Abstract
Background and Aims
Eosinophilic esophagitis (EoE) is an allergic inflammatory disease that leads to esophageal fibrosis and stricture. We have recently shown that in EoE, esophageal epithelial cells undergo an epithelial to mesenchymal transition (EMT), characterized by gain of mesenchymal markers and loss of epithelial gene expression. Whether epithelial cells exposed to profibrotic cytokines can also acquire the functional characteristics of activated myofibroblasts, including migration, contraction, and extracellular matrix deposition, is relevant to our understanding and treatment of EoE-associated fibrogenesis. In the current study, we characterize cell migration, contraction, and collagen production by esophageal epithelial cells that have undergone cytokine-induced EMT in vitro.
Methods and Results
Stimulation of human non-transformed immortalized esophageal epithelial cells (EPC2-hTERT) with profibrotic cytokines TNFα, TGFβ, and IL1β for three weeks led to acquisition of mesenchymal αSMA and vimentin, and loss of epithelial E-cadherin expression. Upon removal of the profibrotic stimulus, epithelial characteristics were partially rescued. TGFβ stimulation had a robust effect upon epithelial collagen production. Surprisingly, TNFα stimulation had the most potent effect upon cell migration and contraction, exceeding the effects of the prototypical profibrotic cytokine TGFβ. IL1β stimulation alone had minimal effect upon esophageal epithelial migration, contraction, and collagen production.
Conclusions
Esophageal epithelial cells that have undergone EMT acquire functional characteristics of activated myofibroblasts in vitro. Profibrotic cytokines exert differential effects upon esophageal epithelial cells, underscoring complexities of fibrogenesis in EoE, and implicating esophageal epithelial cells as effector cells in EoE-associated fibrogenesis.
Introduction
Eosinophilic esophagitis (EoE) is a chronic allergic inflammatory disease characterized by eosinophil infiltration into the esophageal epithelium. The most important clinical complication of EoE is esophageal fibrosis, resulting in progressive dysphagia and recurrent esophageal food impactions requiring urgent endoscopic removal. Unfortunately, very little is currently known about the mechanisms by which fibrosis develops in EoE.
Activated myofibroblasts are the key effector cells in all models of fibrosis [1,2]. In wound healing, tissue strain and cytokine release activate myofibroblasts to begin migration, extracellular matrix (ECM) deposition, and tissue contraction, thus maintaining tissue homeostasis [3]. However, in fibrosis, exaggerated myofibroblast responses result in inappropriate ECM deposition, increased tissue stiffness, and organ dysfunction.
The cellular origin of the myofibroblast is controversial. Line-age–tracing studies in the kidney, lung, and liver have provided conflicting results about the existence of epithelial to mesenchymal transition (EMT), although the most recent evidence suggests that epithelial cells are not a significant source of myofibroblasts in these tissues [4–8]. In EoE, it is presumed that activated myofibroblasts originate from resident fibroblasts in the lamina propria. This is supported by the findings of Aceves et al. who demonstrated marked increases in collagen deposition in the lamina propria of biopsies from patients with EoE compared to normal and GERD controls [9]. Alternatively, we and others have shown epithelial cells from patients with EoE have increased markers of activated myofibroblasts (αSMA and vimentin) and decreased epithelial markers (cytokeratin and E-cadherin) suggesting that one source of the activated myofibroblasts may be epithelial cells [10,11]. We have also shown that epithelial cells in organotypic culture acquire markers of activated myofibroblasts suggesting that EMT may play a role in EoE-associated fibrogenesis [10,11].
Though best known for its roles in development and malignancy [12], EMT is also required for normal tissue homeostasis. During normal embryological development, epithelial cells lose cell-cell connections, become migratory, and eventually form the mesenchyme [13]. Because epithelial cells are capable of transdifferentiation in these settings, it has been postulated that during chronic inflammation, epithelial cells contribute to fibrosis via EMT. As the epithelium is often the site of primary injury and inflammation, it stands to reason that epithelial cells may also function as effector cells in fibrogenesis.
While EMT has been implicated in fibrosis of other organ systems [14–19], definitive evidence for EMT requires in vivo lineage tracing studies. In a bleomycin-induced mouse model of lung fibrosis, Tanjore et al. found that while myofibroblasts of epithelial origin were rare, they did contribute to overall lung fibrosis [20]. However, Chu et al. refuted the notion of EMT in a murine model of hepatic fibrosis by showing a lack of colocalization of epithelial markers with α-smooth muscle actin (α-SMA), a prototypical marker of activated myofibroblasts [21]. Unfortunately, similar in vivo lineage tracing studies to refute or support EMT in EoE are lacking, as there have been no published animal models of EoE-associated fibrogenesis. Furthermore, others have suggested that EMT cannot be defined by marker analysis alone, and must demonstrate acquisition of functional characteristics of activated myofibroblasts, including migration, contraction, and collagen deposition [8,21].
Our previous work, along with that of Kagalwalla et al., has shown that markers of EMT are expressed in esophageal biopsies from subjects with EoE [10,11]. In the current study, we build upon our previous findings and demonstrate that the acquisition of EMT markers by cultured epithelial cells is partially reversible, and that esophageal epithelial cells can acquire functional properties of activated myofibroblasts including migration, contraction, and expression of type I collagen following acquisition of EMT markers.
