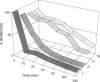
Abstract
Three analogues of the model peptide of sequence IRGERA corresponding to the COOH-terminal residues 130-135 of histone H3 were synthesized, and their antigenicity, immunogenicity, and resistance to trypsin were compared to those of the natural L-peptide. The three analogues correspond to the D-enantiomer, containing only D-residues, and two retro-peptides containing NH-CO bonds instead of natural peptide bonds. The chirality of each residue was maintained in the retro-peptide and inverted in the retro-inverso-peptide. Antibodies to the four peptide analogues were produced by injecting BALB/c mice with peptides covalently coupled to small unilamellar liposomes containing monophosphoryl lipid A. Each of the four peptide analogues induced IgG antibodies of various subclasses. The IgG3 antibodies reacted similarly with the four analogues, whereas antibodies of the IgG1, IgG2a, and IgG2b isotypes showed strong conformational preferences for certain peptides. The retro-inverso-peptide IRGERA mimicked the structure and antigenic activity of the natural L-peptide but not of the D- and retro-peptides, whereas the retro-peptide IRGERA mimicked the D-peptide but not the L- and retro-inverso-peptides. The equilibrium affinity constants (Ka) of three monoclonal antibodies generated against the L- and D-peptides with respect to the four peptide analogues were measured in a biosensor system. Large differences in Ka values were observed when each monoclonal antibody was tested with respect to the four peptides. The use of retro-inverso-peptides to replace natural L-peptides is likely to find many applications in immunodiagnosis and as potential synthetic vaccines.
Full text
PDF
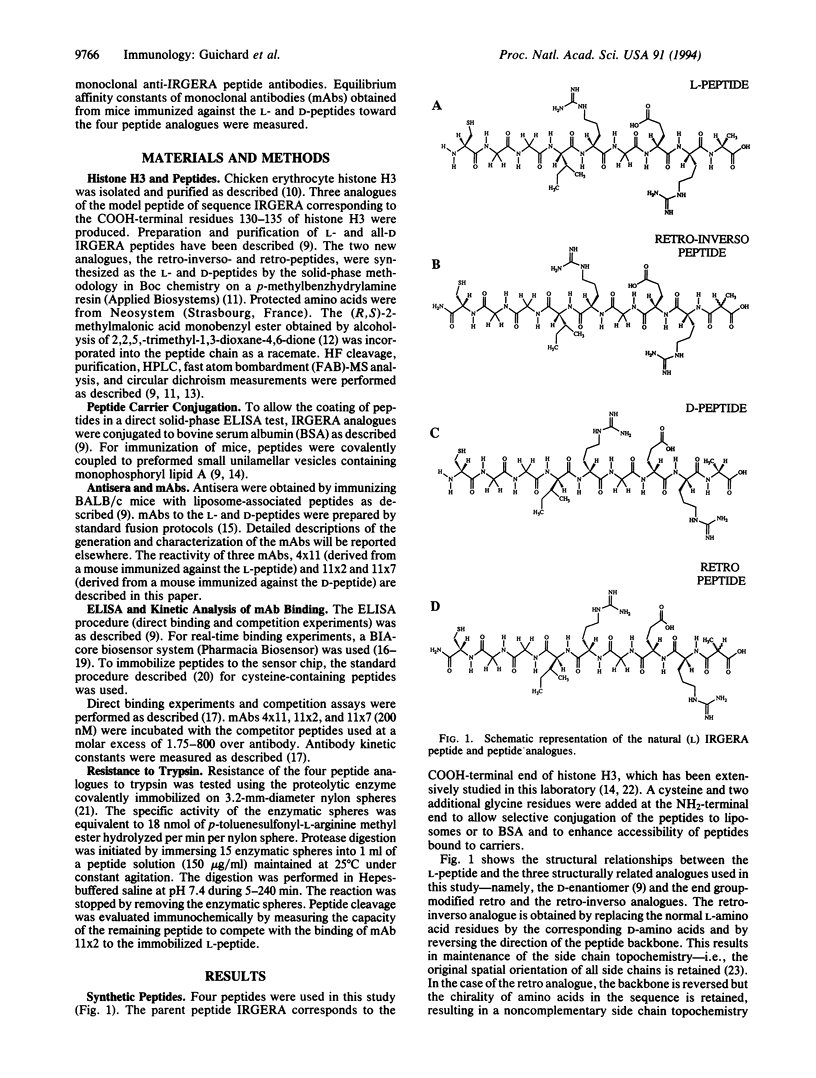
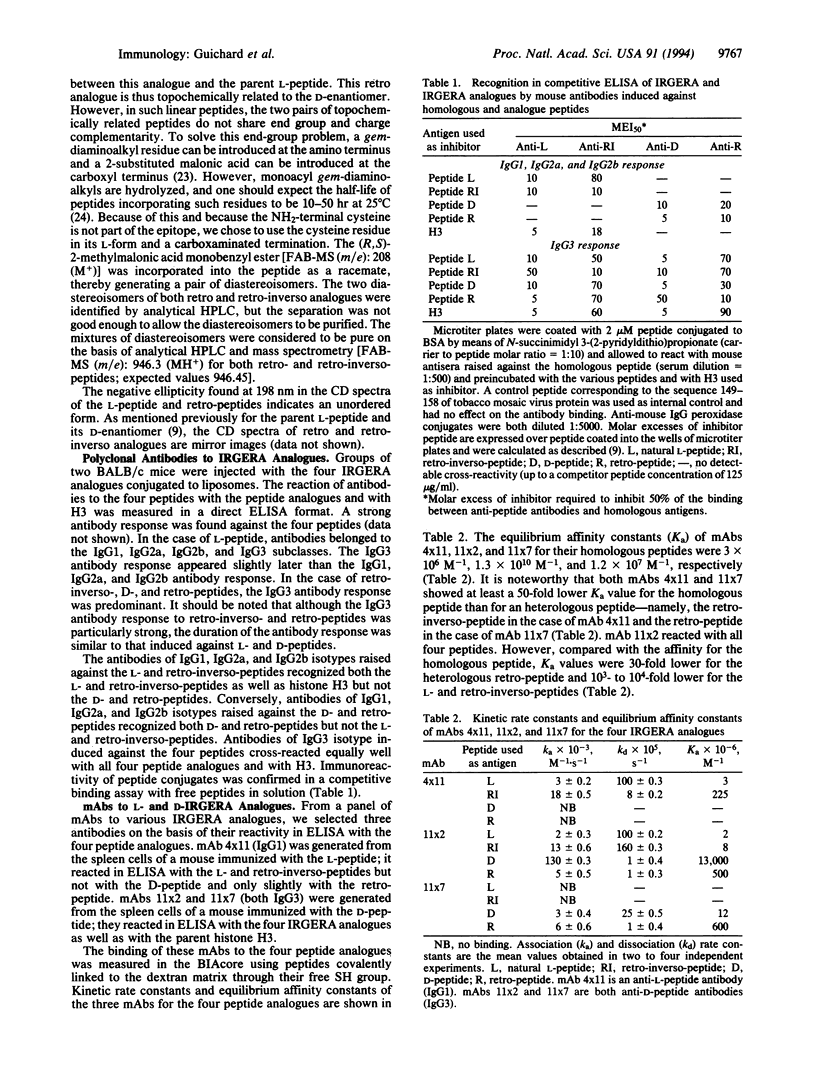
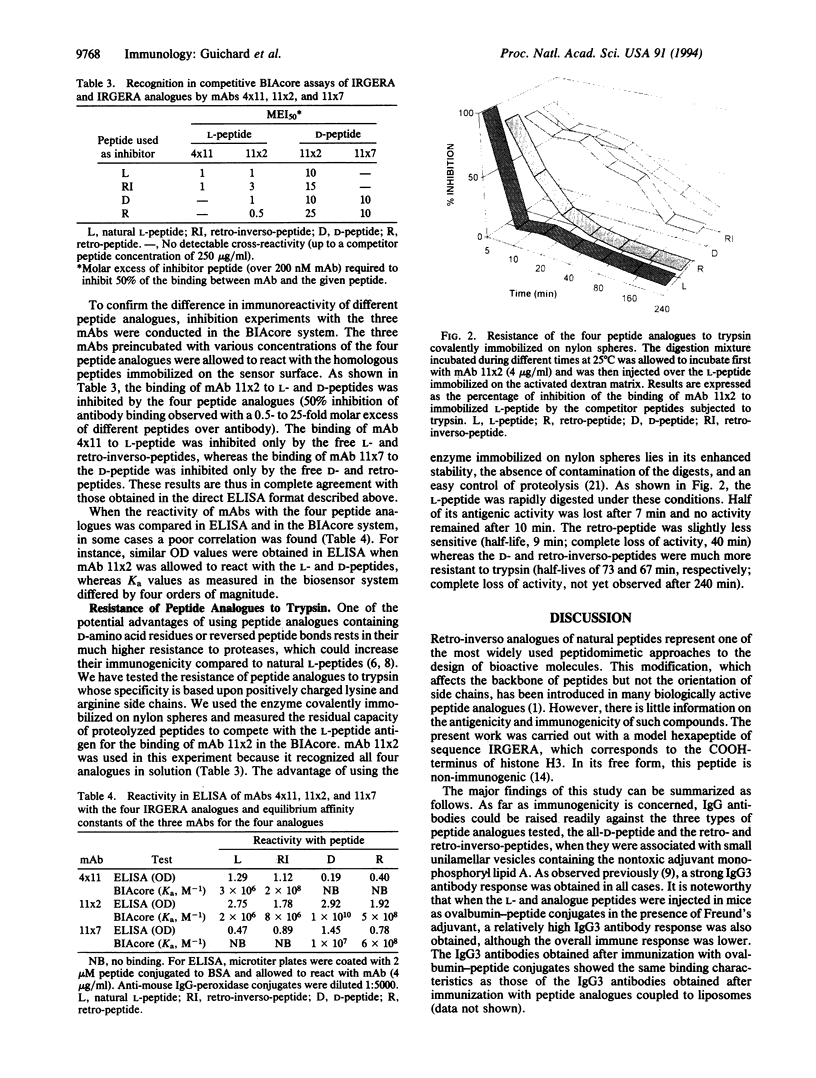

