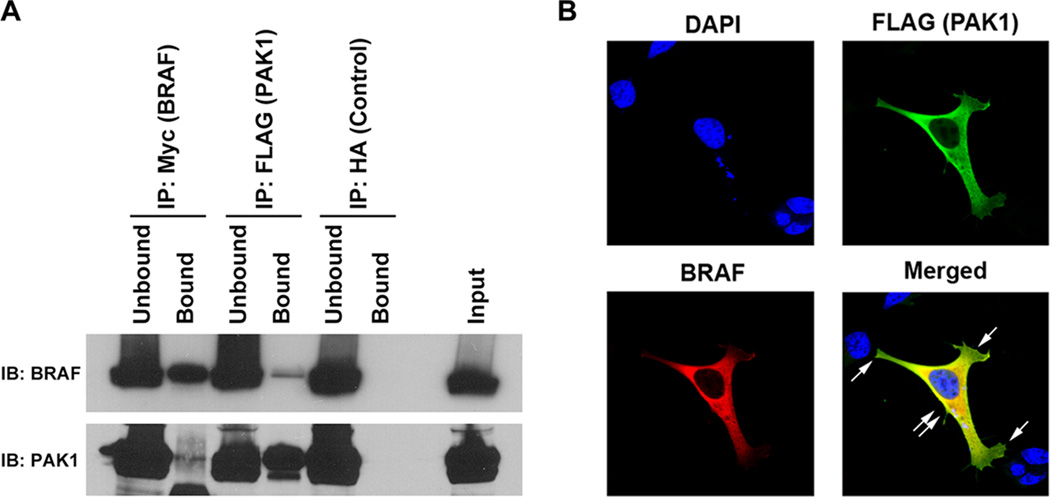
To further characterize the BRAF-PAK interaction, we performed experiments to determine if BRAF and PAK co-localize and/or physically interact. Epitope tagged MYC-BRAF and FLAG-PAK1 were transfected into HEK293 cells and immunoprecipitation (IP) was performed using pre-conjugated FLAG, MYC and HA (control for non-specific binding) beads. Western blot of the precipitated proteins demonstrated that BRAF and PAK1 co-immunoprecipitated in both directions; no non-specific pull-down was identified from the immunoprecipitation using pre-conjugated HA beads (Fig. 5A). To examine whether exogenous BRAF and Group I PAKs co-localize, HEK293 cells were transfected with epitope tagged MYC-BRAF and FLAG-PAK1 cDNAs, stained with DAPI (nuclear), Alexa-488 (FLAG) and Alexa-594 (BRAF), and examined by confocal microscopy immunofluorescence (IF). In these experiments, the exogenous BRAF and PAK1 co-localize mostly in the cytosol (yellow), indicated by double arrows, while PAK alone is identified at the lamellopodia (green), indicated by single arrows (Fig. 5B).
FIGURE 5.
Exogenous BRAF and PAK1 co-immunoprecipitate (IP) and co-localize. A. IP was performed on protein isolated from HEK293 cells that were transfected with cDNAs encoding Myc-tagged wild type BRAF and FLAG-tagged wild type PAK1. The IP using preconjugated anti-Myc and anti-FLAG antibodies demonstrated on Immunoblot (IB) using both the epitope tag and showed that the protein was precipitated and also that a small amount of BRAF precipitated with the FLAG IP and PAK precipitated with the Myc IP. Unbound is IB of the protein in the supernatant prior to washing and Bound is the immunoprecipitated protein. Input is IB of the total protein lysate serving as a size control. Non-specific binding was not identified using the protein precipitated with pre-conjugated anti-HA antibody (Control). B. Co-Transfected HEK293 cells were stained with DAPI (nuclear), Alexa-488 (FLAG) and Alexa-594 (BRAF) and subjected to immunofluorescence with confocal microscopy. Imaging revealed that BRAF and PAK1 co-localized (yellow, double arrow) in the perinuclear region, but not at the lamellopodia, which contained only PAK (single arrow). Data shown are representative of experiments performed on at least three occasions.