Materials and methods
Esophageal epithelial cells
EPC2-hTERT{Harada, 2003 #1166}, an extensively characterized telomerase-immortalized, non-transformed human esophageal epithelial cell line, was used in all experiments. In addition, primary human esophageal epithelial cells were isolated as described [11] from IRB-approved six pediatric esophageal biopsies. Cells were grown at 37 °C in a humidified 5% CO2 incubator and maintained in keratinocyte serum free media (KSFM, Invitrogen, Grand Island, NY) containing human epidermal growth factor (1 ng/mL), bovine pituitary extract (50 μg/mL) and penicillin (100 units/mL) and streptomycin (100 μg/mL). For all experiments, EPC2-hTERT cells were used between passages 36 and 39. Primary cells were used between passages 3–5.
Cytokine stimulation
Cells were seeded in 6-well plates and stimulated in triplicate with one of the following human recombinant cytokines: TNFα (40 ng/mL), TGFβ (10 ng/mL), or IL1β (10 ng/mL). All recombinant cytokines were purchased from R&D Systems (Minneapolis, MN). Media containing individual cytokines was refreshed weekly for 3 weeks. For cytokine rescue experiments, cells were either cultured for an additional week in the presence of individual cytokine, or were cultured in standard media for one week.
Quantitative RT-PCR
RNA was purified using an RNeasy Kit (Qiagen, Valencia, CA) according to manufacturer’s recommendations. RNA samples were reverse transcribed using a high-capacity cDNA reverse transcriptase kit (Applied Biosystems, Foster City, CA). Pre-formulated TaqMan Gene Expression Assays were purchased from Applied Biosystems for human type I collagen (COL1A) (Hs00164004), α-smooth muscle actin (ACTA2) (Hs00426835), vimentin (VIM) (Hs00185584), E-cadherin (CDH1) (Hs01023894), and GAPDH (4352934E). Quantitative RT-PCR was performed using TaqMan Fast Universal PCR Master Mix kit and reactions were performed in triplicate using 96-well optical plates on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). GAPDH was used as an endogenous control to normalize the samples using Ct method of relative quantification, where Ct is the threshold cycle.
Immunofluorescence
EPC2-hTERT cells were seeded in chamber slides and stimulated with relevant cytokines as above. Slides were prepared and stained for α-smooth muscle actin (1:1000) (Sigma, St. Louis, MO) as previously described [11].
Migration assays
Cell migration was assessed by ORIS™ cell migration assays (Platypus Technologies, Madison, WI, USA) and scratch wound healing assays coupled with live cell imaging. EPC2-hTERT cells were first stimulated with TNFα, TGFβ, or IL1β as described above. Media was refreshed weekly for 3 weeks. ORIS™ cell migration assays were used according to the manufacturer’s instructions. In brief, 2.5 × 104 cells were seeded per well into a collagen coated 96-well plate with ORIS™ cell seeding stoppers in place. Following cell adherence for 24 hours, the stoppers were removed, creating a cell-free migration zone where cells were allowed to migrate for another 24 hours. Migrated cells were then labeled with a Calcein fluorescent dye (Sigma, St. Louis, MO) and subjected to quantification of a fluorescence signal intensity using a multi-mode microplate reader (Biotek HT Synergy, Winooski, VT) according to manufacturer’s instructions.
For scratch wound healing assays, EPC2-hTERT cells were seeded in 24-well plates. Following 3 weeks of cytokine stimulation, a 200-μl pipet tip was used to generate a cell free area by scratching each well along the midline. Images of the central scratch and surrounding area were captured every 8 minutes for 24 hours using the Nikon-Eclipse TI-U inverted microscope (Nikon, Melville, NY) and MetaMorph® Microscopy Automation & Image Analysis Software (Molecular Devices, Sunnyvale, CA, USA) and compiled into a movie.
Cell proliferation
BrdU Cell Proliferation Assay (Millipore, Billerica, MA) was performed in parallel to the migration assay, according to the manufacturer’s recommendations.
Contraction assay
Cells were seeded in 10-cm dishes and stimulated with TNFα, TGFβ, or IL1β as described above. Cytokine-stimulated cells were seeded in a 24-well plate at a concentration of 6 × 105 cells per well in a gel containing 10X Eagle’s Minimum Essential Medium (EMEM) (BioWhittaker, Walkersville, MD), FBS (LifeTechnologies, Grand Island, NY), L-glutamine (Cellgro, Manassas, VA), sodium bicarbonate (LifeTechnologies, Grand Island, NY), bovine collagen (Organogenesis, Canton, MA), and Matrigel (BD Biosciences, Bedford, MA). The sides of the gels were detached from the plates following 90 minutes of solidification at 37 °C, and gels were suspended in KSFM. Gels were measured daily, and the diameter of each gel was assessed at the widest point of the gel. After 8 days, the gels were fixed in formalin.
Western blot
Cells were lysed using RIPA buffer containing 0.1% SDS, homogenized by passing lysate through a 20-gauge needle, placed on ice for 45 minutes, and centrifuged at 12000 rpm for 15 minutes at 4 °C. Protein concentrations of the supernatant were determined using a BCA Assay (Thermo Scientific, Rockford, IL USA) according to manufacturer recommendations. 30 μg of protein/sample were separated by gel electrophoresis in a 4–12% Bis-Tris Gel (Life Technologies, CA) and transferred to Immobilon membranes (Millipore, Billerica, MA). Membranes were blocked with a solution of 2.5% albumin and 2.5% non-fat milk in TBST (Cell Signaling Technology, Beverly, MA) and subsequently incubated with rabbit anti-collagen I antibody (1:2500) (Abcam, Cambridge, MA) overnight, followed by a 1-hour incubation with donkey anti-rabbit secondary antibody (1:5000) (Abcam). Blots were developed with WesternBright ECL (Advansta, Menlo Park, CA) and imaged on Blue Ultra AutoRad Film (BioExpress, Kaysville, UT).
