Abstract
Purpose
Adoptive transfer of Epstein Barr Virus (EBV)- and Cytomegalovirus (CMV)-specific cytotoxic T cells (CTLs) genetically modified to express a Chimeric Antigen Receptor (CAR) induces objective tumor responses in clinical trials. In vivo expansion and persistence of these cells is crucial to achieve sustained clinical responses. We aimed to develop an off-the-shelf whole-cell vaccine to boost CAR-redirected virus-specific CTLs in vivo after adoptive transfer. As proof of principle, we validated our vaccine approach by boosting CMV-specific CTLs (CMV-CTLs) engineered with a CAR that targets the GD2 antigen.
Experimental Design
We generated the whole-cell vaccine by engineering the K562 cell line to express the CMV-pp65 protein and the immune stimulatory molecules CD40L and OX40L. Single-cell-derived clones were used to stimulate CMV-CTLs in vitro and in vivo in a xenograft model. We also assessed whether the in vivo boosting of CAR-redirected CMV-CTLs with the whole-cell vaccine enhances the antitumor responses. Finally, we addressed potential safety concerns by including the inducible safety switch caspase9 (iC9) gene in the whole-cell vaccine.
Results
We found that K562 expressing CMV-pp65, CD40L and OX40L effectively stimulates CMV-specific responses in vitro by promoting antigen cross-presentation to professional antigen-presenting cells (APCs). Vaccination also enhances antitumor effects of CAR-redirected CMV-CTLs in xenograft tumor models. Activation of the iC9 gene successfully induces growth arrest of engineered K562 implanted in mice.
Conclusions
Vaccination with a whole-cell vaccine obtained from K562 engineered to express CMV-pp65, CD40L, OX40L and iC9 can safely enhance the antitumor effects of CAR-redirected CMV-CTLs.
Introduction
Chimeric antigen receptor(CAR)-redirected T lymphocytes mediate HLA-independent cytotoxic activity against a variety of human malignancies in preclinical models(1;2). In clinical trials, adoptively transferred CAR-T lymphocytes induce durable tumor regressions when CAR-T cells expand and persist in vivo(3;4). Proliferation and survival of CAR-T cells is strictly dependent on their adequate co-stimulation(3;5;6). Antigen-presenting cells (APCs), such as dendritic cells, that present MHC-restricted antigen epitopes to the T-cell receptor, and express co-stimulatory molecules in a spatially and temporally coordinated fashion, provide the most physiologic T-cell co-stimulation(7). We previously hypothesized that engrafting CARs in virus-specific CTLs (VsCTLs) such as Epstein Barr Virus (EBV)-CTLs or Cytomegalovirus (CMV)-CTLs can recapitulate a physiologic T-cell costimulation of CAR-engineered T cells. VsCTLs expressing a CAR are indeed “dual specific” and can receive a proper co-stimulation by APCs processing and presenting viral epitopes to VsCTL native virus-specific T-cell receptors, while the CAR expression redirects their cytotoxic activity towards tumor cells (8-10).
We validated this strategy in clinical trials, both in the autologous and allogeneic settings. In neuroblastoma patients, we described how autologous EBV-CTLs engineered with a first generation (encoding only the ζ chain moiety) GD2-specific CAR have better initial engraftment compared to autologous polyclonal activated T lymphocytes expressing the same CAR(11). In the context of the allogeneic stem cell transplant, we also showed that donor-derived EBV-CTLs and CMV-CTLs engrafted with a second generation CD19-specific CAR, encoding both the CD28 and ζ chain moieties, can produce antitumor and antiviral activity without causing graft versus host disease(12). However, there were some limitations in both autologous and allogeneic settings. For instance, in neuroblastoma patients, although detectable long-term, autologous GD2-specific CAR-modified EBV-CTLs persisted at a very low frequency in vivo(13). This limited engraftment may indicate that the endogenous presentation of latent EBV antigens, in the absence of virus reactivation, does not promote robust and durable engraftment of the infused CAR-redirected EBV-CTLs. In the allogeneic setting, we found enhanced engraftment of the infused CD19-specific CAR-redirected VsCTLs only in patients who were infused relatively early post-transplant, when higher EBV or CMV viral loads can fully stimulate the infused CAR-redirected VsCTLs through their native T-cell receptors (12). In contrast, engraftment remains suboptimal if the cells are infused late after transplant when the probability of experiencing virus reactivations is rather low(12).
Based on these clinical evidences, we hypothesized that an intentional in vivo vaccine-mediated stimulation of adoptively transferred CAR-modified VsCTLs would produce enhanced engraftment and superior antitumor effect of these cells. We developed a whole-cell vaccine that promotes the cross-presentation of viral epitopes to the native virus-specific T-cell receptors of CAR-redirected VsCTLs. The proposed approach is preferable to a vaccine aimed at boosting CAR-redirected VsCTLs through their CAR specificity, since only APCs processing and presenting viral antigens in the MHC context can fully and physiologically induce T-cell co-stimulation.
A whole-cell vaccine approach based on the administration of irradiated allogeneic immortalized cell lines engineered to express immune-modulatory cytokines such as IL-2 and GM-CSF to cross-present antigens to host APCs has been used in several clinical trials(14-18). Based on these clincial findings, we prepared a whole-cell vaccine by engineering the K562 cell line to stimulate, via antigen cross-presentation, the intrinsic virus-specificity of CAR-modified VsCTLs in vivo. As proof of principle, we selected to engineer the K562 cell line with the CMV-pp65 protein to stimulate CAR-redirected CMV-CTLs (CAR-CMV-CTLs) based on the high frequency of CMV seropositive individuals(19) and the robust evidence that CD8+ T cells specific for the CMV-pp65 protein play a dominant protective role in CMV infections(20).
We envisioned further engineering K562 to express CD40L and OX40L immune stimulatory molecules in order to strengthen the effect of our vaccine. CD40L promotes the maturation of APCs and directly activates CD8+ T cells (21-23), while OX40L promotes the recruitment of CD4+ T cells(24-26), which play an important role in controlling tumor growth in clinical trials of adoptive T-cell transfer(13). We then conducted experiments to show that the K562-derived whole-cell vaccine can safely and effectively stimulate CAR-CMV-CTLs in vitro and in vivo, enhancing their overall antitumor activity.
Material and methods
Cell line
K562, Raji and A459 tumor cells were purchased from American Type Culture Collection (ATCC). K562 and Raji cells were cultured in RPMI1640 (HyClone, Thermo Scientific, Pittsburgh, PA) supplemented with 10% fetal bovine serum (FBS) (HyClone) and 2 mM GlutaMax (Invitrogen, Carlsbad, CA). A549 tumor cell line was cultured in DMEM (Gibco, Invitrogen™, Carlsbad, CA) supplemented with 10% FBS and 2 mM GlutaMax. A459 was single cell cloned based on the expression of the GD2 antigen. The neuroblastoma cell line CHLA-255(27) (kindly provided by Dr Leonid Metelitsa) was derived from a patient. CHLA-255 was cultured in IMDM (Gibco, Invitrogen™, Carlsbad, CA) supplemented with 10% FBS and 2 mM GlutaMax, and we verified that this line retains the surface expression of the target antigen GD2. Cells were maintained in a humidified atmosphere containing 5% CO2 at 37°C. All cell lines were routinely tested to ensure that they were mycoplasma free and authenticated based on short tandem repeats (STR) at MD Anderson Cancer Center (Houston, TX) except for CHLA-255. For the co-culture experiments CHA-255 and Raji cells were transduced with a retroviral vector encoding GFP (>98% GFP+ cells).
