Abstract
Metastasis is one of hallmarks of cancer and a major cause of cancer death. Combatting metastasis is highly challenging. To overcome these difficulties, researchers have focused on physical properties of metastatic cancer cells. Metastatic cancer cells from patients are softer than benign cancer or normal cells. Changes of viscoelasticity of cancer cells are related to the keratin network. Unexpectedly, keratin network is dynamic and regulation of keratin network is important to the metastasis of cancer. Keratin is composed of heteropolymer of type I and II. Keratin connects from the plasma membrane to nucleus. Several proteins including kinases, and protein phosphatases bind to keratin intermediate filaments. Several endogenous compounds or toxic compounds induce phosphorylation and reorganization of keratin network in cancer cells, leading to increased migration. Continuous phosphorylation of keratin results in loss of keratin, which is one of the features of epithelial mesenchymal transition (EMT). Therefore, several proteins involved in phosphorylation and reorganization of keratin also have a role in EMT. It is likely that compounds controlling phosphorylation and reorganization of keratin are potential candidates for combating EMT and metastasis.
Keywords: Metastasis, Viscoelasticity, Phosphorylation of keratin, Reorganization of keratin, Epithelial Mesenchymal Transition, Sphingosylphosphorylcholine
INTRODUCTION
Metastasis is critical hallmark of cancer and contributes to the 90% of cancer death (Hanahan and Weinberg, 2011). Diverse approaches have been attempted to combat the metastasis of cancer. The spot light has been on matrix metalloproteinase inhibitors but the clinical outcome of matrix metalloproteinase inhibitors in most cancer metastasis is poor (Coussens et al., 2002; Pavlaki and Zucker, 2003).
Recently, several researchers investigated physical properties of cancer cells and found that metastatic cancer cells are significantly softer than other benign or normal cells (Cross et al., 2007). This softness of metastatic cancer cells might be useful as diagnostic marker. Measures of physical properties might also be useful as assay methods for new compounds modulating the physical properties of cancer cells using novel devices such as optical stretcher, optical tweezer, and atomic force microscopy (Suresh, 2007).
Because the physical properties and mechanotransduction of cancer cells are crucial in various steps of the metastatic process, control of physical properties of cancer cell may be an effective therapeutic approach for patients suffering cancer (Stroka and Konstantopoulos, 2014).
However, measuring changes of physical properties of cancer cells is not easy to most researchers in pharmacology fields. We are interested in the biological phenomena reflecting the changes of physical properties such as keratin reorganization via phosphorylation, which is changed by sphingosylphosphorylcholine (SPC) and related to viscoelasticity of metastatic cancer cells (Beil et al., 2003). We have studied the underlying molecular mechanisms in keratin 8 (K8) phosphorylation and perinuclear reorganizations of cancer cells for several years. We have reviewed the results of these studies together with the relevant literature.
STRUCTURE AND CHARACTERISTICS OF KERATINS
Epithelial cell keratins are composed of heteropolymer of one type I keratin and one type II keratin proteins (Table 1) (Coulombe and Omary, 2002). Keratin contains a common α-helical rod domain of ∼310 amino acid, sided by non-helical head and tail domains of diverse length and sequence having several phosphorylation sites (Ku et al., 1998; Omary et al., 2006; Loschke et al., 2015) (Fig. 1).
Table 1.
Expression of keratin proteins in epithelial tissues*
| Keratin | Epithelial tissue | Partner |
|---|---|---|
| Type I | ||
| Simple | ||
| K18 | Simple epithelia (e.g. liver, pancreas, colon, lung) | K8, K7 |
| K20 | Simple epithelia, especially gastrointestinal | K8, (K7) |
| Barrier | ||
| K9 | Stratified cornifying epithelia; palm, sole | (K1) |
| K10 | Stratified cornifying epithelia; suprabasal | K1 |
| K12 | Stratified epithelia; cornea | K3 |
| K13 | Stratified epithelia; non-cornifying; suprabasal | K4 |
| K14 | Stratified and complex epithelia; basal | K5 |
| K15 | Stratified epithelia | (K5) |
| K16 | Stratified epithelia; induced during stress, fast turn over; suprabasal | K6a |
| K17 | Stratified epithelia; induced during stress, fast turn over | K6b |
| K19 | Simple and stratified epithelia | K8 |
| K23, K24 | Epithelia | |
| Structural | ||
| K25, K26, K27, K28 | Stratified epithelia; hair follicle sheath | |
| K31, K32, K33a, K33b, K34, K35, K36, K37, K38, K39, K40 | Stratified epithelia; hair, hard structure | |
| Type II | ||
| Simple | ||
| K7, K8 | Simple epithelia | K18 |
| Barrier | ||
| K1 | Stratified cornifying epithelia; suprabasal | K10 |
| K2 | Stratified cornifying epithelia; late suprabasal | (K10) |
| K3 | Stratified epithelia, cornea | K12 |
| K4 | Stratified epithelia; non-cornifying; suprabasal | K13 |
| K5 | Stratified and complex epithelia; basal cells | K14, (K15) |
| K6a | Stratified epithelia; induced during stress, fast turn over | K16 |
| K6b | Stratified epithelia; induced during stress, fast turn over | K17 |
| K6c | Epithelia | |
| K76 | Stratified cornifying epithelia, oral, suprabasal | (K10) |
| K78, K79, K80 | Epithelia | |
| Structural | ||
| K75 | Stratified epithelia; hair follicle | |
| K71, K72, K73, K74 | Stratified epithelia; hair follicle sheath | |
| K81, K82, K83, K84, K85, K86 | Stratified epithelia; hair, hard structure |
Modified from Haines and Lanes, and Loschke (Haines and Lane, 2012; Loschke et al., 2015).
Fig. 1.
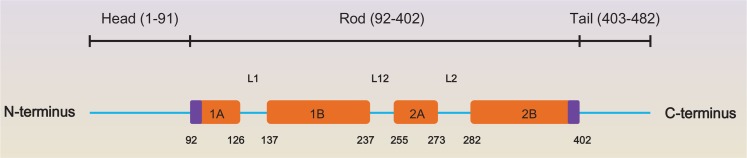
Domain Structure of keratin 8. Keratin proteins are composed of the non-helical N-terminal head- and C-terminal tail-domains as well as the in the middle helical rod-domain (Toivola et al., 2015). The 4 α-helical parts (1A, 1B, 2A and 2B) of the rod domain are combined through the linker domains L1, L12 and L2. The number and domain shown here is K8 based on www.interfil.org. Modified from Toivola et al. (Toivola et al., 2015).
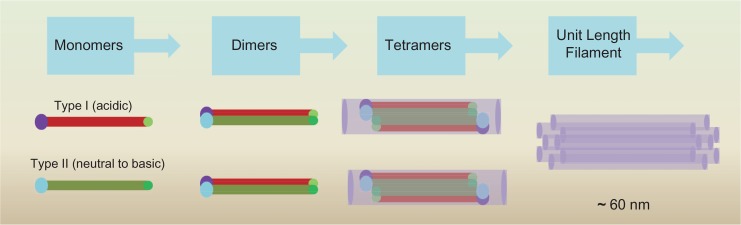
Simple epithelia of liver, intestine, and pancreas, are discovered as pairs of K7, K8, K18, K19, and K20, but the ratio of type I and type II keratins is 1:1 in all cells (Moll et al., 1982; Ku et al., 1999; Toivola et al., 2002). K8 and K18 assemble to form heterodimers in epithelia of gland (Omary et al., 2009; Toivola et al., 2015). Keratins assemble as heterodimers of each of type I and type II keratin monomer, aligned in parallel (Hatzfeld and Weber, 1990; Herrmann and Aebi, 2000; Haines and Lane, 2012). These heterodimers convert to anti-parallel tetramers by overlaying the N-terminal half of rod domains and tetramers then form ‘unit length filaments’ (60 nm in length) (Fig. 2) (Haines and Lane, 2012).
Fig. 2.
Assembly of keratin filaments. The strands are made up of keratin filament proteins. Keratin filament proteins have the same basic structure: they have a globular head at their N-termini, a globular tail at their C termini, and a rod-like α helical domain in between (Haines and Lane, 2012). Two such units can twist each other to shape a “coiled coil” structure (Haines and Lane, 2012). Two of these coiled coils align head-to-tail to form a tetramer. Eight tetramers align end to end to form a unit length filament (32 monomer) (Haines and Lane, 2012). Modified from Haines and Lanel (Haines and Lane, 2012).
