Abstract
Purpose of the review
Microbial dysbiosis in the gut is emerging as a common component in various inflammatory disorders including spondyloarthritis (SpA). The depth of this influence has begun to be realized with next generation sequencing of the gut microbiome providing unbiased assessment of previously uncharted bacterial populations.
Recent findings
Decreased numbers of Firmicutes, a major phyla of gut commensals, especially the species Faecalibacterium prausnitzii and Clostridium leptum have been found in various inflammatory disorders including SpA and IBD, and could be an important link between SpA and gut inflammation. Multiple studies in ankylosing spondylitis, psoriatic arthritis, juvenile SpA and animals models of SpA are revealing common bacterial associations among these diseases as well as IBD.
Summary
We are beginning to appreciate the complex relationship between the gut microbiome and host immune regulation and dysregulation in health and disease. Potentially important differences have been revealed in SpA, but cause and effect relationships remain far from established. Many critical questions remain to be answered before we can apply new knowledge to improve therapeutics in SpA.
Keywords: HLA-B27, microbiome, dysbiosis, ankylosing spondylitis, inflammatory bowel disease
Introduction
Spondyloarthritis (SpA) is a family of immune-mediated inflammatory disorders that includes ankylosing spondylitis (AS), psoriatic arthritis (PsA), juvenile spondyloarthritis (JSpA) and acute anterior uveitis. Undifferentiated SpA is now classified as axial or predominantly peripheral. There is considerable clinical overlap between SpA and inflammatory bowel disease (IBD), with IBD and AS exhibiting shared genetic predisposition and pathogenic mechanisms. IBD has been long associated with alterations in the gut microbiome, which may be primary or secondary factors in disease pathogenesis [1].
Rats overexpressing HLA-B27 spontaneously develop an inflammatory disease exhibiting arthritis and colitis, thus mimicking human SpA [2]. In common with the vast majority of IBD animal models, disease development in this model is microbiota dependent [3]. Rosenbaum and Davey proposed that HLA-B27 alters the intestinal microbiome, which might be the basis for disease predisposition associated with this allele [4]. This concept is supported by theories of a disrupted gut environment in spondyloarthropathy, with altered intestinal permeability perhaps leading to a dysregulated immune response and/or altered dendritic cell function. This may then drive microbial dysbiosis and/or microbiota mediated intestinal inflammation leading to epithelial permeability. Here we review recent developments from studies of the gut microbiome in patients with AS, JSpA and PsA as well as insights obtained from the animal models of SpA.
The gut microbiota
The gut microbiota is the vast microbial community that inhabits our intestine. Microbial cells outnumber host cells by a factor of ten, and collectively harbor 100 fold more genes than the human genome [5]. Staggeringly, an estimated 10% of all the metabolites in humans are thought to have microbial origins [6]. This mutually beneficial relationship offers host nutrients to intestinal commensal bacteria, in return for metabolic and physiological capabilities. Cohabitation with microbes seems to be an ever-evolving process with host microbe crosstalk normally involving regulation of immune activation and inflammation [7]. Although the microbiome varies between individuals, familial and functional similarities are found in the bacterial species represented [8]. Gut microbiome analysis in healthy human populations revealed around 1150 species of bacteria, the majority of which (50–75%) is represented by Firmicutes, followed by Bacteroidetes (10–50%), and Actinobacteria (1–10%), with less that 1% being Proteobacteria [9]. Environmental factors and host genome have both been implicated as contributing to this similarity. The advent of high throughput genome sequencing techniques such as next generation 16S rRNA sequencing has lead to crucial insights into the intestinal metagenome, since more than 70% of the bacterial population including many anaerobes cannot be readily cultured [9]. The ability to routinely obtain an unbiased assessment of gut microbiota has resulted in a more comprehensive view of the gut dysbiosis in SpA patients as well as HLA-B27 driven disease in an animal model. Table 1 refers to studies implicating gut bacteria in human diseases and animal models of spondyloarthropathies.
Table 1.