Endogenous BRAF and PAK1 Co-localize and Co-immunoprecipitate
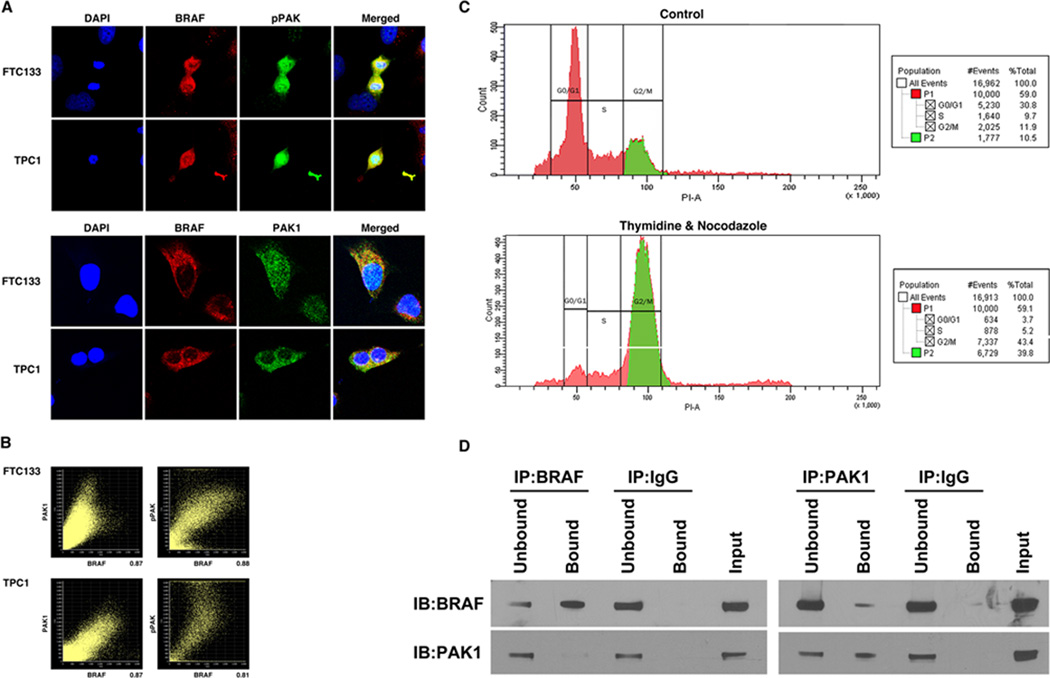
To determine if endogenous BRAF and PAK1 co-localize or co-immunoprecipitate, TPC1 and FTC133 cells with wild-type BRAF were stained with DAPI (nuclear), Alexa-488 (pPAK & PAK1) and Alexa-594 (BRAF) and examined by confocal microscopy. Immunofluorescence (IF) showed that BRAF co-localized with both PAK1 and pPAK, but this was most striking for pPAK and was particularly notable in the cytosol of mitotic cells (Fig. 6A), (low power in Supplemental Figure 1). To measure the degree of co-localization, Pearson’s Coefficient of Correlation (r) was used to analyze the confocal images to quantify a co-localization percentage value. In both cell lines BRAF strongly co-localized with PAK1 and pPAK (Fig. 6B). Protein from TPC1 cells continuously cultured in growth medium was isolated and IP was performed. In these conditions, BRAF and PAK1 did not consistently co-immunoprecipitate (Supplemental Figure 2). Because the IF suggested that BRAF and PAK co-localization occurred mostly in mitotic cells, TPC1 cells were treated for 24 hours with 2mM of Thymidine followed by 24 hours with 100 ng/mL of nocodazole to increase the percentage of cells in G2/M phase (Fig. 6C). Immunoprecipitation and Western blot using the BRAF and PAK1 antibodies demonstrate that endogenous BRAF and PAK1 co-immunoprecipitate in both directions in these conditions although it was more strongly detected when the immunoprecipitation was performing using the PAK antibody (Fig. 6D).
FIGURE 6.