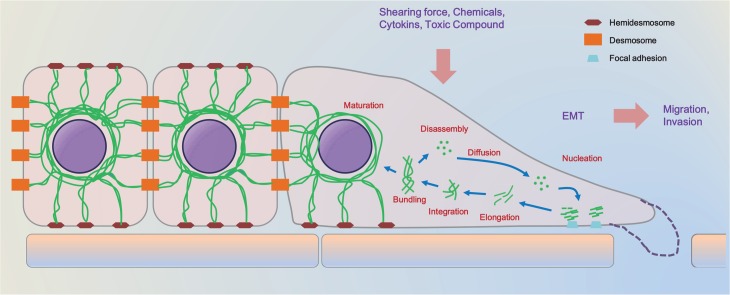
Several situations including diverse stress requires the changes of keratins (Leube et al., 2011). Keratin cycle starts with nucleation of keratin units at the peripheral region of cells including vicinity of focal adhesions (Windoffer et al., 2011). Next, elongation of new keratin units follows actin-dependent movement toward the peripheral keratin network (Windoffer et al., 2011). After consolidation of keratin particles to the keratin network, keratin filaments keep to move toward the rim of nucleus and bundle (Loschke et al., 2015). Parts of keratins break up into several pieces of oligomers that diffuse into the cytosol (Loschke et al., 2015). Other keratins make a perinuclear keratin network and are linked to desmosome and hemidesmosome (Fig. 3) (Windoffer et al., 2011).
Fig. 3.
The keratin cycle. Soluble keratin oligomers congregate into particles in the peripheral region of cells in proximity to focal adhesion sites (nucleation)(Windoffer et al., 2011). These particles grow (elongation) and move toward the cell center in an actin-dependent process (transport) (Windoffer et al., 2011). Subsequently, elongated keratin particles are combined into the peripheral keratin network (integration) (Windoffer et al., 2011). Filament bundling occurs during further centralizing translocation toward the nucleus (transport) (Windoffer et al., 2011). Soluble oligomers set apart (disassembly), diffuse throughout the cytoplasm (diffusion), and are recycled for another turn of keratins formation in the cell periphery (Haines and Lane, 2012). Alternatively, bundled keratin filaments are stabilized (maturation), making the stable perinuclear cage. Modified from Haines and Lanel (Windoffer et al., 2011; Haines and Lane, 2012).
KERATIN IN THE EPITHELIAL CELLS
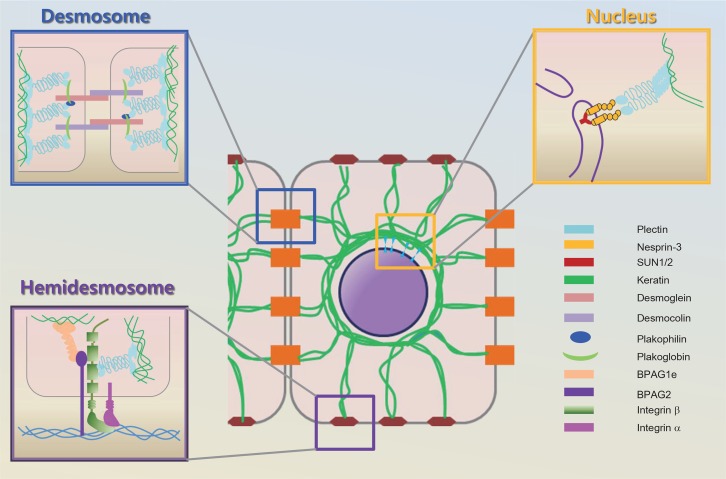
In the epithelia tissues, a network of proteins links the nucleus to membrane of cell through keratin filaments, in which transmembrane proteins gives the ground for cell to cell and cell to extracellular matrix adhesion (Fig. 4) (Omary et al., 2009; Pan et al., 2013).
Fig. 4.
Keratin in epithelial cells. Desmosome junction: Desmosomes link to the keratin filament of cells. Transmembrane desmosomal cadherins, desmoglein and desmocolin, bind placoglobin, the armadillo family protein, which holds the plectin, plakin family member (Fuchs and Raghavan, 2002). The cytoplasmic plaque anchors the keratin intermediate to the desmosome. Hemidesmosome junction: Integrin α and β heterodimers consist of the core of the hemidesmosome, along with BPAG2, a transmembrane protein. BPAG1e and plectin are two hemidesmosomal proteins that are members of the plakin family (Haines and Lane, 2012). They seem to function by connecting the keratin filament to the transmembrane proteins in the hemidesmosome. BPAG1e, bullous pemphigoid antigen 1, epidermal isoform; BPAG2, bullous pemphigoid antigen 2 (Haines and Lane, 2012). Nuclear junction: Nesprin 3 attach to SUN proteins through the perinucelar space and can directly connect to keratin proteins via plectin (Gerlitz and Bustin, 2011). Modified and combined from Fuchs and Raghavan, Gerlitz and Bustin, and Haines and Lanel (Fuchs and Raghavan, 2002; Gerlitz and Bustin, 2011; Haines and Lane, 2012).
Linking to desmosome and hemidesmosome
Keratin is connected to desmosome in the cell to cell adhesion site through desmoplakin (Green and Simpson, 2007). The cadherin family, the desmogleins and desmocollins, join the adhesion point (Getsios et al., 2004; Green and Simpson, 2007). The tails of the cadherins give an association region for the armadillo proteins such as plakoglobin, plakophilins 1–3, and p0071 (Schmidt and Jager, 2005; Green and Simpson, 2007). The carboxy terminal of desmoplakin interacts directly with the amino terminal end of type II keratins (Fig. 4) (Kouklis et al., 1994; Hatsell and Cowin, 2001).
Hemidesmosomes are junction complexes contributing to the adherence of epithelial cells to the basal layer (Borradori and Sonnenberg, 1999). The molecular structure of hemidesmosome is composed of 3 kinds of proteins: the cytoplasmic linker proteins for intermediate filaments at the cytoplasmic leaflet of the plasma membrane, the transmemebrane proteins acting as receptors linking the inside of cell to the proteins of the basal layers (Borradori and Sonnenberg, 1999). Keratin is linked to plectin and BPAG1e at hemidesmosome cell-matrix adhesions (Guo et al., 1995; Green and Simpson, 2007; Pan et al., 2013). The linking of plectin to keratins is required for hemidesmosome assembly (Fig. 4) (Koster et al., 2004). Keratins localize hemidesmosomes and repress migration of cells (Seltmann et al., 2013).
Linking to nucleat envelope
Lamins underlie the inner face of nuclear membrane and also make stable structures within the nucleus interior which contains emerin, lamin B-receptor, and SUN (Sad1 and UNC84 domain containing) 1/2 (Friedl et al., 2011). Nesprins belong to a family of proteins that are mainly known for their position along the nuclear envelope (Mellad et al., 2011).
Nesprins are a core member of the LINC (Linker of Nucleoskeleton and Cytoskeleton) complex that cross over both nuclear membranes to link the cytoplasm and the inside of nucleus (Neumann and Noegel, 2014).
Nesprins interact with SUN proteins through perinuclear space via their KASH (Klarsicht, ANC-1, Syne Homology) domain and directly link to actin filaments (nesprin-1 and -2) and keratins via plectin (nesprin-3) (Padmakumar et al., 2005; Friedl et al., 2011). The cytoplasmic N-terminus of nesprin-3 interacts with plectin, a member of the plakin family of cytoskeletal linker protein (Sonnenberg and Liem, 2007). Nesprin-1 and -2 bind to microtubules via kinesin or dynein (Friedl et al., 2011; Rajgor et al., 2014).
Linking to microfilaments & microtubules
Keratin particles emerge from the vicinity of the plasma membrane, maneuver continuously toward the central part of cell, and consolidate into the peripheral keratin network (Kolsch et al., 2009). These keratin cycles are highly dependent on interaction with actin filament (Pan et al., 2013). Actin depolymerization rapidly triggers keratin intermediate filament formation by turning on keratin related genes (Chang et al., 2014).
Keratin particles also moves fast via microtubules (Liovic et al., 2003). Keratin shows 2 types of motility in cells such as slow, continuous transport of keratin precursor particles of cell, and fast, bidirectional movement of keratin particles (Woll et al., 2005). Type I movement is mediated by actin and type II movement is mediated by microtubule systems (Woll et al., 2005).
Spectroplakins are big cytoskeletal linking proteins that bind to all 3 members of the cytoskeleton such as actin filaments, microtubules, and intermediate filaments (Suozzi et al., 2012). The spectraplakin family is composed of two mammalian genes, MACF1 (Microtubule-Actin Crosslinking Factor 1), and Dst (Dystonin) encoding bullous pemphigoid antigen 1 (Suozzi et al., 2012). BPAG1 connects the keratin network to hemidesmosome of cell to intensify the mechanical strength at the basal layer of the epidermis (Koster et al., 2003; Suozzi et al., 2012).
PHOSPHORYLATION OF KERATINS
A wide range of post-translational modifications have been reported on keratins such as phosphorylation, ubiquitylation, acetylation, glycosylation, and, sumoylation, which seem to control the solubility of keratins in several situations (Omary et al., 2006; Ku et al., 2010; Srikanth et al., 2010; Snider et al., 2011). Recently, a review focuses on post-translational modification of intermediate filament proteins including vimentin and keratin (Snider and Omary, 2014). So we just emphasize phosphorylation of keratin which is key event in perinuclear reorganization of keratin (Beil et al., 2003).
