Abstract
A robust blood biomarker is urgently needed to facilitate early prognosis for those at risk for Alzheimer’s disease (AD). Redox reactive autoantibodies (R-RAAs) represent a novel family of antibodies detectable only after exposure of cerebrospinal fluid (CSF), serum, plasma or immunoglobulin fractions to oxidizing agents. We have previously reported that R-RAA antiphospholipid antibodies (aPLs) are significantly decreased in the CSF and serum of AD patients compared to healthy controls (HCs). These studies were extended to measure R-RAA aPL in serum samples obtained from Alzheimer’s Disease Neuroimaging Initiative (ADNI). Serum samples from the ADNI-1 diagnostic groups from participants with mild cognitive impairment (MCI), AD and HCs were blinded for diagnosis and analyzed for R-RAA aPL by ELISA. Demographics, cognitive data at baseline and yearly follow-up were subsequently provided by ADNI after posting assay data. As observed in CSF, R-RAA aPL in sera from the AD diagnostic group were significantly reduced compared to HC. However, the sera from the MCI population contained significantly elevated R-RAA aPL activity relative to AD patient and/or HC sera. The data presented in this study indicate that R-RAA aPL show promise as a blood biomarker for detection of early AD, and warrant replication in a larger sample. Longitudinal testing of an individual for increases in R-RAA aPL over a previously established baseline may serve as a useful early sero-epidemiologic blood biomarker for individuals at risk for developing dementia of the Alzheimer’s type.
Keywords: ELISA, mild cognitive impairment, neurodegenerative disease, redox-reactive antiphospholipid autoantibodies, serum biomarkers
Introduction
Treating Alzheimer’s disease (AD) before the first appearance of cognitive symptomatology is widely considered to be essential in maximizing therapeutic benefit of compounds currently under development that are aimed at either halting disease progression and/or at least modifying the rate of cognitive decline [1]. The sensitivities of cerebrospinal fluid (CSF) biomarkers and brain imaging technologies to detect early stage AD and progression are improving, but fall short of being used as standard screening techniques. At present there are no established biomarkers in blood that have been replicated in larger studies and have proven useful clinically to identify individuals at risk for developing AD.
Several serum markers have been described which may arise from inflammatory events in the central nervous system in the early course of AD [2–11]. Approaches using serum matrix analysis of multiple analytes [12–17] show promise in developing early detection biomarker panels incorporating both inflammatory and other protein biomarkers in the serum. Analysis of plasma exosomal content for microRNA (miRNA) [18,19] and pathogenic proteins [20,21] are currently undergoing evaluation for early diagnosis of AD.
At present, the fundamental pathophysiological events that give rise to neuronal cell death in AD are unknown. Bruce-Keller and co-workers [22] have reported significant elevations in NADPH oxidase (NOX) activity in the temporal gyri of mild cognitive impairment (MCI) patients. There are also confirmed studies to show that oxidative stress, in both brain and peripheral tissues, is one hallmark of early stage AD in cognitively impaired patients [23,24]. Of special interest are studies that document increased redox-reactive iron in the brains, CSF and peripheral tissues of MCI patients, which correlates with accumulation of free radical damage and parallels closely to the degree of cognitive impairment in these subjects [25].
Recently, it was shown that 92% of all human sera tested contain brain-reactive autoantibodies; with an increased prevalence of brain-reactive antibodies in AD [26]. These data indicate that the humoral immune system is active within the neuropil, and that antibodies readily cross the blood brain barrier (BBB) [27] between the CNS and the blood. Further, in animal models of multiple sclerosis, components of the neuronal cytoskeleton released into the blood during neu-roaxonal loss give rise to neurofilament specific autoantibo-dies [28]. The discovery of the presence of serum α-synuclein autoantibodies in AD and in Dementia with Lewy Bodies (DLB) further reinforces this concept [27]. There is evidence that antiphospholipid (aPL) redox-reactive autoantibodies (R-RAA) are present in both serum and CSF of healthy individuals [29,30]. Certain aPL bind to epitopes on PL in the presence of specific PL-binding plasma proteins; these aPL are designated as aPL-dependent (aPLd). Other aPL autoantibodies bind directly to epitopes on the PL, and are independent of PL-binding plasma proteins (aPLi). The separate activities can be distinguished in the ELISA by using either 10% adult bovine plasma to provide the necessary PL-binding proteins, or 1% BSA as serum diluents [31].
R-RAA aPLi and aPLd shown to be unmasked by treatment with a redox reactive reagent (hemin) include anti-phosphatidylserine (aPS), anti-cardiolipin (aCL), anti-phosphatidy-lethanolamine (aPE) and anti-phosphatidylcholine (aPC), and are present in the CSF from healthy control (HC) individuals, but in comparison are significantly decreased in CSF taken from autopsy-confirmed Alzheimer’s patients (AD) [32,33]. This study was followed by the analysis of serum samples from subjects diagnosed with AD and age-matched HC [31]. R-RAA aPL were significantly reduced in the sera from 16 subjects diagnosed with AD compared to 17 age-matched HC. Furthermore, the data from the serum study were analyzed using classification and regression tree (CART) analysis to identify R-RAA aPL discriminators to classify subjects within the two groups. The ELISA data from two analytes (IgG aPEi and IgM aPEd) were able to predict subjects with AD with 100% specificity and 84% sensitivity [31].