Isolation of peripheral blood mononuclear cells (PBMCs) and generation of dendritic cells (DCs)
PBMCs were isolated from buffy coats (Gulf Coast Regional Blood Center, Houston, TX) or blood donations from healthy donors (under IRB approved protocol, BCM) using Ficoll-Paque (Amersham Biosciences, Piscataway, NJ). Monocytes were obtained from PBMCs by positive magnetic selection with CD14 magnetic beads (Miltenyi Biotec, Auburn, CA). DCs were generated from CD14+ cells cultured in DC media (CellGenix, Antioch, IL) supplemented with Interleukin (IL)-4 (1000 U/mL) and GM-CSF (800 U/mL) (R&D Systems, Minneapolis, MN). On day 5, DCs were matured with IL-6 (1 μg/mL), TNF-α (1 μg/mL), IL-1β (1 μg/mL) and PGE (1 μg/mL) (all from R&D Systems, Inc, Minneapolis, MN) for 48 hours.
K562-derived whole-cell vaccine
The vaccine was generated using the K562 cell line. These cells were transduced with lentiviral vectors encoding either human CD40L or OX40L or pp65/eGFP or the combination CD40L/pp65 or OX40L/pp65. After transduction, single cell clones were obtained. For selected experiments, K562 clones were also genetically modified with a retroviral vector to stably express the inducible caspase-9 suicide gene (iC9)(28).
Generation of autologous phytohemagglutinine-activated T cells (PHA blasts) and lymphoblastoid cell lines (LCLs)
To generate PHA blasts, PBMCs were stimulated with the mitogen phytohemagglutinine-P (PHA-P, 5μg/ml; Sigma-Aldrich, St. Louis, MO). PHA blasts were then expanded in RPMI1640 supplemented with 5% human serum (Valley Biomedical, Winchester, VA) and 2mM Glutamax, and in the presence of IL-2 (100U/ml) (Teceleukin, Chiron Therapeutics, Emeryville, CA). The lymphoblastoid cell lines (LCLs) were generated as previously described(29).
Activation of monocytes by K562-derived whole-cell vaccine
Monocytes were stained with the PKH26 red fluorescent cell linker compound and then co-cultured at a ratio of 5:1 with irradiated K562 labeled with PKH2 green fluorescent cell linker compound (Sigma-Aldrich). After 72 hours, we analyzed the expression of activation/maturation markers in monocytes by flow cytometry, testing the level of expression of CD11c, CD80, CD83 and HLA-DR. Moreover, we monitored the co-culture by a fluorescence microscope.
Generation of retroviral supernatant and transduction of VsCTLs
Retroviral supernatants were produced in 293T cells, as previously described (30). Lentivirus supernatants were produced in 293T cells co-transfected with the lentiviral vector and separated plasmids encoding the VSV-G envelope, gag-pol and REV(31). To generate CMV-CTLs, PBMCs from CMV seropositive donors were stimulated with DCs (20:1) loaded with the CMV-pp65 pepmix (HCMVA, JPT, Berlin, DE) at 5 μM for 2 hours at 37°C in 5% CO2. Cells were then plated in complete media containing RPMI 1640 45%, Clicks medium (Irvine Scientific, Santa Ana, CA) 45%, 10% human AB serum and 2 mM GlutaMax. After 10 days, T cells were re-stimulated with DCs loaded with the same pepmix. After the second round of stimulation, cells were expanded and fed with IL-2 (50 U/mL; Proleukin, Chiron, Emeryville, CA). Three days later, cells were transduced with a retroviral vector encoding a CAR specific for the GD2 antigen and containing the CD28 endodomain (CAR-GD2) using retronectin-coated plates (Takara Bio Inc, Otsu, Shiga, Japan)(12).
Stimulation of PBMCs and CMV-CTLs using K562-derived whole-cell vaccine
PBMCs from seropositive donors were incubated with irradiated K562 (80-100 Gy) at a ratio of 10:1 for 10 - 12 days in the absence of cytokines. Transduced CAR-CMV-CTLs were stimulated weekly with irradiated K562 and autologous CD3-depleted PBMCs at a ratio of 5:1:1 (CTLs:K562:PBMCs CD3-depleted) and fed with IL-2 (50U/ml) twice/week.
IFNγ Enzyme-Linked Immunospot Assay (ELISpot)
The IFNγ ELISpot assay was performed as previously described(8). T cells were plated in triplicate at 105 cells/well with 5 μM of CMV-pp65 pepmix. In all experiments, T cells were also incubated with an irrelevant pepmix, as negative control, or stimulated with 25 ng/mL of phorbol myristate acetate (PMA; Sigma-Aldrich, St Louis, MO) and 1 μg/mL of ionomycin (Iono; Sigma-Aldrich) as positive control. In selected experiments, CAR-CMV-CTLs were tested in ELISpot plates coated with both IFNγ antibody and anti-idiotype antibody (1A7) that induces cross-link of CAR molecules(11).
Flow cytometry
For phenotypic analysis we used CD11c, CD80, CD83, HLA-DR, CD45, CD56, CD19, CD8, CD4, and CD3 mAbs (all from Becton Dickinson, San Jose, CA) conjugated with FITC, PE, PerCP or APC fluorochromes. The expression of CAR-GD2 was detected using the 1A7 Ab. Samples were analyzed with a BD FACScalibur system equipped with the filter set for quadruple fluorescence signals and the CellQuest software (BD Biosciences). For each sample, we analyzed a minimum of 30,000 events. CTLs were also analyzed for binding of specific tetramers. Tetramers were prepared by the Baylor College of Medicine core facility. For each sample, a minimum of 100,000 cells were analyzed.
Chromium-release assay
The cytotoxic activity of T cells was evaluated using a standard 4-hour 51Cr-release assay, as previously described(9). Target cells were incubated in medium alone or in 1% Triton X-100 (Sigma-Aldrich) to determine spontaneous and maximum 51Cr-release, respectively. The mean percentage of specific lysis of triplicate wells was calculated as follows: [(test counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100. The target cells tested included CHLA-255, Raji and PHA blasts loaded with irrelevant or CMV-pp65 pepmixes.
Western blot
Proteins were extracted from 5 × 106 cells, using RIPA lysing buffer (Cell Signaling Technology®, Danvers, MA) supplemented with a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Fifty μg of protein were resolved by SDS-PAGE, transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA) and blocked with 5% (W/V) non-fat dry milk in Tris Buffer Saline (TBS) with 0.1% (V/V) Tween-20. Blots were stained with mouse anti-CMV-pp65 (1:200, clone 1-L-11) (Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-human β-actin (1:10000, clone C4) (Santa Cruz Biotechnology). Blots were washed with TBS containing 0.1% (V/V) Tween-20, stained with horseradish peroxidase conjugated secondary Ab (1:5000, goat anti-mouse sc-2005) (Santa Cruz Biotechnology) and incubated with SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific).