Phosphorylation is a key reaction of keratins, and K1, K8, K18, and K19 are the fully studied among keratin family (Steinert, 1988; Zhou et al., 1999; Omary et al., 2002). Multiple factors such as several stresses, apoptosis, and mitosis, regulate keratin phosphorylation resulting keratin filament reorganization (Ku et al., 1999). Serine is the primary amino acid of phosphorylated keratin (Oshima, 1982; Omary et al., 1998). Tyrosine and threonine are also phosphorylated keratin residues (Feng et al., 1999). Sphingosylphosphorylcholine (SPC)-induced phosphorylation and perinuclear reorganization of keratin are implicated in viscoelasticity of PANC-1 cancer cells (Beil et al., 2003). Therefore, keratin phosphorylation seems to be important in regulating the physical properties of cancer cells. However, it is not yet clear that perinuclear reorganization by phosphorylation is a special event for metastatic cancer or just one step of keratin recycle process. In addition, it is not clear why metastatic cancer cells reveal phenotypes such as the perinuclear reorganized keratin structure.
PLAYERS INVOLVED IN PHOSPHORYLATION AND REORGANIZATION OF KERATINS
Mitogen-activated protein (MAP) kinases
Numerous kinases are involved in phosphorylation of keratins (Snider and Omary, 2014). Phosphorylation of serine residue of keratin leads to disintegration of the stable structure and increased solubility of keratin in the cytoplasm (Omary et al., 1998).
ERK is one of the kinases involved in SPC or leukotriene B4 (LTB4)- evoked K8 phosphorylation and reorganization (Fig. 5, Table 2) (Busch et al., 2012; Park et al., 2012). ERK is also required in acetone extracts from Bupleurm scorzonerifolium-induced K8 phosphorylation in A549 cancer cells (Chen et al., 2005).
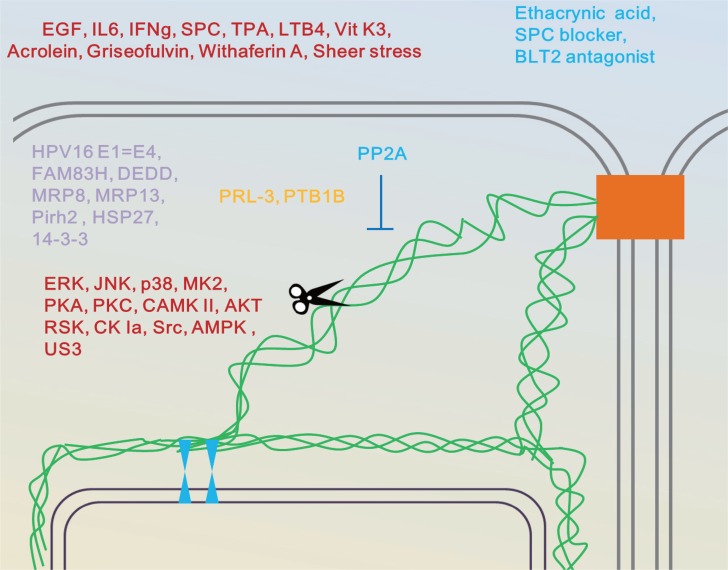
Fig. 5.
Keratin networks as targets and effectors of chemical signals and stresses. Desmosomes locations at which growth factor signaling and force are sensed and transmitted, organize keratin networks (Haines and Lane, 2012). Posttranslational keratin modifications including several kinases and phosphatases enhance keratin network dynamics and the non-polymeric keratin state (Loschke et al., 2015). These modifications evoke weaker cell adhesion, enhanced migration and invasion of epithelial cells. Modified from Loschke et al., (Loschke et al., 2015).
Table 2.
Phosphorylated residues of keratins and kinases involved
No evidence for phosphorylation of residue by indicated kinase but dependent on that.
Serine-73 (Ser-73) of K8 is a residue of phosphorylation by c-Jun N-terminal kinase (JNK) (Fig. 5, Table 2). Furthermore, we found that JNK phosphorylates serine-431 (Ser-431) in SPC-induced phosphorylation and reorganization of K8 (He et al., 2002; Park et al., 2011).
p38 mitogen activated protein kinase (MAPK) is also involved in phosphorylation of Ser-73 induced by treatment with okadaic acid or orthovanadate (Ku et al., 2002a; Woll et al., 2007). p38 MAPK phosphorylates MAPK-activated protein kinase MK2 and phosphorylation of Ser-73 in HT29 cells is dependent on MK2 (Fig. 5, Table 2) (Menon et al., 2010). MK2 also phosphorylates Ser-52 of K18 and Ser-13 of K20 (Menon et al., 2010).
PKA, PKC, and CAMK II
cAMP-dependent protein kinase (PKA) and Ca2+- dependent protein kinase C (PKC) almost exclusively phosphorylates serine of K8 (Fig. 5, Table 2) (Yano et al., 1991). PKA phosphorylates Ser-8, Ser-12, Ser-23, Ser-33, Ser-36, Ser-42, and Ser-50 in the head domain and Ser-416, Ser-423, and Ser-425 in the tail region of K8 (Ando et al., 1996). Protein kinase Cɛ (PKCɛ) phosphorylates K8 at Ser-8 and Ser-23 in thyrotropin-releasing hormone (TRH) -treated GH4C1 cells (Akita et al., 2007). Interestingly, PKCɛ and K8 have perinuclear colocalization under basal conditions and are found in the cell periphery and cell to cell contact region after TRH treatment (Akita et al., 2007). Protein kinase Cδ (PKCδ) phosphorylates Ser-73 of K8 regulating the shear stress-mediated collapse of keratin network in human A549 cells (Ridge et al., 2005). Protein kinase Cζ (PKCζ) phosphorylates Ser-33 of K18 leading to reorganization of keratin proteins induced by shear stress (Sivaramakrishnan et al., 2009). Phosphorylation of Ser-13 of K20 is increased after PKC activation but it is not clear whether PKC phosphorylates Ser-13 of K20 (Zhou et al., 2006). Recently, K8 phosphorylation by PKC is known to a major contributing factor for K8 downregulation in human disc degeneration (Sun et al., 2013).
Calmodulin-dependent protein kinase II (CAMK II) phosphorylates K8 at serine and threonine amino acids (Yano et al., 1991). However, specific sites of phosphorylated residues of K8 by CAMK II were not reported.
AKT and RSK
Predicted phosphorylation sites for Akt exist in several keratins and Akt binds K8 but not K18 (Fig. 5, Table 2) (Paramio et al., 2001; Loschke et al., 2015). In the presence of K8 and K18, K8-Akt interaction is independent of K18 glycosylation and Thr 308-phosphorylation in Akt1 (Ku et al., 2010). Akt1 overexpression also increases K8 and K18 proteins (Fortier et al., 2010b). However, there are no reports on Akt-induced phosphorylation of specific residue(s) of keratin.
K17, a type I keratin, is heavily induced in epidermis after injury, and in psoriasis and cancer (Pan et al., 2011). p90 ribosomal protein S6 kinase 1 (RSK1) phosphorylates Ser-44 residue of K17 of keratinocytes (Fig. 5) (Pan et al., 2011). However, this phosphorylation is not clearly linked to a modification of keratin network.
Casein kinase Iα
Casein kinase Iα (CK-Iα) plays an essential role in the phosphorylation and degradation of β-catenin (Knippschild et al., 2005). Casein kinase Iα (CK-Iα) mediates FAM83H (family with sequence similarity 83 member H)-dependent reorganization of keratin filaments (Kuga et al., 2013). Inhibition of CK-1α is a cause of keratin filament bundling and reverses keratin filament disassembly; but it is not yet known which amino acid residue of K8 or K18 is phosphorylated by CK-Iα. Ser-73 and Ser-431 of K8 and Ser-33 and Ser-52 of K18 are not candidates of substrates of CK-Iα (Fig. 5, Table 2) (Kuga et al., 2013).
Src kinase
Ser-35 of K19, which is a type I keratin, is a well-known residue of phosphorylation (Zhou et al., 1999). Src kinase phosphorylates tyrosine 391 of human K19 (Fig. 5, Table 2) (Zhou et al., 2010). During keratinocytes migration and tissue repair, Src kinase activity is inhibited by wound-induced keratin such as K6a and K6b (Rotty and Coulombe, 2012).
Miscellaneous kinases
The AMP-activated protein kinase (AMPK) is important in the biological response induced by metabolic changes and is turned on by AMP (Velasco et al., 1998). AMPK and 5-aminoimidazole-4-carboxamide ribonucleotide, a AMPK activator phosphorylate K8 and K18 in primary hepatocytes (Fig. 5, Table 2) (Velasco et al., 1998).
