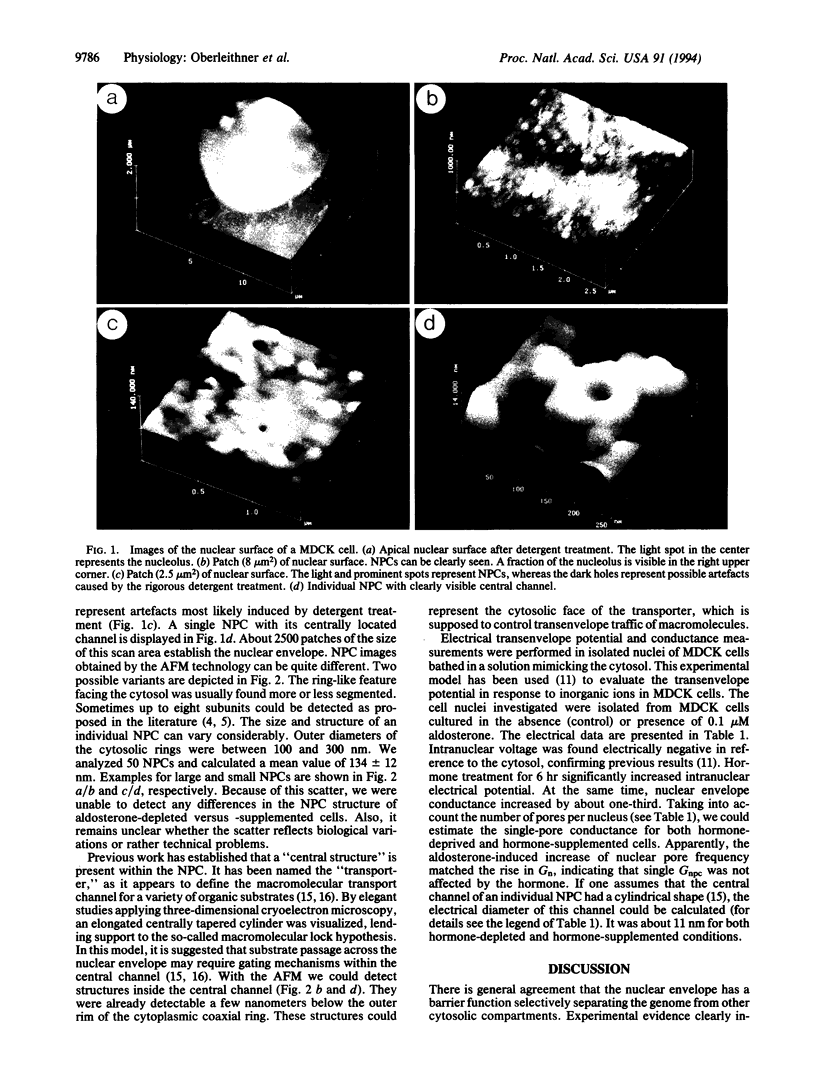
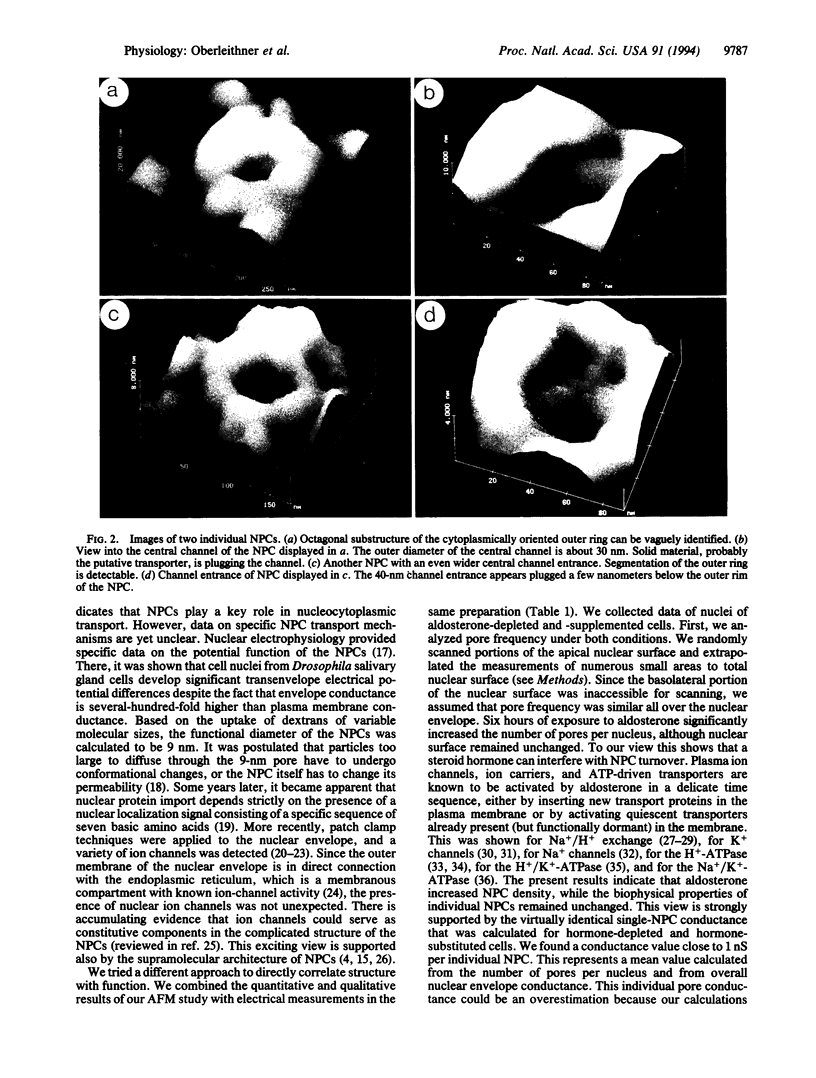
Abstract
In nuclei of renal target cells, aldosterone enhances transcriptional activity followed by the translocation of specific RNA molecules across the nuclear envelope. Trafficking between cell nucleus and cytoplasm occurs via nuclear pore complexes (NPCs) located in the double-layered nuclear envelope. We investigated the nucleocytoplasmic transport route by structure-function analysis at subcellular level in quiescent and aldosterone-stimulated cells. With atomic-force microscopy (AFM) we imaged individual pores of the nuclear surface of cultured kidney cells and related the number of pores per micron2 to nuclear envelope conductance (Gn, per micron2) evaluated electrically by current injection into the isolated nucleus. NPCs were equally distributed resembling "donut-like" structures with outer diameters of 134 +/- 12 nm (n = 50), each equipped with a central channel. Six hours of aldosterone exposure (0.1 microM) increased the number of NPCs per micron 2 of nuclear surface from 7.4 +/- 0.4 to 9.8 +/- 0.4 (n = 12; P < 0.01). At the same time Gn rose from 6900 +/- 520 to 9600 +/- 610 pS/micron2 paralleled by an increase of the intranuclear electrical potential from -2.8 +/- 0.2 to -6.2 +/- 0.4 mV (n = 18; P < 0.01). Assuming that NPCs represent the sole conductive pathway in the nuclear envelope, we calculate a mean single NPC conductance of 932 and 980 pS, in the absence and presence of aldosterone, respectively. We conclude that aldosterone facilitates nucleocytoplasmic transport by increasing the number of NPCs but not by modifying their biophysical properties. Possibly, aldosterone controls similar transport mechanisms in both plasma membrane and nuclear envelope.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akey C. W. Interactions and structure of the nuclear pore complex revealed by cryo-electron microscopy. J Cell Biol. 1989 Sep;109(3):955–970. doi: 10.1083/jcb.109.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey C. W., Radermacher M. Architecture of the Xenopus nuclear pore complex revealed by three-dimensional cryo-electron microscopy. J Cell Biol. 1993 Jul;122(1):1–19. doi: 10.1083/jcb.122.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akey C. W. Visualization of transport-related configurations of the nuclear pore transporter. Biophys J. 1990 Aug;58(2):341–355. doi: 10.1016/S0006-3495(90)82381-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Awqati Q., Norby L. H., Mueller A., Steinmetz P. R. Characteristics of stimulation of H+ transport by aldosterone in turtle urinary bladder. J Clin Invest. 1976 Aug;58(2):351–358. doi: 10.1172/JCI108479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustamante J. O. Nuclear electrophysiology. J Membr Biol. 1994 Mar;138(2):105–112. doi: 10.1007/BF00232638. [DOI] [PubMed] [Google Scholar]