Xenogenic SCID mouse models
Mouse experiments were performed in accordance with Baylor College of Medicine's Animal Husbandry guidelines following IACUC approved protocols. In the first set of experiments, we tested the ability of the K562-derived whole-cell vaccine to stimulate CMV-CTLs from PBMCs collected from healthy CMV-seropositive donors. Fig. 2A summarizes the design of the experiment. Eight to 10 week old NOG/SCID/γc−/− mice (Jackson Lab, Bar Harbor, Maine) received 3 inoculations intraperitoneally (i.p.) and intravenously (i.v.) of 5 × 106 PBMCs(32) and 106 irradiated K562 and were euthanized by day 14 for analysis of immune responses. For the antitumor effects, two models were tested. In the first model, NOG/SCID/γc−/− mice were implanted i.p. with CHLA-255 cells (2.5 × 106), labeled with firefly luciferase and resuspended in Matrigel (Becton Dickinson Biosciences, Franklin Lakes, NJ). Tumor growth was measured by in vivo bioluminescence using the Lumina IVIS in vivo imaging system (Perkin Elmer, Waltham, MA)(33). Five days after tumor inoculation, control and CAR-CMV-CTLs were injected i.p. (10 × 106 cells/mouse). Mice were subsequently vaccinated according to the schedule illustrated in Fig. 2A. IL-2 (1000 U/mouse) was also administered i.p. twice a week for 2 weeks. In the systemic tumor model, NOG/SCID/γc−/− mice were infused via tail injection with GD2+ A459 tumor cells labeled with firefly luciferase (6 × 105 cells). On day 3, mice were injected i.v. with control or CAR-CMV-CTLs (8 × 106 cells/mouse) and vaccinated with K562 as described in Fig. 2A. Tumor growth was monitored by using the Lumina IVIS imaging system. Mice were euthanized when signs of discomfort were detected by the investigator or as recommended by the veterinarian who monitored the mice three times a week or when luciferase signal reached 7.5 × 107 p/sec/cm2/sr. For the validation of the iC9 suicide gene, mice were engrafted with K/CD40L/pp65 and K/OX40L/pp65 clones expressing iC9 and an enhanced firefly luciferase gene(34). After engraftment mice were infused i.p. with the dimerizing drug AP20187 (50 μg/mouse) (Clontech Lab, Mountain View, CA) for two consecutive days. K562 growth was followed by in vivo bioluminescence.
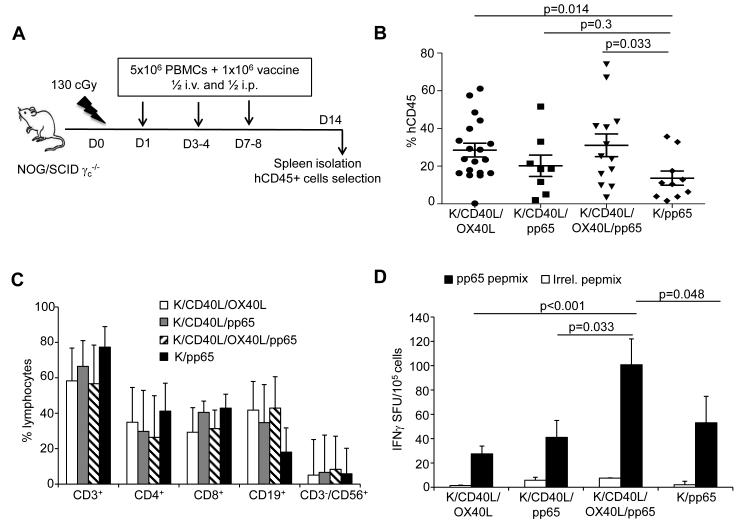
Figure 2. Co-expression of CD40L and OX40L by K562-derived whole-cell vaccine maximizes the stimulation of CMV-CTLs in vivo.
Panel A. Schematic representation of the xenograft mouse model in NOG/SCID/γc−/− mice. Panel B. Engraftment of human CD45+ cells in the spleen, 14 days after vaccination. Panel C. Phenotypic analysis of human CD45+ cells engrafted in the spleen by day 14. Data represent mean ± SD of 8 mice per group. Panel D. Enumeration of the CMV-CTLs in isolated human CD45+ cells as assessed by IFNγ ELISpot in response to CMV-pp65 and irrelevant pepmixes. Data represent mean ± SD of 8 mice per group.
Statistics
Unless otherwise noted, data are summarized as mean ± standard deviation. Student t-test was used to determine statistically significant differences between samples, with P value <0.05 indicating a significant difference. When multiple comparison analyses were required, statistical significance was evaluated by one-way ANOVA. Survival analysis was performed using the Kaplan-Meier method in GraphPad Software (La Jolla, CA). The log-rank test was used to assess statistically significant differences between groups of mice. All P-values <0.05 were considered statistically significant.
Results
K562-derived whole-cell vaccine encoding CMV-pp65 and CD40L stimulates CMV-CTLs in vitro by mediating antigen cross-presentation
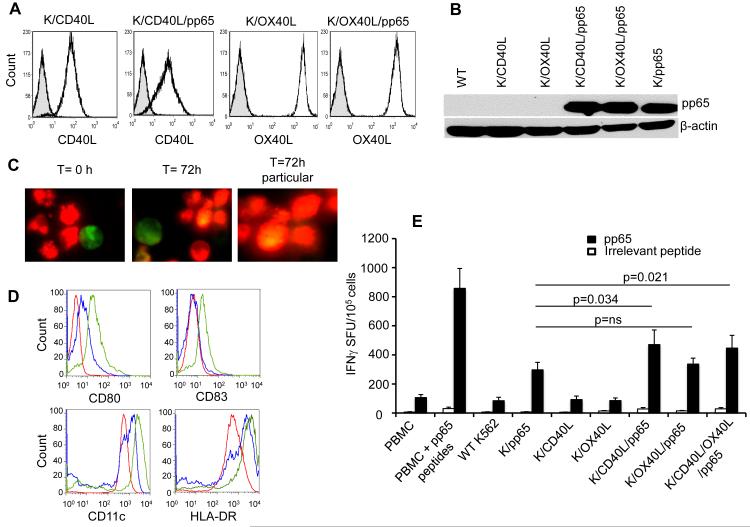
To develop a whole-cell vaccine capable of boosting CMV-CTLs, we engineered the K562 cell line to express CMV-pp65, CD40L and OX40L molecules as follows: CD40L/pp65 (K/CD40L/pp65), OX40L/pp65 (K/OX40L/pp65), CD40L (K/CD40L), OX40L (K/OX40L) or pp65 (K/pp65). K/pp65 also expressed GFP, as a marker of selection. Single cell clones of engineered K562 were used for all the experiments. The expression of CD40L and OX40L was confirmed by FACS analysis (Fig. 1A), while the expression of pp65 was assessed by western blot (Fig. 1B). To ensure in vitro that engineered and irradiated K562 cells promote antigen cross-presentation, we proved that apoptotic bodies derived from irradiated K562 were uptaken by monocytes. As shown in Fig. 1C, freshly isolated monocytes (stained with red fluorescent) were co-cultured for 3 days with either irradiated K/pp65 or K/CD40L/pp65 (stained with green fluorescent). Monocytes engulfed K562-derived apoptotic bodies (stained with yellow fluorescent) and expressed CD80 and CD83, and showed more pronounced up-regulation of CD11c and HLA-DR only in the presence of CD40L (Fig. 1D). OX40L is not known to promote maturation of APCs, therefore it was unsurprising that the effects of K/OX40L/pp65 on the induction of CD80 and CD83 molecules on cultured monocytes were similar to those observed using K/pp65 (Supplementary Fig. 1).