Endogenous BRAF and PAK1 co-immunoprecipitate (IP) and co-localize A. TPC1 and FTC133 cells in continuous growth conditions were stained with DAPI (nuclear), Alexa-488 (PAK1& Thr423 pPAK) and Alexa-594 (BRAF) and subjected to immunofluorescence with confocal microscopy. Imaging revealed that BRAF co-localized with pPAK and PAK1. Low power and additional high power images are shown in Supplemental figure 1. B. In representative FTC133 cells, Person’s coefficient for co-localization of BRAF and PAK1, and BRAF and pPAK were 0.74496 and 0.86779 with percentages of 87% and 88%, respectively. In representative TPC1 cells, Person’s coefficient for co-localization of BRAF and PAK1, and BRAF and pPAK, were 0.83179 and 0.75334 with percentages of 87% and 80%, respectively. C. TPC1 cells were treated with thymidine and nocodazole, which increased the percentage of cells in G2/M phase (39.8%) compared to the untreated control (10.5%). D. Protein was isolated from thymidine and nocodazole treated TPC1 cells and IP was performed using BRAF and PAK1 antibodies, and IgG as a negative control. IB of the precipitated protein demonstrated that BRAF-precipitated protein contained PAK1 and that PAK1-precipitated protein contained BRAF with no non-specific binding with IgG. Unbound is IB of the protein in the supernatant prior to washing and Bound is the immunoprecipitated protein. Input is IB of the total protein lysate to confirm size. Data shown are representative of experiments performed on at least two occasions.
BRAFV600E Expression in Murine Thyroids Increases PAK isoform phosphorylation, activity, and expression
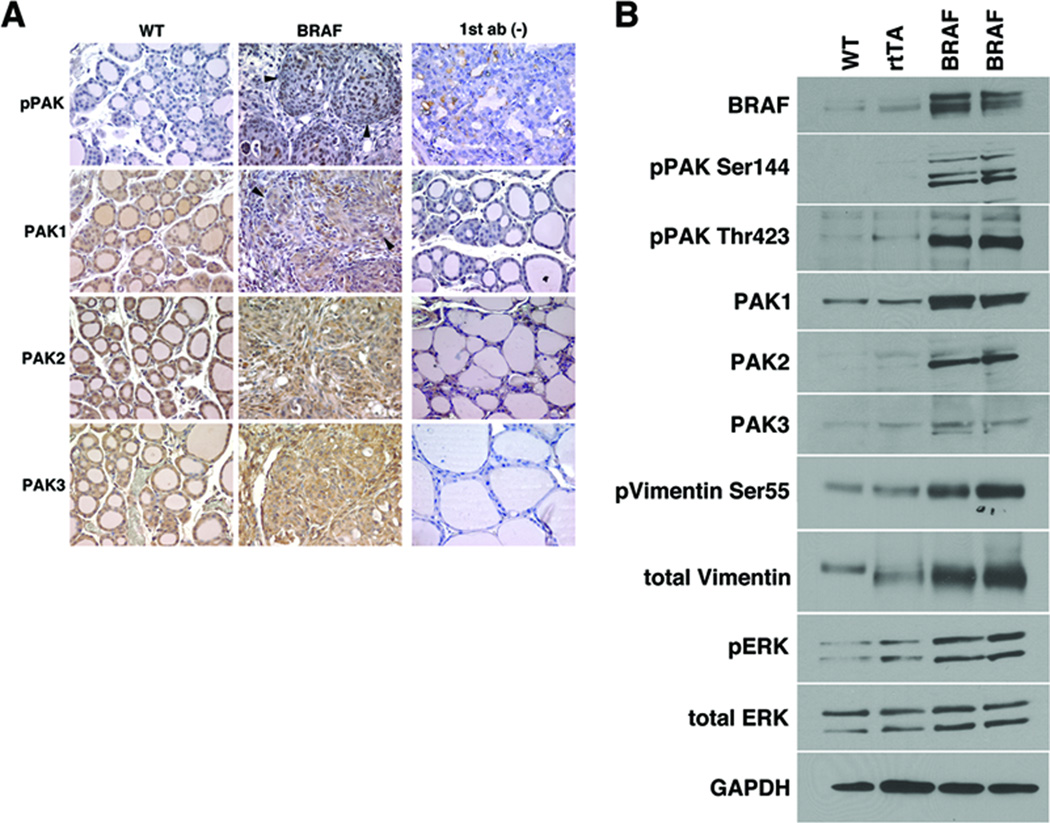
To study the signaling effects of acute activation of BRAF on Group I PAKs in vivo, we obtained paraffin blocks from the thyroid glands of mice with thyroid-specific, doxycycline-inducible BRAFV600E following one week of BRAFV600E overexpression that causes the development of papillary thyroid cancer (Chakravarty et al. 2011). Compared with littermate controls, the thyroid glands from mice with induced BRAFV600E show an increase in immunoactive pPAK Thr423 (Fig. 7A). Total PAK1/2/3 protein expression was similar or increased in the mice. To confirm the immunohistochemistry staining and allow for quantitation, WB was performed on protein isolated from the mouse thyroid glands following BRAF V600E induction and after doxycycline treatment of age matched control wild-type mice and mice expressing the Tg-rTTA construct. In comparison to both controls, the mice with induced BRAF V600E had an increase in immunoactive pPAK Thr423 and pPAK Ser144, p-vimentin Ser55 and pERK consistent with activation of both PAK and MEK pathways (Fig. 7B). Interestingly, PAKs 1 and 2, and vimentin protein levels also were increased. These data suggest that acute activation of BRAF, or BRAF V600E, specifically increases both expression and signaling activation of PAK in vivo. This is consistent with our previously reported data from the invasive fronts of human PTC samples (McCarty et al. 2010).