Microbiome linked with Arthritis and its associated gut inflammation
| Bacteria/Bacterial product | Disease | Reference |
|---|---|---|
| Bacteriodetes spp | Arthritis | [10] |
| Klebsiella pneumoniae | AS and CD | [11] |
| Flagellin | CD | [12] |
| Bacteriodes thetaiotamicron | Colitis | [13] |
| Bacteriodes vulgatus | Colitis | [14] |
| Mycobacteria | Psoriasis | [15] |
| Prevotella copri | RA | [16] |
| Prevotella spp | RA | [17] |
| Chlamydia tracomatis | ReA | [18,19] |
| Salmonella Omp | ReA | [20] |
| Shigella | ReA | [21,22] |
| Yersinia | ReA | [21,22] |
Animal models of SpA
Gut commensals are important for educating our immune system, since animals raised in germ free environments fail to develop lymphoid organs and have muted adaptive immunity [23]. Thus, it is not surprising to think that more subtle differences in microbial communities might influence (or be influenced by) autoimmune or autoinflammatory diseases. In HLA-B27 transgenic rats that develop SpA, inflammatory disease features including arthritis and colitis are absent when animals are derived into a germ free environment [3]. Interestingly, re-introduction of normal flora enables the inflammatory disease to re-establish itself [24]. While these early studies clearly established a role for the gut microbiome in SpA, more recent work has focused on defining the differences. HLA-B27 transgenic rats have a different cecal microbiome as compared to the wild type (non-transgenic) rats [25]. How this affects immune modulation and disease severity is not clear. This study found increases in Prevotellaceae and Rikenellaceae concomitant with development of inflammation in the intestine [25].
Recent murine experiments have demonstrated that overall microbial composition as well as individual species plays an important role in development of inflammatory arthritis. Intestinal segmented filamentous bacteria (SFB) [26] colonization of germ free K/BxN mice was sufficient to drive arthritis development [27]. SFB colonization in the gut induced secondary and tertiary lymphoid tissues to generate IgA and Th17 T cell responses [28]. Notably, SFB antigen presentation by intestinal dendritic cells (CD11c+) is crucial for the development of Th17 cells, evoking a highly SFB-specific Th17 response [26]. These observations provide mechanistic support for earlier research suggesting that mucosal T cells are modulated by gut bacterial components [29], as well as outline the complex interplay between dendritic cells and innate lymphoid cells in regulating intestinal Th17 cell homeostasis. A common feature of SFB and other intestinal microbes which strongly potentiate Th17 responses such as Citrobacter rodentium is their intimate association/attachment to intestinal epithelial cells. This is consistent with the notion that mucosa-associated bacteria may be particularly relevant to IBD and/or SpA pathogenesis. Another bacteria associated with SpA (reactive arthritis), Chlamydia trachomatis, has been associated with induction of IL-23 expression in infected target cells [18]. Polymorphisms in the IL-23 receptor (IL23R) have been associated with AS and IBD [30], and the IL-23 interaction with IL23R promotes the expansion of Th17 cells, and is a direct stimulator of Th17 cytokine production [31]. In the SKG mouse, which is a model of SpA, T cell receptor [32] signaling strength is impaired due to a mutation in ZAP-70. This results in the development and expansion of CD4+ Th17 T cells. When these mice are treated with microbe-associated molecular patterns (MAMPs) such as curdlan, which is a strong inducer of IL-23, there is tremendous Th17 activation and a strong inflammatory response that produces a SpA-like phenotype. Although germfree conditions ameliorate arthritis and ileitis, cohousing SKG mice with WT mice suppressed the ileitis but did not attenuate arthritis, suggesting that host microbiome interactions play a role in IL-23-dependent loss of mucosal function in SKG mice, triggering ileitis in response to curdlan [33].