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al Moudallal Z., Briand J. P., Van Regenmortel M. H. Monoclonal antibodies as probes of the antigenic structure of tobacco mosaic virus. EMBO J. 1982;1(8):1005–1010. doi: 10.1002/j.1460-2075.1982.tb01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuh D., Dubs M. C., Weiss E., Zeder-Lutz G., Van Regenmortel M. H. Determination of kinetic constants for the interaction between a monoclonal antibody and peptides using surface plasmon resonance. Biochemistry. 1992 Jul 14;31(27):6298–6304. doi: 10.1021/bi00142a019. [DOI] [PubMed] [Google Scholar]
- Azimzadeh A., Pellequer J. L., Van Regenmortel M. H. Operational aspects of antibody affinity constants measured by liquid-phase and solid-phase assays. J Mol Recognit. 1992 Mar;5(1):9–18. doi: 10.1002/jmr.300050103. [DOI] [PubMed] [Google Scholar]
- Benkirane N., Friede M., Guichard G., Briand J. P., Van Regenmortel M. H., Muller S. Antigenicity and immunogenicity of modified synthetic peptides containing D-amino acid residues. Antibodies to a D-enantiomer do recognize the parent L-hexapeptide and reciprocally. J Biol Chem. 1993 Dec 15;268(35):26279–26285. [PubMed] [Google Scholar]
- Briand J. P., Barin C., Van Regenmortel M. H., Muller S. Application and limitations of the multiple antigen peptide (MAP) system in the production and evaluation of anti-peptide and anti-protein antibodies. J Immunol Methods. 1992 Dec 8;156(2):255–265. doi: 10.1016/0022-1759(92)90033-p. [DOI] [PubMed] [Google Scholar]
- Briand J. P., Van Dorsselaer A., Raboy B., Muller S. Total chemical synthesis of ubiquitin using BOP reagent: biochemical and immunochemical properties of the purified synthetic product. Pept Res. 1989 Nov-Dec;2(6):381–388. [PubMed] [Google Scholar]
- Chorev M., Rubini E., Gilon C., Wormser U., Selinger Z. Synthesis of partially modified retro-inverso substance P analogues and their biological activity. J Med Chem. 1983 Feb;26(2):129–135. doi: 10.1021/jm00356a003. [DOI] [PubMed] [Google Scholar]
- Dintzis H. M., Symer D. E., Dintzis R. Z., Zawadzke L. E., Berg J. M. A comparison of the immunogenicity of a pair of enantiomeric proteins. Proteins. 1993 Jul;16(3):306–308. doi: 10.1002/prot.340160309. [DOI] [PubMed] [Google Scholar]