Figure 1. K562-based whole-cell vaccine encoding CMV-pp65 and CD40L matures monocytes and stimulates CMV-CTLs in vitro. Panel A.
Expression of CD40L and OX40L in engineered K562. Striped histograms indicate wild type K562 cells. Panel B. Western blot showing the expression of CMV-pp65 in engineered K562. Panel C. Uptake of apoptotic bodies from irradiated K/pp65 and K/CD40L/pp65 by monocytes. Monocytes labeled with PKH26 red fluorescent cell linker compound were co-cultured (5:1 ratio) with irradiated K/CD40L/pp65 labeled with PKH2 green fluorescent cell linker compound. Analysis of fluorescence signals was performed after 72 hours of co-culture using a fluorescence microscope (Olympus IX70). Panel D. Expression of CD80, CD83, CD11c and HLA-DR by monocytes 72 hours after co-culture with irradiated K/pp65 (in blue) and K/CD40L/pp65 (in green). The red line represents the expression of CD80, CD83, CD11c and HLA-DR before the stimulation. Panel E. Frequency of CMV-CTLs assessed by IFNγ ELISpot using the CMV-pp65 pepmix. Data represented mean ± SD of 11 CMV-seropositive donors. Stimulation with an irrelevant pepmix was used as a negative control.
The capacity of the whole-cell vaccine to stimulate ex vivo CMV-CTLs was assessed by co-culturing PBMCs collected from CMV seropositive donors with engineered and irradiated K562 for 10 - 12 days. As positive controls, the same PBMCs were cultured in the presence of CMV-pp65 pepmix. After 10 - 12 days of culture, we found more CD3+CD8+ T cells in K/CD40L/pp65 and K/OX40L/pp65 (22% ± 5%) compared to K/pp65 (14% ± 4%) (p=0.002), and also more CD3+CD4+ T cells (42% ± 9% vs. 33% ± 11%) (p=0.002). The NK cells were 47% ± 15% in K/pp65 and 31% ± 14% in K/CD40L/pp65 and K/OX40L/pp65 (p=0.014) (Table 1). When assayed against CMV-pp65 pepmix, we found that K/pp65 effectively stimulated CMV-CTLs (292 ± 56 IFNγ+ SFU/105 cells) and that the presence of CD40L (K/CD40L/pp65) further enhanced this effect (502 ± 104 IFNγ+ SFU/105 cells) (p=0.034) (Fig. 1E), although not as effectively as the positive control condition in which PBMCs were directly stimulated with CMV-pp65 pepmix (789 ± 130 IFNγ+ SFU/105 cells). The presence of OX40L (K/OX40L/pp65) did not enhance the stimulatory effect observed with K/pp65 (357 ± 40 IFNγ+ SFU/105 cells) (p=ns). The combination of K/CD40L/pp65 and K/OX40L/pp65 did not further increase the frequency of CMV-CTLs (477 ± 91 IFNγ+ SFU/105 cells) (Fig. 1E). Pulsing T cells with an irrelevant pepmix produced negligible IFNγ reactivity (< 30 IFNγ+ SFU/105 cells) (Fig. 1E). Overall, these data indicate that K/CD40L/pp65 can efficiently stimulate CMV-CTLs in vitro from PBMCs collected from seropositive donors.
Table 1.
Phenotype of T cells collected by day 10 – 12 after co-culture with K562-based whole cell vaccine
| CD3+/CD4+ | CD3+/CD8+ | CD3−/56+ | |
|---|---|---|---|
| PBMC/pp65 pepmix | 48% ± 27% | 45% ± 29% | 5% ± 2% |
| K562 wild type | 46% ± 11% | 17% ± 2% | 29% ± 10% |
| K/pp65 | 33% ± 11% | 14% ± 4% | 47% ± 15% |
| K/CD40L | 42% ± 7% | 23% ± 7% | 30% ± 5% |
| K/OX40L | 40% ± 5% | 20% ± 6% | 33% ± 6% |
| K/CD40L/pp65 | 43% ± 11% | 22% ± 3% | 31% ± 13% |
| K/CD40L/pp65 + K/OX40L/pp65 |
42% ± 9% | 22% ± 5% | 31% ± 14% |
CD40L and OX40L expressed by K562-derived whole-cell vaccine cooperate in stimulating CMV-CTLs in vivo
We assessed the capacity of the whole-cell vaccine to stimulate in vivo CMV-CTLs using NOG/SCID/γc−/− mice. Animals were co-infused with freshly isolated PBMCs obtained from CMV-seropositive donors and vaccinated twice with irradiated whole-cell vaccines and PBMCs as a source of APCs. CMV-specific immune responses were measured 7 days after the last vaccination (Fig. 2A). At the time of analysis, human CD45+ cells engrafted in the spleen of mice from all groups, though engraftment was lower in mice vaccinated with K/pp65 as compared to mice vaccinated with K/CD40L/OX40L (p=0.014) or K/CD40L/pp65 and K/OX40L/pp65 (p=0.033) (Fig. 2B). While the immunophenotype of engrafted human CD45+ cells isolated from the spleen showed a similar distribution in CD3+CD4+, CD3+CD8+ and NK cells (Fig. 2C), the antigen specificity of engrafted T cells was significantly different. As shown in Fig. 2D, in all experimental conditions T cells recovered from the spleen of mice vaccinated had detectable CMV-specific IFNγ production. However, the vaccination with combined K/CD40L/pp65 and K/OX40L/pp65 stimulated the highest CMV-specific response (101 ± 21 IFNγ+ SFU/105 cells) compared to controls K/CD40L/OX40L (28 ± 6 IFNγ+ SFU/105 cells) (p<0.001), K/pp65 (53 ± 22 IFNγ+ SFU/105 cells) (p=0.048) and K/CD40L/pp65 (41 ± 14 IFNγ+ SFU/105 cells) (p=0.033). In contrast to the in vitro experiments, in vivo data supported a critical role for the combination of CD40L and OX40L mediated activation in stimulating CMV-CTLs.
