Abstract
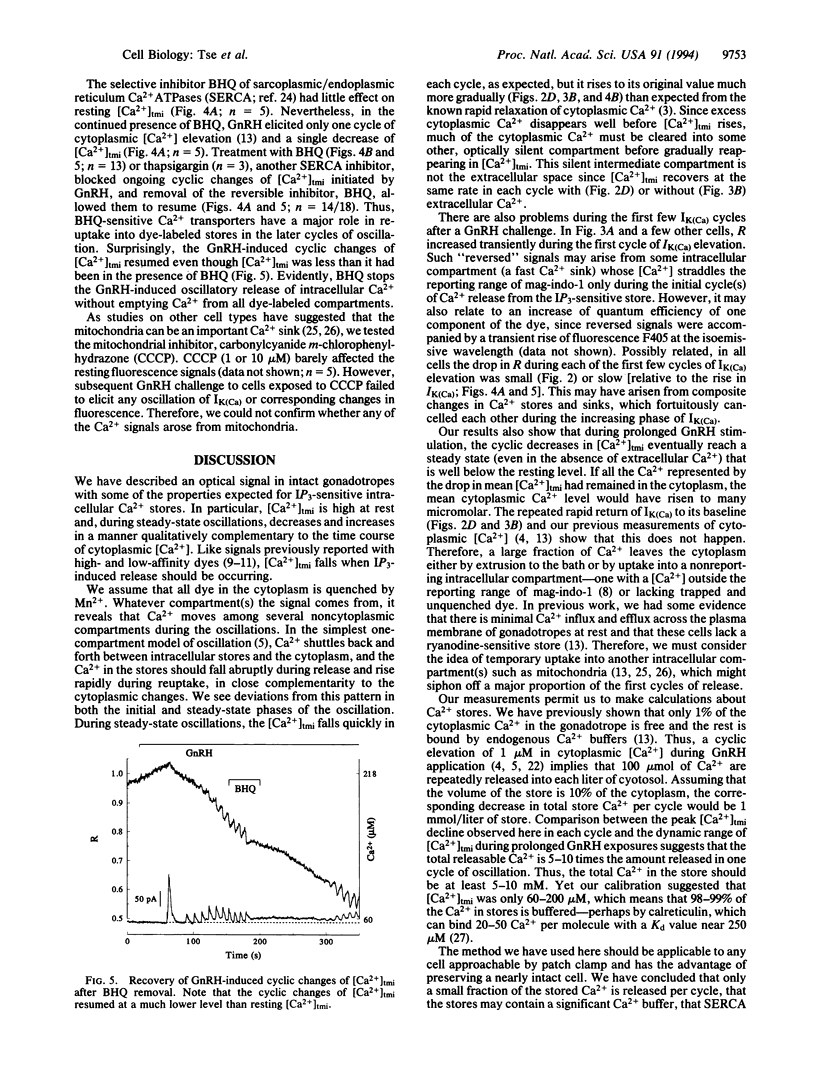
Gonadotropin-releasing hormone induces oscillatory release of Ca2+ from inositol trisphosphate-sensitive stores of gonadotropes. Simultaneously with electrophysiological measures of cytoplasmic [Ca2+], corresponding changes in [Ca2+] within intracellular stores were monitored with a fluorescent dye, mag-indo-1. Each cycle of oscillation released only 10% of the detectable stored Ca2+. Some Ca2+ was recovered by the stores using a mechanism sensitive to inhibitors of intracellular Ca2+ ATPases, and much of the remainder was temporarily and rapidly pumped into other intracellular compartments or out of the cell. The dynamics of Ca2+ oscillations are thus more complex than a repeated emptying and refilling of a single compartment. The free concentrations measured show that intracellular Ca2+ store compartments contain strong Ca2+ buffers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Intracellular calcium mobilization by inositol 1,4,5-trisphosphate: intracellular movements and compartmentalization. Cell Calcium. 1993 Mar;14(3):185–200. doi: 10.1016/0143-4160(93)90066-f. [DOI] [PubMed] [Google Scholar]
- Fewtrell C. Ca2+ oscillations in non-excitable cells. Annu Rev Physiol. 1993;55:427–454. doi: 10.1146/annurev.ph.55.030193.002235. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hofer A. M., Machen T. E. Technique for in situ measurement of calcium in intracellular inositol 1,4,5-trisphosphate-sensitive stores using the fluorescent indicator mag-fura-2. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2598–2602. doi: 10.1073/pnas.90.7.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall J. M., Dormer R. L., Campbell A. K. Targeting aequorin to the endoplasmic reticulum of living cells. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1008–1016. doi: 10.1016/0006-291x(92)92304-g. [DOI] [PubMed] [Google Scholar]
- Kukuljan M., Stojilković S. S., Rojas E., Catt K. J. Apamin-sensitive potassium channels mediate agonist-induced oscillations of membrane potential in pituitary gonadotrophs. FEBS Lett. 1992 Apr 13;301(1):19–22. doi: 10.1016/0014-5793(92)80201-q. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Li Y. X., Rinzel J., Keizer J., Stojilković S. S. Calcium oscillations in pituitary gonadotrophs: comparison of experiment and theory. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):58–62. doi: 10.1073/pnas.91.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenson M. Y., Cuccaro M. L., Jacobs L. S. Calcium release from pituitary secretory granules: modulation by thiols, disulfides, and dihydropyridine calcium channel blockers. Endocrinology. 1990 May;126(5):2671–2678. doi: 10.1210/endo-126-5-2671. [DOI] [PubMed] [Google Scholar]
- Michalak M., Milner R. E., Burns K., Opas M. Calreticulin. Biochem J. 1992 Aug 1;285(Pt 3):681–692. doi: 10.1042/bj2850681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard-Rooney D. C., Hajnóczky G., Seitz M. B., Schneider T. G., Thomas A. P. Imaging of inositol 1,4,5-trisphosphate-induced Ca2+ fluxes in single permeabilized hepatocytes. Demonstration of both quantal and nonquantal patterns of Ca2+ release. J Biol Chem. 1993 Nov 5;268(31):23601–23610. [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993 Oct 29;262(5134):744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Short A. D., Klein M. G., Schneider M. F., Gill D. L. Inositol 1,4,5-trisphosphate-mediated quantal Ca2+ release measured by high resolution imaging of Ca2+ within organelles. J Biol Chem. 1993 Dec 5;268(34):25887–25893. [PubMed] [Google Scholar]
- Stojilković S. S., Kukuljan M., Tomić M., Rojas E., Catt K. J. Mechanism of agonist-induced [Ca2+]i oscillations in pituitary gonadotrophs. J Biol Chem. 1993 Apr 15;268(11):7713–7720. [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Hanley M. R. Pharmacological tools for perturbing intracellular calcium storage. Methods Cell Biol. 1994;40:65–89. doi: 10.1016/s0091-679x(08)61110-3. [DOI] [PubMed] [Google Scholar]
- Tse A., Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992 Jan 24;255(5043):462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- Tse A., Hille B. Role of voltage-gated Na+ and Ca2+ channels in gonadotropin-releasing hormone-induced membrane potential changes in identified rat gonadotropes. Endocrinology. 1993 Apr;132(4):1475–1481. doi: 10.1210/endo.132.4.8384989. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Almers W., Hille B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science. 1993 Apr 2;260(5104):82–84. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994 Jun 15;477(Pt 3):511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse F. W., Iwata A., Almers W. Membrane flux through the pore formed by a fusogenic viral envelope protein during cell fusion. J Cell Biol. 1993 May;121(3):543–552. doi: 10.1083/jcb.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]



