Abstract
An alkyl aryl ether bond formation reaction between phenols and primary and secondary alcohols with PhenoFluor has been developed. The reaction features a broad substrate scope and tolerates many functional groups, and substrates that are challenging for more conventional ether bond forming processes may be coupled. A preliminary mechanistic study indicates reactivity distinct from conventional ether bond formation.
Keywords: alkyl aryl ethers, C—O bond formation, fluorine, Mitsunobu reaction, PhenoFluor
Ethers are common,[1] yet their synthesis is often challenging. Simple ethers are readily made through the Williamson ether synthesis, which was developed more than 150 years ago,[2] but even for moderately complex ethers, such as those derived from secondary alcohols, the Williamson synthesis often fails owing to low reactivity and undesired side reactions, such as elimination.[2] Transition-metal-catalyzed or -mediated cross-coupling reactions, exemplified by Ullmann,[3] Chan–Lam–Evans,[4] and Buchwald–Hartwig couplings,[5] are powerful methods for ether bond formation. Functionally complex secondary alcohols, however, are challenging substrates for these transition-metal-catalyzed reactions as well. The direct coupling between phenols and alcohols for alkyl aryl ether bond formation is appealing and orthogonal to other cross-coupling approaches. The Mitsunobu reaction has been developed for this purpose, and a large substrate scope has been demonstrated.[6, 7] However, several substrate classes, such as salicylaldehydes, are not tolerated, and general alcohol–alcohol cross-couplings are deemed challenging owing to the high pKa values of typical alcohols.[8] Likewise, the direct coupling of phenols and 2,2,2-trifluoroethanol in a Mitsunobu reaction has not been reported. Herein, we report a new approach to alkyl aryl ether formation through the use of the reagent PhenoFluor (Scheme 1). The reaction provides a conceptually different, promising reactivity profile, possibly enabled by the tight ion pairing of key intermediates. Our strategy distinguishes itself by operational simplicity—phenols and alcohols can be used directly—and a large substrate scope, which includes salicylaldehydes and 2,2,2-trifluoroethanol. An example of selective glycosylation is also shown, further demonstrating the potential synthetic applications.
Scheme 1.
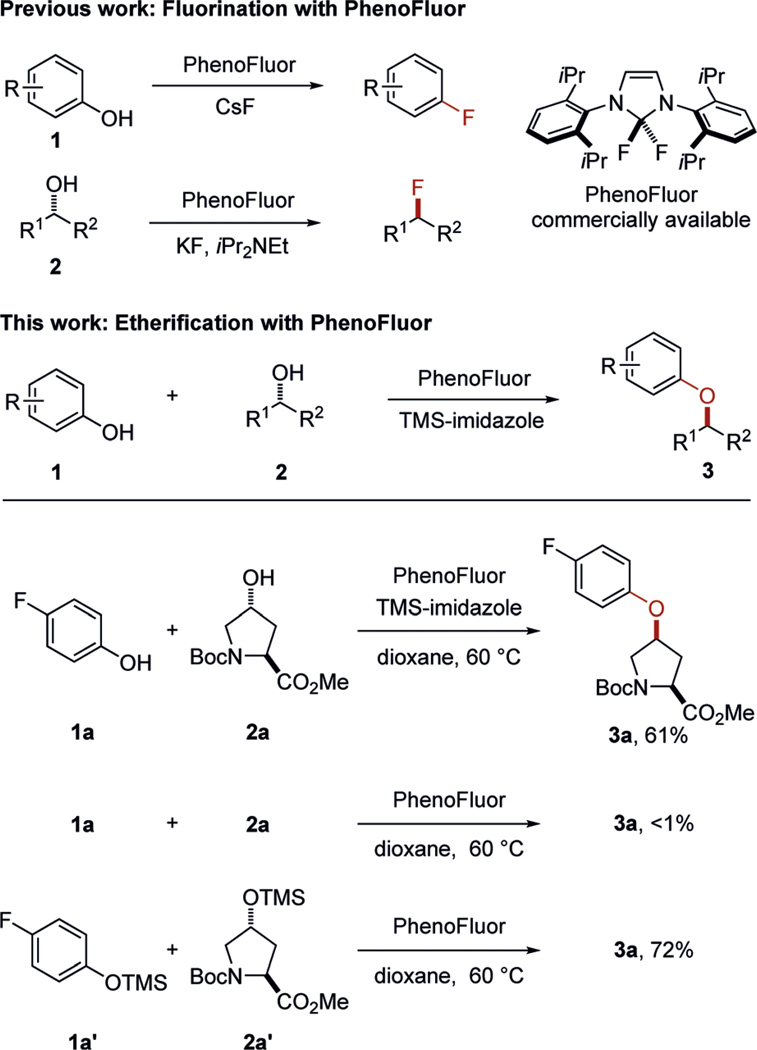
Fluorination and etherification reactions with PhenoFluor. Boc = tert-butyloxycarbonyl, TMS = trimethylsilyl.