Virus-specificity of “dual-specific” CAR-CMV-CTLs is boosted in vitro by the K562-derived whole-cell vaccine
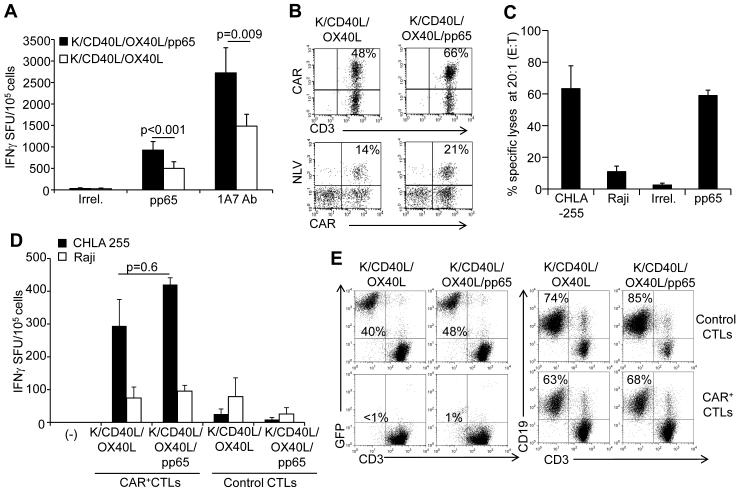
To assess whether the whole-cell vaccines can be used to boost “dual-specific” CAR-CMV-CTLs, we generated CMV-CTLs as previously described(10;12) and engrafted them with the CAR-GD2. The transduction efficiency of CMV-CTLs exposed to the retroviral supernatant encoding the CAR-GD2 ranged between 35% and 65%, as detected by flow cytometry. CAR-CMV-CTLs were then stimulated twice, one week apart, with engineered and irradiated K562 and autologous PBMCs (as a source of APCs), and assessed for phenotype and IFNγ production by ELISpot. CAR-CMV-CTLs stimulated with combined K/CD40L/pp65 and K/OX40L/pp65 showed a significant enrichment in specific precursors responding to the CMV-pp65 pepmix as assessed by IFNγ ELISpot assay (1397 ± 212 IFNγ+ SFU/105 cells) compared to CTLs stimulated with control K/CD40L/OX40L (749 ± 146 IFNγ+ SFU/105 cells) (p<0.001) (Fig. 3A). Similarly, CAR-restricted responses, measured after stimulation with the anti-idiotype 1A7 Ab that cross-links CAR-GD2 molecules, significantly increased in CAR-CMV-CTLs stimulated with K/CD40L/pp65 and K/OX40L/pp65 (2819 ± 452 IFNγ+ SFU/105 cells) compared to CTLs stimulated with control K/CD40L/OX40L (1610 ± 267 IFNγ+ SFU/105 cells)(p=0.009) (Fig. 3A). In HLA-A2+ donors, phenotypic analysis confirmed a significant enrichment in NLV-tetramer+ and CAR+ CTLs after stimulations with K/CD40L/pp65 and K/OX40L/pp65 (Fig 3B).
Figure 3. Virus-specificity of “dual specific” CAR-CMV-CTLs is stimulated by K562-derived whole-cell vaccine in vitro.
In these experiments we compared the effector function of CAR-CMV-CTLs stimulated in vitro with control K/CD40L plus K/OX40L and K/CD40L/pp65 plus K/OX40L/pp65. Panel A. Enumeration of IFNγ-producing cells by ELISpot in response to the CMV-pp65 pepmix or the 1A7 Ab that cross-links the CAR-GD2. Data summarize means ± SD of 9 donors. Panel B. Detection of CAR and NLV-tetramer in CAR-CMV-CTLs by flow cytometry in a representative HLA-A2+ donor. While the CAR staining detects all CAR-CMV-CTLs, the tetramer only identifies CAR-CMV-CTLs specific for one single epitope (NLV) in the context of one haplotype (HLA-A2.01). Panel C. Cytotoxic activity (51Cr-release assay at a 20:1 effector:target ratio) against CHLA-255 neuroblastoma cells (GD2+ cells) and Raji lymphoma cells (GD2- cells). PHA blasts pulsed with CMV-pp65 or irrelevant pepmixes were also used as target cells. Data summarize mean ± SD of 4 donors. Panel D. Frequency of IFNγ-producing cells in response to CHLA-255 and Raji at 1:1 effector:target ratio. Data summarize mean ± SD of 3 donors. Panel E. Antitumor activity of CAR-CMV-CTLs in co-culture experiments against CHLA-255 (GD2+ cells) (right panels) and Raji cells (GD2- cells) (left panels). Both CHLA-255 and Raji cells were transduced with a retroviral vector encoding GFP. Tumor cells and CAR-CMV-CTLs were plated at 1:1 ratios, and CAR-CMV-CTLs (CD3+ cells) and tumor cells (GFP+ cells) were quantified by flow cytometry after 4 days of co-culture. Representative of 4 different donors.
We explored the retained effector function of CAR-CMV-CTLs stimulated in vitro with K/CD40L/pp65 and K/OX40L/pp65 against CMV-pp65+ target and neuroblastoma GD2+ cells through their native TCRs and CAR, respectively. In a standard 51Cr-release assay, CAR-CMV-CTLs showed cytotoxic activity against the GD2+ target (CHLA-255) (63% ± 14%) and pp65-pepmix loaded PHA blasts (59% ± 3%) (at 20:1 E:T ratio), but not against the GD2– target cell line (Raji) or PHA blasts loaded with an irrelevant pepmix (Fig. 3C and Suppl. Fig. 1). Control CMV-CTLs not expressing the CAR showed no activity against CHLA-255 (data not shown). Similar results were obtained by measuring IFNγ production in ELISpot assays. We plated CTLs and tumor cells at a ratio of 1:1, and after 24 hours CAR-CMV-CTLs stimulated with K/CD40L/pp65 and K/OX40L/pp65 in response to CHLA-255 showed a trend for a higher IFNγ production (421 ± 21 IFNγ+ SFU/105 cells) as compared to CAR-CMV-CTLs stimulated with K/CD40L/OX40L (295 ± 81 IFNγ+ SFU/105 cells)(p=0.6) (Fig. 3D). Reactivity against Raji cells (GD2– targets) was low in all experimental conditions. In co-culture experiments in which CTLs and tumor cells were plated at a 1:1 ratio and cultured for 4 days, CAR-CMV-CTLs retained their capacity to eliminate CHLA-255 but not Raji (Fig. 3E). Overall, these data indicate that CAR-CMV-CTLs stimulated with K/CD40L/pp65 and K/OX40L/pp65 retain their selective specificities for CMV-pp65 and GD2 antigens.
Vaccination with K562-derived whole-cell vaccine encoding CMV-pp65, CD40L and OX40L increases the antitumor effect of “dual specific” CAR-CMV-CTLs
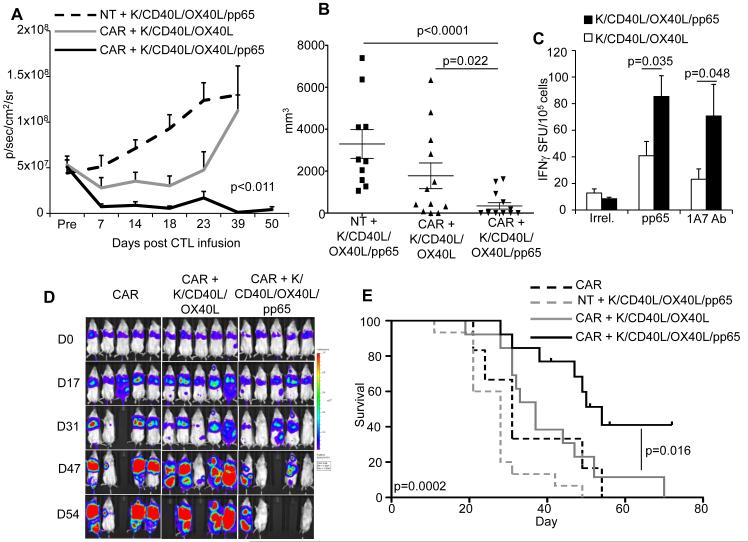
To assess if vaccination with the K562-derived whole-cell vaccine increases the antitumor effects of CAR-CMV-CTLs, NOG/SCID/γc−/− mice were implanted i.p. with CHLA-255 cells labeled with firefly luciferase. Five days after tumor implant, mice received i.p. control or CAR-CMV-CTLs followed by the vaccination schedule illustrated in Fig. 2A. Mice vaccinated with the K/CD40L/pp65 and K/OX40L/pp65 combination controlled tumor growth significantly better by day 50 than mice vaccinated with K/CD40L/OX40L (p=0.011) (Fig. 4A). Tumor control was CAR-mediated, since tumors grew despite vaccinations with K/CD40L/pp65 and K/OX40L/pp65 in mice infused with control CMV-CTLs (Fig. 4A). We selected day 50 to stop the experiment and to assess macroscopically for the presence of tumor at the time of euthanasia. We found that while only 2 out of 17 (12%) mice vaccinated with K/CD40L/OX40L were tumor free, 8 out of 17 (47%) mice were tumor free in the group vaccinated with K/CD40L/pp65 and K/OX40L/pp65. In addition, tumors were significantly smaller in mice vaccinated with K/CD40L/pp65 and K/OX40L/pp65 compared to mice receiving control CMV-CTLs (p<0.0001) or CAR-CMV-CTLs and control K/CD40L/OX40L vaccine (p=0.022) (Fig. 4B). Human CD45+ T cells recovered from the spleen of mice vaccinated with combined K/CD40L/pp65 and K/OX40L/pp65 also showed the highest frequency of CMV-CTLs (85 ± 16 IFNγ+ SFU/105 cells) compared to mice vaccinated with the control K/CD40L/OX40L (41 ± 11 IFNγ+ SFU/105 cells) (p=0.035). We measured CAR-dependent immune responses after stimulation with the anti-idiotype 1A7 Ab. Similar to above, responses were increased in T cells recovered from the spleen of mice vaccinated with combined K/CD40L/pp65 and K/OX40L/pp65 (71 ± 24 IFNγ+ SFU/105 cells) compared to mice vaccinated with control K/CD40L/OX40L (23 ± 8 IFNγ+ SFU/105 cells)(p=0.048) (Fig 4C).
