Abstract
Purpose
Developmental dyslexia (DD) is commonly thought to arise from phonological impairments. However, an emerging perspective is that a more general procedural learning deficit, not specific to phonological processing, may underlie DD. The current study examined if individuals with DD are capable of extracting statistical regularities across sequences of passively experienced speech and nonspeech sounds. Such statistical learning is believed to be domain-general, to draw upon procedural learning systems, and to relate to language outcomes.
Method
DD and control groups were familiarized with a continuous stream of syllables or sine-wave tones, the ordering of which was defined by high or low transitional probabilities across adjacent stimulus pairs. Participants subsequently judged two 3-stimulus test items with either high or low statistical coherence as being the most similar to the sounds heard during familiarization.
Results
As with control participants, the DD group was sensitive to the transitional probability structure of the familiarization materials as evidenced by above-chance performance. However, the performance of participants with DD was significantly poorer than controls across linguistic and nonlinguistic stimuli. In addition, reading-related measures were significantly correlated with statistical learning performance of both speech and nonspeech material.
Conclusion
Results are discussed in light of procedural learning impairments among participants with DD.
Fundamental language skills, such as reading, are learned early in life and are culturally dependent. One psychological process that has been suggested to be involved in learning to read is the ability to detect statistical regularities. Reading involves the mapping between phonology, the sounds of the language, and orthography, their arbitrary visual forms. The correspondence between phonology and orthography is complex in languages such as English. For example, the vowel “e” is pronounced as /ε/ in a word such as bed but as /i/ when paired with an “a” as in bead. Although many of these correspondences are taught explicitly in learning to read, learning models emphasize that emergent statistical regularities among phonology–orthography correspondences are also important (McClelland & Patterson, 2002; Seidenberg & McClelland, 1989). Because the number of phonology–orthography correspondences is vast (more than 1,000 rules according to the dual route cascade model of reading; Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001), effective learners may rely upon emergent statistical regularities to support explicit instruction.
Consistent with this claim, Arciuli and Simpson (2012) observed that reading abilities among both adults and children are highly correlated with the ability to extract visual statistical structure from the environment. In a similar manner, a recent study demonstrated that reading proficiency among native American-English adult second-language-learners of Hebrew is positively correlated with the ability to extract structure from the environment (Frost, Siegelman, Narkiss, & Afek, 2013). To be specific, the ability to track regularities in a continuous stream of visual shapes correlates with performance on tasks that monitor the assimilation of the structure of Hebrew words via morphological priming. In a similar manner, sensitivity to statistical regularities is also found to contribute to language proficiency. Evans, Saffran, and Robe-Torres (2009) have demonstrated that the ability to track regularities is positively correlated with vocabulary growth among children. Taken together, these independent observations suggest that sensitivity to statistical regularities may impact reading development by enabling detection of probabilistic correspondences among letters and phonemes and the detection of regularities that exist among letters (transitional probabilities between letters). Moreover, it may enhance vocabulary growth, which, in turn, can contribute to reading performance (Biemiller, 2003).
Developmental Dyslexia
Developmental dyslexia (DD) is one of the most frequent neurodevelopmental disorders. It is characterized by selective impairment in reading skill acquisition despite conventional instruction, adequate intelligence, and sociocultural opportunity. DD is fairly widespread, but its exact prevalence is uncertain (estimates range from 5% to 17% of school-age children in the United States; Shaywitz & Shaywitz, 2005). It is more frequently reported in males (Démonet, Taylor, & Chaix, 2004), and its frequency differs across languages (Lindgren, De Renzi, & Richman, 1985). The typical presenting symptoms of DD are difficulties in reading, writing, and spelling as well as deficits in word identification and phonological decoding (Vellutino, Fletcher, Snowling, & Scanlon, 2004). Although progress has been made in the past decades, the underlying cognitive and biological mechanisms of DD are still under extensive debate (for a review, see Démonet et al., 2004).
Phonological Deficit Account
The classic approach views DD as stemming from a phonological deficit (Snowling, 2000). By this account, dyslexia is presumed to arise from a deficit in direct access to and manipulation of phonemic language units retrieved from long-term declarative memory (Snowling, 2000). Indeed, phonological impairments are among the central symptoms associated with DD (Ramus et al., 2003). Phonological awareness (sensitivity to the sound structure in a word), verbal short-term memory, and lexical retrieval are impaired in DD (Vellutino et al., 2004). However, an accumulating body of evidence demonstrates that people with DD have a wide range of nonlinguistic deficits. These include impairments in motor functions (Fawcett & Nicolson, 1995; Nicolson & Fawcett, 1994) and attention deficits (Facoetti, Paganoni, Turatto, Marzola, & Mascetti, 2000; Helland & Asbjørnsen, 2000) as well as procedural learning impairments (Lum, Ullman, & Conti-Ramsden, 2013). One difficulty for the phonological hypothesis is its inability to account for these additional impairments (Brookes, Nicolson, & Fawcett, 2007; Démonet et al., 2004). Furthermore, phonological impairments also are observed in individuals who do not have DD (Morris et al., 1998). It is possible that phonological deficits may be symptoms arising from another underlying cause.
Procedural Learning Deficit Account
An emerging perspective in dyslexia research is that difficulty in phonology, reading, writing, and spelling skills among people with DD may be related to a more general learning deficit not specific to phonological processing or to speech materials (Nicolson & Fawcett, 2011). According to this perspective, DD may be related to selective disruption of the procedural learning system that subserves the learning and control of established sensorimotor and cognitive habits, skills, and procedures (Alvarez & Squire, 1994). This deficit is posited to stem from dysfunction within one or more of the brain areas related to this system (e.g., the prefrontal cortex around Broca's area, the parietal cortex, and the subcortical structures, including the basal ganglia and the cerebellum). By this perspective, a procedural learning impairment disrupts automatization of skill and knowledge, which may potentially affect grapheme–phoneme conversion, word recognition, verbal working memory, and learning orthographic regularities, thereby contributing to reading impairment. In addition, this approach suggests that procedural learning system impairment in DD may lead to mild motor and articulatory problems that result in impoverished representations of the phonological characteristics of speech and concomitant difficulties in grapheme–phoneme conversion and in learning to read. Because the procedural learning system involves a network of multiple brain regions, this perspective postulates that it is possible to observe a range of manifestations of DD (Nicolson & Fawcett, 2010). For example, individuals with DD may exhibit both reading and syntactic impairments.
In support of this perspective, several neuroimaging studies report cerebellar impairment in individuals with dyslexia (Nicolson et al., 1999; Rae et al., 1998). Recent research has found that the right cerebellum anatomy best discriminates between typical readers and individuals with dyslexia (Pernet, Poline, Démonet, & Rousselet, 2009). Other studies have observed atypical basal ganglia activity in those with dyslexia (Brunswick, McCrory, Price, Frith, & Frith, 1999; Kita et al., 2013; Paulesu et al., 1996). Furthermore, behavioral research demonstrates impairments among individuals with DD on a variety of tasks believed to be subserved by the procedural learning system, such as implicit learning tasks. Children and adults with DD exhibit impaired performance on common implicit learning paradigms, such as the serial reaction time task (Gabay, Schiff, & Vakil, 2012c; Menghini, Hagberg, Caltagirone, Petrosini, & Vicari, 2006; Pugh et al., 2014; Stoodley, Harrison, & Stein, 2006; Vicari et al., 2005; Vicari, Marotta, Menghini, Molinari, & Petrosini, 2003) and artificial grammar learning (Pavlidou & Williams, 2014). Furthermore, implicit motor learning skills among those with DD have been found to be more fragile, to be less resistant to interference (Gabay, Schiff, & Vakil, 2012b), and to consolidate less effectively (Gabay, Schiff, & Vakil, 2012a; Hedenius et al., 2013) compared to implicit learning among typical readers.
Several studies have found intact procedural learning in those with DD (Kelly, Griffiths, & Frith, 2002; Rüsseler, Gerth, & Münte, 2006). However, a recent meta-analysis on procedural learning in DD indicates this intact procedural learning performance can arise from compensatory declarative learning mechanisms that may mask procedural learning deficits in DD (Lum et al., 2013). In support of this possibility, experiments with older participants or studies using simple (deterministic) sequential structures are more likely to be contaminated by compensation via declarative processes during procedural learning tasks. In considering these factors in a meta-analysis of the literature, Lum et al. (2013) concluded procedural learning is impaired in DD.