Animal models of IBD and SpA have also provided novel insight at anti-inflammatory pathways elicited by the intestinal microbiota. The mucosal lining of the lumen has emerged as an important component of host-microbe interaction. Epithelial fucosylation helps promote commensal colonization, at the same time resisting pathogens in the mucosal lining [34]. Another emerging area is the action of short chain fatty acids (SCFAs), fermentation products of gut microbes whose production is enriched in mucus degrading bacteria [35]. One such SCFA, butyrate, regulates intestinal permeability [36]. Low doses of butyrate enhance barrier function, although high doses increase intestinal permeability, probably secondary to cell death [37]. Honda and colleagues demonstrated that gnotobiotic mice colonized with Clostridium leptum and Clostridium coccoides have enhanced accumulation of Tregs in colonic lamina propria. They showed that Clostridium groups activate colonic intestinal epithelial cells to produce TGF-β and other Treg-inducing molecules [38,39]. Administration of diets that are rich in SCFA like butyrate to mice, or the administration of butyrate itself to naïve CD4+ cells, can promote their differentiation to colonic Tregs [40,41]. The myriad reported anti-inflammatory effects of SCFA also extend to imparting anti-inflammatory effects on intestinal antigen presenting cells (APC) [42] Potential therapeutic effects warrant further scrutiny in SpA animal models or patient populations.[43].
Ankylosing Spondylitis
A recent study [44], revealed distinct microbial colonization in the terminal ileum of a small number of patients with AS, using healthy individuals as controls. There was an increase in the abundance of Lachnospiraceae, Ruminococcaceae, and Prevotellaceae in AS patients. Interestingly, these bacterial species are also observed in the DSS-induced colitis model of mice [45] Although the authors saw a decreased abundance of Streptococcus and Actinomyces in comparison to the control population, they did not see any differences in the bacteria normally associated with reactive arthritis or even Klebsiella species, which have been hypothesized to trigger AS [46].
A study comparing certain gut microbes in AS patients with age matched controls revealed an increase in sulphate reducing Bacteriodes in patients [47]. In a follow up study with AS patients and healthy controls, these authors reported that reduced levels of IL-10 production upon stimulation of their PBMCs with autologous Bacteroides [48]. Previous studies in HLA-B27 transgenic rats [24] also reported that recolonization of the gut of germfree animals with Bacteroides lead to gut inflammation, whereas Lactobacillus and fusiform bacteria did not result in inflammatory lesions. Klebsiella pneumonia, long hypothesized to be involved in the pathogenesis of AS based in part on higher serum levels of IgA antibodies [49] could not be confirmed by others [50].
Treatment of HLA-B27 transgenic rats with SpA using prebiotics (compounds that induce growth or activity of commensals) has shown some benefit for colitis [13], raising hope for future therapies aimed at altering the gut microbiome. There are a number of mechanisms by which HLA-B27 might alter the microbiome. For example, human monocytic cells expressing HLA-B27 exhibit impaired handling of Salmonella [51], and exhibit reduced proliferative capacity against LPS, suggesting that intracellular effects of HLA-B27 might be involved in shaping the intestinal microbiome. One study reported that of 104 patients with spondyloarthropathies that were tested, polymorphisms in NOD2 were frequent in SpA patients with chronic gut inflammation (comparable to Crohn’s patients), whereas, in SpA patients with acute gut inflammation or without gut inflammation, NOD2 polymorphisms were similar to the control population [52]. Studies with HLA-B27 transgenic animals that are resistant to SpA associated gut inflammation might be helpful in resolving these scenarios.
Juvenile SpA
Patients with a form of juvenile SpA classified as enthesitis-related arthritis (ERA), exhibit decreased abundance of Clostridium leptum [53] similar to AS patients [47]. Another member of the Clostridales family known as Fecalibacterium prausnitzii was also decreased in patients with juvenile SpA compared to healthy controls. Despite these differences, serum IgA and IgG antibody against F. prausnitzii and B. fragilis were similar between controls and patients. There is also some evidence of a cellular immune response to the outer membrane protein of Salmonella typhimurium in juvenile SpA patients compared to healthy controls [54]. Intriguingly, study of the microbiota of juvenile SpA patients revealed that patients could be stratified into two distinct clusters, one dominated by Bacteroides genus members, the other by Akkermansia muciniphila. The fact these may represent distinct disease subtypes remains an enticing possibility and serves to highlight that this approach may also be extended to the spectrum of SpA-like diseases.