Statistical analysis
A two-tailed Student’s t-test was used for analysis of Figs. 1,3 and 4 and a one-way ANOVA and post-hoc comparison with Bonferroni was used to analyze Fig. 2. A p value ≤ 0.05 was considered to be statistically significant.
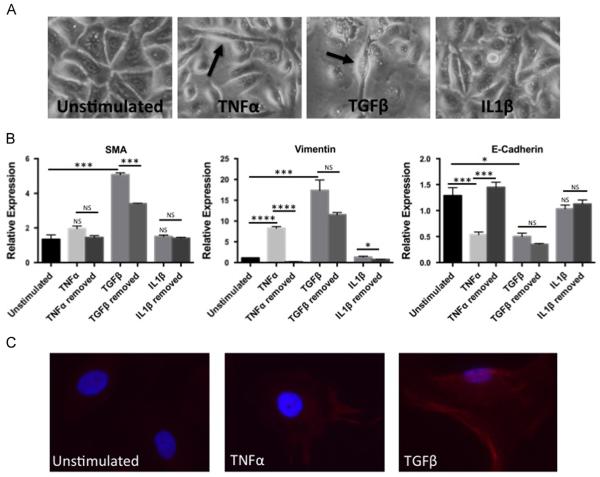
Fig. 1.
Proinflammatory cytokines induce markers of epithelial to mesenchymal transition (EMT) in human esophageal epithelial cells in a partially reversible fashion. (A) Morphologic changes in EPC2-hTERT cells stimulated with TNFα, TGFβ, and IL1β compared to unstimulated cells. Arrows highlight representative spindle-shaped cells. Pictures were taken at 100X. (B) mRNA expression of αSMA, vimentin and E-cadherin in EPC2-hTERT cells stimulated in triplicate following 4-week continuous stimulation with TNFα, TGFβ, or IL1β compared to 3 weeks of cytokine stimulation and 1 week of cytokine-free rescue. *p<0.05, ***p<0.001, ****p<0.0001, NS=not significant. Results presented are representative of at least 3 individual experiments. (C) αSMA staining in EPC2-hTERT cells compared to unstimulated cells. Blue indicates nuclear DAPI staining, red indicates αSMA staining. Pictures were taken at 400X.
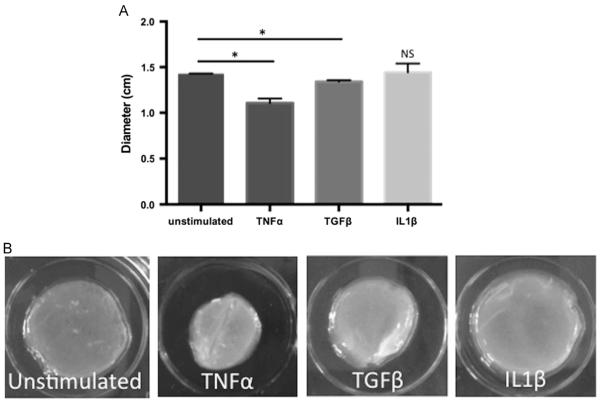
Fig. 3.
Stimulation of human esophageal epithelial cells with TNFα and TGFβ enhances contraction of collagen gels. (A) Average diameter of EPC2-hTERT-embedded collagen gels after stimulation with profibrotic cytokines. Results represent average diameters of 3 separate experiments all performed in triplicate. *p<0.05 (B) Representative formalin-fixed collagen gels following 8 days of contraction.
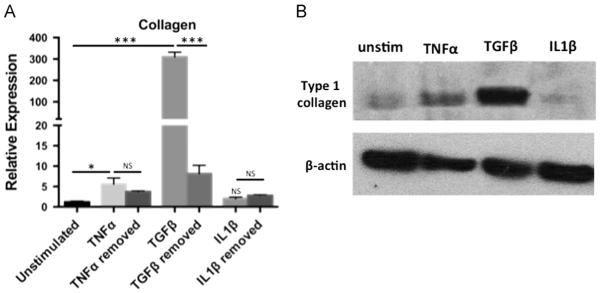
Fig. 4.
Stimulation of EPC2-hTERT cells with TNFα and TGFβ enhances expression and secretion of type I collagen. (A) Quantification of mRNA expression of type 1 collagen following either 4 weeks of continuous cytokine stimulation compared to 3 weeks of stimulation and 1 week of cytokine-free rescue. *p<0.05, ***p<0.001 (B) Type I collagen immunoblot of EPC2-hTERT cell lysates following individual cytokine stimulation.