- Feldmann M., June C. H., McMichael A., Maini R., Simpson E., Woody J. N. T-cell-targeted immunotherapy. Immunol Today. 1992 Mar;13(3):84–85. doi: 10.1016/0167-5699(92)90146-X. [DOI] [PubMed] [Google Scholar]
- Friede M., Muller S., Briand J. P., Van Regenmortel M. H., Schuber F. Induction of immune response against a short synthetic peptide antigen coupled to small neutral liposomes containing monophosphoryl lipid A. Mol Immunol. 1993 Apr;30(6):539–547. doi: 10.1016/0161-5890(93)90028-a. [DOI] [PubMed] [Google Scholar]
- Harper M., Lema F., Boulot G., Poljak R. J. Antigen specificity and cross-reactivity of monoclonal anti-lysozyme antibodies. Mol Immunol. 1987 Feb;24(2):97–108. doi: 10.1016/0161-5890(87)90081-2. [DOI] [PubMed] [Google Scholar]
- Jönsson U., Fägerstam L., Ivarsson B., Johnsson B., Karlsson R., Lundh K., Löfås S., Persson B., Roos H., Rönnberg I. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. Biotechniques. 1991 Nov;11(5):620–627. [PubMed] [Google Scholar]
- Michalon P., Couturier R., Bender K., Hecker H., Marion C. Structural analysis of Trypanosoma brucei brucei chromatin by limited proteolysis. An electrical-birefringence study. Eur J Biochem. 1993 Sep 1;216(2):387–394. doi: 10.1111/j.1432-1033.1993.tb18156.x. [DOI] [PubMed] [Google Scholar]
- Milton R. C., Milton S. C., Kent S. B. Total chemical synthesis of a D-enzyme: the enantiomers of HIV-1 protease show reciprocal chiral substrate specificity [corrected]. Science. 1992 Jun 5;256(5062):1445–1448. doi: 10.1126/science.1604320. [DOI] [PubMed] [Google Scholar]
- Muller S., Isabey A., Couppez M., Plaue S., Sommermeyer G., Van Regenmortel M. H. Specificity of antibodies raised against triacetylated histone H4. Mol Immunol. 1987 Jul;24(7):779–789. doi: 10.1016/0161-5890(87)90062-9. [DOI] [PubMed] [Google Scholar]
- Neimark J., Briand J. P. Development of a fully automated multichannel peptide synthesizer with integrated TFA cleavage capability. Pept Res. 1993 Jul-Aug;6(4):219–228. [PubMed] [Google Scholar]