Psoriatic arthritis (PsA)
Many patients with psoriasis and psoriatic arthritis (PsA) have associated subclinical gut inflammation [55]. Decreased bacterial diversity due to lower abundances of several taxa were demonstrated [56]. The authors found Coprococcus to be inversely associated with psoriasis with or without arthritis (PsA), whereas a decline in relative abundance of Ruminococcus and Akkermansia were unique to PsA. This is of particular interest since Ruminococcus are also reduced in abundance in patients with IBD [57]. Moreover, the decreased abundance of Akkermansia in PsA contrasts to that of juvenile SpA, indicating distinct microbes may also drive the etiology of these diseases.
Another gut commensal found in healthy populations, Alistipes, was lower in abundance in both PsA [56] and Crohn’s disease [57]. Many of these microorganisms play a role in degrading mucus and producing SCFAs that influence gut homeostasis. A hallmark of dysbiosis in these individuals may be a loss of commensals, disrupting immune homeostasis.
Intestinal Permeability and SpA
Disruption of the intestinal epithelium has profound implications for the loss of mucosal tolerance. Further to the epithelium’s role in providing physical and chemical barriers between microbe and host, the provision of mucus and other metabolites (e.g. fucose) to support the colonization of commensals is well described. While a number of studies support increased intestinal permeability in SpA patients [58], mirroring IBD populations [59], this is not a universal observation [60]. Nonetheless, it is conceivable that transient or sub-clinical mucosal lesions may significantly disrupt local barrier integrity without overt systemic changes in intestinal permeability. The ‘chicken-egg’ dichotomy of inflammation and barrier function remains unresolved. Either local inflammation drives damage to the epithelium itself or dysbiotic changes that do not favor epithelial fitness (e.g. the loss of SCFA-producing bacteria), or a disrupted epithelium promotes a breakdown of mucosal homeostasis with resulting inflammation and dysbiosis (Figure 1). Therefore, events in SpA leading to increased intestinal permeability may be spatio-temporally linked. Currently, HLA-B27 transgenic rats that develop subclinical and overt IBD provide a robust model to dissect some of these details. These transgenic animals exhibit background strain-dependent disease activity and severity, with Fischer (F344) animals exhibiting the most severe disease and Lewis animals less severely affected (manuscript in preparation). In contrast HLA-B27 transgenic rats with the Dark Agouti (DA) background remain disease free, providing an opportunity to determine genetic and environmental factors that control gut inflammation.
Figure 1.
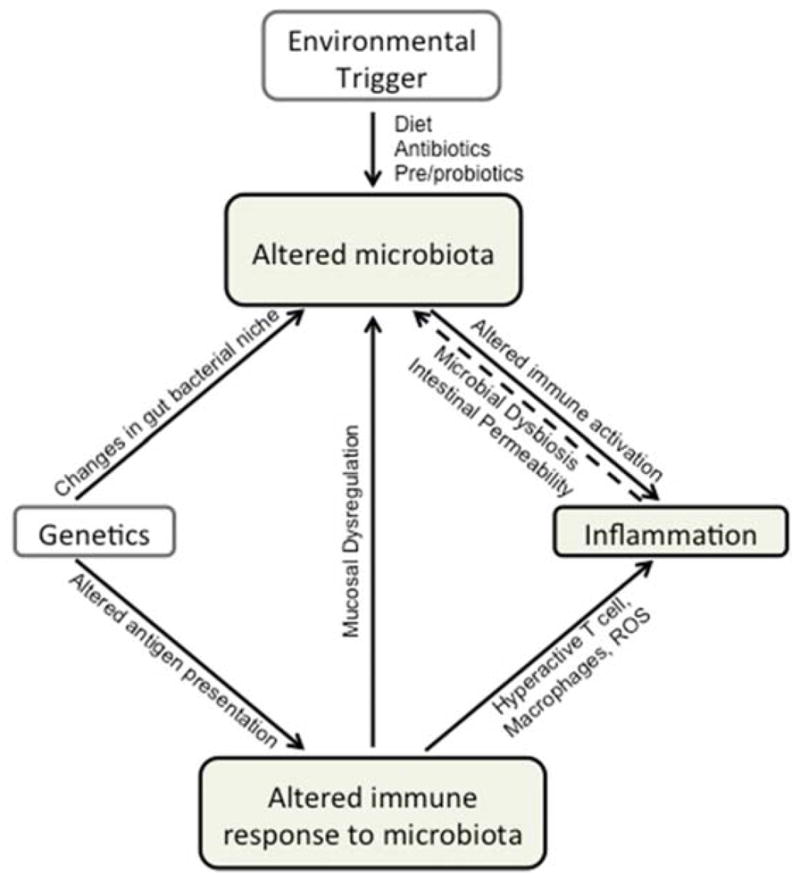
Host genetics, environmental triggers or inflammation may all trigger changes to the intestinal microbiota (dysbiosis). Importantly, changes to the intestinal microbiota itself may cause or contribute to inflammation. Host genetics may either create niches that promote dysbiosis, or directly alter immune responses to the ‘normal’ microbiota. These altered immune responses may manifest in hyper-active innate and adaptive immune responses that promote inflammation. Due to intimate epithelial-microbiota interactions, dysbiosis may also disrupt barrier function and intestinal homeostasis leading to inflammation, a process itself that may impair barrier integrity. Environmental triggers of dysbiosis are incompletely understood, but include diet and antibiotic use.