US3 is a specific serine/threonine protein kinase found in herpes simplex virus (Murata et al., 2002; Koyanagi et al., 2014). US3 protein kinase directly phosphorylates K17 (Fig. 5, Table 2) (Murata et al., 2002). However, there are no reports on US3 or AMPK-induced phosphorylation of specific residue(s) of keratin.
OTHER PLAYES IN KERATIN PHOSPHORYLATION AND REORGANIZATION
Protein phosphatase
Several kinases are reportedly implicated in the SPC-induced phosphorylation of K8. For example, ERK and JNK are involved in SPC-induced K8 phosphorylation (Park et al., 2011; Busch et al., 2012). So common upstream regulator of ERK and JNK might be important in SPC-induced K8 phosphorylation. Protein phosphatase-2A (PP2A) dephosphorylated phospho ERK and phospho JNK (Fig. 5) (He et al., 2002; Hu et al., 2009). PP2A directly dephosphorylates K8 during hyposmotic stress in HT29 cells (Tao et al., 2006). PP2A also maintains the structure and interactions of hepatic keratin intermediate filaments (Toivola et al., 1997). PP2A down regulation is also involved in LTB4-evoked phosphorylation of K8 at Ser-431 (Park et al., 2012).
Phosphatase of regenerating liver-3 (PRL-3) belongs to the PRL protein tyrosine phosphatase family and highly PRL-3 expressed cancer cells demonstrate reduction of K8 phosphorylation, especially at the front of invasion and metastasis to liver (Fig. 5) (Mizuuchi et al., 2009). Especially, loss of plakophilin 3 results in an increase in PRL3 levels promoting K8 dephosphorylation of HCT116 cells (Khapare et al., 2012).
Pharmacological inhibition of the protein-tyrosine phosphatase PTP1B increases phosphorylation of Tyr-267 of K8, decreases solubility, and increases K8 filament bundling, whereas PTP1B overexpression has the opposite effects (Fig. 5) (Snider et al., 2013).
It seems that effects on K8 structure and stability by phosphorylation of serine differ from those of tyrosine phosphorylation. Further study is needed to elucidate the role of different phosphorylated keratins on structure and reorganization.
Miscellaneous binding partner of keratins
High-risk human papillomaviruses (HPV) such as HPV16, are the major cause of cervical cancer and one of HPV16 proteins, E1–E4 binds to keratins leading to keratin network disorganization (Fig. 5) (Wang et al., 2004). Albatross exists with keratin filaments in nonpolarized epithelial cells and keratins stabilize the Albatross protein (Fig. 5) (Sugimoto et al., 2008). A newly identified keratin-associated protein, FAM83H regulates the filamentous state of keratins and the C-terminal region of FAM83H interacts with keratins (Fig. 5) (Kuga et al., 2013).
Death effector domain with DNA binding protein (DEDD), is present mostly as mono- or diubiquitinated form, and diubiquitinated DEDD bind to the K8 and K18 (Fig. 5) (Lee et al., 2002). Migration inhibitory factor-related protein 8 (MRP8) and MRP14, may be implicated in Ca2+-induced keratins reorganization in TR146 human squamous cell carcinoma (Fig. 5) (Goebeler et al., 1995). p53-induced ubiquitin-protein ligase (Pirh2), binds to K8 and K18 and phosphorylation of either Pirh2 or K8 and K18, influences their binding (Fig. 5) (Duan et al., 2009).
Association of small heat shock proteins (HSP) with intermediate filament including keratins, may regulate filament interactions in cellular networks. For example, the chaperone HSP27 affects assembly dynamics and organization of K8 and K18 cytoskeleton through direct keratin interactions (Fig. 5) (Perng et al., 1999; Kayser et al., 2013; Loschke et al., 2015).
14-3-3 protein binds to several kinases of signal transduction (Liao and Omary, 1996). 14-3-3 proteins also interact with phosphorylated form of keratin in simple epithelia during the course of cell cycle and plays a role of cofactor for solubilization of keratins (Liao and Omary, 1996). Ser-33 phosphorylation of K18 influences binding of K18 to 14-3-3 proteins in the course of mitosis and interaction of K18 with 14-3-3 proteins regulates keratin filaments and mitotic progression of hepatic cells (Fig. 5) (Ku et al., 2002b).
INDUCERS OF PHOSPHORYLATION AND REORGANIZATION OF KERATINS
Growth factor & cytokines
Epidermal growth factor (EGF) leads to phosphorylation of keratin in rat hepatocyte before keratin reorganization (Fig. 5) (Baribault et al., 1989). EGF-induced K8 phosphorylation happens at Ser-23 of head domain and Ser-431 of tail domain (Ku and Omary, 1997).
Interleukin-6 (IL-6) significantly up-regulates K8 and K18 in intestinal epithelial cells such as Caco2-BBE (brush border expressing) cell line and IL-6 evoked K8 phosphorylation at serine residue (Fig. 5) (Wang et al., 2007b). IL-6 protect intestinal barrier via K8/K18 in compromised condition (Wang et al., 2007b).
K17, the myoepithelial keratin, is expressed in psoriasis but is not present in healthy skin (Komine et al., 1996). Increased production of interferon gamma (IFNγ) induces the expression of K17 by activating transcription factor STAT1 (Komine et al., 1996). However, it is not clear whether IFNγ induces phosphorylation and reorganization of K17.
12-O-Tetradecanoylphorbol-13-acetate & LTB4
Exposure of the hepatocytes to 12-O-tetradecanoyl-phorbol-13-acetate (TPA) (150 nM), a typical activator of protein kinase C, leads to phosphorylation of K8 but not K18 (Cadrin et al., 1992). Recently, we found that transglutaminase-2 plays important role in TPA-induced K8 phosphorylation and reorganization (Fig. 6) (Lee et al., 2014). Our data show that LTB4 is an inducer of K8 phosphorylation and that ERK is involved in LTB4-induced phosphorylation and reorganization of K8 in pancreatic cancer cells. LTB4 receptor 2 (BLT2) receptor mediates effects of LTB4 via PP2A down-regulation (Fig. 5) (Park et al., 2012).
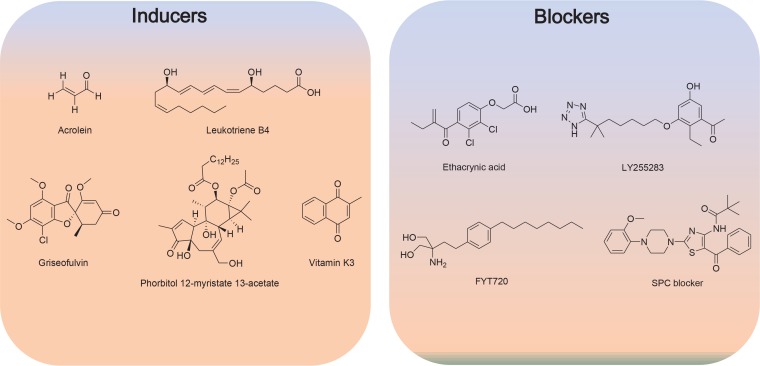
Fig. 6.
Structure of inducers and blockers of keratin phosphorylation and reorganization.
Chemical compounds & others including physical stresses
Treatment of several human breast cancer cells including MCF7, T47D, SKBR3 with vitamin K3 (50–100 μM) leads to K8 phosphorylation at Ser-73 via MEK (MAPK/ERK kinase) 1/2 signaling (Fig. 6) (Scott et al., 2005).
Acrolein is a primary mediator of pulmonary edema and induces phosphorylation of K8 at Ser-73 in bronchiolar lung epithelia (Fig. 6) (Burcham et al., 2014).
Griseofulvin induces Mallory-Denk bodies in hepatocytes of mice (Fortier et al., 2010a). In this mice model, griseofulvin induces phosphorylation of K8 (Ser-79, Ser-436) and K18 (Ser-33) (Fig. 6).
Pervanadate, tyrosine phosphatase inhibitor, induces phosphorylation of tyrosine residue in K8, and K19, but not K18 via p38 MAP kinase (Feng et al., 1999). This process appears independent of ERK kinase pathway.
Withaferin A (WFA) binds to the vimentin and modifies perinuclear aggregates of intermediates filaments including keratin (Grin et al., 2012).
Compressive loads induce K8 phosphorylation in human disc generation by activating protein kinase C (Sun et al., 2013). Shear stress also evokes reorganization of the keratin network via the phosphorylation of K8 by PKCζ (Sivaramakrishnan et al., 2009). Heat stress or rotavirus infection induced phosphorylation of K8 in human colonic cell line HT29 (Liao et al., 1995).