Precisely how procedural learning impairments relate to the reading deficits of DD is not yet well understood. Refining the details of procedural learning specifically affected in DD remains an important research objective. As noted above, some have hypothesized that impaired procedural learning may interfere with skill automation or articulation, in turn leading to impoverished phonological representations that do not effectively support learning to read (Nicolson & Fawcett, 2011). This conceptualization emphasizes the motor and skill-acquisition functions associated with procedural learning systems (Doyon & Benali, 2005; Doyon, Penhune, & Ungerleider, 2003; Ungerleider, Doyon, & Karni, 2002). Consistent with this perspective, the majority of implicit learning studies in those with DD have focused on procedural learning in the motor domain. This has arisen partly from efforts to examine if procedural learning impairments in DD are general and not specifically tied to language processing. Whereas impaired motor procedural learning in DD is consistent with theoretical claims of a general learning impairment not specific to phonological processing or to language development (Brookes et al., 2007; Fawcett & Nicolson, 1995; Howard, Howard, Japikse, & Eden, 2006; Lum et al., 2013; Stoodley et al., 2006; Stoodley, Ray, Jack, & Stein, 2008), the very breadth of these tasks means that the precise definition of procedural that is impaired in dyslexia remains underspecified (Song, 2009). In this regard, it is noteworthy that accumulating evidence implicates the procedural learning system in nonmotor behavior, including learning, perceptual, and linguistic processing (Middleton & Strick, 2000; Seger, 2008; Strick, Dum, & Fiez, 2009). For example, procedural learning has been implicated in sequence formation (Goschke, Friederici, Kotz, & Van Kampen, 2001), probabilistic category learning (Shohamy, Myers, Onlaor, & Gluck, 2004), and perceptual categorization (Maddox & Ashby, 2004; Seger, 2008). It has also been closely tied to formation of grammar rules (Ullman, 2001). In better understanding the nature of procedural learning impairment in dyslexia, it will be especially important to examine aspects of procedural learning that may be relevant to the language domain. This will allow us to better understand how the ubiquitous phonological deficits in DD may arise from a general procedural learning impairment.
Statistical Learning
To this end, we investigated whether individuals with DD are impaired at detecting statistical regularities in the order of syllables or tones across a continuous stream of sound (Saffran, Aslin, & Newport, 1996). The classic statistical learning (SL) paradigm measures the ability to detect boundaries between groups of elements on the basis of the sequential probabilities among the elements (Saffran, Johnson, Aslin, & Newport, 1999). In a typical SL task, participants hear a continuous acoustic stream of syllables, such as tupirogolabubidakupadoti. Within the stream, syllables are ordered such that transitional probabilities between some pairs of syllables are higher (1.0, la always precedes bu) whereas others are lower (.33, either ro, ku, or ti may precede go). Immediately after passively listening to these acoustic streams, participants are able to distinguish between sound sequences with higher transitional probabilities and those with lower transitional probabilities (Saffran et al., 1996, 1999; Saffran, Newport, Aslin, Tunick, & Barrueco, 1997).
This sensitivity to the transitional probability between sound sequences is thought to support word segmentation in fluent speech because words would be expected to have higher transitional probabilities among syllables (pre regularly precedes tty as in pretty) than sequences that cross word boundaries (tyba in the English phrase pretty baby). Nonetheless, sensitivity to transitional probabilities across continuous input appears to be domain general in that it is observed for nonspeech tones (Saffran et al., 1999), music (Tillmann & McAdams, 2004), and visual shapes (Turk-Browne, Jungé, & Scholl, 2005), in addition to syllables.
SL is believed to be a form of implicit learning because learning proceeds in the absence of intention to learn (Perruchet & Pacton, 2006). Another criteria for implicit learning is that learning appears to be inaccessible to consciousness (Reber, 1967). However, there is a great debate about if implicit learning tasks, including SL tasks (Saffran et al., 1997), can actually occur without consciousness (Shanks, 2003).
Similar to other implicit learning tasks (such as the serial reaction time task), SL is evident for both simple and higher-order structures (Fiser & Aslin, 2001; Newport & Aslin, 2004). Moreover, neuroimaging evidence is consistent with engagement of the procedural learning system during SL tasks (Karuza et al., 2013; Turk-Browne, Scholl, Chun, & Johnson, 2009). Although both implicit learning tasks and SL tasks pursue the objective of investigating general learning mechanisms under unsupervised learning situations, there are several important differences. Whereas other implicit learning tasks (such as the serial reaction time task and artificial grammar learning tasks) are believed to rely of the formation of chunks (Buchner, Steffens, & Rothkegel, 1998; Pothos & Bailey, 2000), SL is believed to involve statistical computations (Buchner et al., 1998). It is still not clear whether statistical computations and chunk formation are independent processes or if one process is based on another (Thiessen, Kronstein, & Hufnagle, 2013). One possibility is that chunks are inferred from the results of (unconscious) statistical computations. Another possibility is that (perhaps conscious) chunks are formed from the outset and then evolve as a result of basic associative learning principles (for a review, see Perruchet & Pacton, 2006). Furthermore, in contrast with other implicit learning tasks (such as artificial grammar learning tasks), SL tasks involve continuous input, which has to be segmented into discrete units, thus perhaps more resembling some of the demands encountered in language acquisition. These potential differences between SL tasks and other implicit learning tasks (and, by association, procedural learning), and the potential contribution of SL to language acquisition highlight the importance of examining SL in participants with DD.
In the present study, we examined SL among participants with DD and controls across stimulus materials created with speech syllables and with nonspeech tones. The phonological deficit account of DD is consistent with the expectation that DD listeners will perform poorly on SL tasks composed of continuous streams of syllables because DD would reduce the accuracy with which participants perceive or encode the phonological input during familiarization. However, if individuals with DD have a general impairment that impacts procedural learning tasks more generally, then DD performance should be impaired relative to controls on both speech and nonspeech SL tasks.
We also examined the relationship between SL performance and individual reading ability. Previous research has demonstrated that reading abilities are associated with visual SL performance among typical readers (Arciuli & Simpson, 2012). On the basis of this, we expect to observe correlations between standardized reading tests and SL performance. We also expect that there may be correlations between SL and other reading-related skills factors, such as rapid naming. According to the procedural learning hypothesis, procedural learning impairment disrupts reading by affecting automatization. As such, there should be an association between the ability to automatically associate stimuli, such as letters, with their appropriate names (measured by rapid automatized naming tasks; Denckla & Rudel, 1976) and SL performance. Note also that this theory predicts problems in naming that are not confined to reading-related tasks. To be specific, individuals with DD are expected to be impaired in naming all stimulus types (e.g., colors) and not only in naming that requires grapheme–phoneme decoding (e.g., letters; Fawcett & Nicolson, 1994). Thus, we examined whether SL abilities would be related to the ability to automatically name alphanumeric materials (letters or numbers) or to rapid automatic naming of any category (such as digits, letters, objects, colors).
General Method
Participants
Sixteen volunteers with DD and an equal number of control volunteers participated. All were native English-speaking university students in the area of Pittsburgh, PA, who had no reported signs of sensory or neurological deficits, including attention deficit hyperactive disorder. All came from families of middle to high socioeconomic status. Diagnosis of a comorbid developmental learning disability was an exclusion criterion. A well-documented history of dyslexia was the inclusion criterion for the dyslexia group: (a) Each individual received a formal diagnosis of dyslexia by a qualified psychologist, (b) each individual's diagnosis was verified by the diagnostic and therapeutic center at their university, and (c) each individual was receiving accommodations in educational settings. The control group was age matched with the DD group, with no reading problems and the same level of cognitive ability (as measured by Raven's Standard Progressive Matrices; Raven, Court, & Raven, 1992). The inclusion criterion for the control group was a lack of history of learning disabilities as well as performing at or above average on standardized measures of reading. Written informed consent was obtained from all participants. The study was approved by the Psychology Research Ethics Committee of CMU, and it was conducted in accordance with the Declaration of Helsinki.