Studies in HLA-B27 rats indicate intestinal inflammation and impaired barrier function occur concurrently [61]. Thus development of barrier dysfunction, dysbiosis and inflammation may be tightly linked both temporally and spatially. HLA-B27 expression is known to cause an unfolded protein response (UPR) in APC triggered by protein accumulation and misfolding [62]. Although not detectable in ileal biopsies [63], it is possible that individuals with HLA-B27 have a stress response in a subset of inflammatory cells. This could lead to either a disruption of the epithelial barrier, a local inflammatory response or both leading culminating to the loss of barrier function and the loss of oral tolerance. It is conceivable that increased translocation of microbial products may prime the development of spondylitis-inducing immune cells which subsequently migrate to the periphery. Moreover, given that microbial products may induce peripheral inflammation themselves, e.g. the curdlan/SKG model described above or endotoxin-induced uveitis (EIU), translocated microbial products may contribute to the inflammatory cascade at extra-intestinal sites [32,64].
Conclusions
Understanding the complexity and dynamic nature of the gut microbiome and its role in inflammatory disorders including SpA is a work in progress. During homeostasis, host microbe interactions in the gut guide the normal development of host immune response, whereas microbial dysbiosis is implicated in disease pathogenesis. Currently, the broad spectrum of disease observed in both SpA patient populations and in animal models (e.g. the strain specific development of disease in HLA-B27 transgenic rats) provides an opportunity to dissect genetic, environment and microbiota-specific differences that underline SpA pathogenesis. Enticingly, antibiotic treatment, probiotic and prebiotic delivery and even fecal transplant are all examples of how the microbiota may be readily manipulated. Moreover, the identification of SpA-associated microbiota phenotypes may aid in the diagnosis or prognosis of HLA-B27 dependent disease. In summary, microbiome research has the potential to revolutionize research, diagnosis and treatment of spondyloarthritis.
Key Points.
Microbial dysbiosis of gut commensals has been implicated in SpA and may provide an important link between SpA and gut inflammation.
Epithelial permeability, either as cause or effect of gut inflammation has been implicated in loss of mucosal tolerance.
Genetic factors and environmental triggers can concomitantly influence the gut microbiome promoting disease.
HLA-B27 transgenic rats with SpA develop subclinical or overt IBD and represent a robust model to dissect these interactions.
Cause and effect relationships between microbial dysbiosis and SpA may lead to the development of novel therapeutic approaches.
Acknowledgments
Financial support and sponsorship
The Intramural Research Program Grant Z01 AR041184 to Dr. Robert A. Colbert, NIAMS (NIH), supported this work.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest relevant to this work.
Contributor Information
Tejpal Gill, Pediatric Translational Research Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Mark Asquith, Division of Arthritis and Rheumatic Diseases, Oregon Health & Science University.
James T. Rosenbaum, Division of Arthritis and Rheumatic Diseases, Oregon Health & Science University and Legacy Devers Eye Institute.