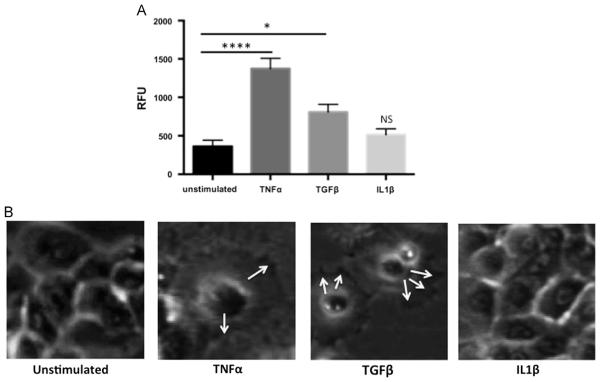
Fig. 2.
Stimulation of human esophageal epithelial cells with TNFα and TGFβ enhances cell migration. (A) Relative fluorescent units (RFUs) were measured and compared to fluorescence of unstimulated cells. *p<0.05, ****p<0.0001. (B) Filopodia and ruffled edges of EPC2-hTERT cells stimulated with TNFα and TGFβ, compared to unstimulated cells and cells treated with IL1β. Arrows indicate distinct filopodia. Pictures are still-shots from our live-action supplementary figures.
Results
Inducible expression of EMT markers in human esophageal epithelial cells can be partially reversed in vitro
We have previously shown that stimulation of the EPC2-hTERT cells with combinations of TNFα, IL1β, and TGFβ leads to increased expression of mesenchymal markers and reduction in expression of epithelial genes, consistent with EMT11. We now sought to determine the effects of each individual cytokine upon the development of EMT, and to address a key question regarding reversibility of EMT in vitro. EPC2-hTERT cells were stimulated with individual cytokines TNFα, TGFβ, or IL1β for 3 weeks, followed by 1 week of cytokine-free “rescue”. The 3-week time point was selected based on the findings of Ohashi et al., who previously showed that esophageal epithelial cells undergo maximal TGFβ-induced transition to spindle-like morphology after 21 days of cytokine exposure in vitro[22].
Following three weeks of stimulation, both TGFβ and TNFα stimulated EPC2-hTERT cells acquired spindle-like cell morphology (Fig. 1A). In contrast, cells stimulated with IL1β retained their epithelial, cuboidal morphology. Quantification of mesenchymal and epithelial mRNA gene expression supported the observed morphologic changes in EPC2-hTERT cells. Expression of αSMA (Fig. 1B), the prototypical marker of activated myofibroblasts, was significantly induced by stimulation with TGFβ, and withdrawal of TGFβ significantly reduced α-SMA expression. Though not statistically significant, TNFα increased αSMA expression; following withdrawal of TNFα, αSMA levels returned to baseline. Stimulation with IL1β did not alter αSMA expression. Similarly, the mesenchymal marker vimentin (Fig. 1B) was also induced by stimulation with TGFβ, with a trend toward normalization following withdrawal of TGFβ for one week, though not statistically significant. TNFα significantly induced vimentin expression, and expression of vimentin returned to baseline following removal of TNFα.
Although TGFβ suppressed E-cadherin expression (Fig. 1B), expression of this epithelial gene remained suppressed following removal of TGFβ. In contrast, TNFα also significantly suppressed E-cadherin expression, though in a reversible fashion. IL1β did not affect E-cadherin expression or cell morphology in our model system.
In addition to PCR confirmation of EMT, we stained EPC2-hTERT monolayers for αSMA. In the unstimulated state, there is no αSMA staining (Fig. 1C), however when stimulated with TGFβ or TNFα, there is a clear increase in αSMA. Stimulation with IL1β did not show significant change in αSMA (data not shown) in agreement of the lack of αSMA mRNA induction (Fig. 1B).
We have further stimulated independent esophageal epithelial cells grown in primary culture with either TGFβ or TNFα. Unlike EPC2-hTERT, most cells (>95%) were found to be either completely killed (n = 2) or to have underwent growth arrest (n=4) exhibiting flat and enlarged cell morphology consistent with premature senescence as we have described previously in EPC2 and other primary human esophageal epithelial cells [23–25]. Within senescent cells, there appeared to be a small subset of cells (<3–5%) displaying spindle-shaped morphology, suggesting that EMT was induced in response to either TGFβ or TNFα stimulation (data not shown); however, the presence of majority of senescent-like cells precluded documentation of EMT by qRT-PCR assays. Thus, our current model system for EMT may be limited in primary culture without genetic manipulations.
Stimulation of human esophageal epithelial cells with TGFβ and TNFα, but not IL1β, induces epithelial cell migration
EMT is characterized by the loss of the epithelial adhesion protein E-cadherin and subsequent cellular acquisition of migratory properties [26–28]. To determine whether profibrotic cytokine may stimulate cell migration, we treated EPC2-hTERT cells with or without TGFβ, TNFα or IL1β for three weeks for the ORIS™ cell migration assays.
Both TGFβ and TNFα-treated epithelial cells demonstrated significantly increased fluorescence intensity compared to unstimulated control cells (Fig. 2A), denoting increased migration. On the other hand, there was no significant change in fluorescence in IL1β treated cells. To determine whether enhanced cell proliferation contributed to the increase in fluorescence, we quantified BrdU uptake under the same conditions, and detected no significant differences in BrdU uptake within the migration zone (data not shown), suggesting that increased fluorescence was most likely secondary to enhanced migration.