All participants underwent a series of cognitive tests (see Table 1 for detailed descriptions) to evaluate general intelligence (as measured by Raven's Progressive Matrices), verbal working memory (as measured by the forward and backward Digit Span subtest from the Wechsler Adult Intelligence Scale, Wechsler, 1997), rapid automatized naming (Wolf & Denckla, 2005), and phonological awareness (Spoonerism Test; adapted from Brunswick et al., 1999). In addition, all participants performed both untimed and timed (fluency) tests of word reading and decoding skills. Participants performed the Word Identification and Word Attack subtests from the Woodcock Reading Mastery Tests–Revised (Woodcock, 1987). In addition, participants performed the Sight Word Efficiency, Forms A+B (i.e., rate of word identification), and Phonemic Decoding Efficiency, Forms A+B (i.e., rate of decoding pseudowords), subtests from the Test of Word Reading Efficiency (Torgesen, Wagner, & Rashotte, 1999). Results are shown in Table 2.
Table 1.
Psychometric tests.
| The following tests were administered according to the test manual instructions: |
| 1. Raven's Standard Progressive Matrices (Raven, Court, & Raven, 1992): Nonverbal intelligence was assessed by the Raven's Progressive Matrices test. This task requires participants to choose an item from the bottom of the figure that would complete the pattern at the top. The maximum raw score is 60. Test reliability coefficient is .9. |
| 2. Digit Span subtest from the Wechsler Adult Intelligence Scale (Wechsler, 1997): In this task, participants are required to recall the names of the digits presented auditorily in the order they were presented, with a maximum of total raw score of 28. Task administration is discontinued after a failure to recall two trials with a similar length of digits. Test reliability coefficient is .9. |
| 3. Rapid Automatized Naming (RAN; Wolf & Denckla, 2005): The tasks require oral naming of rows of visually presented exemplars drawn from a constant category (RAN colors, RAN categories, RAN numerals, and RAN letters). It requires not only the retrieval of a familiar phonological code for each stimulus, but also coordination of phonological and visual (color) or orthographic (alphanumeric) information quickly in time. The reliability coefficient of these tests ranges from .98 to .99. |
| 4. Woodcock Reading Mastery Tests Word Identification and Word Attack subtests (Woodcock, 1987). The Word Identification subtest measures participants' ability to accurately pronounce printed English words, ranging from high to low frequency of word occurrence, with a maximum total raw score of 106. Test reliability coefficient is .97. The Word Attack subtest assesses participants' ability to read pronounceable nonwords varying in complexity, with a maximum total raw score of 45. Test reliability coefficient is .87. Task administration is discontinued when six consecutive words are read incorrectly. |
| 5. Sight Word Efficiency and Phonemic Decoding Efficiency subtests from the Test of Word Reading Efficiency (Torgesen et al., 1999): The Sight Word Efficiency (i.e., rate of word identification) and Phonemic Decoding Efficiency (i.e., rate of decoding pseudowords) subtests were used to measure reading rate. The test contains two timed measures of real-word reading and pseudoword decoding. Participants are required to read the words aloud as quickly and accurately as possible. The score reflects the total number of words/nonwords read correctly in a fixed 45-s interval. Task administration is discontinued after 45 s. Sight Word Efficiency maximum raw score is 108. Phonemic Decoding Efficiency maximum raw score is 65. Test–retest reliability coefficients for these subtests are .91 and .90, respectively. |
| 6. Spoonerism Test (adapted from Brunswick et al., 1999): This test assesses the participants' ability to segment single-syllable words and then to synthesize the segments to provide new words. For example, the word pair basket lemon becomes lasket bemon. The maximum raw score is 12. |
Table 2.
Demographic and psychometric data of developmental dyslexia (DD) and control groups.
| Measure | DD group M (SD) | Range | Control group M (SD) | Range | p | Cohen's d |
|---|---|---|---|---|---|---|
| Age (in years) | 21.56 (4.89) | 18–35 | 22.12 (2.94) | 18–30 | ns | 0.13 |
| Raven's SPM | 56.93 (2.9) | 51–60 | 58 (2.3) | 52–60 | ns | 0.4 |
| DSa (combined) | 10.25 (2.86) | 5–16 | 13.68 (2.44) | 8–18 | < .01 | 1.29 |
| RAN objectsa | 103.87 (19.12) | 74–129 | 117.81 (10.13) | 100–133 | < .05 | 0.91 |
| RAN colorsa | 98.31 (13.74) | 80–124 | 112.75 (5.11) | 103–121 | < .01 | 1.39 |
| RAN numbersa | 106.12 (5.4) | 95–113 | 114 (3.91) | 107–120 | < .01 | 1.67 |
| RAN lettersa | 102.56 (6.1) | 85–111 | 111.93 (4.95) | 102–117 | < .01 | 1.808 |
| WRMT-R WIa | 98.37 (5.004) | 92–113 | 111.18 (7.74) | 100–126 | < .01 | 1.96 |
| WRMT-R WAa | 96 (8.73) | 82–115 | 115 (12.07) | 100–137 | < .01 | 1.803 |
| TOWRE SW (A+B)a | 97.31 (8.2) | 81–112 | 114.94 (8.82) | 100–127 | < .01 | 2.07 |
| TOWRE PD (A+B)a | 90 (9.12) | 72–112 | 114.37 (9.94) | 100–127 | <.01 | 2.55 |
| Spoonerism time | 148.43 (71.67) | 82–368 | 92.93 (30.5) | 63–150 | < .01 | 1.007 |
| Spoonerism accuracy | 8.12 (3.66) | 1–12 | 11 (2.21) | 4–12 | < .05 | 0.95 |
Note. SPM = Standard Progressive Matrices; RAN = Rapid Automatized Naming; DS = Digit Span subtest from the Wechsler Adult Intelligence Scale; WRMT-R WI = Woodcock Reading Mastery Tests–Revised Word Identification subtest; WRMT-R WA = Woodcock Reading Mastery Tests–Revised Word Attack subtest; TOWRE SW = Test of Word Reading Efficiency Sight Word Efficiency subtest; TOWRE PD = Test of Word Reading Efficiency Phonemic Decoding Efficiency subtest.
Standard scores (whereby smaller numbers are expected for DD group); other scores are raw scores.
The DD and control groups did not differ in age or general intelligence. However, compared to the control group, the DD group exhibited a clear profile of reading disability conforming to the symptomatology of DD. They differed significantly from the control group on word reading and decoding skills in both rate and accuracy measures (see Table 2). In addition, the DD group showed characteristic deficits in the three major phonological domains: phonological awareness (spoonerisms), verbal short-term memory (Digit Span subtest), and rapid naming (rapid automatized naming task).
Note that all participants in the DD group were high-functioning university students with dyslexia. Prior studies of dyslexia revealed that such participants exhibit average performance on standardized reading tests (including that of low-frequency words, such as word identification from the Woodcock Reading Mastery Test–Revised) but nevertheless differ significantly from matched control groups and continue to present phonological problems that can be assessed by phonological tests, such as the Spoonerism Test (Wilson & Lesaux, 2001). Our participants with dyslexia fit this profile. Each individual had received a former diagnosis of dyslexia by a qualified psychologist. The DD group differed significantly from the control group in all literacy measures and exhibited phonological processing impairments (as indicted by the Spoonerism Test) despite average performance on standardized tests. This profile is clearly indicative of a sample of adults with dyslexia.
Materials
Speech Materials
The first aim was to examine if individuals with DD are sensitive to transitional probabilities across sequences of concatenated speech syllables. The stimulus materials were identical to those used by Saffran et al. (1996). The speech stream contained four consonants (/p/, /t/, /b/, /d/) and four vowels (/a/, /ae/, /i/, /u/) combined into four trisyllabic nonsense words (tupiro, golabu, bidaku, padoti). These trisyllabic stimuli were generated by a speech synthesizer in a monotone female voice at the rate of 270 syllables per minute, such that each syllable had an average duration of approximately 222 ms. Participants heard each of the four nonsense words 45 times (180 total words) in pseudorandom order over the course of 2 min of passive exposure, with the stipulations that the same word never occurred twice in a row and that transitions between words occurred approximately equally often. The synthesizer produced no acoustic cues to word boundaries, resulting in a continuous stream of sounds. All syllables were produced in a monotonic 200-Hz fundamental frequency with unchanging rate of speech and no pauses between words or syllables. The only cues for word boundaries were the transitional probabilities between pairs of syllables, which were higher within nonsense words (1.0) than between nonsense words (.33).