- Bustamante J. O. Restricted ion flow at the nuclear envelope of cardiac myocytes. Biophys J. 1993 Jun;64(6):1735–1749. doi: 10.1016/S0006-3495(93)81545-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H. J., Wolff E. K., Gould S. A., Dixon Northern B., Peterson C. M., Hansma P. K. Imaging cells with the atomic force microscope. J Struct Biol. 1990 Oct-Dec;105(1-3):54–61. doi: 10.1016/1047-8477(90)90098-w. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Price T. M., Forbes D. J. Inhibition of in vitro nuclear transport by a lectin that binds to nuclear pores. J Cell Biol. 1987 Feb;104(2):189–200. doi: 10.1083/jcb.104.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanover J. A. The nuclear pore: at the crossroads. FASEB J. 1992 Mar;6(6):2288–2295. doi: 10.1096/fasebj.6.6.1312045. [DOI] [PubMed] [Google Scholar]
- Harvey B. J. Energization of sodium absorption by the H(+)-ATPase pump in mitochondria-rich cells of frog skin. J Exp Biol. 1992 Nov;172:289–309. doi: 10.1242/jeb.172.1.289. [DOI] [PubMed] [Google Scholar]
- Hinshaw J. E., Carragher B. O., Milligan R. A. Architecture and design of the nuclear pore complex. Cell. 1992 Jun 26;69(7):1133–1141. doi: 10.1016/0092-8674(92)90635-p. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., Hansma P. K. Atomic force microscopy for high-resolution imaging in cell biology. Trends Cell Biol. 1992 Jul;2(7):208–213. doi: 10.1016/0962-8924(92)90248-l. [DOI] [PubMed] [Google Scholar]
- Innocenti B., Mazzanti M. Identification of a nucleo-cytoplasmic ionic pathway by osmotic shock in isolated mouse liver nuclei. J Membr Biol. 1993 Jan;131(2):137–142. doi: 10.1007/BF02791322. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., KANNO Y. The electrical conductance and potential across the membrane of some cell nuclei. J Cell Biol. 1963 Feb;16:421–425. doi: 10.1083/jcb.16.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke A. J., Weiger T. M., Matzke M. A. Detection of a large cation-selective channel in nuclear envelopes of avian erythrocytes. FEBS Lett. 1990 Oct 1;271(1-2):161–164. doi: 10.1016/0014-5793(90)80397-2. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Cohn J., Malter H. Ion channels in the nuclear envelope. Nature. 1990 Feb 22;343(6260):764–767. doi: 10.1038/343764a0. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., Innocenti B., Rigatelli M. ATP-dependent ionic permeability on nuclear envelope in in situ nuclei of Xenopus oocytes. FASEB J. 1994 Feb;8(2):231–236. doi: 10.1096/fasebj.8.2.7509760. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Giebisch G., Geibel J. Imaging the lamellipodium of migrating epithelial cells in vivo by atomic force microscopy. Pflugers Arch. 1993 Dec;425(5-6):506–510. doi: 10.1007/BF00374878. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Schuricht B., Wünsch S., Schneider S., Püschel B. Role of H+ ions in volume and voltage of epithelial cell nuclei. Pflugers Arch. 1993 Apr;423(1-2):88–96. doi: 10.1007/BF00374965. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Steigner W., Silbernagl S., Vogel U., Gstraunthaler G., Pfaller W. Madin-Darby canine kidney cells. III. Aldosterone stimulates an apical H+/K+ pump. Pflugers Arch. 1990 Jul;416(5):540–547. doi: 10.1007/BF00382687. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Weigt M., Westphale H. J., Wang W. Aldosterone activates Na+/H+ exchange and raises cytoplasmic pH in target cells of the amphibian kidney. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1464–1468. doi: 10.1073/pnas.84.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Wünsch S., Schneider S. Patchy accumulation of apical Na+ transporters allows cross talk between extracellular space and cell nucleus. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):241–245. doi: 10.1073/pnas.89.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Panté N., Aebi U. The nuclear pore complex. J Cell Biol. 1993 Sep;122(5):977–984. doi: 10.1083/jcb.122.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pácha J., Frindt G., Antonian L., Silver R. B., Palmer L. G. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol. 1993 Jul;102(1):25–42. doi: 10.1085/jgp.102.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmacher M., Tillamnn R. W., Fritz M., Gaub H. E. From molecules to cells: imaging soft samples with the atomic force microscope. Science. 1992 Sep 25;257(5078):1900–1905. doi: 10.1126/science.1411505. [DOI] [PubMed] [Google Scholar]
- Schmid A., Gögelein H., Kemmer T. P., Schulz I. Anion channels in giant liposomes made of endoplasmic reticulum vesicles from rat exocrine pancreas. J Membr Biol. 1988 Sep;104(3):275–282. doi: 10.1007/BF01872329. [DOI] [PubMed] [Google Scholar]
- Verrey F., Schaerer E., Zoerkler P., Paccolat M. P., Geering K., Kraehenbuhl J. P., Rossier B. C. Regulation by aldosterone of Na+,K+-ATPase mRNAs, protein synthesis, and sodium transport in cultured kidney cells. J Cell Biol. 1987 May;104(5):1231–1237. doi: 10.1083/jcb.104.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella S., Guerra L., Helmle-Kolb C., Murer H. Aldosterone actions on basolateral Na+/H+ exchange in Madin-Darby canine kidney cells. Pflugers Arch. 1992 Oct;422(1):9–15. doi: 10.1007/BF00381507. [DOI] [PubMed] [Google Scholar]
- Wang W. H., Henderson R. M., Geibel J., White S., Giebisch G. Mechanism of aldosterone-induced increase of K+ conductance in early distal renal tubule cells of the frog. J Membr Biol. 1989 Nov;111(3):277–289. doi: 10.1007/BF01871012. [DOI] [PubMed] [Google Scholar]
- Zasloff M. tRNA transport from the nucleus in a eukaryotic cell: carrier-mediated translocation process. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6436–6440. doi: 10.1073/pnas.80.21.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]