Figure 4. Vaccination with K562-derived whole-cell vaccine expressing CMV-pp65, CD40L and OX40L enhances antitumor effects of CAR-CMV-CTLs in vivo.
Panel A. NOG/SCID/γc−/− mice engrafted i.p. with the neuroblastoma cell line CHLA-255 labeled with firefly luciferase were infused i.p. with control or CAR-CMV-CTLs and vaccinated. The graph summarizes tumor bioluminescence. Summary of CMV-CTL line prepared from 4 donors: 15 mice (control CMV-CTLs plus K/CD40L/pp65 and K/OX40L/pp65), 17 mice (CAR-CMV-CTLs plus K/CD40L and K/OX40L) and 17 mice (CAR-CMV-CTLs plus K/CD40L/pp65 and K/OX40L/pp65) were used per group. Panel B. Mice euthanized were analyzed for the presence of macroscopic tumors. The graph summarizes the volume of the tumor collected in the different groups. Panel C. Enumeration of the CMV-CTLs in the isolated human CD45+ cells from the spleen as assessed by IFNγ ELISpot in response to CMV-pp65 and irrelevant pepmixes or the 1A7 Ab that cross-links the CAR-GD2. Data represent mean ± SD. Panel D. Mice were inoculated i.v. with the GD2+ lung carcinoma cell line A459 labeled with Firefly luciferase. Mice were then infused i.v. with control or CAR-CMV-CTLs and vaccinated. Tumor bioluminescence was then measured overtime. The graph is representative of one of 4 experiments using CMV-CTLs from 4 donors. Panel E. Kaplan-Meier analysis of tumor-bearing mice. Summary of CMV-CTL lines prepared from 4 donors: 15 mice (control CMV-CTLs plus K/CD40L/pp65 and K/OX40L/pp65), 13 mice (CAR-CMV-CTLs plus K/CD40L and K/OX40L), 13 mice (CAR-CMV-CTLs plus K/CD40L/pp65 and K/OX40L/pp65) and 8 mice (CAR-CMV-CTLs alone) were used per group.
We further validated the vaccination approach in a systemic model. For these experiments, NOG/SCID/γc−/− mice were infused i.v. with a single cell derived clone of the A459 tumor cell line that expresses GD2 and rapidly metastasizes upon lung engraftment. Tumor cells were labeled with firefly luciferase to measure tumor bioluminescence in vivo. In this model, control and CAR-CMV-CTLs were infused i.v. and the vaccination was performed as described in Fig. 2A. As illustrated in Fig. 4D, in this systemic model, vaccination with combined K/CD40L/pp65 and K/OX40L/pp65 also induced better control of tumor growth by CAR-CMV-CTLs, which translated into significantly improved overall survival (p=0.016) (Fig. 4E). Altogether, these data indicate that vaccination with combined K562-derived whole-cell vaccine K/CD40L/pp65 and K/OX40L/pp65 improves the antitumor effects of CAR-CMV-CTLs in xenograft models.
Activation of the iC9 suicide gene expressed by the K562-derived whole-cell vaccine abrogates their tumorigenicity
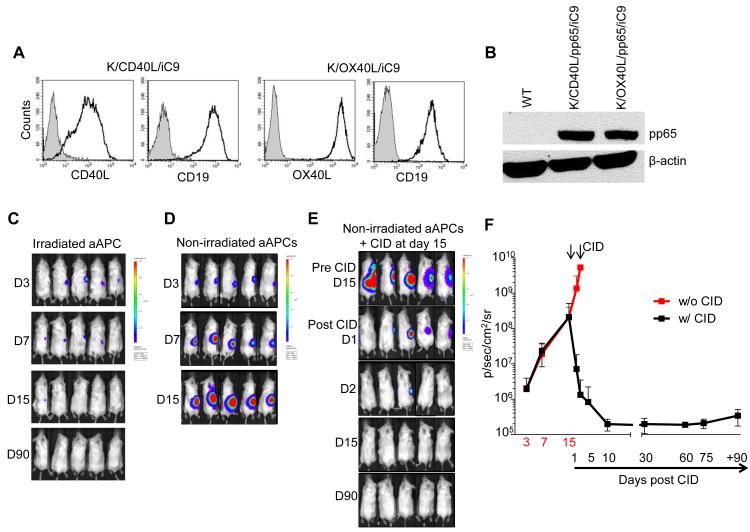
For a potential clinical application, we sought to ensure the safety of this approach in vivo as the whole-cell vaccine is derived from a tumor cell line. For these experiments, K/CD40L/pp65 and K/OX40L/pp65 were labeled with an enhanced firefly luciferase that allows visualizing fewer than 10 cells in a mouse(35). Irradiation of K/CD40L/pp65 and K/OX40L/pp65 before inoculation into NOG/SCID/γc−/− mice completely abrogated the cells’ tumorigenicity. As shown in Fig. 5C, when K/CD40L/pp65 and K/OX40L/pp65 were irradiated at 80-100 Gy before infusion, tumor growth was completely prevented in mice observed for more than 90 days. As an extra precaution, and to guarantee the safety of the vaccination, we further engineered the K562 cell line with the inducible suicide iC9 that also expresses a truncated form of CD19 as a selectable marker(27). Single cell clones were selected based on the expression of CD19 (Fig. 5A,B). Cells were inoculated subcutaneously without irradiation (4 × 106 cells) into NOG/SCID/γc−/− mice. By day 15 after engraftment, mice received i.p. AP20187 (50 μg/mouse) for two consecutive days. Mice monitored for more than 90 days did not develop the tumor (Fig. 5D,E,F). Overall, these data indicate that the safety of the vaccination with K/CD40L/pp65 and K/OX40L/pp65 can be further assured through the incorporation of the iC9 suicide gene.
Figure 5. Activation of the iC9 suicide gene eliminates engrafted K562-derived whole-cell vaccine in vivo.