Two words (tupiro, bidaku) for which transitional probabilities among pairs of syllables were high (1.0) during familiarization, and two part-words (syllable sequences that occur across word boundaries during familiarization and therefore have lower transitional probabilities across syllables: tigola, bupado) were used as test items. In each trial, participants heard one word test item and one part-word test item and selected which sounded more familiar. Each word was paired with each part-word twice, and the order of item presentation was counterbalanced across pairings (such that one time the word was presented first and the other time the part-word was presented first) to create eight test items. Preliminary pilot data (N = 8) indicated that 2 min of exposure to the synthesized speech was sufficient to elicit significant learning among typically developing university students (with no history of learning disability) as indicated by above-chance performance on the forced-choice test items (M = 82%).
Nonspeech Materials
A second set of materials modeled the statistical structure of the speech materials, with nonlinguistic tones replacing the syllables. These materials were identical to those of Saffran et al. (1999). Each syllable comprising the speech materials was replaced with a unique tone (e.g., BU = D#). Each of the four trisyllabic nonsense words (e.g., bidaku) from the novel speech stream thereby was translated into a sequence of three tones (e.g., D#, F, E) with no silence between tones. The tone triads were concatenated without pauses in a pseudorandom order to generate a continuous stream. The order of the high transitional probability, statistically coherent tone-words was identical to the order of the words in the linguistic stimuli, resulting in a statistical structure identical to the syllable materials.
The 12 tones were drawn from an octave ranging between A at 440 Hz and G# at 831 Hz (A, 440 Hz; A#, 466 Hz; B, 494 Hz; C, 523 Hz; C#, 554 Hz; D, 587 Hz; D#, 622 Hz; E, 659 Hz; F, 698 Hz; F#, 740 Hz; G, 784 Hz; G#, 831 Hz). The tones were pure sine waves synthesized in Adobe Audition. On the basis of previous research, each tone was 330 ms (Saffran et al., 1999). Note that this is slightly longer than the duration of each syllable in the speech materials; prior research suggests that adults require tonal stimuli to be presented somewhat more slowly than speech stimuli to achieve equivalent performance. As such, although participants heard the same number of tone-words (180 total; 45 of each of the four triads) as in the speech materials, the overall duration of familiarization with the tone materials was longer (2 min and 50 s).
The test items were structured in the same way as the test items for the speech materials: eight two-alternative forced-choice items included both tonal words and tonal part-words. In the test phase, each trial consisted of a pair of tone triads. One was a tone-word from the familiarization stream with high (1.0 tone-to-tone transitional probabilities) statistical coherence. The other straddled a tone-word boundary in the familiarization stream and had low transitional probabilities (.33) within the triad. The test items included two tone-words (ADE and BFG) and two part-words (D#G#A# and F#CC#); each tone-word was paired with each part-word twice, with order counterbalanced. Preliminary pilot data (N = 8) suggested that the 2 min and 50 s of exposure to the synthesized tone stream was sufficient to elicit learning among typically developing university students (with no history of learning disability) as indicated by above-chance performance on the forced-choice test items (M = 82%).
Procedure
All testing took place in sound-attenuated booths with participants wearing headphones (Beyer, DT-150) while seated directly in front of a computer monitor. Stimulus presentation and the recording of response time and accuracy were controlled by a computer program (E-Prime; Schneider, Eschman, & Zuccolotto, 2002).
All participants first completed a familiarization phase, during which they listened passively to a sequence of sounds. Immediately after the familiarization phase, participants completed a two-alternative forced-choice test consisting of eight trials. Each trial consisted of a pair of stimuli: one word and one part-word. The participant's task was to judge which of the two sounds was most similar to the sounds heard during familiarization by pressing 1 or 2 on the computer keyboard to indicate the first or second sound. Trial order was randomized.
Each participant completed a familiarization segment and a forced-choice test for both speech and nonspeech materials. The order of the speech and nonspeech tasks was counterbalanced across participants. Analyses revealed no main effects or interactions with the order in which the two tasks were performed.
Results
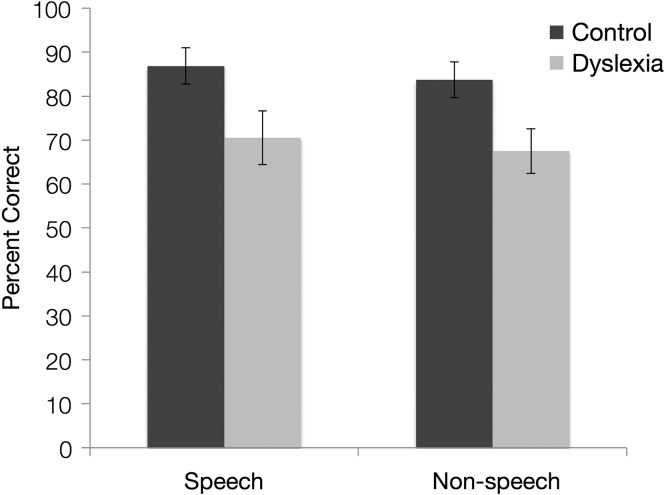
A mixed-model analysis of variance (ANOVA) with group (DD vs. controls) as a between-subjects factor and SL task (speech vs. nonspeech) as a within-subject factor, and accuracy across test trials as the dependent measure, was conducted. Results are presented in Figure 1. There was a main effect of group, F(1, 30) = 10.366, p = .003, ηp2 = .256, indicating that the DD group performed significantly less accurately (M = 69%) than the control group (M = 85%). There was no main effect of SL task and no interaction, F < 1.
Figure 1.
Average test trial accuracy of DD and control groups after familiarization with speech and nonspeech sequences. Error bars represent standard errors.
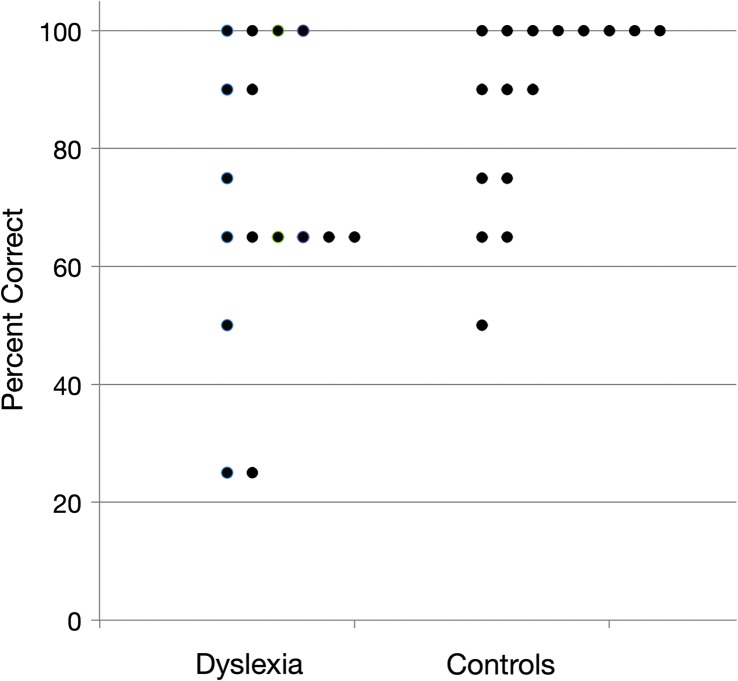
Further analyses were conducted to investigate whether accuracy at test in recognizing statistically coherent words from familiarization was above chance level (50%). Results for the speech materials are presented in Figure 2. Single-sample two-tailed t tests indicated that both groups exhibited SL at above-chance (50%) levels for speech materials. Overall, the DD group achieved 70% accuracy on test trials, t(15) = 3.35, p < .01, whereas the control group achieved 86% accuracy, t(15) = 8.85, p < .01.
Figure 2.
A scatter plot of test trial accuracy of the DD and control participants on the speech task.