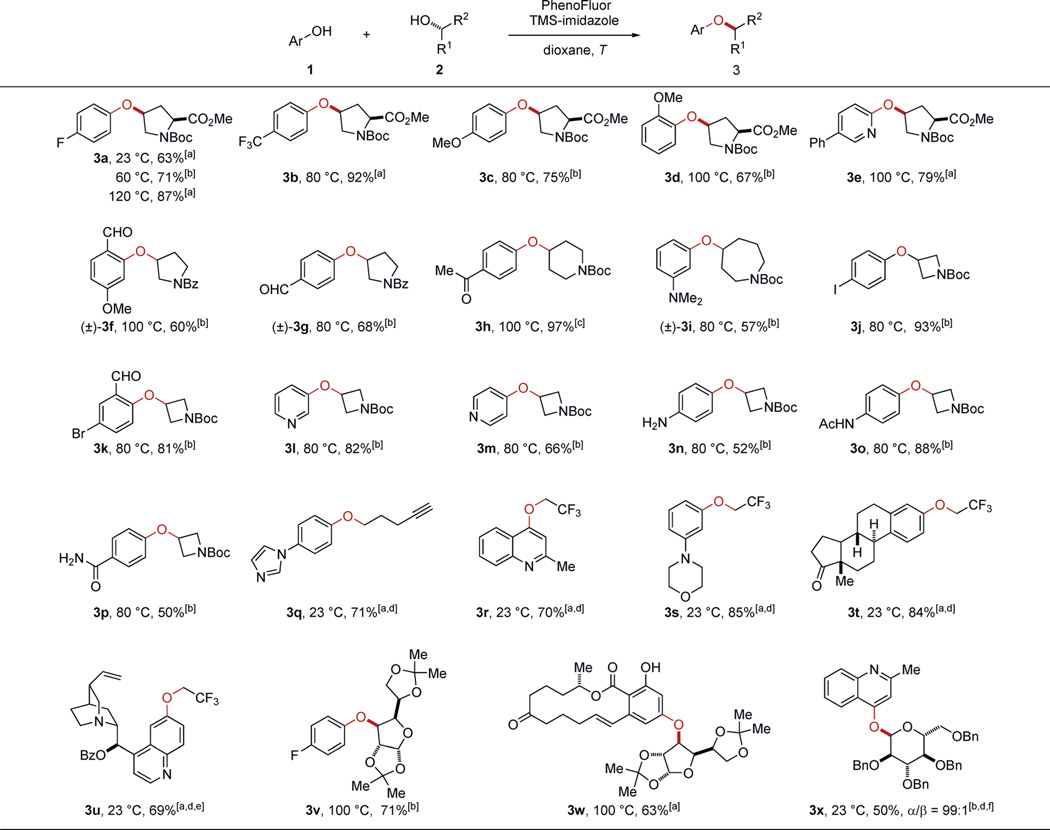
PhenoFluor is a commercially available deoxyfluorination reagent for both alcohols and phenols, with a larger demonstrated substrate scope than any other deoxyfluorination reagent.[9] We speculated that instead of fluoride, phenolates or alcoholates might act as nucleophiles to provide ethers if the fluoride ions were effectively trapped by silanes.[10] The reaction of an equimolar mixture of alcohol 2a and 4-fluorophenol with PhenoFluor and TMS-imidazole (2 equiv) in dioxane at 60°C for 21 hours afforded ether 3a in 61% yield (Scheme 1; for reaction optimization, see the Supporting Information). Without the addition of TMS-imidazole, less than 1% of ether 3a was formed, and deoxyfluorination of alcohol 2a was observed. The yield of the ether bond formation could be increased by using the silyl ethers of phenol 1a and alcohol 2a, but for operational simplicity, we opted to develop the ether bond formation directly from phenols and alcohols.