While the discrimination between HC and AD by serum R-RAA aPL measurement provides the opportunity to identify a novel biomarker for assessing disease progression, the goal of the current study was to identify clinically useful serum biomarkers that appear prior to onset of cognitive decline. For this reason, serum samples from subjects diagnosed with MCI were included in the present study.
Methods
Serum samples
A pilot study was initiated with coded serum samples from 18 subjects assigned to each of three diagnostic groups (n = 6) by the Alzheimer’s Disease Neuroimaging Initiative (ADNI, see Appendix) (HC, MCI and AD). The samples were received on dry ice, and stored at −80 °C until tested. On completion of ELISA analysis of the R-RAA data from these sera, subject diagnostic group assignment information were obtained from the ADNI and matched to the ELISA data to determine if a predictive relationship between serum R-RAA aPL and cognitive status would justify validation in an independent follow-up with a larger sample set.
The follow-up study was initiated with coded serum samples from 90 subjects, assigned by ADNI to three diagnostic groups (n = 30) (HC, MCI and AD). After the R-RAA assay results from the blinded serum samples were released to ADNI, the R-RAA aPL data used in the preparation of this manuscript were matched to the participant diagnosis, demographic data and MMSE scores by accessing the ADNI sample database (adni.loni.usc.edu).
Sample preparation
Pilot study
Hemin chloride (Fe3+Cl) at 80–90% purity (Sigma Chemical Co., St. Louis, MO) was used to prepare a 112 mM solution of hematin (Fe3+OH) stock solution by dissolving hemin powder in 1 M NaOH with gentle stirring for 2 h, and then filtering through a Whatman #1 filter followed by storage at 4 °C. The concentration of the hematin solution was determined from the extinction coefficient of the monomer absorption maxima (385 nm) of 5840 M−1cm−1 [34], and remained stable for a period of at least 4 months.
The coded ADNI serum samples (0.5 ml) were thawed and divided equally into two aliquots. Immediately before hemin treatment of the serum either hematin in 1 M NaOH or 1 M control NaOH solution were slowly added to stirred buffers containing 20 mM Tris, 151 mM NaC1, 3 mM NaN3 (TBS), pH 7.8. The final pH of the hemin or control buffers was adjusted to 7.8 and 0.1 ml aliquots of thawed serum samples were mixed with 0.9 ml of hemin and control buffer. The samples were incubated for 20 h on a rocking platform at 36°C then stored at −80 °C until ELISA analysis.
Follow-up study
Based on the encouraging results obtained in the pilot study, the ADNI provided samples for a follow-up study (vide infra). During the period between these studies, we had the opportunity to more carefully evaluate protocols and methods to optimize conditions for processing serum samples for R-RAA aPL analysis. A more pure source of hemin (≥99%, Frontier Scientific, Logan, UT) replaced the Sigma product used in the pilot studies. With each change in methodology, bridging studies using a set of serum samples from five HC donors frozen in multiple aliquots were used to determine the rank order OD output in each aPL ELISA assay. The R-RAA preparation protocol previously described [35] was used with modifications.
Aliquots of the 90 ADNI serum samples were thawed and treated with Cleanascite™ (Biotech Support Group, Inc., North Brunswick, NJ) at a serum: Cleanascite™ ratio of 4:1 v/v in 2 ml micro centrifuge tubes with gentle rocking at 37 °C for 10 min followed by centrifugation for 1 min at 16 000 g. Treatment with Cleanascite™ removes lipoproteins from the serum, which have been shown to bind and undergo oxidation by hemin [36]. The supernatants were carefully aspirated, and divided into two aliquots for treatment with or without hemin as before. A hematin solution (130 mM) in 1 M NaOH was made from the Frontier hemin powder, using the spectro-photometric analysis described for the pilot study to determine the concentration. A 0.45 μm Acrodisc PDF filter (Pall Life Sciences, Port Washington, NY) was used for the filtration step. An aliquot of the stock hematin solution was diluted in TBS and the pH adjusted to 7.8 with 1 M HCl (final hemin concentration = 1.28 mM). A control buffer without hemin was prepared using the same volume of 1 M NaOH and adjusted to pH 7.8 as above. Serum aliquots from each subject were separately diluted 1:15 v/v in the hemin and control TBS buffers, gently rocked for 3 h at 37 °C, then stored frozen at −80 °C until ELISA analysis.