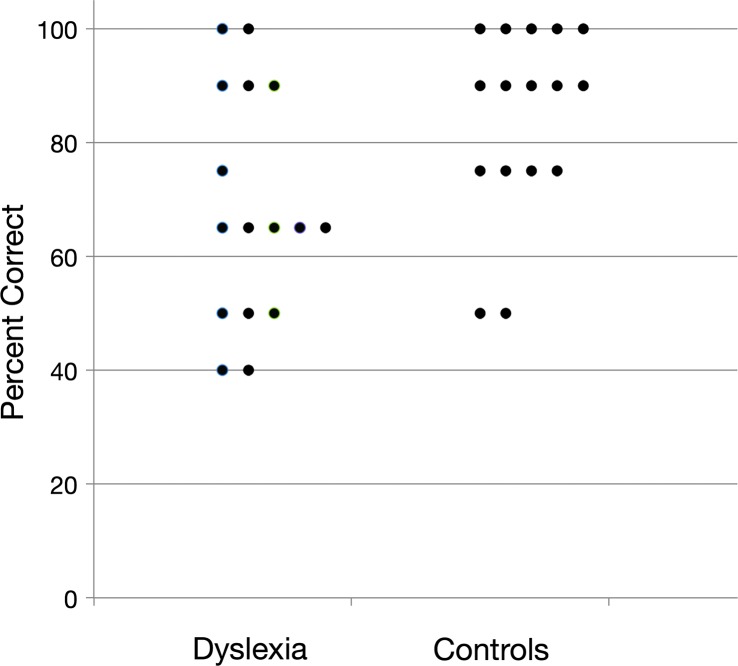
Results for nonspeech materials are presented in Figure 3. Single-sample two-tailed t tests indicated that both DD and control groups learned above chance level (50%). The DD group achieved 67% accuracy on test trials, t(15) = 3.44, p < .01, whereas the control group achieved 83% accuracy, t(15) = 8.27, p < .01.
Figure 3.
A scatter plot of test trial accuracy of the DD and control participants on the nonspeech task.
It is well established that participants can present performance variability during SL tasks and that participants without impairment can show learning at or below chance level. These participants are not excluded from analyses (Saffran et al., 1999; Schapiro, Gregory, Landau, McCloskey, & Turk-Browne, 2014) as in the above-mentioned ANOVA involving the full data set from all DD and control participants, because it is important to document this within- and between-group variability. The fact that more participants with than without dyslexia exhibited learning at or below chance level strengthens the argument that individuals with DD have difficulties in extracting statistical regularities. Nevertheless, we conducted an identical ANOVA excluding DD and control participants who were at or below chance level at least in one of the two SL tasks (seven DD, three control participants). Group differences were significant, F(1, 20) = 4.79, p = .041, ηp2 = .18, with DD learners presenting less SL learning (M = 80.1) compared with controls (M = 89.07).
Relationship Between SL and Individual Reading Ability
In addition to the group analysis reported above, we also examined the relationship between SL performance (accuracy across test trials) and reading ability as measured by four standardized tests: the (a) Word Identification and (b) Word Attack subtests from the Woodcock Reading Mastery Test–Revised and the (c) Sight Word Efficiency, Forms A+B (i.e., rate of word identification) and (d) Phonemic Decoding Efficiency, Forms A+B (i.e., rate of decoding pseudowords) subtests from the Test of Word Reading Efficiency. This analysis was motivated by previous research examining the relationship between visual SL and individual reading ability (Arciuli & Simpson, 2012). Examination of the correlations allowed us to investigate the relationship between individual reading ability and SL independent of diagnostic category. We also examined the correlation between SL performance and rapid automatized naming, because the procedural learning deficit account predicts a positive correlation between learning performance and the ability to automatically form visual–verbal associations.
Results are presented in Table 3. The performance on most reading standardized tests was positively correlated with both speech and nonspeech SL performance. To be specific, the Phonemic Decoding efficiency score (which measures the ability to read phonetically regular pseudowords quickly and accurately) as well as the Sight Word efficiency score (which measures the ability to read real words quickly and accurately) were both correlated with speech and nonspeech SL performance. Also the Word Attack score (which measures the ability to read pronounceable nonwords) was positively correlated with speech SL. Furthermore, the scores of the rapid automatized naming task (which measures the ability to automatically associate stimulus with their names) were positively correlated with both speech and nonspeech SL performance. This association was evident for most categories and was not confined to those that required grapheme–phoneme decoding, such as letters.
Table 3.
Correlation coefficients between speech and nonspeech statistical learning (SL) performance of the DD and control groups and psychometric tests.
| Measurement | Speech SL | Nonspeech SL |
|---|---|---|
| Standardized reading tests | ||
| WRMT-R WI | .193 | .281 |
| WRMT-R WA | .420** | .243 |
| TOWRE SW (A+B) | .370* | .355* |
| TOWRE PD (A+B) | .511** | .306* |
| Rapid naming | ||
| RAN objects | .221 | .397* |
| RAN colors | .439* | .345* |
| RAN numbers | .394* | .358* |
| RAN letters | .324* | .306* |
| Phonological awareness | ||
| Spoonerism time | −.285 | −.166 |
| Spoonerism accuracy | .340* | .054 |
| Verbal working memory | ||
| DS | .261 | .240 |
| Cognitive ability | ||
| Raven's SPM | −.147 | .037 |
Note. WRMT-R WI = Woodcock Reading Mastery Tests–Revised Word Identification subtest; WRMT-R WA = Woodcock Reading Mastery Tests–Revised Word Attack subtest; TOWRE SW = Test of Word Reading Efficiency Sight Word Efficiency subtest; TOWRE PD = Test of Word Reading Efficiency Phonemic Decoding Efficiency subtest; RAN = rapid automatized naming task; DS = Digit Span subtest from the Wechsler Adult Intelligence Scale; SPM = Standard Progressive Matrices.
p < .05,
p < .01.
Discussion
The purpose of the present research was to examine SL of transitional probabilities across speech and nonspeech materials among individuals with DD. Control listeners with normal reading abilities exhibited SL comparable to that observed in previous studies (Saffran et al., 1996, 1999). Their postfamiliarization recognition of statistically coherent triads of both syllables and tones was above chance and was statistically equivalent across sound types. It should be noted that this equivalence was achieved, on the basis of prior research (Saffran et al., 1999), via small methodological differences in the presentation rate of the speech and nonspeech materials. The DD group also learned both speech and nonspeech materials at above-chance levels. However, relative to control participants, the DD group performed more poorly. Learners with DD were equally impaired on sequential statistical learning for both speech and nonspeech materials. The fact that learners with DD exhibited general impairment across stimulus types suggests that speech and nonspeech materials do not represent a substantially different learning challenge for those with DD. Rather, it suggests a general impairment in extracting statistical structure from the acoustic environment. As a group, participants with DD performed above chance level on both SL tasks. This lessens the possibility that difficulties in processing linguistic/syntactic information are the driving force behind the poorer SL observed for individuals with DD, compared to control participants. The above-chance performance may also suggest that procedural learning abilities in high-functioning adults with DD are not absent but impaired or less efficient, compared with typical readers. Indeed, procedural learning impairment across different paradigms typically is measured as a degree of performance difference relative to controls. Several studies have demonstrated impaired (rather than absent) procedural learning in high-functioning adults with DD (Gabay, Vakil, Schiff, & Holt, 2015; Howard et al., 2006). An underlying dysfunction of procedural learning in DD may result in difficulty in extracting regularities, thereby disrupting the typical course of reading acquisition. Without an efficient learning system that can detect statistical regularities, individuals with DD may face greater difficulty in reading that is not entirely based on explicit instruction. Future studies should explore if longer exposure durations to statistical regularities might resolve the learning gap between the two groups. In addition, it is important to remember that our study involves a group of high-achieving young adults with dyslexia. It may be suggested that our results are all the stronger for being clearly evident in this group. Nevertheless, caution is warranted in extending these results broadly; further evidence will be necessary to determine if SL impairments (or even greater SL deficits, such as learning at or below chance level) will be observed in samples of people with dyslexia who are not high-functioning university students, such as children.