FIGURE 7.
pPAK expression is increased by acute expression of BRAFV600E in murine thyroid glands. A. Slide sections of thyroid glands isolated from mice following induction of thyroid-specific doxycycline-inducible BRAFV600E demonstrated increased levels of immunoactive Thr423 phosphorylated PAK compared to wild-type control littermates. Total levels of PAK1/2/3 were similar between mice with expression of BRAFV600E versus control. Negative controls (omitting the primary antibody) are shown. B. WB of protein was isolated from the thyroid glands of doxycycline (dox) treated mice with thyroid-specific inducible BRAFV600E and dox-treated wild type and rTTA-expressing control mice. Induction of BRAF V600E resulted in increased levels of phosphorylated PAK, total PAKs 1 and 2, and as well as PAK and MEK specific phosphorylation of vimentin and ERK, respectively.
DISCUSSION
In the present study, we report a functional interaction between BRAF, a critical oncogene in thyroid cancer, and PAK, a key regulator of cell motility and tumorigenesis. Loss of BRAF expression in thyroid cancer cells results in reduced PAK function in vitro and BRAFV600E induction in thyroid cells in vitro and in vivo leads to PAK activation. The data further demonstrate that PAK is not regulated by MEK in thyroid cancer cells, suggesting a MEK independent BRAF-PAK signaling connection. Finally, we demonstrate for the first time that BRAF and PAK1 physically interact in both exogenous expression and endogenous systems. Interestingly, the physical interaction between endogenous wild type BRAF and PAK1 appears to be enhanced in mitosis although the precise nature of the interaction and whether they occur as part of a larger protein complex requires further study. Recent data support the assertion of a role for PAK in MAP kinase-pathway mediated tumor formation in vitro and in vivo. It was recently demonstrated that PAK1−/− and PAK+/− mice are relatively resistant to RAS driven squamous cell carcinomas (SCC) (Chow, et al. 2012E). PAK1 has been shown to be particularly critical for MAPK activation in BRAF WT melanoma and in breast cancer by linking MET activation to MAPK signaling (Ong et al. 2011; Shrestha et al. 2012). This has been shown to be related to PAK-mediated phosphorylation of CRAF. In thyroid cells, even in the absence of an activating mutation, BRAF is the dominant RAF isoform mediating upstream-activated oncogenic pathways (Mitsutake, et al. 2006).
In all three tested thyroid cancer cell lines, two with wild type BRAF (TPC1 and FTC133) and one heterozygous for the BRAFV600E mutation (BCPAP), BRAF suppression reduced PAK activity. The interaction between WTBRAF and PAK was then further explored in the co-IP and IF studies and the proteins were shown to interact. The mice with acute overexpression of BRAF V600E in the thyroid display both increased expression of PAK 1 and 2 and PAK phosphorylation (Figure 7B). This model is one of acute activation and overexpression and is a robust system to study acute signaling effects. Experiments in mice that stably express a single copy of BRAF V600E in the thyroid glands are ongoing. Further experiments are required to determine if the BRAF/PAK protein complex, and mechanism of BRAF-regulated PAK activity differs for BRAFV600E versus wild-type BRAF.