Robert A. Colbert, Pediatric Translational Research Branch, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
References
- 1.Strober W. Impact of the gut microbiome on mucosal inflammation. Trends Immunol. 2013;34:423–430. doi: 10.1016/j.it.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taurog JD, Maika SD, Satumtira N, Dorris ML, McLean IL, Yanagisawa H, Sayad A, Stagg AJ, Fox GM, Le O’Brien A, et al. Inflammatory disease in HLA-B27 transgenic rats. Immunol Rev. 1999;169:209–223. doi: 10.1111/j.1600-065x.1999.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 3.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, Hammer RE. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbaum JT, Davey MP. Time for a gut check: evidence for the hypothesis that HLA-B27 predisposes to ankylosing spondylitis by altering the microbiome. Arthritis Rheum. 2011;63:3195–3198. doi: 10.1002/art.30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 6.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes E, Li JV, Marchesi JR, Nicholson JK. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16:559–564. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- 10.Mielants H, Veys EM, Cuvelier C, De Vos M, Goemaere S, De Clercq L, Schatteman L, Gyselbrecht L, Elewaut D. The evolution of spondyloarthropathies in relation to gut histology. III. Relation between gut and joint. J Rheumatol. 1995;22:2279–2284. [PubMed] [Google Scholar]
- 11.Ebringer A, Heathcote G, Baker J, Waller M, Shanks GD, Edstein MD. Evaluation of the safety and tolerability of a short higher-dose primaquine regimen for presumptive anti-relapse therapy in healthy subjects. Trans R Soc Trop Med Hyg. 2011;105:568–573. doi: 10.1016/j.trstmh.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Targan SR, Landers CJ, Yang H, Lodes MJ, Cong Y, Papadakis KA, Vasiliauskas E, Elson CO, Hershberg RM. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 13.Hoentjen F, Welling GW, Harmsen HJ, Zhang X, Snart J, Tannock GW, Lien K, Churchill TA, Lupicki M, Dieleman LA. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11:977–985. doi: 10.1097/01.mib.0000183421.02316.d5. [DOI] [PubMed] [Google Scholar]
- 14.Rath HC, Wilson KH, Sartor RB. Differential induction of colitis and gastritis in HLA-B27 transgenic rats selectively colonized with Bacteroides vulgatus or Escherichia coli. Infect Immun. 1999;67:2969–2974. doi: 10.1128/iai.67.6.2969-2974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rambukkana A, Das PK, Witkamp L, Yong S, Meinardi MM, Bos JD. Antibodies to mycobacterial 65-kDa heat shock protein and other immunodominant antigens in patients with psoriasis. J Invest Dermatol. 1993;100:87–92. doi: 10.1111/1523-1747.ep12354979. [DOI] [PubMed] [Google Scholar]
- 16.Bernard NJ. Rheumatoid arthritis: Prevotella copri associated with new-onset untreated RA. Nat Rev Rheumatol. 2014;10:2. doi: 10.1038/nrrheum.2013.187. [DOI] [PubMed] [Google Scholar]
- 17*.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. This study highlights the gut dysbiosis in patients with psoriatic arthritis and found this microbial profile similar to IBD characterized by a reduction in Akkermansia, Ruminococcus, and Pseudobutyrivibrio. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, Gaston JS. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci U S A. 2010;107:17698–17703. doi: 10.1073/pnas.1011736107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter JD, Hudson AP. Reactive arthritis: clinical aspects and medical management. Rheum Dis Clin North Am. 2009;35:21–44. doi: 10.1016/j.rdc.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Shasany AK, Aggarwal A, Sinha S, Sisodia BS, Khanuja SP, Misra R. Low molecular weight proteins of outer membrane of Salmonella typhimurium are immunogenic in Salmonella induced reactive arthritis revealed by proteomics. Clin Exp Immunol. 2007;148:486–493. doi: 10.1111/j.1365-2249.2007.03362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leirisalo-Repo M. Reactive arthritis. Scand J Rheumatol. 2005;34:251–259. doi: 10.1080/03009740500202540. [DOI] [PubMed] [Google Scholar]