Scratch wound healing assays coupled with live cell imaging were further performed to characterize patterns of cell migration (Supplemental Data S1–4). In contrast to untreated epithelial cells, which remained relatively motionless and retained cell-cell contact, TGFβ and TNFα stimulated epithelial cells moved independently. The presence of filopodia and a “ruffled” leading edge was seen in TGFβ and TNFα treated epithelial cells (Fig. 2B). In contrast, IL1β treated cells mostly retained cell-cell contact, and moved together en bloc. Notably, cells stimulated with TNFα appeared to more completely fill in the scratch defect compared to TGFβ-stimulated cells (Supplementary Data S2-3).
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.yexcr.2014.08.026.
Cultured esophageal epithelial cells become contractile following acquisition of EMT markers
In the constitutive state, epithelial cells have very little contractile ability within a collagen gel. Liu et al. found when bronchial epithelial cells were embedded within a collagen matrix, epithelial cells displayed less contraction compared to bronchial fibroblasts. The epithelial cells only exhibited contraction when placed on top of the gels [29]. We hypothesized that epithelial cells which had undergone EMT, based upon enhanced αSMA expression, could acquire the ability to contract within a collagen gel, similar to fibroblasts. Following 3 weeks of stimulation by individual cytokines, we quantified cell contraction within a collagen matrix over a period of one week. As shown in Fig. 3A-B, cells stimulated with TNFα or TGFβ contracted significantly more than the unstimulated control, and TNFα stimulated cells were most contractile. Cells treated with IL1β did not exhibit any significant contraction.
TNFα and TGFβ induce expression of type I collagen by human esophageal epithelial cells in vitro
Collagen production is an important function of activated myofibroblasts, aiding in normal wound healing and contributing to enhanced tissue stiffness in fibrosis. We quantified the expression and secretion of type I collagen in EPC2-hTERT cells either stimulated for 4 weeks or stimulated for 3 weeks followed by 1 week of cytokine-free rescue. Stimulation with TGFβ, and to a lesser degree, TNFα, resulted in a significant increase in collagen mRNA expression (Fig. 4A) compared to unstimulated controls, which was reversible upon removal of TGFβ. Collagen expression was not induced by IL1β stimulation. Expression of type I collagen protein was also assessed using immunoblots of cytokine-stimulated EPC2-hTERT cell lysates (Fig. 4B), confirming mRNA expression results.
Discussion
In this study, we show that stimulation with TGFβ and TNFα, but not IL1β, leads to the acquisition of markers of EMT in cultured esophageal epithelial cells, and that withdrawal of the proinflammatory stimulus leads to partial reversal of these distinct gene expression patterns. Most importantly, our study addresses a key concept central to current controversies in EMT regarding the function of epithelial cells following the acquisition of markers of EMT. Specifically, we show for the first time that in addition to the gain of mesenchymal markers and loss of epithelial markers in vitro, esophageal epithelial cells can attain phenotypic features of activated myofibroblasts, including migration, collagen synthesis, and contractility.
We previously demonstrated that competitive inhibition of TNFα and IL1β signaling prevented changes in both epithelial morphology and expression of E-cadherin in epithelial cells cultured in the presence of fibroblast-derived cytokines. In the current study, we evaluated the effect of individual recombinant cytokines upon acquisition of EMT markers, and further determined whether expression of these markers was reversible in vitro. Few studies have explored the reversibility of EMT in the setting of fibrosis. In a rat model of EMT in the kidney, Arnoni et al. demonstrated that while progression of fibrosis could be halted using an angiotensin I receptor antagonist, the existing fibrosis could not be reversed [30]. In EoE, Kagalwalla et al. quantified vimentin and cytokeratin expression in patient biopsies before and after clinical treatment, and found that EMT scores normalized to the levels of control patients post-treatment [10]. Consistent with the Kagalwalla study, we found that induction of mesenchymal markers could be partially reversed by withdrawal of cytokine stimulation. Although TGFβ stimulation led to reversible inductions in mesenchymal genes, TGFβ-induced suppression of E-cadherin was not reversible upon withdrawal of the proinflammatory stimulus for 1 week. Surprisingly, TNFα had a similar effect upon mesenchymal gene expression, yet TNFα induced E-cadherin suppression was reversible in this model. The signaling pathways which regulate cytokine-mediated EMT are complex, and others have reported that re-expression of E-cadherin following TGFβ induced EMT first requires inhibition of signaling pathways to decrease mesenchymal gene expression [31]. It is possible that a longer time period in unstimulated media might have led to more complete recovery of E-cadherin expression. However, our results suggest that the recovery of the epithelial phenotype lags behind loss of mesenchymal gene expression. The limited reversal in EMT upon cytokine withdrawal may be accounted for by EMT-promoting factors such as IL-6 and IL-8 which are produced by the mesenchymal cells. Such possibility is currently under investigation by purifying both mesenchymal and epithelial subsets of cells.
In our previous report, we noted the importance of IL1β in a cross-talk model between esophageal epithelial cells and esophageal fibroblasts. When stimulated with conditioned media from esophageal epithelial cells, esophageal fibroblasts produced both IL1β and TNFα, but not TGFβ [11]. Subsequent stimulation of esophageal epithelial cells with this fibroblast conditioned media led to features of EMT, which could be prevented by competitive inhibition of IL1β or TNFα signaling. In contrast, the current study does not implicate IL1β alone in the development of EMT. These discrepancies highlight the importance of other possible soluble mediators and cell types that may play a role in EoE-associated fibrosis in vivo. Indeed, the proinflammatory and profibrotic cytokine milieu in EoE is poorly understood, and involves complex interactions between a number of cell types including epithelial cells, fibroblasts, and infiltrating inflammatory cells. While IL1β alone may not induce EMT in this model, future studies will be required to identify additional chemokines that may act synergistically with IL1β.
