Abstract
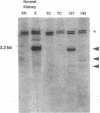
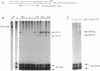
Mutational inactivation and allelic loss of the von Hippel-Lindau (VHL) gene appear to be causal events for the majority of spontaneous clear-cell renal carcinomas. We now show that hypermethylation of a normally unmethylated CpG island in the 5' region provides another potentially important mechanism for inactivation of the VHL gene in a significant portion of these cancers. This hypermethylation was found in 5 of 26 (19%) tumors examined. Four of these had lost one copy of VHL while one retained two heavily methylated alleles. Four of the tumors with VHL hypermethylation had no detectable mutations, whereas one had a missense mutation in addition to hypermethylation of the single retained allele. As would be predicted for the consequence of methylation in this 5' CpG island, none of the 5 tumors expressed the VHL gene. In contrast, normal kidney and all tumors examined with inactivating VHL gene mutations but no CpG island methylation had expression. In a renal cell culture line, treatment with 5-aza-2'-deoxycytidine resulted in reexpression of the VHL gene. These findings suggest that aberrant methylation of CpG islands may participate in the tumor-suppressor gene inactivations which initiate or cause progression of common human cancers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anglard P., Trahan E., Liu S., Latif F., Merino M. J., Lerman M. I., Zbar B., Linehan W. M. Molecular and cellular characterization of human renal cell carcinoma cell lines. Cancer Res. 1992 Jan 15;52(2):348–356. [PubMed] [Google Scholar]
- Antequera F., Boyes J., Bird A. High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell. 1990 Aug 10;62(3):503–514. doi: 10.1016/0092-8674(90)90015-7. [DOI] [PubMed] [Google Scholar]
- Bartolomei M. S., Webber A. L., Brunkow M. E., Tilghman S. M. Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes Dev. 1993 Sep;7(9):1663–1673. doi: 10.1101/gad.7.9.1663. [DOI] [PubMed] [Google Scholar]
- Baylin S. B., Makos M., Wu J. J., Yen R. W., de Bustros A., Vertino P., Nelkin B. D. Abnormal patterns of DNA methylation in human neoplasia: potential consequences for tumor progression. Cancer Cells. 1991 Oct;3(10):383–390. [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Brooks J. D., Bova G. S., Marshall F. F., Isaacs W. B. Tumor suppressor gene allelic loss in human renal cancers. J Urol. 1993 Oct;150(4):1278–1283. doi: 10.1016/s0022-5347(17)35760-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Gehrke C. W., Kuo K. C., Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988 Mar 1;48(5):1159–1161. [PubMed] [Google Scholar]
- Ferguson-Smith A. C., Sasaki H., Cattanach B. M., Surani M. A. Parental-origin-specific epigenetic modification of the mouse H19 gene. Nature. 1993 Apr 22;362(6422):751–755. doi: 10.1038/362751a0. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Vogelstein B., Hamilton S. R., Feinberg A. P. Hypomethylation of DNA from benign and malignant human colon neoplasms. Science. 1985 Apr 12;228(4696):187–190. doi: 10.1126/science.2579435. [DOI] [PubMed] [Google Scholar]
- Greger V., Passarge E., Höpping W., Messmer E., Horsthemke B. Epigenetic changes may contribute to the formation and spontaneous regression of retinoblastoma. Hum Genet. 1989 Sep;83(2):155–158. doi: 10.1007/BF00286709. [DOI] [PubMed] [Google Scholar]
- Issa J. P., Vertino P. M., Wu J., Sazawal S., Celano P., Nelkin B. D., Hamilton S. R., Baylin S. B. Increased cytosine DNA-methyltransferase activity during colon cancer progression. J Natl Cancer Inst. 1993 Aug 4;85(15):1235–1240. doi: 10.1093/jnci/85.15.1235. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Wolkowicz M. J., Rideout W. M., 3rd, Gonzales F. A., Marziasz C. M., Coetzee G. A., Tapscott S. J. De novo methylation of the MyoD1 CpG island during the establishment of immortal cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6117–6121. doi: 10.1073/pnas.87.16.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautiainen T. L., Jones P. A. DNA methyltransferase levels in tumorigenic and nontumorigenic cells in culture. J Biol Chem. 1986 Feb 5;261(4):1594–1598. [PubMed] [Google Scholar]