The equivalent degree of impairment in SL for speech syllables and nonspeech tones among the present DD listeners is difficult to reconcile with theories positing that DD arises due to impairments in phonological representation or processing (Snowling, 2000). Whereas impaired phonological processing in DD may be expected to affect SL across speech materials, a more general disruption of SL across both speech and nonspeech materials is problematic. However, inasmuch as SL of transitional probabilities presents an implicit learning challenge that makes demands on the procedural learning system, the common impairment across speech and nonspeech materials is consistent with theories suggesting a domain-general impairment in procedural learning in DD (Nicolson & Fawcett, 2011; Ullman, 2004). Indeed, previous research has shown that individuals with DD are significantly impaired on a variety of implicit learning tasks that require chunk formation, such as sequence learning (Gabay et al., 2012c; Howard et al., 2006; Lum et al., 2013; Stoodley et al., 2006, 2008; Vicari et al., 2005) as well as artificial grammar learning (Pavlidou & Williams, 2014). The current experiments extend those prior results with evidence that participants with DD are impaired at SL, a form of implicit learning that relies upon different processes, such as statistical computations, and is thought to be directly related to language acquisition. Further support for the procedural learning account could be pursued with tests of whether SL for nonauditory stimuli, such as sequentially presented shapes (e.g., Baldwin, Andersson, Saffran, & Meyer, 2008; Fiser & Aslin, 2002), is impaired in DD. Although studies demonstrate a positive relationship between visual SL and reading among unimpaired adult and child readers (Arciuli & Simpson, 2012), to date, research has not examined visual SL abilities among individuals with DD.
The impairment of SL among participants with DD and the observed positive correlation between reading fluency and SL performance support theoretical claims of a link between implicit learning and reading. To be specific, both speech and nonspeech SL performance were positively related to phonological decoding efficiency and sight word efficiency. Note that each of these correlated measures is an estimate of reading fluency, not accuracy. We predict a stronger association between SL performance and fluency—compared with accuracy—measures because of the adult college-level sample of the current study. Shaywitz (1998) has suggested that, over the course of development, readers with DD become more accurate, but reading remains slower, effortful, and nonautomatic. Thus, Shaywitz argues, timed reading measures (speed) must be used for the diagnosis of DD at the level of college or professional school. Because timed reading measures are the best predictors of DD among the type of high-functioning university students with reading difficulties that we tested, the relationship of these measures to SL impairment is supporting evidence of the relationship of SL to DD. This observation could also be considered with regard to the fact that in the present data an accuracy measure (for example, the Word Attack task) was correlated only with performance on speech SL and not with performance on nonspeech SL. It may be that fluency measures provide a finer, more sensitive assay of DD impairment among the university-level adults we tested here. This possibility should be considered with caution due to the modest sample size employed in the current study, but it informs future research.
How Might Atypical SL Affect Linguistic Development?
The observation that adults with DD are less efficient at tracking transitional probabilities across acoustic elements suggests a number of possible routes to the poor language outcomes that typify DD. First, impairment in the ability to learn from statistical structure in the acoustic environment may influence lexical development directly by reducing the ability to extract word forms from fluent speech. This could lead to a smaller vocabulary and more difficulty in processing lexical items. Given that lexical development interacts with phonological development (Stoel-Gammon, 2011), one possibility is that inefficiency in SL may result in smaller or less robust vocabularies, which could affect the resolution of phonological representations. This could produce phonological processing deficits characteristic of DD. This relationship might also present in the opposite direction with poor phonological skills affecting vocabulary development (Aguiar & Brady, 1991). Independent of the casual relationship between phonological processing and vocabulary, research has demonstrated slow lexical development in children with DD (Lyytinen & Lyytinen, 2004; Scarborough, 1990). As an alternative, impaired SL might influence learning to read directly by reducing sensitivity to the statistical regularities that exist among letters and phonemes, including the discovery of phonological rules. Last, the acquisition of phonological categories involves two important processes. Listeners must discover functional units of language from a continuous sound stream without a priori knowledge of the temporal time window that characterizes these units (segmentation). Moreover, because the detailed acoustics of these units varies across instances as a function of a preceding context, different talkers, and speech rates, listeners must generalize from highly variably experienced acoustics to new exemplars (categorization). The current study demonstrated impairment in SL that could support segmentation, which, in turn, could lead to problems in acquisition of phonetic categories among learners with DD. Future studies should also explore how DD learners cope with speech category learning and how it affects the resolution of phonological representations. As we noted above, the present results are consistent with a general procedural learning impairment among individuals with DD. Because language learning is likely to place many demands on the procedural learning system beyond segmentation of fluent acoustic streams, poor SL performance among individuals with DD may be but one of a larger constellation of impairments arising from a more general cause.
Although the present data do not definitively determine the nature of the relationship of DD and SL, they are significant in that they establish for the first time that participants with DD are poorer at extracting statistical regularities from fluent sound streams than are typical readers. The fact that this impairment is general and not specific to phonological processing is of particular importance. The results are consistent with evidence that general procedural learning is impaired in those with DD and support the possibility that tracking transitional probabilities of elements within acoustic streams is related to reading competence.
Implications for Domain-General Versus Domain-Specific Debate
SL is believed to involve a domain-general mechanism by which the cognitive system discovers the underlying distributional properties of the input. However, current evidence suggests that SL emerges from local computations carried out within a modality and via a multidomain neurocognitive system that either modulates or operates on inputs from modality-specific representations (for reviews, see Conway & Pisoni, 2008; Frost, Armstrong, Siegelman, & Christiansen, 2015). As such, problems with SL could stem from both modality-constrained learning processes and global, higher-order learning processes. For example, previous research has shown that two sequences of statistical regularities can be learned simultaneously across modalities as well as within a modality, as long as vocabularies do not share the same perceptual dimensions (for example, two different sequential structures from the same modality: tone vs. nonwords or visual shapes vs. color). However, learning suffers when the two within-modality sequences share the same perceptual dimension (Conway & Christiansen, 2006).
Examination of statistical learning in populations with reading impairment could inform us about the domain-general versus domain-specific debate. A general deficit across sensory modalities or different perceptual dimensions would be in favor of a domain-general system acting upon statistical regularities. Our current investigation was not designed to empirically test this question. However, the fact that SL impairments among individuals with DD were not confined to speech materials implies an impairment affecting domain-general learning processes. Targeted research examining SL among impaired readers for sequences within and across modalities with perceptual dimensions that are orthogonal or overlapping will be informative in relating how domain-general deficits may affect language and reading development.
Acknowledgment
This work was supported by the National Institutes of Health Grant R01DC004674 to L.L.H.
Funding Statement
This work was supported by the National Institutes of Health Grant R01DC004674 to L.L.H.