PHOSPHORYLATION AND REORGANIZATION OF KERATINS IN CANCER
Several reports support an active role of keratins as versatile regulators in carcinogenesis (Karantza, 2011). However, roles of phosphorylation of keratin in carcinogenesis and metastasis are controversial. For example, loss of K8 Ser-73 and Ser-431 phosphorylation is also observed in human oral squamous cell carcinoma (OSCC) tissues evaluated by immunohistochemistry, in which dephosphorylation greatly associated with size, and progression of the tumor (Alam et al., 2011). Moreover, overexpression of K8 and K18 is related to up-regulation of histone type 2 H2aa3 and keratin reorganization may accelerate cancerous transformation of glutathione S-transferase P-form positive foci in the course of rat hepatocarcinogenesis (Kakehashi et al., 2009). Similarly, K19 expression in human hepatocellular carcinoma is correlated with increased invasiveness and metastasis (Govaere et al., 2014).
On the other hand, loss of K8 and K18 leads to increased collective emigration and invasiveness of breast cancer cells (Fortier et al., 2010a). Similarly, SPC evokes a perinuclear reorganization of keratin proteins via phosphorylation of Ser-431 of K8, and increased migration of human pancreatic cancer cells (Beil et al., 2003). JNK and ERK phosphorylates K8 at Ser-431, and stimulate the perinuclear reorganization of keratin resulting enhanced migration (Busch et al., 2012; Park et al., 2011).
The probable differences in results might be by use of different kinds of cells and methods (Windoffer et al., 2011).
Epithelial-mesencymal transition (EMT) is an important event that permit a polarized epithelial cell, to experience numerous biochemical conversions to deduce a mesenchymal phenotype of cell including increased migration, invasiveness, and significantly elevated resistance to apoptosis (Kalluri and Neilson, 2003).
Loss of keratin by phosphorylation is one of hallmarks in EMT (Kalluri and Weinberg, 2009). Therefore it is plausible that players implicated in perinuclear reorganization of keratin by phosphorylation are also involved in EMT. Accordingly, Tgase-2 involved in SPC or TPA-induced K8 phosphorylation and reorganization, is also implicated in TGF-β1-induced EMT (Park et al., 2013). ERK1/2, JNK and p38 are involved in phosphorylation of keratins and also TGF-β1-induced EMT (Park et al., 2013; Zhao et al., 2015). RKS2 involved in keratin phosphorylation, are involved in macrophage-stimulating protein-induced EMT (Ma et al., 2011).
Several phosphatases involved in dephosphorylation of keratins are also implicated in process of EMT. PRL-3 or PTP1B involved in keratin dephosphorylation also induced EMT (Wang et al., 2007a; Hiraga et al., 2013). In contrast, PP2A, DEDD, and AMPK reverses EMT (Lv et al., 2012; Bhardwaj et al., 2014; Chou et al., 2014; Kim et al., 2015). Therefore, several players in keratin phosphorylation seems have an important role in EMT and new target identification in keratin phosphorylation and reorganization might be new targets for controling EMT and metastasis.
New opportunity of compounds regulating the phosphorylation and reorganization of keratins
Modulation of keratin phosphorylation and reorganization is potential new way for controlling EMT and metastasis of cancer (Beil et al., 2003). Apparently, several kinase inhibitors including MAP kinase, might be used as agents for reducing phosphorylation and subsequent reorganization of keratins leading to EMT suppression. We attempted to identify compounds affecting the keratin phosphorylation and reorganization using SPC as inducer. We found that ethacrynic acid, a well-known diuretic, inhibits SPC-induced K8 phosphorylation, reorganization, and migration via Tgase-2 inhibition (Byun et al., 2013). We reported that BLT2 participates in the LTB4-induced K8 phosphorylation, reorganization and migration and LY255283 suppressed LTB4-induced phosphorylation and reorganization of keratins (Park et al., 2012). Therefore, BLT2 antagonists and Tgase-2 inhibitors might be new tools for controlling EMT and metastasis.
We also developed SPC blocker based on structure of SPC. Several compounds derived from SPC, suppressed SPC-induced K8 phosphorylation, reorganization and migration (Lee et al., 2014). We also screened microbial extracts and found that some microbial extracts suppress SPC-induced migration using SPC-induced migration of PANC-1 cells (Kang et al., 2011).
However, additional inducers released from tumor microenvironment that affect keratin phosphorylation and reorganization have not been identified. If several factors are released from tumor microenvironment and induced keratin phosphorylation and reorganization, blocking the common pathway would be an optimal strategy. Hence PP2A activator or inducers also might be good candidate for controlling keratin reorganization by dephosphorylating the phosphor serine residue of keratins or phosphorylated kinases (active forms) involved in phosphorylation of keratins.
CONCLUSION
Metastatic cancer cell is much softer than non-metastatic cancer cells (Cross et al., 2007). Viscoelasticity of cancer cells is related to keratin architecture. So elucidating new players to regulate the keratin phosphorylation and reorganization might provide new targets for suppressing the metastasis. Furthermore, novel compounds modulating the phosphorylation and reorganization of keratin might be a new hope for fighting against metastasis of cancer.
Acknowledgments
This study was supported by grants from the Korea Healthcare Technology R&D project (no. A101836), and the Basic Science Research Program, through the NRF (NRF-2014R1A2A1A01004016).
REFERENCES
- Akita Y, Kawasaki H, Imajoh-Ohmi S, Fukuda H, Ohno S, Hirano H, Ono Y, Yonekawa H. Protein kinase C epsilon phosphorylates keratin 8 at Ser8 and Ser23 in GH4C1 cells stimulated by thyrotropin-releasing hormone. FEBS J. 2007;274:3270–3285. doi: 10.1111/j.1742-4658.2007.05853.x. [DOI] [PubMed] [Google Scholar]
- Alam H, Gangadaran P, Bhate AV, Chaukar DA, Sawant SS, Tiwari R, Bobade J, Kannan S, D’Cruz AK, Kane S, Vaidya MM. Loss of keratin 8 phosphorylation leads to increased tumor progression and correlates with clinico-pathological parameters of OSCC patients. PLoS One. 2011;6:e27767. doi: 10.1371/journal.pone.0027767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S, Tokui T, Yano T, Inagaki M. Keratin 8 phosphorylation in vitro by cAMP-dependent protein kinase occurs within the amino- and carboxyl-terminal end domains. Biochem Biophys Res Commun. 1996;221:67–71. doi: 10.1006/bbrc.1996.0546. [DOI] [PubMed] [Google Scholar]
- Baribault H, Blouin R, Bourgon L, Marceau N. Epidermal growth factor-induced selective phosphorylation of cultured rat hepatocyte 55-kD cytokeratin before filament reorganization and DNA synthesis. J Cell Biol. 1989;109:1665–1676. doi: 10.1083/jcb.109.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beil M, Micoulet A, von Wichert G, Paschke S, Walther P, Omary MB, Van Veldhoven PP, Gern U, Wolff-Hieber E, Eggermann J, Waltenberger J, Adler G, Spatz J, Seufferlein T. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat Cell Biol. 2003;5:803–811. doi: 10.1038/ncb1037. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Singh S, Srivastava SK, Arora S, Hyde SJ, Andrews J, Grizzle WE, Singh AP. Restoration of PPP2CA expression reverses epithelial-to-mesenchymal transition and suppresses prostate tumour growth and metastasis in an orthotopic mouse model. Br. J. Cancer. 2014;110:2000–2010. doi: 10.1038/bjc.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–418. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Raso A, Henry PJ. Airborne acrolein induces keratin-8 (Ser-73) hyperphosphorylation and intermediate filament ubiquitination in bronchiolar lung cell monolayers. Toxicology. 2014;319:44–52. doi: 10.1016/j.tox.2014.02.010. [DOI] [PubMed] [Google Scholar]
- Busch T, Armacki M, Eiseler T, Joodi G, Temme C, Jansen J, von Wichert G, Omary MB, Spatz J, Seufferlein T. Keratin 8 phosphorylation regulates keratin reorganization and migration of epithelial tumor cells. J Cell Sci. 2012;125:2148–2159. doi: 10.1242/jcs.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun HJ, Kang KJ, Park MK, Lee HJ, Kang JH, Lee EJ, Kim YR, Kim HJ, Kim YW, Jung KC, Kim SY, Lee CH. Ethacrynic acid inhibits sphingosylphosphorylcholine-induced keratin 8 phosphorylation and reorganization via transglutaminase-2 inhibition. Biomol Ther. 2013;21:338–342. doi: 10.4062/biomolther.2013.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadrin M, McFarlane-Anderson N, Aasheim LH, Kawahara H, Franks DJ, Marceau N, French SW. Differential phosphorylation of CK8 and CK18 by 12-O-tetradecanoyl-phorbol-13-acetate in primary cultures of mouse hepatocytes. Cell Signal. 1992;4:715–722. doi: 10.1016/0898-6568(92)90052-A. [DOI] [PubMed] [Google Scholar]