Ether bond formation proceeds at 23°C, but higher yields are obtained at elevated temperature. The reaction tolerates a variety of functional groups, including aldehydes, alkenes, alkynes, amines, amides, esters, halides, and ketones (Table 1). Both electron-poor and -rich phenols are suitable substrates. Substituted salicylaldehydes cannot be used to access ethers with alcohols in Mitsunobu reactions owing to the condensation of the aldehyde with Huisgen zwitterions, the key intermediates in Mitsunobu reactions.[8] However, under our current cross-coupling reaction conditions, products (±)-3 f and 3k were obtained in 60% and 81% yield, respectively. Furthermore, pyridinols and quinolinols are also suitable substrates (see 3e, 3l, 3m, 3r, and 3u).
Table 1.
Cross-coupling of phenols and alcohols.
 |
1/2/PhenoFluor/TMS-imidazole=1.0:2.0:2.0:4.0.
1/2/PhenoFluor/TMS-imidazole=1.5:1.0:1.5:3.0.
1/2/PhenoFluor/TMS-imidazole= 1.0:3.0:3.0:6.0.
TMSNEt2 was used instead of TMS-imidazole.
A yield of 85% was obtained when the reaction was carried out at 80 °C.
Toluene was used as the solvent instead of dioxane.
Bn=benzyl, Bz=benzoyl.
Primary and secondary alcohols, including β,β-branched alcohols, can be employed. Alkyl trifluoroethyl ethers can be made through Mitsunobu reactions,[11] but the synthesis of aryl trifluoroethyl ethers in Mitsunobu reactions has not been reported.[12–14] As shown in Table 1, ether bond formation of phenols and 2,2,2-trifluoroethanol proceeded with Pheno- Fluor at 23°C (3r–3u). Moreover, lactols also undergo PhenoFluor-mediated C–O bond formation: 2,3,4,6-Tetra-O-benzyl-d-galactose, for example, undergoes glycosylation with 99:1 α/β selectivity (3x);[15] glycosylation under Mitsunobu conditions afforded 3x in 30:70 α/β selectivity (see the Supporting Information).
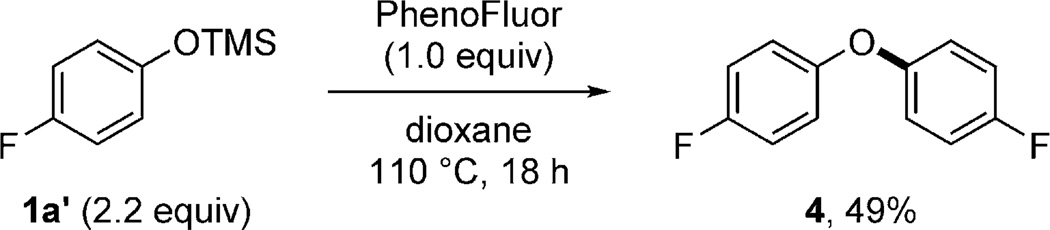
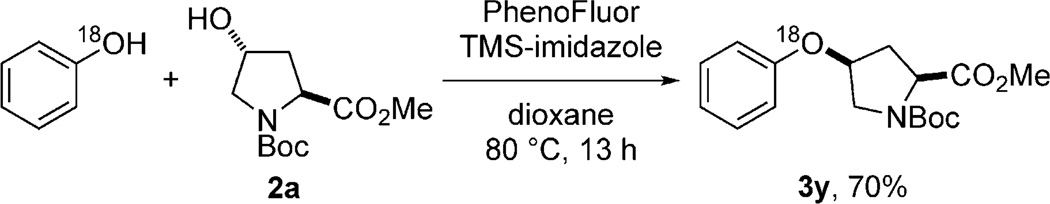
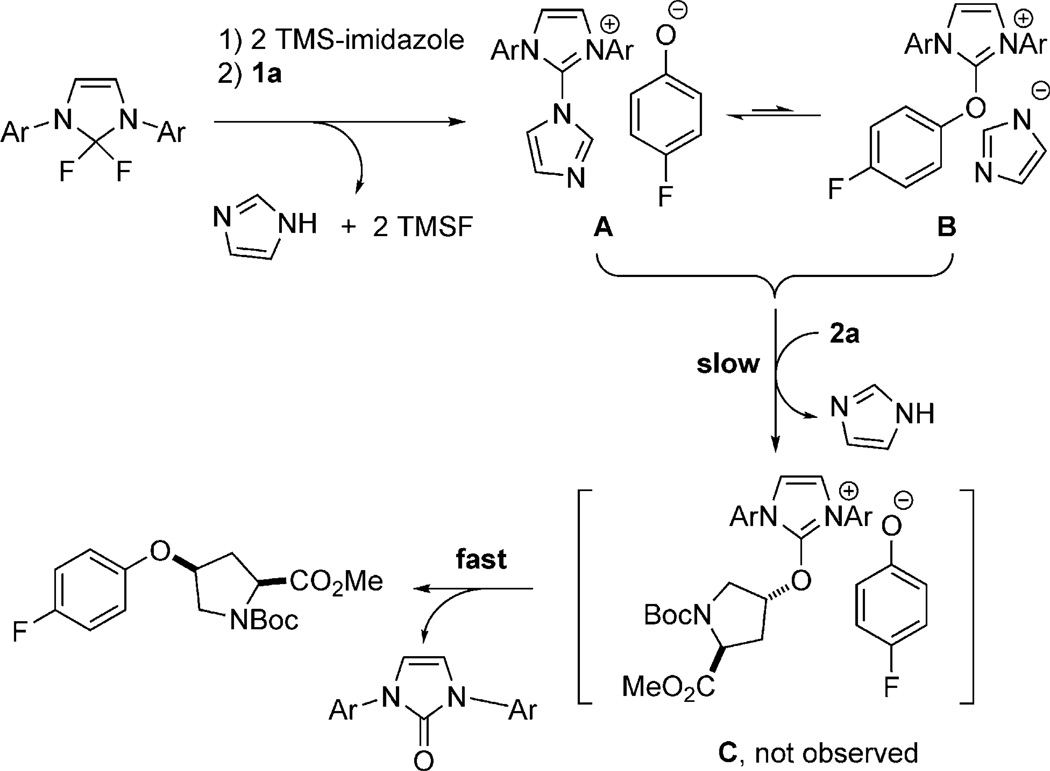