References
- Aguiar L., & Brady S. (1991). Vocabulary acquisition and reading ability. Reading and Writing, 3, 413–425. [Google Scholar]
- Alvarez P., & Squire L. R. (1994). Memory consolidation and the medial temporal lobe: A simple network model. Proceedings of the National Academy of Sciences, 91, 7041–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciuli J., & Simpson I. C. (2012). Statistical learning is related to reading ability in children and adults. Cognitive Science, 36, 286–304. [DOI] [PubMed] [Google Scholar]
- Baldwin D., Andersson A., Saffran J., & Meyer M. (2008). Segmenting dynamic human action via statistical structure. Cognition, 106, 1382–1407. [DOI] [PubMed] [Google Scholar]
- Biemiller A. (2003). Vocabulary: Needed if more children are to read well. Reading Psychology, 24, 323–335. [Google Scholar]
- Brookes R. L., Nicolson R. I., & Fawcett A. J. (2007). Prisms throw light on developmental disorders. Neuropsychologia, 45, 1921–1930. [DOI] [PubMed] [Google Scholar]
- Brunswick N., McCrory E., Price C., Frith C., & Frith U. (1999). Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke's Wortschatz? Brain, 122, 1901–1917. [DOI] [PubMed] [Google Scholar]
- Buchner A., Steffens M. C., & Rothkegel R. (1998). On the role of fragmentary knowledge in a sequence learning task. The Quarterly Journal of Experimental Psychology, 51(A), 251–281. [Google Scholar]
- Coltheart M., Rastle K., Perry C., Langdon R., & Ziegler J. (2001). DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review, 108, 204–256. [DOI] [PubMed] [Google Scholar]
- Conway C. M., & Christiansen M. H. (2006). Statistical learning within and between modalities pitting abstract against stimulus-specific representations. Psychological Science, 17, 905–912. [DOI] [PubMed] [Google Scholar]
- Conway C. M., & Pisoni D. B. (2008). Neurocognitive basis of implicit learning of sequential structure and its relation to language processing. Annals of the New York Academy of Sciences, 1145, 113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Démonet J.-F., Taylor M. J., & Chaix Y. (2004). Developmental dyslexia. The Lancet, 363, 1451–1460. [DOI] [PubMed] [Google Scholar]
- Denckla M. B., & Rudel R. G. (1976). Rapid ‘automatized’ naming (R.A.N.): Dyslexia differentiated from other learning disabilities. Neuropsychologia, 14, 471–479. [DOI] [PubMed] [Google Scholar]
- Doyon J., & Benali H. (2005). Reorganization and plasticity in the adult brain during learning of motor skills. Current Opinion in Neurobiology, 15, 161–167. [DOI] [PubMed] [Google Scholar]
- Doyon J., Penhune V., & Ungerleider L. G. (2003). Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia, 41, 252–262. [DOI] [PubMed] [Google Scholar]
- Evans J. L., Saffran J. R., & Robe-Torres K. (2009). Statistical learning in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 52, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facoetti A., Paganoni P., Turatto M., Marzola V., & Mascetti G. G. (2000). Visual-spatial attention in developmental dyslexia. Cortex, 36, 109–123. [DOI] [PubMed] [Google Scholar]
- Fawcett A. J., & Nicolson R. I. (1994). Naming speed in children with dyslexia. Journal of Learning Disabilities, 27, 641–646. [DOI] [PubMed] [Google Scholar]
- Fawcett A. J., & Nicolson R. I. (1995). Persistent deficits in motor skill of children with dyslexia. Journal of Motor Behavior, 27, 235–240. [Google Scholar]
- Fiser J., & Aslin R. N. (2001). Unsupervised statistical learning of higher-order spatial structures from visual scenes. Psychological Science, 12, 499–504. [DOI] [PubMed] [Google Scholar]
- Fiser J., & Aslin R. N. (2002). Statistical learning of higher-order temporal structure from visual shape sequences. Journal of Experimental Psychology: Learning, Memory, and Cognition, 28, 458–467. [DOI] [PubMed] [Google Scholar]
- Frost R., Armstrong B. C., Siegelman N., & Christiansen M. H. (2015). Domain generality versus modality specificity: The paradox of statistical learning. Trends in Cognitive Sciences, 19, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R., Siegelman N., Narkiss A., & Afek L. (2013). What predicts successful literacy acquisition in a second language? Psychological Science, 24, 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay Y., Schiff R., & Vakil E. (2012a). Attentional requirements during acquisition and consolidation of a skill in normal readers and developmental dyslexics. Neuropsychology, 26, 744–757. [DOI] [PubMed] [Google Scholar]
- Gabay Y., Schiff R., & Vakil E. (2012b). Dissociation between online and offline learning in developmental dyslexia. Journal of Clinical and Experimental Neuropsychology, 34, 279–288. [DOI] [PubMed] [Google Scholar]
- Gabay Y., Schiff R., & Vakil E. (2012c). Dissociation between the procedural learning of letter names and motor sequences in developmental dyslexia. Neuropsychologia, 50, 2435–2441. [DOI] [PubMed] [Google Scholar]
- Gabay Y., Vakil E., Schiff R., & Holt L.L. (2015) Probabalistic category learning in developmental dyslexia: Evidence from feedback and paired-associate weather prediction tasks. Neuropsychology [Epub ahead of print]. PMID: 25730732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T., Friederici A. D., Kotz S. A., & Van Kampen A. (2001). Procedural learning in Broca's aphasia: Dissociation between the implicit acquisition of spatio-motor and phoneme sequences. Journal of Cognitive Neuroscience, 13, 370–388. [DOI] [PubMed] [Google Scholar]
- Hedenius M., Persson J., Alm P. A., Ullman M. T., Howard J. H. Jr., Howard D. V., & Jennische M. (2013). Impaired implicit sequence learning in children with developmental dyslexia. Research in Developmental Disabilities, 34, 3924–3935. [DOI] [PubMed] [Google Scholar]
- Helland T., & Asbjørnsen A. (2000). Executive functions in dyslexia. Child Neuropsychology, 6, 37–48. [DOI] [PubMed] [Google Scholar]
- Howard J. H., Howard D. V., Japikse K. C., & Eden G. F. (2006). Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia, 44, 1131–1144. [DOI] [PubMed] [Google Scholar]
- Karuza E. A., Newport E. L., Aslin R. N., Starling S. J., Tivarus M. E., & Bavelier D. (2013). The neural correlates of statistical learning in a word segmentation task: An fMRI study. Brain and Language, 127, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. W., Griffiths S., & Frith U. (2002). Evidence for implicit sequence learning in dyslexia. Dyslexia, 8, 43–52. [DOI] [PubMed] [Google Scholar]
- Kita Y., Yamamoto H., Oba K., Terasawa Y., Moriguchi Y., Uchiyama H., … Inagaki M. (2013). Altered brain activity for phonological manipulation in dyslexic Japanese children. Brain, 136, 3696–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S. D., De Renzi E., & Richman L. C. (1985). Cross-national comparisons of developmental dyslexia in Italy and the United States. Child Development, 56, 1404–1417. [PubMed] [Google Scholar]
- Lum J. A., Ullman M. T., & Conti-Ramsden G. (2013). Procedural learning is impaired in dyslexia: Evidence from a meta-analysis of serial reaction time studies. Research in Developmental Disabilities, 34, 3460–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyytinen P., & Lyytinen H. (2004). Growth and predictive relations of vocabulary and inflectional morphology in children with and without familial risk for dyslexia. Applied Psycholinguistics, 25, 397–411. [Google Scholar]
- Maddox W. T., & Ashby F. G. (2004). Dissociating explicit and procedural-learning based systems of perceptual category learning. Behavioural Processes, 66, 309–332. [DOI] [PubMed] [Google Scholar]
- McClelland J. L., & Patterson K. (2002). Rules or connections in past-tense inflections: What does the evidence rule out? Trends in Cognitive Sciences, 6, 465–472. [DOI] [PubMed] [Google Scholar]
- Menghini D., Hagberg G. E., Caltagirone C., Petrosini L., & Vicari S. (2006). Implicit learning deficits in dyslexic adults: An fMRI study. Neuroimage, 33, 1218–1226. [DOI] [PubMed] [Google Scholar]
- Middleton F. A., & Strick P. L. (2000). Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Research Reviews, 31, 236–250. [DOI] [PubMed] [Google Scholar]
- Morris R. D., Stuebing K. K., Fletcher J. M., Shaywitz S. E., Lyon G. R., Shankweiler D. P., … Shaywitz B. A. (1998). Subtypes of reading disability: Variability around a phonological core. Journal of Educational Psychology, 90, 347–373. [Google Scholar]
- Newport E. L., & Aslin R. N. (2004). Learning at a distance: I. Statistical learning of non-adjacent dependencies. Cognitive Psychology, 48, 127–162. [DOI] [PubMed] [Google Scholar]
- Nicolson R., & Fawcett A. (2010). Dyslexia, learning, and the brain. Cambridge, MA: MIT Press. [Google Scholar]