- 22.Townes CL, Ali A, Robson W, Pickard R, Hall J. Tolerance of bacteriuria after urinary diversion is linked to antimicrobial peptide activity. Urology. 2011;77:509 e501–508. doi: 10.1016/j.urology.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25**.Lin P, Bach M, Asquith M, Lee AY, Akileswaran L, Stauffer P, Davin S, Pan Y, Cambronne ED, Dorris M, et al. HLA-B27 and human beta2-microglobulin affect the gut microbiota of transgenic rats. PLoS One. 2014;9:e105684. doi: 10.1371/journal.pone.0105684. This study demonstrated for the first time, an association of altered cecal microbiota with HLA-B27 expression in transgenic rats. The changes included increse in Prevotella spp. and decrease in Rikenellaceae relative abundances in B27 TG animals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. This study brings out the complexity of barrier surface immune homeostatis and the role of segmented filamentous bacteria in inducing the Th17cells. Also, explains the role of dendritic cells and innate lymphoid cells in rgulation of Th17 cells in lamina propria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Lecuyer E, Rakotobe S, Lengline-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, et al. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–620. doi: 10.1016/j.immuni.2014.03.009. This study demonstrates segmented filamentous bacteria mediated Th17 responses inside and ouside of the gut organized lymphoid tissue. [DOI] [PubMed] [Google Scholar]
- 29.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis BS, Hoytema van Konijnenburg DP, Grivennikov SI, Mucida D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity. 2014;41:244–256. doi: 10.1016/j.immuni.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Rehaume LM, Mondot S, Aguirre de Carcer D, Velasco J, Benham H, Hasnain SZ, Bowman J, Ruutu M, Hansbro PM, McGuckin MA, et al. ZAP-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in SKG mice. Arthritis Rheumatol. 2014;66:2780–2792. doi: 10.1002/art.38773. This study associated the T cell receptor signal strength with the body’s immune responses to microbial content and curdlan response in SKG mice. This elucidates the influence of host genetics on microbes and host microbe relationship. [DOI] [PubMed] [Google Scholar]
- 34.Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, Bogatyrev SR, Ismagilov RF, Pamer EG, Turnbaugh PJ, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. 2014;514:638–641. doi: 10.1038/nature13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng L, He Z, Chen W, Holzman IR, Lin J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61:37–41. doi: 10.1203/01.pdr.0000250014.92242.f3. [DOI] [PubMed] [Google Scholar]
- 37.Atarashi K, Honda K. Microbiota in autoimmunity and tolerance. Curr Opin Immunol. 2011;23:761–768. doi: 10.1016/j.coi.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 40.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 42.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 44*.Costello ME, Ciccia F, Willner D, Warrington N, Robinson PC, Gardiner B, Marshall M, Kenna TJ, Triolo G, Brown MA. Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 2014 doi: 10.1002/art.38967. This study decribed a discrete microbial signature in the terminal Ileum of the AS patients. This is based on increase in families Lachnospiraceae, Veillonellaceae, Prevotellaceae, Porphyromonadaceae, and Bacteroidaceae. [DOI] [PubMed] [Google Scholar]
- 45.Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2011;17:917–926. doi: 10.1002/ibd.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ebringer R, Cooke D, Cawdell DR, Cowling P, Ebringer A. Ankylosing spondylitis: klebsiella and HL-A B27. Rheumatol Rehabil. 1977;16:190–196. doi: 10.1093/rheumatology/16.3.190. [DOI] [PubMed] [Google Scholar]
- 47.Stebbings S, Munro K, Simon MA, Tannock G, Highton J, Harmsen H, Welling G, Seksik P, Dore J, Grame G, et al. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology (Oxford) 2002;41:1395–1401. doi: 10.1093/rheumatology/41.12.1395. [DOI] [PubMed] [Google Scholar]
- 48.Stebbings SM, Taylor C, Tannock GW, Baird MA, Highton J. The immune response to autologous bacteroides in ankylosing spondylitis is characterized by reduced interleukin 10 production. J Rheumatol. 2009;36:797–800. doi: 10.3899/jrheum.080964. [DOI] [PubMed] [Google Scholar]