Panel A. Characterization of the clones by flow cytometry analysis. Gray areas indicate wild type K562 cells. Panel B. Western blot showing the expression of CMV-pp65 in the clones expressing the iC9 transgene. Panels C and D. NOG/SCID/γc−/− mice were inoculated subcutaneously with irradiated (C) or non-irradiated (D) K562-derived whole-cell vaccine expressing the iC9 gene and labeled with an enhanced firefly luciferase. Tumor growth was measured by in vivo imaging. Panel E. Effects of the administration of the chemical inducer of dimerization (CID) AP20187 on the growth of engineered vaccine.
Discussion
We previously reported that the infusion of EBV-CTLs and CMV-CTLs expressing a CAR promotes objective tumor regressions in clinical trials(11-13). However, in vivo expansion and persistence of these cells remain suboptimal likely because, in the absence of significant amounts of viral load, the co-stimulation provided by endogenous APCs processing and presenting latent viral antigens is insufficient to promote robust engraftment of CAR-redirected VsCTLs once infused. Here, we developed a strategy that can achieve the necessary engraftment. We have generated a vaccination approach using a K562-derived whole-cell vaccine and demonstrated that the antitumor effect of adoptively transferred CAR-redirect CMV-CTLs is enhanced when these CTLs are boosted in vivo by the vaccine.
Vaccination is the most common modality to induce both humoral and cellular immune responses. In the absence of clinically approved vaccines to induce cellular immune responses to either EBV or CMV, several experimental vaccination approaches for a clinical translation can be envisioned. These include DNA-plasmids(36), peptides(37) and ex vivo expanded and antigen-loaded DCs(38). However, each of these approaches has limitations that are primarily due to low immunogenicity (DNA-plasmid vaccine)(39), toxicity caused by the strong adjuvants included in the vaccine preparation (peptide vaccine)(40), and significant variability of the biologic characteristics of the final product and manufacturing costs (DC-based vaccine). Based on these limitations, we elected to generate an off-the-shelf whole-cell vaccine to boost in vivo adoptively transferred CAR-CMV-CTLs.
Autologous and allogeneic whole-cell vaccines consisting of tumor cells genetically manipulated to express GM-CSF or other cytokines, chemokines and immune stimulatory molecules have been used in clinical trials to promote cross-presentation of tumor-associated antigen to APCs in vivo(14;15;17;41-43). Based on this evidence, we proposed to engineer the very well characterized tumor cell line K562 to express the highly immunogenic CMV-pp65 protein. We thus created a whole-cell vaccine to administer to patients infused with CMV-CTLs expressing a tumor-specific CAR.
Our data demonstrate that the ectopic expression of the viral protein pp65 by K562 can be efficiently used to transfer the protein, likely in the form of apoptotic bodies, to APCs that can then process and present pp65 epitopes in the context of the appropriate MHC molecules. This approach, when applied directly in vivo to boost adoptively transferred CAR-CMV-CTLs, has the advantage of delivering preformed antigens to APCs without the need for the in vivo protein synthesis required by DNA-plasmid vaccines. In addition, such a cell-based vaccine easily can be further engineered to express other molecules to enhance immune responses. In our specific case, we selected CD40L and OX40L. We and others have used CD40L expression in the past to generate autologous vaccines for hematological malignancies to induce the up-regulation of the co-stimulatory molecules CD80 and CD86 in leukemic cells through the CD40-CD40L pathway(43-45). Here, we demonstrated that CD40L expressed in the whole-cell vaccine is essential in promoting the expression of CD80 and CD83 in monocytes engulfing apoptotic bodies. Control vaccine producing pp65 but lacking CD40L is indeed less efficient in that regard. Since CD80 and CD83 are upregulated in mature DCs to initiate immune responses(23;46), CD40L expressed by the whole-cell vaccine seems to accomplish the crucial step of APC maturation upon antigen processing.
We also included OX40L in the K562-derived whole-cell vaccine. As illustrated by our data, OX40L does not play a role in inducing the expression of CD80 and CD83 by monocytes engulfing apoptotic bodies. As a consequence, K/OX40L/pp65 are not superior to control K/pp65 in boosting CMV-CTLs. We have previously combined CD40L and OX40L molecules/signaling showing that they mediate enhanced potency of an autologous leukemia vaccine(47). Consistent with that experience, we did not show an advantage in combining both CD40L and OX40L in short-term experiments in vitro, since OX40L mostly delivers critical late accessory signals that augment the proliferation and survival of memory CD4+ T cells(48;49). However, the combination CD40L and OX40L within the whole-cell vaccine showed clear benefits in in vivo experiments. We found in mice a more profound increase of CMV-CTLs when both CD40L and OX40L were incorporated within the whole-cell vaccine suggesting the critical role of CD4 in boosting CMV-CTL responses. As a consequence, when CMV-CTLs are expressing a CAR, boosting in vivo their native virus-specificity with the combination K/CD40L/pp65 and K/OX40L/pp65 showed better antitumor effects in two models of xenogenic solid tumors. Since K562 are also known to stimulate the proliferation of natural killer cells (NKs), we found in vitro and in vivo that the boosting with K562-derived whole-cell vaccine induced the expansion of NKs in addition to CAR-CMV-CTLs. While no description of increased NKs have been reported using K562/GM-CSF cells in patients(17), considering the antitumor effects of NKs, the in vivo boosting of this cell subset by the K562-derived whole-cell vaccine may be beneficial.
Finally, we also addressed the potential safety concerns raised by using tumor cells as a vaccine. Autologous and allogeneic tumor cell lines have been safely used in multiple large clinical trials, suggesting that irradiation before inoculation abrogates their growth. Despite this apparent safety, however, a lethal acute respiratory distress syndrome and severe eosinophilia were reported in a patient vaccinated with irradiated autologous myeloblasts admixed with GM-CSF secreting K562 (http://oba.od.nih.gov/oba/RAC/meetings/Dec2011/RAC_Minutes_12-11.pdf). We found in animals that irradiation abolishes the growth of our K562 engineered with pp65, CD40L and OX40L. However, we also demonstrated that an additional safety mechanism can be implemented by further engineering these cells to express the iC9 suicide gene. Activation of iC9 by a small molecule halts the growth of live (deliberately non irradiated) engineered K562 implanted in mice and since iC9 has been validated in a clinical trial it can be used efficiently in the context of a vaccine approach(27).
In conclusion, we demonstrated that a K562-derived whole-cell vaccine can safely enhance the antitumor effects of adoptively transferred CAR-CMV-CTLs. Due to the high flexibility of the whole-cell vaccine, K562 can be properly engineered to express other immunogenic antigens derived from other viruses and provide other relevant molecules to activate the immune system.
Supplementary Material
Translational Relevance.
T cells recognizing viral antigens such as Epstein Barr Virus (EBV) and Cytomegalovirus (CMV) acquire tumor specificity when genetically modified to express a Chimeric Antigen Receptor (CAR). Prolonged expansion and persistence of adoptively transferred tumor-specific T cells in vivo is a critical step in achieving sustained clinical responses. Here, we provide data showing that a K562-based whole-cell vaccine generated to express the viral antigen CMV-pp65 and immune stimulatory molecules CD40L and OX40L enhances the antitumor effects of CMV-CTLs expressing a CAR by boosting their intrinsic virus-specificity.
Acknowledgements
The authors would like to thank Dr Brian Rabinovich from MD Anderson Cancer Center, Houston, TX for providing the enhanced firefly luciferase gene and Catherine Gillespie from the Center for Cell and Gene Therapy for the manuscript editing.