Epithelial cell migration is important for tissue homeostasis, with roles in embryogenesis and normal wound healing. However, epithelial and mesenchymal cells have distinct migratory capabilities. Epithelial cells cluster together, facilitated by cell-cell adhesion molecules including E-cadherin, and migrate together through collective epithelial movement [32]. In contrast, mesenchymal cell movement is dynamic, and mesenchymal cells move individually through extension of filopodia and pseudopodia (Fig. 2B) [33]. Consistent with this, both TNFα and TGFβ, which caused cells to appear dissociated from the monolayer, with a fibroblast appearance, had substantial effects upon cell migration (Fig. 2, Supplementary data S2-3). In contrast, IL1β stimulated cells appeared clustered together, and moved en bloc (Supplementary data S4).
Supplementary material related to this article can be found online at http://dx.doi.org/10.1016/j.yexcr.2014.08.026
While fibroblast contraction in collagen gels has been well characterized in models of skin and lung fibrosis [34,35], contraction of transdifferentiated esophageal epithelial cells in collagen matrices has not been previously described. Of the three tested cytokines, TNFα had the most significant effect upon collagen gel contraction, exceeding that of TGFβ. This was unexpected, as αSMA is considered to be the major driver of tissue force exertion and contraction [36,37], and TNFα induced less αSMA compared to TGFβ stimulated epithelial cells. The effects of TNFα in fibrogenesis are unclear, as its effects are cell-type and organ specific. In alveolar epithelial cells that have undergone EMT, TNFα alone causes collagen gel contraction, but not to the same degree as TGFβ. In dermal fibroblasts, on the other hand, TNFα inhibits the synthesis of type I collagen [38,39]. In contrast, TNFα promotes production of ECM by murine intestinal myofibroblasts in vitro. Notably, others have shown that TNFα stimulation of the murine 3T3 fibroblast cell line stimulated the expression of TGFβ [40]. Thus, while stimulation of esophageal epithelial cells with TGFβ induced higher levels of mesenchymal genes compared to TNFα, it is possible that TNFα may similarly exert a more potent effect upon myofibroblast-like function via upregulation of additional profibrotic genes, including TGFβ.
Despite elegant lineage tracing studies in other models [20], persistent skepticism surrounding EMT is based upon the lack of clinically significant collagen production by epithelial cells which have undergone an EMT. Epithelial cells lack constitutive expression of extracellular matrix proteins, and thus have not been proposed as a cellular source of collagen in EoE. For the first time, we show that cultured esophageal epithelial cells can secrete type I collagen, under the influence of TGFβ and, to a lesser degree, TNFα (Fig. 4). Other models have previously highlighted the potential importance of epithelial-derived collagen. In a bleomycin model of murine lung injury, Yang et al. showed that activated lung epithelial cells produce mesenchymal proteins that provoke lamina propria fibrosis and that conditional alveolar epithelial deletion of type I collagen led to reduced bleomycin-induced lamina propria fibrosis compared to genotype controls, suggesting that epithelial derived collagen promotes fibrosis by enhancing fibroblast activation in the lamina propria [41]. During embryologic development, corneal epithelial cells secrete collagen to form a primary stroma, into which migrating neural crest cells differentiate into fibroblasts to produce a secondary stroma [42]. Both the Yang and Bard studies suggest that even small amounts of epithelial derived collagen may drive lamina propria fibrosis, and underscore the importance of determining the role of the epithelial cells in synthesis of collagen and other ECM components in EoE.
The most unexpected result of our study was that the functional consequences of TNFα stimulation equaled or exceeded those of TGFβ in our model system with regard to both epithelial migration and contraction. Increased expression of TNFα has been reported in esophageal biopsies from EoE subjects [43], though little is known about its specific role in EoE pathobiology and fibrogenesis. TNFα antagonists are emerging as treatments for several fibrotic diseases including Crohn disease [44] and systemic sclerosis [45]. A small case series of infliximab used in adult EoE subjects was recently reported, which showed a reduction in symptoms and tissue eosinophilia in 1 of 3 subjects [46]. It is important to note that this was a small clinical pilot study, which did not specifically examine effects upon EMT. Larger trials will be required to determine whether infliximab is effective in EoE, and specifically whether this overlooked cytokine is important in esophageal epithelial remodeling in vivo.
Enhanced migration, contraction, and collagen deposition are integral parts of myofibroblast function, and both lamina propria fibrosis and dysfunctional esophageal smooth muscle contribute to clinically significant reductions in esophageal distensibility [47–49].
While the major myofibroblast burden likely resides in the subepithelial compartment in EoE, our study implicates the epithelial compartment as a source of myofibroblasts in EoE-associated tissue dysfunction. Thus, further understanding the potential consequences of EMT and fibrogenesis within the epithelial compartment may lead to novel treatment and preventative strategies for patients affected by this significant disease.
Supplementary Material
Acknowledgements
This work was supported by the Joint Penn-CHOP Center in Digestive, Liver and Pancreatic Medicine and the NIH/NIDDK P30 Center in Molecular Studies in Digestive and Liver Diseases (NIH-P30-DK050306) and its core facilities (Molecular Pathology and Imaging Core, Molecular Biology/Gene Expression Core, Transgenic and Chimeric Mouse Core, and Cell Culture Core).