- Nicolson R. I., & Fawcett A. J. (1994). Comparison of deficits in cognitive and motor skills among children with dyslexia. Annals of Dyslexia, 44, 147–164. [DOI] [PubMed] [Google Scholar]
- Nicolson R. I., & Fawcett A. J. (2011). Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex, 47, 117–127. [DOI] [PubMed] [Google Scholar]
- Nicolson R. I., Fawcett A. J., Berry E. L., Jenkins I. H., Dean P., & Brooks D. J. (1999). Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. The Lancet, 353, 1662–1667. [DOI] [PubMed] [Google Scholar]
- Paulesu E., Frith U., Snowling M., Gallagher A., Morton J., Frackowiak R. S., & Frith C. D. (1996). Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain, 119, 143–157. [DOI] [PubMed] [Google Scholar]
- Pavlidou E. V., & Williams J. M. (2014). Implicit learning and reading: Insights from typical children and children with developmental dyslexia using the artificial grammar learning (AGL) paradigm. Research in Developmental Disabilities, 35, 1457–1472. [DOI] [PubMed] [Google Scholar]
- Pernet C. R., Poline J. B., Démonet J.-F., & Rousselet G. A. (2009). Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neuroscience, 10, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruchet P., & Pacton S. (2006). Implicit learning and statistical learning: One phenomenon, two approaches. Trends in Cognitive Sciences, 10, 233–238. [DOI] [PubMed] [Google Scholar]
- Pothos E. M., & Bailey T. M. (2000). The role of similarity in artificial grammar learning. Journal of Experimental Psychology: Learning, Memory, and Cognition, 26, 847–862. [DOI] [PubMed] [Google Scholar]
- Pugh K. R., Frost S. J., Rothman D. L., Hoeft F., Del Tufo S. N., Mason G. F., … Landi N. (2014). Glutamate and choline levels predict individual differences in reading ability in emergent readers. The Journal of Neuroscience, 34, 4082–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C., Lee M. A., Dixon R. M., Blamire A. M., Thompson C. H., Styles P., … Stein J. F. (1998). Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. The Lancet, 351, 1849–1852. [DOI] [PubMed] [Google Scholar]
- Ramus F., Rosen S., Dakin S. C., Day B. L., Castellote J. M., White S., & Frith U. (2003). Theories of developmental dyslexia: Insights from a multiple case study of dyslexic adults. Brain, 126, 841–865. [DOI] [PubMed] [Google Scholar]
- Raven J., Court J. H., & Raven J. (1992). Standard Progressive Matrices. Oxford, England: Oxford Psychologists Press. [Google Scholar]
- Reber A. S. (1967). Implicit learning of artificial grammars. Journal of Verbal Learning and Verbal Behavior, 6, 855–863. [Google Scholar]
- Rüsseler J., Gerth I., & Münte T. F. (2006). Implicit learning is intact in adult developmental dyslexic readers: Evidence from the serial reaction time task and artificial grammar learning. Journal of Clinical and Experimental Neuropsychology, 28, 808–827. [DOI] [PubMed] [Google Scholar]
- Saffran J. R., Aslin R. N., & Newport E. L. (1996, December 13). Statistical learning by 8-month-old infants. Science, 274, 1926–1928. [DOI] [PubMed] [Google Scholar]
- Saffran J. R., Johnson E. K., Aslin R. N., & Newport E. L. (1999). Statistical learning of tone sequences by human infants and adults. Cognition, 70, 27–52. [DOI] [PubMed] [Google Scholar]
- Saffran J. R., Newport E. L., Aslin R. N., Tunick R. A., & Barrueco S. (1997). Incidental language learning: Listening (and learning) out of the corner of your ear. Psychological Science, 8, 101–105. [Google Scholar]
- Scarborough H. S. (1990). Very early language deficits in dyslexic children. Child Development, 61, 1728–1743. [PubMed] [Google Scholar]
- Schapiro A. C., Gregory E., Landau B., McCloskey M., & Turk-Browne N. B. (2014). The necessity of the medial temporal lobe for statistical learning. Journal of Cognitive Neuroscience, 26, 1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W., Eschman A., & Zuccolotto A. (2002). E-Prime reference guide. Pittsburgh, PA: Psychology Software Tools. [Google Scholar]
- Seger C. A. (2008). How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neuroscience & Biobehavioral Reviews, 32, 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M. S., & McClelland J. L. (1989). A distributed, developmental model of word recognition and naming. Psychological Review, 96, 523–568. [DOI] [PubMed] [Google Scholar]
- Shanks D. R. (2003). Attention and awareness in “implicit” sequence learning. Advances in Consciousness Research, 48, 11–42. [Google Scholar]
- Shaywitz S. E. (1998). Dyslexia. New England Journal of Medicine, 338, 307–312. [DOI] [PubMed] [Google Scholar]
- Shaywitz S. E., & Shaywitz B. A. (2005). Dyslexia (specific reading disability). Biological Psychiatry, 57, 1301–1309. [DOI] [PubMed] [Google Scholar]
- Shohamy D., Myers C., Onlaor S., & Gluck M. (2004). Role of the basal ganglia in category learning: How do patients with Parkinson's disease learn? Behavioral Neuroscience, 118, 676–686. [DOI] [PubMed] [Google Scholar]
- Snowling M. J. (2000). Dyslexia. Oxford, England: Blackwell. [Google Scholar]
- Song S. (2009). Consciousness and the consolidation of motor learning. Behavioural Brain Research, 196, 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoel-Gammon C. (2011). Relationships between lexical and phonological development in young children. Journal of Child Language, 38(1), 1–34. [DOI] [PubMed] [Google Scholar]
- Stoodley C. J., Harrison E. P., & Stein J. F. (2006). Implicit motor learning deficits in dyslexic adults. Neuropsychologia, 44, 795–798. [DOI] [PubMed] [Google Scholar]
- Stoodley C. J., Ray N. J., Jack A., & Stein J. F. (2008). Implicit learning in control, dyslexic, and garden‐variety poor readers. Annals of the New York Academy of Sciences, 1145, 173–183. [DOI] [PubMed] [Google Scholar]
- Strick P. L., Dum R. P., & Fiez J. A. (2009). Cerebellum and nonmotor function. Annual Review of Neuroscience, 32, 413–434. [DOI] [PubMed] [Google Scholar]
- Thiessen E. D., Kronstein A. T., & Hufnagle D. G. (2013). The extraction and integration framework: A two-process account of statistical learning. Psychological Bulletin, 139, 792–814. [DOI] [PubMed] [Google Scholar]
- Tillmann B., & McAdams S. (2004). Implicit learning of musical timbre sequences: Statistical regularities confronted with acoustical (dis)similarities. Journal of Experimental Psychology: Learning, Memory, and Cognition, 30, 1131–1142. [DOI] [PubMed] [Google Scholar]
- Torgesen J. K., Wagner R., & Rashotte C. (1999). Test of Word Reading Efffciency. Austin, TX: Pro-Ed. [Google Scholar]
- Turk-Browne N. B., Jungé J. A., & Scholl B. J. (2005). The automaticity of visual statistical learning. Journal of Experimental Psychology: General, 134, 552–564. [DOI] [PubMed] [Google Scholar]
- Turk-Browne N. B., Scholl B. J., Chun M. M., & Johnson M. K. (2009). Neural evidence of statistical learning: Efficient detection of visual regularities without awareness. Journal of Cognitive Neuroscience, 21, 1934–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman M. T. (2001). A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience, 2, 717–726. [DOI] [PubMed] [Google Scholar]
- Ullman M. T. (2004). Contributions of memory circuits to language: The declarative/procedural model. Cognition, 92, 231–270. [DOI] [PubMed] [Google Scholar]
- Ungerleider L. G., Doyon J., & Karni A. (2002). Imaging brain plasticity during motor skill learning. Neurobiology of Learning and Memory, 78, 553–564. [DOI] [PubMed] [Google Scholar]
- Vellutino F. R., Fletcher J. M., Snowling M. J., & Scanlon D. M. (2004). Specific reading disability (dyslexia): What have we learned in the past four decades? Journal of Child Psychology and Psychiatry, 45, 2–40. [DOI] [PubMed] [Google Scholar]
- Vicari S., Finzi A., Menghini D., Marotta L., Baldi S., & Petrosini L. (2005). Do children with developmental dyslexia have an implicit learning deficit? Journal of Neurology, Neurosurgery & Psychiatry, 76, 1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari S., Marotta L., Menghini D., Molinari M., & Petrosini L. (2003). Implicit learning deficit in children with developmental dyslexia. Neuropsychologia, 41, 108–114. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1997). WAIS-III, Wechsler Adult Intelligence Scale: Administration and scoring manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Wilson A. M., & Lesaux N. K. (2001). Persistence of phonological processing deficits in college students with dyslexia who have age-appropriate reading skills. Journal of Learning Disabilities, 34, 394–400. [DOI] [PubMed] [Google Scholar]
- Wolf M., & Denckla M. B. (2005). RAN/RAS: Rapid automatized naming and rapid alternating stimulus tests. Austin, TX: Pro-Ed. [Google Scholar]
- Woodcock R. W. (1987). Woodcock Reading Mastery Tests–Revised. Circle Pines, MN: AGS. [Google Scholar]