Mechanistically, we believe that the ether bond formation reported here is distinct from conventional ether bond forming reactions. For example, diaryl ether bond formation was found to occur when 1 equivalent of PhenoFluor was treated with 2.2 equivalents of the aryl silyl ether 1a′(Scheme 2); diaryl ethers cannot be accessed through conventional Mitsunobu reactions by an SN2 pathway. An 18O labeling experiment established that the oxygen atom contained in the ether product originates from the phenol (Scheme 3). Moreover, we established that ether bond formation proceeds with inversion at the carbinol center (see the Supporting Information). In situ 19F NMR and HRMS monitoring of the reaction revealed the presence of ion pairs A and B, but not C, which leads us to propose a slow formation of ion pair C, followed by fast nucleophilic displacement to form the desired product (Scheme 4). Given the similarities between the PhenoFluor-mediated deoxyfluorination[9a,b] and the ether bond formation reported here, we believe that the formation of tight ion pairs is of particular relevance for PhenoFluor-mediated reactivity, and may be, at least in part, responsible for the efficient C–O bond formation and the large substrate scope.
Scheme 2.
Diaryl ether formation.
Scheme 3.
The 18O label originates from phenol.
Scheme 4.
Proposed mechanism. Ar=2,6-diisopropylphenyl.
In conclusion, we have developed a novel alkyl aryl ether synthesis from alcohols and phenols with PhenoFluor. The presented ether bond formation has a large substrate scope and can provide ethers that are challenging to obtain with conventional Mitsunobu reactions. We believe that the distinct PhenoFluor-based reactivity shown here may contribute to the development of more general ether bond formation reactions.
Supplementary Material
Footnotes
We thank Teppei Fujimoto for the synthesis of PhenoFluor, Filippo Sladojevich for helpful discussions, and the NIH (NIH-NIGMS GM088237) for financial support.