- Chang TH, Huang HD, Ong WK, Fu YJ, Lee OK, Chien S, Ho JH. The effects of actin cytoskeleton perturbation on keratin intermediate filament formation in mesenchymal stem/stromal cells. Biomaterials. 2014;35:3934–3944. doi: 10.1016/j.biomaterials.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Chen YL, Lin SZ, Chang WL, Cheng YL, Harn HJ. Requirement for ERK activation in acetone extract identified from Bupleurum scorzonerifolium induced A549 tumor cell apoptosis and keratin 8 phosphorylation. Life Sci. 2005;76:2409–2420. doi: 10.1016/j.lfs.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Chou CC, Lee KH, Lai IL, Wang D, Mo X, Kulp SK, Shapiro CL, Chen CS. AMPK reverses the mesenchymal phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a signaling axis. Cancer Res. 2014;74:4783–4795. doi: 10.1158/0008-5472.CAN-14-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe PA, Omary MB. ‘Hard’ and ‘soft’ principles defining the structure, function and regulation of keratin intermediate filaments. Curr Opin Cell Biol. 2002;14:110–122. doi: 10.1016/S0955-0674(01)00301-5. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- Cross SE, Jin YS, Rao J, Gimzewski JK. Nanomechanical analysis of cells from cancer patients. Nat Nanotechnol. 2007;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- Duan S, Yao Z, Zhu Y, Wang G, Hou D, Wen L, Wu M. The Pirh2-keratin 8/18 interaction modulates the cellular distribution of mitochondria and UV-induced apoptosis. Cell Death Differ. 2009;16:826–837. doi: 10.1038/cdd.2009.12. [DOI] [PubMed] [Google Scholar]
- Feng L, Zhou X, Liao J, Omary MB. Pervanadate-mediated tyrosine phosphorylation of keratins 8 and 19 via a p38 mitogen-activated protein kinase-dependent pathway. J Cell Sci. 1999;112:2081–2090. doi: 10.1242/jcs.112.13.2081. [DOI] [PubMed] [Google Scholar]
- Fortier AM, Riopel K, Desaulniers M, Cadrin M. Novel insights into changes in biochemical properties of keratins 8 and 18 in griseofulvin-induced toxic liver injury. Exp Mol Pathol. 2010a;89:117–125. doi: 10.1016/j.yexmp.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Fortier AM, Van Themsche C, Asselin E, Cadrin M. Akt isoforms regulate intermediate filament protein levels in epithelial carcinoma cells. FEBS Lett. 2010b;584:984–988. doi: 10.1016/j.febslet.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K, Lammerding J. Nuclear mechanics during cell migration. Curr Opin Cell Biol. 2011;23:55–64. doi: 10.1016/j.ceb.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002;3:199–209. doi: 10.1038/nrg758. [DOI] [PubMed] [Google Scholar]
- Gerlitz G, Bustin M. The role of chromatin structure in cell migration. Trends Cell Biol. 2011;21:6–11. doi: 10.1016/j.tcb.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Goebeler M, Roth J, van den Bos C, Ader G, Sorg C. Increase of calcium levels in epithelial cells induces translocation of calcium-binding proteins migration inhibitory factor-related protein 8 (MRP8) and MRP14 to keratin intermediate filaments. Biochem J. 1995;309:419–424. doi: 10.1042/bj3090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govaere O, Komuta M, Berkers J, Spee B, Janssen C, de Luca F, Katoonizadeh A, Wouters J, van Kempen LC, Durnez A, Verslype C, De Kock J, Rogiers V, van Grunsven LA, Topal B, Pirenne J, Vankelecom H, Nevens F, van den Oord J, Pinzani M, Roskams T. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63:674–685. doi: 10.1136/gutjnl-2012-304351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Grin B, Mahammad S, Wedig T, Cleland MM, Tsai L, Herrmann H, Goldman RD. Withaferin a alters intermediate filament organization, cell shape and behavior. PLoS One. 2012;7:e39065. doi: 10.1371/journal.pone.0039065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-X. [DOI] [PubMed] [Google Scholar]
- Haines RL, Lane EB. Keratins and disease at a glance. J Cell Sci. 2012;125:3923–3928. doi: 10.1242/jcs.099655. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hatsell S, Cowin P. Deconstructing desmoplakin. Nat Cell Biol. 2001;3:E270–272. doi: 10.1038/ncb1201-e270. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Weber K. The coiled coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratins: use of site-specific mutagenesis and recombinant protein expression. J Cell Biol. 1990;110:1199–1210. doi: 10.1083/jcb.110.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T, Stepulak A, Holmstrom TH, Omary MB, Eriksson JE. The intermediate filament protein keratin 8 is a novel cytoplasmic substrate for c-Jun N-terminal kinase. J Biol Chem. 2002;277:10767–10774. doi: 10.1074/jbc.M111436200. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opin Cell Biol. 2000;12:79–90. doi: 10.1016/S0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- Hiraga R, Kato M, Miyagawa S, Kamata T. Nox4-derived ROS signaling contributes to TGF-beta-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013;33:4431–4438. [PubMed] [Google Scholar]
- Hu X, Wu X, Xu J, Zhou J, Han X, Guo J. Src kinase up-regulates the ERK cascade through inactivation of protein phosphatase 2A following cerebral ischemia. BMC Neurosci. 2009;10:74. doi: 10.1186/1471-2202-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakehashi A, Inoue M, Wei M, Fukushima S, Wanibuchi H. Cytokeratin 8/18 overexpression and complex formation as an indicator of GST-P positive foci transformation into hepatocellular carcinomas. Toxicol Appl Pharmacol. 2009;238:71–79. doi: 10.1016/j.taap.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI200320530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Park MK, Kim HJ, Kim Y, Lee CH. Isolation of soil microorganisms having antibacterial activity and antimigratory effects on sphingosylphosphorylcholine-induced migration of PANC-1 cells. Toxicol Res. 2011;27:241–246. doi: 10.5487/TR.2011.27.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karantza V. Keratins in health and cancer: more than mere epithelial cell markers. Oncogene. 2011;30:127–138. doi: 10.1038/onc.2010.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser J, Haslbeck M, Dempfle L, Krause M, Grashoff C, Buchner J, Herrmann H, Bausch AR. The small heat shock protein Hsp27 affects assembly dynamics and structure of keratin intermediate filament networks. Biophys J. 2013;105:1778–1785. doi: 10.1016/j.bpj.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khapare N, Kundu ST, Sehgal L, Sawant M, Priya R, Gosavi P, Gupta N, Alam H, Karkhanis M, Naik N, Vaidya MM, Dalal SN. Plakophilin3 loss leads to an increase in PRL3 levels promoting K8 dephosphorylation, which is required for transformation and metastasis. PLoS One. 2012;7:e38561. doi: 10.1371/journal.pone.0038561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kim HJ, Park MK, Kang GJ, Byun HJ, Lee H, Lee CH. Cardamonin suppresses TGF-beta1-induced epithelial mesenchymal transition via restoring protein phosphatase 2A expression. Biomol Ther. 2015;23:141–148. doi: 10.4062/biomolther.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippschild U, Gocht A, Wolff S, Huber N, Lohler J, Stoter M. The casein kinase 1 family: participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Kolsch A, Windoffer R, Leube RE. Actin-dependent dynamics of keratin filament precursors. Cell Motil. Cytoskeleton. 2009;66:976–985. doi: 10.1002/cm.20395. [DOI] [PubMed] [Google Scholar]
- Komine M, Freedberg IM, Blumenberg M. Regulation of epidermal expression of keratin K17 in inflammatory skin diseases. J Invest Dermatol. 1996;107:569–575. doi: 10.1111/1523-1747.ep12582820. [DOI] [PubMed] [Google Scholar]
- Koster J, Geerts D, Favre B, Borradori L, Sonnenberg A. Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly. J Cell Sci. 2003;116:387–399. doi: 10.1242/jcs.00241. [DOI] [PubMed] [Google Scholar]
- Kouklis PD, Hutton E, Fuchs E. Making a connection: direct binding between keratin intermediate filaments and desmosomal proteins. J Cell Biol. 1994;127:1049–1060. doi: 10.1083/jcb.127.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi N, Imai T, Arii J, Kato A, Kawaguchi Y. Role of herpes simplex virus 1 Us3 in viral neuroinvasiveness. Microbiol Immunol. 2014;58:31–37. doi: 10.1111/1348-0421.12108. [DOI] [PubMed] [Google Scholar]