It is of interest that in human melanoma cells the absence of BRAFV600E mutation is reported to be associated with increased PAK signaling through CRAF and to predict response to PAK inhibition (Ong et al. 2011). Moreover, in melanoma cells, the BRAFV600E-specific kinase inhibitor vemurafinib did not inhibit PAK phosphorylation of MEK in cells homozygous for BRAFV600E, suggesting that that mutant BRAF may not signal through PAK as efficiently in these cells (Ong et al. 2011). However, to our knowledge, the role of wild-type BRAF on PAK activity or of BRAFV600E beyond pharmacological kinase inhibition has not yet been reported in melanoma. In the present study, thyroid cancer cells with wild-type BRAF or that are heterozygous for BRAFV600E demonstrate BRAF expression-dependent PAK activation. The difference between the two sets of results may be due to the loss of both kinase and scaffold functions in the knock-down model, the presence of WT BRAF in the thyroid systems, and/or cell autonomous differences in response to BRAF inhibitors [as has been previously described (Montero-Conde et al. 2013)]. Interestingly, it has been reported recently that melanoma cells heterozygous for BRAF V600E display different responses to vemurafinib compared with cells that are homozygous for the mutant allele (Sapkota, et al. 2013).
It also is important to recognize the centrality of BRAF activity in PTC tumorigenesis setting of activated wild type BRAF (via RAS mutations and RET/PTC rearrangements for example) or through BRAF mutations. This is supported by the relative mutual exclusivity of these oncogenes and the importance of BRAF signaling for their oncogenic functions (Melillo et al. 2005). Because activation of PAK is known to most significantly at the invasive fronts of tumors (Vasko and Saji 2007), it is possible that PAK signaling plays a role in the progression of MAPK-activated PTCs in general but the mechanisms may vary dependent on the mode of pathway activation.
While the co-IP and co-localization experiments indicate that BRAF and PAK1 physically interact, the precise nature or requirements for this interaction have not yet been elucidated. It seems likely that both proteins are part of a larger complex since BRAF signaling is facilitated through kinase suppressor of Ras 1 (KSR1) and other scaffold proteins (McKay, et al. 2009). Further evidence that strengthens this possibility is that scans using STRING v9.1 (Franceschini A 2013) of both BRAF and PAK1 do not predict direct binding sites. Of interest are recent data demonstrating that PAK can function as a scaffold by binding signaling molecules to its N-terminus domain. Specifically, PAK1 was shown to directly bind to both PDK1 and AKT, and to facilitate AKT membrane recruitment and PDK1 phosphorylation (Higuchi et al. 2008). In addition, PAK scaffolding has been reported to facilitate CRAF-MEK1 signaling (Wang et al. 2013). In the present study, the inhibitor PID did not reduce levels of pERK. This was unexpected and suggests either cell type or condition-specific differences (low confluence vs high) from the prior studies. Thus, it is possible that PAK binds to a BRAF-associated complex leading to their functional interaction but that the precise downstream effects may depend on other components of the complex. Current studies are ongoing to determine the nature of their interaction.
Another question regarding BRAF and PAKs relationship was whether BRAF could directly phosphorylate Group I PAKs. We evaluated this in preliminary studies using in vitro kinase assays in which PAK1 was a substrate for activated BRAF. In these experiments, PAK1 was not phosphorylated by BRAF (data not shown), however not all PAK isoforms were evaluated and other members of the potential BRAF-PAK complex may be needed to facilitate such the event. Further evidence opposing BRAF phosphorylation of PAK comes from the Human Protein Reference Database program (Keshava Prasad TS 2009), which did not identify a BRAF phosphorylation motif on PAK1. Taken together, it seems unlikely that PAK1 (Wang et al. 2013) is directly phosphorylated by BRAF. The lack of a cooperative effect on migration by dually inhibiting MEK and PAK, and the lack of PAK inhibition by inhibiting MEK in those same conditions, suggests that either both pathways can maximally inhibit cell motility alone or that both signaling pathways merge downstream in the thyroid cell systems that were studied. PAKs have previously been shown to regulate MEK1 by phosphorylating ser298, thus a common downstream pathway seems possible (Slack-Davis et al. 2003). Finally, the role of other PAK isoforms on BRAF interactions has not been tested in detail to date; we focused on PAK1 due to our prior studies demonstrating that this is the primary isoform that regulates migration of thyroid cancer cells (McCarty et al. 2010).
In summary, we have identified a functional physical interaction between BRAF and PAK1 that regulates cell motility. The presence of the pathway is supported by activation and inhibition studies, co-localization and co-immunoprecipitation studies, and is supported by in vivo data. While further studies are needed to fully characterize the protein complex between BRAF and PAK, the presence of this signaling pathway is relevant for BRAF mediated tumor formation, progression, and perhaps resistance to therapeutic inhibitors in thyroid cancer.