References
- 1.a) Patai S. The Chemistry of the Ether Linkage. London: Interscience Publishers; 1967. [Google Scholar]; b) Buckingham J. Dictionary of Natural Products. Cambridge, MA: University Press; 1994. [Google Scholar]
- 2.a) Williamson A. Justus Liebigs Ann. Chem. 1851;77:37. [Google Scholar]; b) March J. March’s Advanced Organic Chemistry, Reaction, Mechanisms and Structure. 5th ed. New York: Wiley; 2001. p. 477. [Google Scholar]
- 3.a) Kunz K, Scholz U, Ganzer D. Synlett. 2003:2428. [Google Scholar]; b) Ma D, Cai Q. Acc. Chem. Res. 2008;41:1450. doi: 10.1021/ar8000298. [DOI] [PubMed] [Google Scholar]; c) Monnier F, Taillefer M. Angew. Chem. Int. Ed. 2009;48:6954. doi: 10.1002/anie.200804497. Angew. Chem. 2009, 121, 7088. [DOI] [PubMed] [Google Scholar]; d) Wang Y, Zeng J, Cui X. Youji Huaxue. 2010;30:181. [Google Scholar]; e) Naidu AB, Jaseer EA, Sekar G. J. Org. Chem. 2009;74:3675. doi: 10.1021/jo900438e. [DOI] [PubMed] [Google Scholar]; f) Wolter M, Nordmann G, Job GE, Buchwald SL. Org. Lett. 2002;4:973. doi: 10.1021/ol025548k. [DOI] [PubMed] [Google Scholar]; g) Ullmann F. Ber. Dtsch. Chem. Ges. 1903;36:2382. [Google Scholar]; h) Ullmann F. Ber. Dtsch. Chem. Ges. 1904;37:853. [Google Scholar]
- 4.a) Qiao JX, Lam PYS. Synthesis. 2011:829. [Google Scholar]; b) Rao KS, Wu T-S. Tetrahedron. 2012;68:7735. [Google Scholar]; c) Chan DMT, Monaco KL, Wang R-P, Winters MP. Tetrahedron Lett. 1998;39:2933. [Google Scholar]; d) Evans DA, Katz JL, West TR. Tetrahedron Lett. 1998;39:2937. [Google Scholar]; e) Lam PYS, Clark CG, Saubern S, Adams J, Winters MP, Chan DMT, Combs A. Tetrahedron Lett. 1998;39:2941. [Google Scholar]; f) El Khatib M, Molander GA. Org. Lett. 2014;16:4944. doi: 10.1021/ol5024689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Surry DS, Buchwald SL. Chem. Sci. 2011;2:27. doi: 10.1039/C0SC00331J. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Hartwig JF. Acc. Chem. Res. 2008;41:1534. doi: 10.1021/ar800098p. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wu X, Fors BP, Buchwald SL. Angew. Chem. Int. Ed. 2011;50:9943. doi: 10.1002/anie.201104361. Angew. Chem. 2011, 123, 10117. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Vorogushin AV, Huang X, Buchwald SL. J. Am. Chem. Soc. 2005;127:8146. doi: 10.1021/ja050471r. [DOI] [PubMed] [Google Scholar]; e) Mann G, Hartwig JF. J. Am. Chem. Soc. 1996;118:13109. [Google Scholar]; f) Mann G, Incarvito C, Rheingold AL, Hartwig JF. J. Am. Chem. Soc. 1999;121:3224. [Google Scholar]; g) Kataoka N, Shelby Q, Stambuli JP, Hartwig JF. J. Org. Chem. 2002;67:5553. doi: 10.1021/jo025732j. [DOI] [PubMed] [Google Scholar]
- 6.For reviews on the Mitsunobu reaction, see: Swamy KCK, Kumar NNB, Balaraman E, Kumar KVPP. Chem. Rev. 2009;109:2551. doi: 10.1021/cr800278z. But TYS, Toy PH. Chem. Asian J. 2007;2:1340. doi: 10.1002/asia.200700182. Hughes DL. Org. React. 1992;42:335.
- 7.Several reductive etherifications of carbonyl compounds with alcohols or phenols have been reported; see: Sassaman MB, Prakash GKS, Olah GA, Donald P, Loker KB. Tetrahedron. 1988;44:3771. Hatakeyama S, Mori H, Kitano K, Yamada H, Nishizawa M. Tetrahedron Lett. 1994;35:4367. Evans PA, Cui J, Gharpure SJ, Hinkle RJ. J. Am. Chem. Soc. 2003;125:11456. doi: 10.1021/ja036439j. Yang W-C, Lu X-A, Kulkarni SS, Hung S-C. Tetrahedron Lett. 2003;44:7837. Iwanami K, Seo H, Tobita Y, Oriyama T. Synthesis. 2005;183 Barluenga J, Tomas-Gamasa M, Aznar F, Valdes C. Angew. Chem. Int. Ed. 2010;49:4993. doi: 10.1002/anie.201001704. Angew. Chem., 2010, 122, 5113. Gharpure SJ, Prasad JVK. Eur. J. Org. Chem. 2013:2076.