- Ku NO, Azhar S, Omary MB. Keratin 8 phosphorylation by p38 kinase regulates cellular keratin filament reorganization: modulation by a keratin 1-like disease causing mutation. J Biol Chem. 2002a;277:10775–10782. doi: 10.1074/jbc.M107623200. [DOI] [PubMed] [Google Scholar]
- Ku NO, Liao J, Omary MB. Phosphorylation of human keratin 18 serine 33 regulates binding to 14-3-3 proteins. EMBO J. 1998;17:1892–1906. doi: 10.1093/emboj/17.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Michie S, Resurreccion EZ, Broome RL, Omary MB. Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc Natl Acad Sci USA. 2002b;99:4373–4378. doi: 10.1073/pnas.072624299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Omary MB. Phosphorylation of human keratin 8 in vivo at conserved head domain serine 23 and at epidermal growth factor-stimulated tail domain serine 431. J Biol Chem. 1997;272:7556–7564. doi: 10.1074/jbc.272.11.7556. [DOI] [PubMed] [Google Scholar]
- Ku NO, Toivola DM, Strnad P, Omary MB. Cytoskeletal keratin glycosylation protects epithelial tissue from injury. Nat Cell Biol. 2010;12:876–885. doi: 10.1038/ncb2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Zhou X, Toivola DM, Omary MB. The cytoskeleton of digestive epithelia in health and disease. Am J Physiol. 1999;277:G1108–1137. doi: 10.1152/ajpgi.1999.277.6.G1108. [DOI] [PubMed] [Google Scholar]
- Kuga T, Kume H, Kawasaki N, Sato M, Adachi J, Shiromizu T, Hoshino I, Nishimori T, Matsubara H, Tomonaga T. A novel mechanism of keratin cytoskeleton organization through casein kinase Ialpha and FAM83H in colorectal cancer. J Cell Sci. 2013;126:4721–4731. doi: 10.1242/jcs.129684. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Park MK, Kim HJ, Kang JH, Kim YR, Kang GJ, Byun HJ, Lee CH. 12-O-tetradecanoylphorbol-13-acetate induces keratin 8 phosphorylation and reorganization via expression of transglutaminase-2. Biomol Ther. 2014;22:122–128. doi: 10.4062/biomolther.2014.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Schickling O, Stegh AH, Oshima RG, Dinsdale D, Cohen GM, Peter ME. DEDD regulates degradation of intermediate filaments during apoptosis. J Cell Biol. 2002;158:1051–1066. doi: 10.1083/jcb.200112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leube RE, Moch M, Kolsch A, Windoffer R. “Panta rhei”: Perpetual cycling of the keratin cytoskeleton. Bioarchitecture. 2011;1:39–44. doi: 10.4161/bioa.1.1.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Lowthert LA, Omary MB. Heat stress or rotavirus infection of human epithelial cells generates a distinct hyperphosphorylated form of keratin 8. Exp Cell Res. 1995;219:348–357. doi: 10.1006/excr.1995.1238. [DOI] [PubMed] [Google Scholar]
- Liao J, Omary MB. 14-3-3 proteins associate with phosphorylated simple epithelial keratins during cell cycle progression and act as a solubility cofactor. J Cell Biol. 1996;132:345–357. doi: 10.1083/jcb.133.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liovic M, Mogensen MM, Prescott AR, Lane EB. Observation of keratin particles showing fast bidirectional movement colocalized with microtubules. J Cell Sci. 2003;116:1417–1427. doi: 10.1242/jcs.00363. [DOI] [PubMed] [Google Scholar]
- Loschke F, Seltmann K, Bouameur JE, Magin TM. Regulation of keratin network organization. Curr. Opin. Cell Biol. 2015;32C:56–64. doi: 10.1016/j.ceb.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Lv Q, Hua F, Hu ZW. DEDD, a novel tumor repressor, reverses epithelial-mesenchymal transition by activating selective autophagy. Autophagy. 2012;8:1675–1676. doi: 10.4161/auto.21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Guin S, Padhye SS, Zhou YQ, Zhang RW, Wang MH. Ribosomal protein S6 kinase (RSK)-2 as a central effector molecule in RON receptor tyrosine kinase mediated epithelial to mesenchymal transition induced by macrophage-stimulating protein. Mol. Cancer. 2011;10:66. doi: 10.1186/1476-4598-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellad JA, Warren DT, Shanahan CM. Nesprins LINC the nucleus and cytoskeleton. Curr Opin Cell Biol. 2011;23:47–54. doi: 10.1016/j.ceb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Menon MB, Schwermann J, Singh AK, Franz-Wachtel M, Pabst O, Seidler U, Omary MB, Kotlyarov A, Gaestel M. p38 MAP kinase and MAPKAP kinases MK2/3 cooperatively phosphorylate epithelial keratins. J Biol Chem. 2010;285:33242–33251. doi: 10.1074/jbc.M110.132357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi E, Semba S, Kodama Y, Yokozaki H. Down-modulation of keratin 8 phosphorylation levels by PRL-3 contributes to colorectal carcinoma progression. Int. J. Cancer. 2009;124:1802–1810. doi: 10.1002/ijc.24111. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Murata T, Goshima F, Nishizawa Y, Daikoku T, Takakuwa H, Ohtsuka K, Yoshikawa T, Nishiyama Y. Phosphorylation of cytokeratin 17 by herpes simplex virus type 2 US3 protein kinase. Microbiol Immunol. 2002;46:707–719. doi: 10.1111/j.1348-0421.2002.tb02755.x. [DOI] [PubMed] [Google Scholar]
- Neumann S, Noegel AA. Nesprins in cell stability and migration. Adv Exp Med Biol. 2014;773:491–504. doi: 10.1007/978-1-4899-8032-8_22. [DOI] [PubMed] [Google Scholar]
- Omary MB, Ku NO, Liao J, Price D. Keratin modifications and solubility properties in epithelial cells and in vitro. Subcell Biochem. 1998;31:105–140. [PubMed] [Google Scholar]
- Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119:1794–1805. doi: 10.1172/JCI37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary MB, Ku NO, Tao GZ, Toivola DM, Liao J. “Heads and tails” of intermediate filament phosphorylation: multiple sites and functional insights”. Trends Biochem Sci. 2006;31:383–394. doi: 10.1016/j.tibs.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Omary MB, Ku NO, Toivola DM. Keratins: guardians of the liver. Hepatology. 2002;35:251–257. doi: 10.1053/jhep.2002.31165. [DOI] [PubMed] [Google Scholar]
- Oshima RG. Developmental expression of murine extra-embryonic endodermal cytoskeletal proteins. J Biol Chem. 1982;257:3414–3421. [PubMed] [Google Scholar]
- Padmakumar VC, Libotte T, Lu W, Zaim H, Abraham S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou I. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- Pan X, Hobbs RP, Coulombe PA. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr Opin Cell Biol. 2013;25:47–56. doi: 10.1016/j.ceb.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Kane LA, Van Eyk JE, Coulombe PA. Type I keratin 17 protein is phosphorylated on serine 44 by p90 ribosomal protein S6 kinase 1 (RSK1) in a growth- and stress-dependent fashion. J Biol Chem. 2011;286:42403–42413. doi: 10.1074/jbc.M111.302042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramio JM, Segrelles C, Ruiz S, Jorcano JL. Inhibition of protein kinase B (PKB) and PKCzeta mediates keratin K10-induced cell cycle arrest. Mol Cell Biol. 2001;21:7449–7459. doi: 10.1128/MCB.21.21.7449-7459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Lee HJ, Shin J, Noh M, Kim SY, Lee CH. Novel participation of transglutaminase-2 through c-Jun N-terminal kinase activation in sphingosylphosphorylcholine-induced keratin reorganization of PANC-1 cells. Biochim. Biophys. Acta. 2011;1811:1021–1029. doi: 10.1016/j.bbalip.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Park MK, Park Y, Shim J, Lee HJ, Kim S, Lee CH. Novel involvement of leukotriene B(4) receptor 2 through ERK activation by PP2A down-regulation in leukotriene B(4)-induced keratin phosphorylation and reorganization of pancreatic cancer cells. Biochim. Biophys. Acta. 2012;1823:2120–2129. doi: 10.1016/j.bbamcr.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Park MK, You HJ, Lee HJ, Kang JH, Oh SH, Kim SY, Lee CH. Transglutaminase-2 induces N-cadherin expression in TGF-beta1-induced epithelial mesenchymal transition via c-Jun-N-terminal kinase activation by protein phosphatase 2A down-regulation. Eur. J. Cancer. 2013;49:1692–1705. doi: 10.1016/j.ejca.2012.11.036. [DOI] [PubMed] [Google Scholar]
- Pavlaki M, Zucker S. Matrix metalloproteinase inhibitors (MMPIs): the beginning of phase I or the termination of phase III clinical trials. Cancer Metastasis Rev. 2003;22:177–203. doi: 10.1023/A:1023047431869. [DOI] [PubMed] [Google Scholar]
- Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112(Pt 13):2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Rajgor D, Mellad JA, Soong D, Rattner JB, Fritzler MJ, Shanahan CM. Mammalian microtubule P-body dynamics are mediated by nesprin-1. J Cell Biol. 2014;205:457–475. doi: 10.1083/jcb.201306076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge KM, Linz L, Flitney FW, Kuczmarski ER, Chou YH, Omary MB, Sznajder JI, Goldman RD. Keratin 8 phosphorylation by protein kinase C delta regulates shear stress-mediated disassembly of keratin intermediate filaments in alveolar epithelial cells. J Biol Chem. 2005;280:30400–30405. doi: 10.1074/jbc.M504239200. [DOI] [PubMed] [Google Scholar]
- Rotty JD, Coulombe PA. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J Cell Biol. 2012;197:381–389. doi: 10.1083/jcb.201107078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Jager S. Plakophilins--hard work in the desmosome, recreation in the nucleus? Eur J Cell Biol. 2005;84:189–204. doi: 10.1016/j.ejcb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Scott GK, Atsriku C, Kaminker P, Held J, Gibson B, Baldwin MA, Benz CC. Vitamin K3 (menadione)-induced oncosis associated with keratin 8 phosphorylation and histone H3 arylation. Mol Pharmacol. 2005;68:606–615. doi: 10.1124/mol.105.013474. [DOI] [PubMed] [Google Scholar]
- Seltmann K, Roth W, Kroger C, Loschke F, Lederer M, Huttelmaier S, Magin TM. Keratins mediate localization of hemidesmosomes and repress cell motility. J Invest Dermatol. 2013;133:181–190. doi: 10.1038/jid.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Schneider JL, Sitikov A, Goldman RD, Ridge KM. Shear stress induced reorganization of the keratin intermediate filament network requires phosphorylation by protein kinase C zeta. Mol. Biol. Cell. 2009;20:2755–2765. doi: 10.1091/mbc.E08-10-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider NT, Omary MB. Post-translational modifications of intermediate filament proteins: mechanisms and functions. Nat Rev Mol Cell Biol. 2014;15:163–177. doi: 10.1038/nrm3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider NT, Park H, Omary MB. A conserved rod domain phosphotyrosine that is targeted by the phosphatase PTP1B promotes keratin 8 protein insolubility and filament organization. J Biol Chem. 2013;288:31329–31337. doi: 10.1074/jbc.M113.502724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider NT, Weerasinghe SV, Iniguez-Lluhi JA, Herrmann H, Omary MB. Keratin hypersumoylation alters filament dynamics and is a marker for human liver disease and keratin mutation. J Biol Chem. 2011;286:2273–2284. doi: 10.1074/jbc.M110.171314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A, Liem RK. Plakins in development and disease. Exp Cell Res. 2007;313:2189–2203. doi: 10.1016/j.yexcr.2007.03.039. [DOI] [PubMed] [Google Scholar]
- Srikanth B, Vaidya MM, Kalraiya RD. O-GlcNAcylation determines the solubility, filament organization, and stability of keratins 8 and 18. J Biol Chem. 2010;285:34062–34071. doi: 10.1074/jbc.M109.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert PM. The dynamic phosphorylation of the human intermediate filament keratin 1 chain. J Biol Chem. 1988;263:13333–13339. [PubMed] [Google Scholar]
- Stroka KM, Konstantopoulos K. Physical biology in cancer. 4. Physical cues guide tumor cell adhesion and migration. Am J Physiol Cell Physiol. 2014;306:C98–C109. doi: 10.1152/ajpcell.00289.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Inoko A, Shiromizu T, Nakayama M, Zou P, Yonemura S, Hayashi Y, Izawa I, Sasoh M, Uji Y, Kaibuchi K, Kiyono T, Inagaki M. The keratin-binding protein Albatross regulates polarization of epithelial cells. J Cell Biol. 2008;183:19–28. doi: 10.1083/jcb.200803133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Guo YS, Yan SJ, Wan ZY, Gao B, Wang L, Liu ZH, Gao Y, Samartzis D, Lan LF, Wang HQ, Luo ZJ. CK8 phosphorylation induced by compressive loads underlies the downregulation of CK8 in human disc degeneration by activating protein kinase C. Lab Invest. 2013;93:1323–1330. doi: 10.1038/labinvest.2013.122. [DOI] [PubMed] [Google Scholar]
- Suozzi KC, Wu X, Fuchs E. Spectraplakins: master orchestrators of cytoskeletal dynamics. J Cell Biol. 2012;197:465–475. doi: 10.1083/jcb.201112034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao GZ, Toivola DM, Zhou Q, Strnad P, Xu B, Michie SA, Omary MB. Protein phosphatase-2A associates with and dephosphorylates keratin 8 after hyposmotic stress in a site- and cell-specific manner. J Cell Sci. 2006;119:1425–1432. doi: 10.1242/jcs.02861. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Boor P, Alam C, Strnad P. Keratins in health and disease. Curr. Opin. Cell Biol. 2015;32C:73–81. doi: 10.1016/j.ceb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Goldman RD, Garrod DR, Eriksson JE. Protein phosphatases maintain the organization and structural interactions of hepatic keratin intermediate filaments. J Cell Sci. 1997;110:23–33. doi: 10.1242/jcs.110.1.23. [DOI] [PubMed] [Google Scholar]
- Toivola DM, Zhou Q, English LS, Omary MB. Type II keratins are phosphorylated on a unique motif during stress and mitosis in tissues and cultured cells. Mol. Biol. Cell. 2002;13:1857–1870. doi: 10.1091/mbc.01-12-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco G, Gomez del Pulgar T, Carling D, Guzman M. Evidence that the AMP-activated protein kinase stimulates rat liver carnitine palmitoyltransferase I by phosphorylating cytoskeletal components. FEBS Lett. 1998;439:317–320. doi: 10.1016/S0014-5793(98)01400-8. [DOI] [PubMed] [Google Scholar]
- Wang H, Quah SY, Dong JM, Manser E, Tang JP, Zeng Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007a;67:2922–2926. doi: 10.1158/0008-5472.CAN-06-3598. [DOI] [PubMed] [Google Scholar]
- Wang L, Srinivasan S, Theiss AL, Merlin D, Sitaraman SV. Interleukin-6 induces keratin expression in intestinal epithelial cells: potential role of keratin-8 in interleukin-6-induced barrier function alterations. J Biol Chem. 2007b;282:8219–8227. doi: 10.1074/jbc.M604068200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Griffin H, Southern S, Jackson D, Martin A, McIntosh P, Davy C, Masterson PJ, Walker PA, Laskey P, Omary MB, Doorbar J. Functional analysis of the human papillomavirus type 16 E1=E4 protein provides a mechanism for in vivo and in vitro keratin filament reorganization. J Virol. 2004;78:821–833. doi: 10.1128/JVI.78.2.821-833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windoffer R, Beil M, Magin TM, Leube RE. Cytoskeleton in motion: the dynamics of keratin intermediate filaments in epithelia. J Cell Biol. 2011;194:669–678. doi: 10.1083/jcb.201008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woll S, Windoffer R, Leube RE. Dissection of keratin dynamics: different contributions of the actin and microtubule systems. Eur J Cell Biol. 2005;84:311–328. doi: 10.1016/j.ejcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Woll S, Windoffer R, Leube RE. p38 MAPK-dependent shaping of the keratin cytoskeleton in cultured cells. J Cell Biol. 2007;177:795–807. doi: 10.1083/jcb.200703174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Tokui T, Nishi Y, Nishizawa K, Shibata M, Kikuchi K, Tsuiki S, Yamauchi T, Inagaki M. Phosphorylation of keratin intermediate filaments by protein kinase C, by calmodulin-dependent protein kinase and by cAMP-dependent protein kinase. Eur J Biochem. 1991;197:281–290. doi: 10.1111/j.1432-1033.1991.tb15909.x. [DOI] [PubMed] [Google Scholar]
- Zhao L, Geng H, Liang ZF, Zhang ZQ, Zhang T, Yu X, Zhong CY. Benzidine induces epithelial-mesenchymal transition in human uroepithelial cells through ERK1/2 pathway. Biochem Biophys Res Commun. 2015;459:643–649. doi: 10.1016/j.bbrc.2015.02.163. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Cadrin M, Herrmann H, Chen CH, Chalkley RJ, Burlingame AL, Omary MB. Keratin 20 serine 13 phosphorylation is a stress and intestinal goblet cell marker. J Biol Chem. 2006;281:16453–16461. doi: 10.1074/jbc.M512284200. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Snider NT, Liao J, Li DH, Hong A, Ku NO, Cartwright CA, Omary MB. Characterization of in vivo keratin 19 phosphorylation on tyrosine-391. PLoS One. 2010;5:e13538. doi: 10.1371/journal.pone.0013538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Liao J, Hu L, Feng L, Omary MB. Characterization of the major physiologic phosphorylation site of human keratin 19 and its role in filament organization. J Biol Chem. 1999;274:12861–12866. doi: 10.1074/jbc.274.18.12861. [DOI] [PubMed] [Google Scholar]