Supplementary Material
Acknowledgments
FUNDING: This work was supported by the NIH Grant (P01CA124570) to MDR, National Institute Of General Medical Sciences of the NIH (under Award T32GM068412 to CMK), and Grants P30CA016058 and S10RR025443
Footnotes
CONFLICT OF INTEREST: The authors have no conflict of interest to declare.
REFERENCES
- Arozarena I, Sanchez-Laorden B, Packer L, Hidalgo-Carcedo C, Hayward R, Viros A, Sahai E, Marais R. Oncogenic BRAF Induces Melanoma Cell Invasion by Downregulating the cGMP-Specific Phosphodiesterase PDE5A. Cancer Cell. 2011;19:45–57. doi: 10.1016/j.ccr.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Beeser A, Chernoff J. Production and use of a cell permeable inhibitor of group A Paks (TAT-PID) to analyze signal transduction. Methods. 2005;37:203–207. doi: 10.1016/j.ymeth.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, Bollag G, Kolesnick R, Thin TH, Rosen N, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–4711. doi: 10.1172/JCI46382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HY, Jubb AM, Koch JN, Jaffer ZM, Stepanova D, Campbell DA, Duron SG, O'Farrell M, Cai Q, Klein-Szanto AJ, et al. p21-activated kinase 1 is required for efficient tumor formation and progression in a Ras-mediated skin cancer model. Cancer Res. 2012E;2012:14. doi: 10.1158/0008-5472.CAN-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H, Yusof AM, Kothandaraman S, Saji M, Wang C, Kumar K, Milum K, Carleton M, Pan X, Ringel MD, et al. Localization of CaSR Antagonists in CaSR-expressing Medullary Thyroid Cancer. J Clin Endocrinol Metab. 2013;98:E1722–E1729. doi: 10.1210/jc.2013-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabien N, Fusco A, Santoro M, Barbier Y, Dubois PM, Paulin C. Description of a human papillary thyroid carcinoma cell line. Morphologic study and expression of tumoral markers. Cancer. 1994;73:2206–2212. doi: 10.1002/1097-0142(19940415)73:8<2206::aid-cncr2820730828>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: diagnostic and clinical implications. Best Pract Res Clin Endocrinol Metab. 2008;22:955–969. doi: 10.1016/j.beem.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaiz C, Chernoff J, Ammoun S, Peterson JR, Hanemann CO. PAK kinase regulates Rac GTPase and is a potential target in human schwannomas. Exp Neurol. 2009;218:137–144. doi: 10.1016/j.expneurol.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini ASD, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A, Mills S, Swarup R, Bennett M, Pridmore T. Colocalization of fluorescent markers in confocal microscope images of plant cells. Nature Protocols. 2008;3:619–628. doi: 10.1038/nprot.2008.31. [DOI] [PubMed] [Google Scholar]
- Goretzki PE, Frilling A, Simon D, Roeher HD. Growth regulation of normal thyroids and thyroid tumors in man. Recent Results Cancer Res. 1990;118:48–63. doi: 10.1007/978-3-642-83816-3_6. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Onishi K, Kikuchi C, Gotoh Y. Scaffolding function of PAK in the PDK1-Akt pathway. Nat Cell Biol. 2008;10:1356–1364. doi: 10.1038/ncb1795. [DOI] [PubMed] [Google Scholar]
- Huang WER. Constitutive activation of Mek1 by mutation of serine phosphorylation sites. PNAS. 1994;91:8960–8963. doi: 10.1073/pnas.91.19.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshava Prasad TSGR, Kandasamy K, Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R, Shafreen B, Venugopal A, Balakrishnan L, Marimuthu A, Banerjee S, Somanathan DS, Sebastian A, Rani S, Ray S, Harrys Kishore CJ, Kanth S, Ahmed M, Kashyap MK, Mohmood R, Ramachandra YL, Krishna V, Rahiman BA, Mohan S, Ranganathan P, Ramabadran S, Chaerkady R, Pandey A. Human Protein Reference Database--2009 update. Nucleic Acids Res. 2009;37:767–772. doi: 10.1093/nar/gkn892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE, Jr, Vasko VV, Saji M, et al. Phase II Trial of Sorafenib in Metastatic Thyroid Cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, Refetoff S, Nikiforov YE, Fagin JA, Mitsutake N, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- Knauf JA, Sartor MA, Medvedovic M, Lundsmith E, Ryder M, Salzano M, Nikiforov YE, Giordano TJ, Ghossein RA, Fagin JA. Progression of BRAF-induced thyroid cancer is associated with epithelial-mesenchymal transition requiring concomitant MAP kinase and TGFbeta signaling. Oncogene. 