- 8.Girard M, Murphy P, Tsou NN. Tetrahedron Lett. 2005;46:2449. [Google Scholar]
- 9.a) Tang P, Wang W, Ritter T. J. Am. Chem. Soc. 2011;133:11482. doi: 10.1021/ja2048072. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sladojevich F, Arlow SI, Tang P, Ritter T. J. Am. Chem. Soc. 2013;135:2470. doi: 10.1021/ja3125405. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Fujimoto T, Becker F, Ritter T. Org. Process Res. Dev. 2014;18:1041. doi: 10.1021/op500121w. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Campbell MG, Ritter T. Org. Process Res. Dev. 2014;18:474. doi: 10.1021/op400349g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Si–F bond is strong (BDEs: Si–F:135 kcal mol−1; C–F: 116 kcal mol−1; Si–O: 110 kcal mol−1); see: Sanderson RT. Chemical Bonds and Bond Energy. New York: Academic Press; 1976.
- 11.For examples, see: Sebesta DP, O’Rourke SS, Pieken WA. J. Org. Chem. 1996;61:361. Gueyrard D, Rollin P, Nga TTT, Ourévitch M, Bégué J-P, Bonnet-Delpon D. Carbohydr. Res. 1999;318:171. Szabó D, Bonto A-M, Kövesdi I, Gömöry Á, Rábai J. J. Fluorine Chem. 2005;126:641.
- 12.For the importance of aryl trifluoroethyl ethers in medicinal chemistry and synthetic chemistry, see: Bégué J-P, Bonnet-Delpon D. In: Bioorganic and Medicinal Chemistry of Fluorine. Legros J, translator. Hoboken: Wiley; 2008. translated from French to English. Yang Q, Njardarson JT. Tetrahedron Lett. 2013;54:7080. doi: 10.1016/j.tetlet.2013.10.097.
- 13.For the direct coupling of phenols and trifluoroethanol, see: Laatsch H. Z. Naturforsch B. 1985;40B:534. Hawthorne SB, Miller DJ, Nivens DE, White DC. Anal. Chem. 1992;64:405.
- 14.For the synthesis of aryl trifluoroethyl ethers with transitionmetal-catalyzed coupling reactions, see: Vuluga D, Legros J, Crousse B, Bonnet-Delpon D. Eur. J. Org. Chem. 2009:3513. doi: 10.1021/jo9012699. Quach TD, Batey RA. Org. Lett. 2003;5:1381. doi: 10.1021/ol034454n. Keegstra MA, Peters THA, Brandsma L. Tetrahedron. 1992;48:3633. and Ref. [4a]. For SNAr reactions of 2,2,2-trifluoroethanol with electron-poor arenes, see: Gupton JT, Idoux JP, Colon C, Rampi R. Synth. Commun. 1982;12:695. Idoux JP, Madenwald ML, Garcia BS, Chu DL, Gupton JT. J. Org. Chem. 1985;50:1876. Tejero I, Huertas I, Gonzalez-Lafont A, Lluch JM, Marquet J. J. Org. Chem. 2005;70:1718. doi: 10.1021/jo048354m. Vlasov VM. Russ. Chem. Rev. 2003;72:681. For the Williamson synthesis of aryl trifluoroethyl ethers, see: Kamal A, Pratap TB, Ramana KV, Ramana AV, Babu AH. Tetrahedron Lett. 2002;43:7353. Yang Q, Njardarson JT, Draghici C, Li F. Angew. Chem. Int. Ed. 2013;52:8648. doi: 10.1002/anie.201304624. Angew. Chem. 2013, 125, 8810.
- 15.For examples of the glycosylation of phenols, see: Li Y, Mo H, Lian G, Yu B. Carbohydr. Res. 2012;363:14. doi: 10.1016/j.carres.2012.09.025. Yamanoi T, Yamazaki I. Tetrahedron Lett. 2001;42:4009. Yamanoi T, Fujioka A, Inazu T. Bull. Chem. Soc. Jpn. 1994;67:1488. Jensen KJ, Meldal M, Bock K. J. Chem. Soc. Perkin Trans. 1. 1993:2119. Briner K, Vasella A. Helv. Chim. Acta. 1990;73:1764. Matsumoto T, Katsuki M, Suzuki K. Chem. Lett. 1989:437. Vaccaro WD, Sher R, Davis HR., Jr Bioorg. Med. Chem. Lett. 1998;8:35. doi: 10.1016/s0960-894x(97)10185-8.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.