- 49.Tani Y, Tiwana H, Hukuda S, Nishioka J, Fielder M, Wilson C, Bansal S, Ebringer A. Antibodies to Klebsiella, Proteus, and HLA-B27 peptides in Japanese patients with ankylosing spondylitis and rheumatoid arthritis. J Rheumatol. 1997;24:109–114. [PubMed] [Google Scholar]
- 50.Stone MA, Payne U, Schentag C, Rahman P, Pacheco-Tena C, Inman RD. Comparative immune responses to candidate arthritogenic bacteria do not confirm a dominant role for Klebsiella pneumonia in the pathogenesis of familial ankylosing spondylitis. Rheumatology (Oxford) 2004;43:148–155. doi: 10.1093/rheumatology/keg482. [DOI] [PubMed] [Google Scholar]
- 51.Penttinen MA, Heiskanen KM, Mohapatra R, DeLay ML, Colbert RA, Sistonen L, Granfors K. Enhanced intracellular replication of Salmonella enteritidis in HLA-B27-expressing human monocytic cells: dependency on glutamic acid at position 45 in the B pocket of HLA-B27. Arthritis Rheum. 2004;50:2255–2263. doi: 10.1002/art.20336. [DOI] [PubMed] [Google Scholar]
- 52.Laukens D, Peeters H, Marichal D, Vander Cruyssen B, Mielants H, Elewaut D, Demetter P, Cuvelier C, Van Den Berghe M, Rottiers P, et al. CARD15 gene polymorphisms in patients with spondyloarthropathies identify a specific phenotype previously related to Crohn’s disease. Ann Rheum Dis. 2005;64:930–935. doi: 10.1136/ard.2004.028837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53*.Stoll ML, Kumar R, Morrow CD, Lefkowitz EJ, Cui X, Genin A, Cron RQ, Elson CO. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther. 2014;16:486. doi: 10.1186/s13075-014-0486-0. This study found relative dercease in Faecalibacterium prausnitzii inenthesitis related arthritis patients as compared to controls. It also identified loss of Akermansia muciniphila and Bacteroides in a subset of patients provinding further evidence of IBD and SpA overlap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh YP, Singh AK, Aggarwal A, Misra R. Evidence of cellular immune response to outer membrane protein of Salmonella typhimurium in patients with enthesitis-related arthritis subtype of juvenile idiopathic arthritis. J Rheumatol. 2011;38:161–166. doi: 10.3899/jrheum.100542. [DOI] [PubMed] [Google Scholar]
- 55.Scarpa R, Manguso F, D’Arienzo A, D’Armiento FP, Astarita C, Mazzacca G, Ayala F. Microscopic inflammatory changes in colon of patients with both active psoriasis and psoriatic arthritis without bowel symptoms. J Rheumatol. 2000;27:1241–1246. [PubMed] [Google Scholar]
- 56.Scher JU, Ubeda C, Artacho A, Attur M, Isaac S, Reddy SM, Marmon S, Neimann A, Brusca S, Patel T, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Jarnerot G, Tysk C, Jansson JK, Engstrand L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139:1844–1854. e1841. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Gonzalez O, Cantero-Hinojosa J, Paule-Sastre P, Gomez-Magan JC, Salvatierra-Rios D. Intestinal permeability in patients with ankylosing spondylitis and their healthy relatives. Br J Rheumatol. 1994;33:644–647. doi: 10.1093/rheumatology/33.7.644. [DOI] [PubMed] [Google Scholar]
- 59.Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 60.Kuiper S, van Pelt J, Verheesen PE, Rentsch HU, Stockbrugger R, van der Linden SM. Patients with ankylosing spondylitis and healthy relatives do not show increased small intestinal permeability with the lactulose-mannitol test. Clin Exp Rheumatol. 1993;11:413–416. [PubMed] [Google Scholar]
- 61.Kerr SW, Wolyniec WW, Filipovic Z, Nodop SG, Braza F, Winquist RJ, Noonan TC. Repeated measurement of intestinal permeability as an assessment of colitis severity in HLA-B27 transgenic rats. J Pharmacol Exp Ther. 1999;291:903–910. [PubMed] [Google Scholar]
- 62.Colbert RA, Tran TM, Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol. 2014;57:44–51. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciccia F, Accardo-Palumbo A, Rizzo A, Guggino G, Raimondo S, Giardina A, Cannizzaro A, Colbert RA, Alessandro R, Triolo G. Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation. Ann Rheum Dis. 2014;73:1566–1574. doi: 10.1136/annrheumdis-2012-202925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286:611–613. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]