Funding Sources
1F32DK100088 (to A.B.M.), R01DK087789 (to M.L.W.), APFED Hope Award (to M.L.W.), Grosvenor Fund Management (to M.L.W.), R01DK077005 (to H.N.), K26 RR032714 (to H.N.), and Pennsylvania CURE Program Grant (to H.N.).
REFERENCES
- [1].Phan SH. Biology of Fibroblasts and Myofibroblasts. Proceedings of the American Thoracic Society. 2008;5:334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- [4].Kisseleva T, Brenner DA. Is it the end of the line for the EMT? Hepatology. 2011;53:1433–1435. doi: 10.1002/hep.24312. [DOI] [PubMed] [Google Scholar]
- [5].Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J. Clin. Invest. 2011;121:468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J. Am. Soc. Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- [7].Morbini P, Inghilleri S, Campo I, et al. Incomplete expression of epithelial-mesenchymal transition markers in idiopathic pulmonary fibrosis. Pathol. Res. Pract. 2011;207:559–567. doi: 10.1016/j.prp.2011.06.006. [DOI] [PubMed] [Google Scholar]
- [8].Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aceves SS, Newbury RO, Dohil R, et al. Esophageal remodeling in pediatric eosinophilic esophagitis. Journal of Allergy and Clinical Immunology. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- [10].Kagalwalla AF, Akhtar N, Woodruff SA, et al. Eosinophilic esophagitis: Epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. Journal of Allergy and Clinical Immunology. 2012;129:1387–1396. e7. doi: 10.1016/j.jaci.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Muir AB, Lim DM, Benitez AJ, et al. Esophageal epithelial and mesenchymal cross-talk leads to features of epithelial to mesenchymal transition in vitro. Exp. Cell Res. 2013;319:850–859. doi: 10.1016/j.yexcr.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Findlay VJ, Wang C, Watson DK, et al. Epithelial-to-mesenchymal transition and the cancer stem cell phenotype: insights from cancer biology with therapeutic implications for colorectal cancer. Cancer Gene Ther. 2014 doi: 10.1038/cgt.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thiery JP, Acloque H, Huang RYJ, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [14].Zeisberg M, Kalluri R. Fibroblasts emerge via epithelial-mesenchymal transition in chronic kidney fibrosis. Front. Biosci. 2008;13:6991–6998. doi: 10.2741/3204. [DOI] [PubMed] [Google Scholar]
- [15].Balli D, Ustiyan V, Zhang Y, et al. Foxm1 transcription factor is required for lung fibrosis and epithelial-to-mesenchymal transition. EMBO J. 2013;32:231–244. doi: 10.1038/emboj.2012.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Flier SN, Tanjore H, Kokkotou EG, et al. Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. Journal of Biological Chemistry. 2010;285:20202–20212. doi: 10.1074/jbc.M110.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takahashi E, Inoue T, Fujimoto T, et al. Epithelial mesenchymal transition-like phenomenon in trabecular meshwork cells. Exp. Eye Res. 2014;118:72–79. doi: 10.1016/j.exer.2013.11.014. [DOI] [PubMed] [Google Scholar]
- [18].Murdoch CE, Chaubey S, Zeng L, et al. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through pro-inflammatory effects and endothelial-mesenchymal transition. J. Am. Coll. Cardiol. 2014 doi: 10.1016/j.jacc.2014.02.572. [DOI] [PubMed] [Google Scholar]
- [19].Sonnylal S, Xu S, Jones H, et al. Connective tissue growth factor causes EMT-like cell fate changes in vivo and in vitro. J. Cell. Sci. 2013;126:2164–2175. doi: 10.1242/jcs.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tanjore H, Xu XC, Polosukhin VV, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 2009;180:657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chu AS, Diaz R, Hui J-J, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ohashi S, Natsuizaka M, Wong GS, et al. Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res. 2010;70:4174–4184. doi: 10.1158/0008-5472.CAN-09-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harada H, Nakagawa H, Oyama K, et al. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol. Cancer Res. 2003;1:729–738. [PubMed] [Google Scholar]
- [24].Ohashi S, Natsuizaka M, Naganuma S, et al. A NOTCH3-mediated squamous cell differentiation program limits expansion of EMT-competent cells that express the ZEB transcription factors. Cancer Res. 2011;71:6836–6847. doi: 10.1158/0008-5472.CAN-11-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kagawa S, Natsuizaka M, Whelan KA, et al. Cellular senescence checkpoint function determines differential Notch1-dependent oncogenic and tumor-suppressor activities. Oncogene. 2014 doi: 10.1038/onc.2014.169. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huber O, Bierkamp C, Kemler R. Cadherins and catenins in development. Curr. Opin. Cell Biol. 1996;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- [27].Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- [28].Cano A, Gamallo C, Kemp CJ, et al. Expression pattern of the cell adhesion molecules. E-cadherin, P-cadherin and alpha 6 beta 4 intergrin is altered in pre-malignant skin tumors of p53-deficient mice. Int. J. Cancer. 1996;65:254–262. doi: 10.1002/(SICI)1097-0215(19960117)65:2<254::AID-IJC21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [29].Liu X, Umino T, Cano M, et al. Human bronchial epithelial cells can contract type I collagen gels. Am. J. Physiol. 1998;274:L58–65. doi: 10.1152/ajplung.1998.274.1.L58. [DOI] [PubMed] [Google Scholar]