- Latif F., Tory K., Gnarra J., Yao M., Duh F. M., Orcutt M. L., Stackhouse T., Kuzmin I., Modi W., Geil L. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993 May 28;260(5112):1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- Li E., Beard C., Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993 Nov 25;366(6453):362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Makos M., Nelkin B. D., Chazin V. R., Cavenee W. K., Brodeur G. M., Baylin S. B. DNA hypermethylation is associated with 17p allelic loss in neural tumors. Cancer Res. 1993 Jun 15;53(12):2715–2718. [PubMed] [Google Scholar]
- Makos M., Nelkin B. D., Lerman M. I., Latif F., Zbar B., Baylin S. B. Distinct hypermethylation patterns occur at altered chromosome loci in human lung and colon cancer. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1929–1933. doi: 10.1073/pnas.89.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makos M., Nelkin B. D., Reiter R. E., Gnarra J. R., Brooks J., Isaacs W., Linehan M., Baylin S. B. Regional DNA hypermethylation at D17S5 precedes 17p structural changes in the progression of renal tumors. Cancer Res. 1993 Jun 15;53(12):2719–2722. [PubMed] [Google Scholar]
- Nelkin B. D., Przepiorka D., Burke P. J., Thomas E. D., Baylin S. B. Abnormal methylation of the calcitonin gene marks progression of chronic myelogenous leukemia. Blood. 1991 Jun 1;77(11):2431–2434. [PubMed] [Google Scholar]
- Ogawa O., Kakehi Y., Ogawa K., Koshiba M., Sugiyama T., Yoshida O. Allelic loss at chromosome 3p characterizes clear cell phenotype of renal cell carcinoma. Cancer Res. 1991 Feb 1;51(3):949–953. [PubMed] [Google Scholar]
- Ohtani-Fujita N., Fujita T., Aoike A., Osifchin N. E., Robbins P. D., Sakai T. CpG methylation inactivates the promoter activity of the human retinoblastoma tumor-suppressor gene. Oncogene. 1993 Apr;8(4):1063–1067. [PubMed] [Google Scholar]
- Presti J. C., Jr, Rao P. H., Chen Q., Reuter V. E., Li F. P., Fair W. R., Jhanwar S. C. Histopathological, cytogenetic, and molecular characterization of renal cortical tumors. Cancer Res. 1991 Mar 1;51(5):1544–1552. [PubMed] [Google Scholar]
- Presti J. C., Jr, Reuter V. E., Cordon-Cardo C., Mazumdar M., Fair W. R., Jhanwar S. C. Allelic deletions in renal tumors: histopathological correlations. Cancer Res. 1993 Dec 1;53(23):5780–5783. [PubMed] [Google Scholar]
- Riggs A. D., Pfeifer G. P. X-chromosome inactivation and cell memory. Trends Genet. 1992 May;8(5):169–174. doi: 10.1016/0168-9525(92)90219-t. [DOI] [PubMed] [Google Scholar]
- Sakai T., Toguchida J., Ohtani N., Yandell D. W., Rapaport J. M., Dryja T. P. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. Am J Hum Genet. 1991 May;48(5):880–888. [PMC free article] [PubMed] [Google Scholar]
- Shuin T., Kondo K., Torigoe S., Kishida T., Kubota Y., Hosaka M., Nagashima Y., Kitamura H., Latif F., Zbar B. Frequent somatic mutations and loss of heterozygosity of the von Hippel-Lindau tumor suppressor gene in primary human renal cell carcinomas. Cancer Res. 1994 Jun 1;54(11):2852–2855. [PubMed] [Google Scholar]
- Stöger R., Kubicka P., Liu C. G., Kafri T., Razin A., Cedar H., Barlow D. P. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell. 1993 Apr 9;73(1):61–71. doi: 10.1016/0092-8674(93)90160-r. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Tumor suppressor genes. Science. 1991 Nov 22;254(5035):1138–1146. doi: 10.1126/science.1659741. [DOI] [PubMed] [Google Scholar]
- Zbar B., Brauch H., Talmadge C., Linehan M. Loss of alleles of loci on the short arm of chromosome 3 in renal cell carcinoma. 1987 Jun 25-Jul 1Nature. 327(6124):721–724. doi: 10.1038/327721a0. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Nelkin B. D., Silverman A., Ehrlich G., Poiesz B., Baylin S. B. The short arm of chromosome 11 is a "hot spot" for hypermethylation in human neoplasia. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5693–5697. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Nelkin B. D., Celano P., Yen R. W., Falco J. P., Hamilton S. R., Baylin S. B. High expression of the DNA methyltransferase gene characterizes human neoplastic cells and progression stages of colon cancer. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3470–3474. doi: 10.1073/pnas.88.8.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hout A. H., van den Berg E., van der Vlies P., Dijkhuizen T., Störkel S., Oosterhuis J. W., de Jong B., Buys C. H. Loss of heterozygosity at the short arm of chromosome 3 in renal-cell cancer correlates with the cytological tumour type. Int J Cancer. 1993 Feb 1;53(3):353–357. doi: 10.1002/ijc.2910530302. [DOI] [PubMed] [Google Scholar]