2011;30:3153–3163. doi: 10.1038/onc.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurebayashi J, Tanaka K, Otsuki T, Moriya T, Kunisue H, Uno M, Sonoo H. All-Trans-Retinoic Acid Modulates Expression Levels of Thyroglobulin and Cytokines in a New Human Poorly Differentiated Papillary Thyroid Carcinoma Cell Line, KTC-1. J Clin Endocrinol Metab. 2000;85:2889–2896. doi: 10.1210/jcem.85.8.6732. [DOI] [PubMed] [Google Scholar]
- Landa I, Ganly I, Chan TA, Mitsutake N, Matsuse M, Ibrahimpasic T, Ghossein RA, Fagin JA. Frequent Somatic TERT Promoter Mutations in Thyroid Cancer: Higher Prevalence in Advanced Forms of the Disease. J Clin Endocrinol Metab. 2013E doi: 10.1210/jc.2013-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Robinson MA, Harrison SC. The active conformation of the PAK1 kinase domain. Structure. 2005;13:769–778. doi: 10.1016/j.str.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Liu X, Qu S, Liu R, Sheng C, Shi X, Zhu G, Murugan AK, Guan H, Yu H, Wang Y, et al. TERT Promoter Mutations and Their Association with BRAF V600E Mutation and Aggressive Clinicopathological Characteristics of Thyroid Cancer. J Clin Endocrinol Metab jc. 2014;2013:4048. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QLYF, Frautschy SA, Cole GM. PAK in Alzheimer disease, Huntington disease and X-linked mental retardation. Cell Logist. 2012;2:117–125. doi: 10.4161/cl.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrodouli EOE, Koc M, Andera L, Sasazuki T, Shirasawa S, Pintzas A. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study. Mol Cancer. 2011;10 doi: 10.1186/1476-4598-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty SK, Saji M, Zhang X, Jarjoura D, Fusco A, Vasko VV, Ringel MD. Group I p21-activated kinases regulate thyroid cancer cell migration and are overexpressed and activated in thyroid cancer invasion. Endocr Relat Cancer. 2010;17:989–999. doi: 10.1677/ERC-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay MM, Ritt DA, Morrison DK. Signaling dynamics of the KSR1 scaffold complex. Proc Natl Acad Sci U S A. 2009;106:11022–11027. doi: 10.1073/pnas.0901590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo RM, Castellone MD, Guarino V, De Falco V, Cirafici AM, Salvatore G, Caiazzo F, Basolo F, Giannini R, Kruhoffer M, et al. The RET/PTC-RAS-BRAF linear signaling cascade mediates the motile and mitogenic phenotype of thyroid cancer cells. J Clin Invest. 2005;115:1068–1081. doi: 10.1172/JCI22758. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mitsutake N, Miyagishi M, Mitsutake S, Akeno N, Mesa C, Jr, Knauf JA, Zhang L, Taira K, Fagin JA. BRAF mediates RET/PTC-induced mitogen-activated protein kinase activation in thyroid cells: functional support for requirement of the RET/PTC-RAS-BRAF pathway in papillary thyroid carcinogenesis. Endocrinology. 2006;147:1014–1019. doi: 10.1210/en.2005-0280. [DOI] [PubMed] [Google Scholar]
- Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3:520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucera C, Lawler J, Parangi S. BRAFV600E and Microenvironment in Thyroid Cancer: A Functional Link to Drive Cancer Progression. Cancer Research. 2011;71:2417–2422. doi: 10.1158/0008-5472.CAN-10-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong CC, Jubb AM, Haverty PM, Zhou W, Tran V, Truong T, Turley H, O'Brien T, Vucic D, Harris AL, et al. Targeting p21-activated kinase 1 (PAK1) to induce apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2011;108:7177–7182. doi: 10.1073/pnas.1103350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porchia LM, Guerra M, Wang YC, Zhang Y, Espinosa AV, Shinohara M, Kulp SK, Kirschner LS, Saji M, Chen CS, et al. 2-Amino-N-{4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phe nyl} Acetamide (OSU-03012), a Celecoxib Derivative, Directly Targets p21-Activated Kinase. Mol Pharmacol. 2007;72:1124–1131. doi: 10.1124/mol.107.037556. [DOI] [PubMed] [Google Scholar]