Funding
This work was supported in part by R01 CA142636 National Institutes of Health-NCI, W81XWH-10-10425 Department of Defense, Technology/Therapeutic Development Award.
Footnotes
Conflict of Interest
GD and BS are investigators in a collaborative research grant between the Center for Cell and Gene Therapy and Celgene to develop genetically modified T cells.
Authorship
G.D., B.S. designed the research. I.C., G.W., and B.S. performed the experiments. B.C.B. and M.S.W. provided technical assistance. G.D., B.S. I.C. analyzed the data and wrote the manuscript.
Reference List
- 1.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014 Jan;257(1):107–26. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011 Aug 10;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014 Oct 16;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med. 2014 Feb 19;6(224):224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011 May 2;121(5):1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992 Dec 24;71(7):1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 8.Rossig C, Bollard CM, Nuchtern JG, Rooney CM, Brenner MK. Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood. 2002 Mar 15;99(6):2009–16. doi: 10.1182/blood.v99.6.2009. [DOI] [PubMed] [Google Scholar]
- 9.Savoldo B, Rooney CM, Di Stasi A, Abken H, Hombach A, Foster AE, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30{zeta} artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007 Oct 1;110(7):2620–30. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Micklethwaite KP, Savoldo B, Hanley PJ, Leen AM, mmler-Harrison GJ, Cooper LJ, et al. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood. 2010 Apr 1;115(13):2695–703. doi: 10.1182/blood-2009-09-242263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008 Nov;14(11):1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013 Oct 24;122(17):2965–73. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011 Dec 1;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borrello I, Pardoll D. GM-CSF-based cellular vaccines: a review of the clinical experience. Cytokine Growth Factor Rev. 2002 Apr;13(2):185–93. doi: 10.1016/s1359-6101(01)00034-x. [DOI] [PubMed] [Google Scholar]
- 15.Nemunaitis J, Jahan T, Ross H, Sterman D, Richards D, Fox B, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006 Jun;13(6):555–62. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- 16.Rousseau RF, Haight AE, Hirschmann-Jax C, Yvon ES, Rill DR, Mei Z, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003 Mar 1;101(5):1718–26. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 17.Smith BD, Kasamon YL, Kowalski J, Gocke C, Murphy K, Miller CB, et al. K562/GM-CSF immunotherapy reduces tumor burden in chronic myeloid leukemia patients with residual disease on imatinib mesylate. Clin Cancer Res. 2010 Jan 1;16(1):338–47. doi: 10.1158/1078-0432.CCR-09-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J. 2010 Jul;16(4):304–10. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006 Nov 1;43(9):1143–51. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- 20.Wills MR, Carmichael AJ, Mynard K, Jin X, Weekes MP, Plachter B, et al. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996 Nov;70(11):7569–79. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoenberger SP, Toes RE, van d, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998 Jun 4;393(6684):480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 22.Schoenberger SP, Jonges LE, Mooijaart RJ, Hartgers F, Toes RE, Kast WM, et al. Efficient direct priming of tumor-specific cytotoxic T lymphocyte in vivo by an engineered APC. Cancer Res. 1998 Jul 15;58(14):3094–100. [PubMed] [Google Scholar]
- 23.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van KC, Durand I, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994 Oct 1;180(4):1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001 Dec 15;167(12):6804–11. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg AD, Evans DE, Thalhofer C, Shi T, Prell RA. The generation of T cell memory: a review describing the molecular and cellular events following OX40 (CD134) engagement. J Leukoc Biol. 2004 Jun;75(6):962–72. doi: 10.1189/jlb.1103586. [DOI] [PubMed] [Google Scholar]
- 26.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000 Sep 15;165(6):3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 27.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011 Nov 3;365(18):1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CA, Ng CY, Heslop HE, Holladay MS, Richardson S, Turner EV, et al. Production of genetically modified Epstein-Barr virus-specific cytotoxic T cells for adoptive transfer to patients at high risk of EBV-associated lymphoproliferative disease. J Hematother. 1995 Apr;4(2):73–9. doi: 10.1089/scd.1.1995.4.73. [DOI] [PubMed] [Google Scholar]
- 29.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006 Dec 1;108(12):3890–7. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996 Apr 12;272(5259):263–7. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 31.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005 Nov;12(5):933–41. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Koo GC, Hasan A, O'Reilly RJ. Use of humanized severe combined immunodeficient mice for human vaccine development. Expert Rev Vaccines. 2009 Jan;8(1):113–20. doi: 10.1586/14760584.8.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perna SK, Pagliara D, Mahendravada A, Liu H, Brenner MK, Savoldo B, et al. Interleukin-7 mediates selective expansion of tumor-redirected cytotoxic T lymphocytes (CTLs) without enhancement of regulatory T-cell inhibition. Clin Cancer Res. 2014 Jan 1;20(1):131–9. doi: 10.1158/1078-0432.CCR-13-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabinovich BA, Ye Y, Etto T, Chen JQ, Levitsky HI, Overwijk WW, et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14342–6. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tey SK, Dotti G, Rooney CM, Heslop HE, Brenner MK. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol Blood Marrow Transplant. 2007 Aug;13(8):913–24. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008 Oct;9(10):776–88. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009 Nov 5;361(19):1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 38.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010 Jul 29;363(5):411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 39.McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003 Jun;9(6):729–35. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3(7):e2636. doi: 10.1371/journal.pone.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salgia R, Lynch T, Skarin A, Lucca J, Lynch C, Jung K, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003 Feb 15;21(4):624–30. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 42.Bowman L, Grossmann M, Rill D, Brown M, Zhong WY, Alexander B, et al. IL-2 adenovector-transduced autologous tumor cells induce antitumor immune responses in patients with neuroblastoma. Blood. 1998 Sep 15;92(6):1941–9. [PubMed] [Google Scholar]
- 43.Biagi E, Rousseau R, Yvon E, Schwartz M, Dotti G, Foster A, et al. Responses to human CD40 ligand/human interleukin-2 autologous cell vaccine in patients with B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2005 Oct 1;11(19 Pt 1):6916–23. doi: 10.1158/1078-0432.CCR-05-0484. [DOI] [PubMed] [Google Scholar]
- 44.Rousseau RF, Biagi E, Dutour A, Yvon ES, Brown MP, Lin T, et al. Immunotherapy of high-risk acute leukemia with a recipient (autologous) vaccine expressing transgenic human CD40L and IL-2 after chemotherapy and allogeneic stem cell transplantation. Blood. 2005 Oct 25; doi: 10.1182/blood-2005-03-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000 Nov 1;96(9):2917–24. [PubMed] [Google Scholar]
- 46.Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999 Jul 5;190(1):125–33. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biagi E, Dotti G, Yvon E, Lee E, Pule M, Vigouroux S, et al. Molecular transfer of CD40 and OX40 ligands to leukemic human B cells induces expansion of autologous tumor-reactive cytotoxic T lymphocytes. Blood. 2005 Mar 15;105(6):2436–42. doi: 10.1182/blood-2004-07-2556. [DOI] [PubMed] [Google Scholar]
- 48.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004 Jun;4(6):420–31. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg AD. OX40: targeted immunotherapy--implications for tempering autoimmunity and enhancing vaccines. Trends Immunol. 2002 Feb;23(2):102–9. doi: 10.1016/s1471-4906(01)02127-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.