- [30].Arnoni CP, Maquigussa E, Passos CS, et al. Inhibition of cellular transdifferentiation by losartan minimizes but does not reverse type 2 diabetes-induced renal fibrosis. J Renin Angiotensin Aldosterone Syst. 2014 doi: 10.1177/1470320313497817. (1470320313497817) [DOI] [PubMed] [Google Scholar]
- [31].Das S, Becker BN, Hoffmann FM, et al. Complete reversal of epithelial to mesenchymal transition requires inhibition of both ZEB expression and the Rho pathway. BMC Cell Biol. 2009;10:94. doi: 10.1186/1471-2121-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hirashima T, Hosokawa Y, Iino T, et al. On fundamental cellular processes for emergence of collective epithelial movement. Biol Open. 2013;2:660–666. doi: 10.1242/bio.20134523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev. Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- [34].Shannon DB, McKeown STW, Lundy FT, et al. Phenotypic differences between oral and skin fibroblasts in wound contraction and growth factor expression. Wound Repair Regen. 2006;14:172–178. doi: 10.1111/j.1743-6109.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- [35].Kim S-Y, Lee J-H, Kim HJ, et al. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. AJP: Lung Cellular and Molecular Physiology. 2012;302:L891–908. doi: 10.1152/ajplung.00288.2011. [DOI] [PubMed] [Google Scholar]
- [36].Saika S, Yamanaka O, Flanders KC, et al. Epithelial-mesenchymal transition as a therapeutic target for prevention of ocular tissue fibrosis. Endocr Metab Immune Disord Drug Targets. 2008;8:69–76. doi: 10.2174/187153008783928343. [DOI] [PubMed] [Google Scholar]
- [37].Hinz B, Phan SH, Thannickal VJ, et al. The Myofibroblast. The American Journal of Pathology. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mauviel A, Daireaux M, Rédini F, et al. Tumor necrosis factor inhibits collagen and fibronectin synthesis in human dermal fibroblasts. FEBS Lett. 1988;236:47–52. doi: 10.1016/0014-5793(88)80283-7. [DOI] [PubMed] [Google Scholar]
- [39].Mauviel A, Heino J, Kähäri VM, et al. Comparative effects of interleukin-1 and tumor necrosis factor-alpha on collagen production and corresponding procollagen mRNA levels in human dermal fibroblasts. J. Invest. Dermatol. 1991;96:243–249. doi: 10.1111/1523-1747.ep12462185. [DOI] [PubMed] [Google Scholar]
- [40].Sullivan DE, Ferris M, Nguyen H, et al. TNF-alpha induces TGF-beta1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. J. Cell. Mol. Med. 2009;13:1866–1876. doi: 10.1111/j.1582-4934.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yang J, Velikoff M, Canalis E, et al. Activated alveolar epithelial cells initiate fibrosis through autocrine and paracrine secretion of connective tissue growth factor. AJP: Lung Cellular and Molecular Physiology. 2014;306:L786–96. doi: 10.1152/ajplung.00243.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bard JB, Hay ED. The behavior of fibroblasts from the developing avian cornea. Morphology and movement in situ and in vitro. J. Cell Biol. 1975;67:400–418. doi: 10.1083/jcb.67.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Persad R, Huynh HQ, Hao L, et al. Angiogenic remodeling in pediatric EoE is associated with increased levels of VEGF-A, angiogenin, IL-8, and activation of the TNF-α-NFκB pathway. Journal of Pediatric Gastroenterology and Nutrition. 2012;55:251–260. doi: 10.1097/MPG.0b013e31824b6391. [DOI] [PubMed] [Google Scholar]
- [44].Pallotta N, Barberani F, Hassan N-A, et al. Effect of infliximab on small bowel stenoses in patients with Crohn’s disease. World J. Gastroenterol. 2008;14:1885–1890. doi: 10.3748/wjg.14.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Phumethum V, Jamal S, Johnson SR. Biologic therapy for systemic sclerosis: a systematic review. J. Rheumatol. 2011;38:289–296. doi: 10.3899/jrheum.100361. [DOI] [PubMed] [Google Scholar]
- [46].Straumann A, Bussmann C, Conus S, et al. Anti-TNF-alpha (infliximab) therapy for severe adult eosinophilic esophagitis. J. Allergy Clin. Immunol. 2008;122:425–427. doi: 10.1016/j.jaci.2008.06.012. [DOI] [PubMed] [Google Scholar]
- [47].Rieder F, Nonevski I, Ma J, et al. T-Helper 2 Cytokines, Transforming Growth Factor β1, and Eosinophil Products Induce Fibrogenesis and Alter Muscle Motility in Patients With Eosinophilic Esophagitis. Gastroenterology. 2014;146:1266–1277. e9. doi: 10.1053/j.gastro.2014.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Aceves SS, Chen D, Newbury RO, et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-β1, and increase esophageal smooth muscle contraction. J. Allergy Clin. Immunol. 2010;126:1198–204. e4. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- [49].Nicodème F, Hirano I, Chen J, et al. Esophageal Distensibility as a Measure of Disease Severity in Patients With Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology. 2013;11:1101–1107. e1. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.