- Radu MSG, Kosoff R, Chernoff J. PAK signalling during the development and progression of cancer. Nat Rev Cancer. 2014;14:13–25. doi: 10.1038/nrc3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider LF, Oladimeji P, Diakonova M. PAK1 Regulates Breast Cancer Cell Invasion through Secretion of Matrix Metalloproteinases in Response to Prolactin and Three-Dimensional Collagen IV. Mol Endocrinol. 2013;27:1048–1064. doi: 10.1210/me.2012-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesco-Eizaguirre G, Rodriguez I, De la Vieja A, Costamagna E, Carrasco N, Nistal M, Santisteban P. The BRAFV600E Oncogene Induces Transforming Growth Factor {beta} Secretion Leading to Sodium Iodide Symporter Repression and Increased Malignancy in Thyroid Cancer. Cancer Res. 2009;69:8317–8325. doi: 10.1158/0008-5472.CAN-09-1248. [DOI] [PubMed] [Google Scholar]
- Ringel MD. Molecular markers of aggressiveness of thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2009;16:361–366. doi: 10.1097/MED.0b013e32832ff2cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel MD, Hayre N, Saito J, Saunier B, Schuppert F, Burch H, Bernet V, Burman KD, Kohn LD, Saji M. Overexpression and Overactivation of Akt in Thyroid Carcinoma. Cancer Res. 2001;61:6105–6111. [PubMed] [Google Scholar]
- Sapkota B, Hill CE, Pollack BP. Vemurafenib enhances MHC induction in BRAF homozygous melanoma cells. Oncoimmunology. 2013;2:e22890. doi: 10.4161/onci.22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweppe RE, Klopper JP, Korch C, Pugazhenthi U, Benezra M, Knauf JA, Fagin JA, Marlow L, Copland JA, Smallridge RC, et al. Deoxyribonucleic Acid Profiling Analysis of 40 Human Thyroid Cancer Cell Lines Reveals Cross-Contamination Resulting in Cell Line Redundancy and Misidentification. J Clin Endocrinol Metab. 2008;83:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha Y, Schafer EJ, Boehm JS, Thomas SR, He F, Du J, Wang S, Barretina J, Weir BA, Zhao JJ, et al. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene. 2012;31:3397–3408. doi: 10.1038/onc.2011.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack-Davis J, Eblen S, Zecevic M, Boerner S, Tarcsafalvi A, Diaz H, Marshall M, Weber M, Parsons J, Catling A. PAK1 phosphorylation of MEK1 regulates fibronectin-stimulated MAPK activation. The Journal of Cell Biology. 2003;162:281–291. doi: 10.1083/jcb.200212141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko V, Espinosa AV, Scouten W, He H, Auer H, Liyanarachchi S, Larin A, Savchenko V, Francis GL, de la Chapelle A, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007;104:2803–2808. doi: 10.1073/pnas.0610733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fu M, Wang L, Liu J, Li Y, Brakebusch C, Mei Q. p21-Activated Kinase 1 (PAK1) Can Promote ERK Activation in a Kinase-independent Manner. J Biol Chem. 2013;288:20093–20099. doi: 10.1074/jbc.M112.426023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whale A, Hashim FN, Fram S, Jones GE, Wells CM. Signalling to cancer cell invasion through PAK family kinases. Front Biosci. 2011;16:849–864. doi: 10.2741/3724. [DOI] [PubMed] [Google Scholar]
- Wong RM, Bresee C, Braunstein GD. Comparison with published systems of a new staging system for papillary and follicular thyroid carcinoma. Thyroid. 2013;23:566–574. doi: 10.1089/thy.2012.0181. [DOI] [PubMed] [Google Scholar]
- Xing M. BRAF Mutation in Papillary Thyroid Cancer: Pathogenic Role, Molecular Bases, and Clinical Implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- Xing MAA, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O'Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinchuk V, Zinchuk O, Okada T. Quantitative Colocalization Analysis of Multicolor Confocal Immunofluorescence Microscopy Images: Pushing Pixels to Explore Biological Phenomena. Acta Histochem Cytochem. 2007;40:101–111. doi: 10.1267/ahc.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.