- Papadouli I., Potamianos S., Hadjidakis I., Bairaktari E., Tsikaris V., Sakarellos C., Cung M. T., Marraud M., Tzartos S. J. Antigenic role of single residues within the main immunogenic region of the nicotinic acetylcholine receptor. Biochem J. 1990 Jul 1;269(1):239–245. doi: 10.1042/bj2690239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richalet-Sécordel P. M., Deslandres A., Plaué S., You B., Barré-Sinoussi F., Van Regenmortel M. H. Cross-reactive potential of rabbit antibodies raised against a cyclic peptide representing a chimeric V3 loop of HIV-1 gp120 studied by biosensor technique and ELISA. FEMS Immunol Med Microbiol. 1994 Jun;9(1):77–87. doi: 10.1111/j.1574-695X.1994.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Stenberg M., Nygren H. Kinetics of antigen-antibody reactions at solid-liquid interfaces. J Immunol Methods. 1988 Oct 4;113(1):3–15. doi: 10.1016/0022-1759(88)90376-6. [DOI] [PubMed] [Google Scholar]
- Stockhaus J., Nagatani A., Halfter U., Kay S., Furuya M., Chua N. H. Serine-to-alanine substitutions at the amino-terminal region of phytochrome A result in an increase in biological activity. Genes Dev. 1992 Dec;6(12A):2364–2372. doi: 10.1101/gad.6.12a.2364. [DOI] [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D., Laursen R. A. A D-antifreeze polypeptide displays the same activity as its natural L-enantiomer. FEBS Lett. 1993 Feb 8;317(1-2):31–34. doi: 10.1016/0014-5793(93)81485-i. [DOI] [PubMed] [Google Scholar]
- Zeder-Lutz G., Altschuh D., Denery-Papini S., Briand J. P., Tribbick G., Van Regenmortel M. H. Epitope analysis using kinetic measurements of antibody binding to synthetic peptides presenting single amino acid substitutions. J Mol Recognit. 1993 Jun;6(2):71–79. doi: 10.1002/jmr.300060205. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen D. R., von Holt C. A new procedure for the isolation and fractionation of histones. FEBS Lett. 1971 May 20;14(5):333–337. doi: 10.1016/0014-5793(71)80294-6. [DOI] [PubMed] [Google Scholar]