aPL ELISA
The thawed hemin-treated and buffer control-treated samples were separately diluted in 1% bovine serum albumin (BSA) or 10% adult bovine plasma (ABP) as previously described [31] to a final serum dilution of 1:100 for ELISA analyses. The buffers supplemented with ABP provide PL-binding plasma proteins that after binding to the PL undergo conformational changes that expose the target for aPL autoantibodies [37]. The plasma proteins which bind to cardiolipin (CL) are β2-glycoprotein I and prothrombin, whereas phosphatidyletha-nolamine (PE) is bound by high and low molecular weight kininogens [38]. aPL dependent upon PL-binding plasma proteins are designated in this study as aPLd. In the BSA supplemented buffer, the autoantibodies recognize the phospholipid independent of plasma proteins [39]. These aPL are designated as aPLi in this study. Three autoantibody isotypes (IgG, IgM and IgA) of aPLd and aPLi were assessed for reactivity against phosphatidylserine (PS), CL, PE and phosphatidylcholine (PC). All samples were tested in triplicate. For the pilot study, the ELISA methodology was conducted exactly as previously described [31]. The colorimetric readout from the ELISA assay was performed by incubation at 37 °C until OD of the standard positive control aPL reached ~1.0. R-RAA aPL activity was expressed as the OD difference between hemin-treated and buffer control-treated values for each sample.
For the follow-up study, dilutions of each aPL positive control were used to construct a calibration curve on each ELISA plate. As before, the colorimetric readout from the ELISA assay was performed by incubation at 37 °C until OD of the highest standard positive control aPL reached ~1.0. The values for the hemin-treated and buffer control-treated samples were interpolated from a standard curve constructed using a second-order polynomial to fit the positive control data points. The R-RAA activity (in interpolated OD units) was expressed as the OD difference between hemin-treated and buffer control-treated values for each sample. As there is no absolute method to quantify the R-RAA aPL ELISA results, the ordinates are scaled to “interpolated OD units”.
Statistical analysis of data
Statistical analysis of R-RAA aPL OD values in the three groups of subjects (HC, MCI and AD) were performed using one way ANOVA, with Tukey post hoc tests of all pairs [40]. Significant p values are indicated in Figures 1 and 2. R-RAA aPL OD values analyzed by ANOVA passed the test of homogeneity of variance, with the exception of R-RAA aPCi in the follow-up study. In this case, following a Johnson transformation of the OD data, equal variance was achieved [40].
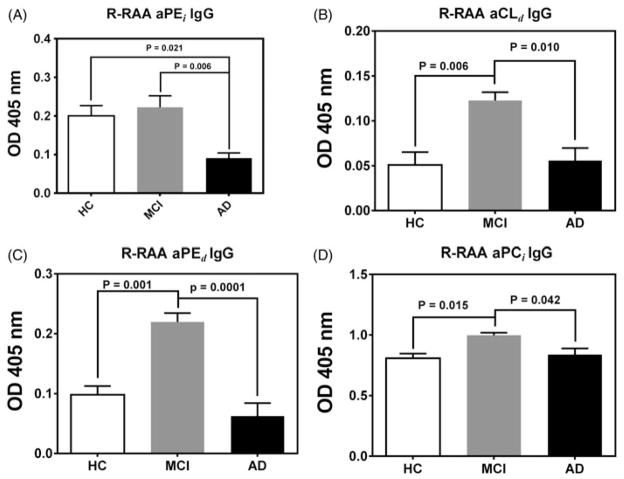
Figure 1.
ADNI pilot study (six subjects/group. Data = Mean ± SEM). Hemin unmasked redox reactive autoantibody (R-RAA) aPL activity in sera from healthy control subjects (HC, white bars) and subjects diagnosed either with Mild Cognitive Impairment (MCI, grey bars) or Alzheimer’s Disease (AD, black bars); as assessed by standardized cognitive tests (see text for details). ELISA OD values were normalized to positive control OD values (~1.0) from qualified lots of anti-phospholipid antisera within each ELISA assay. Intrinsic antibody activity (OD values of the same serum sample prepared in the absence of hemin and analyzed identically) is subtracted from the hemin-treated OD values to give the R-RAA aPL OD units for the serum samples reported in the figure.
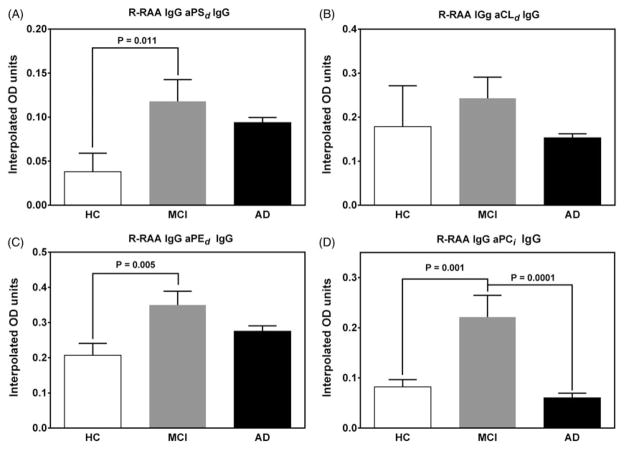
Figure 2.
Hemin unmasked redox reactive autoantibody (R-RAA) activity in sera from healthy control subjects (HC, white bars) and subjects diagnosed either with Mild Cognitive Impairment (MCI, grey bars) or Alzheimer’s Disease (AD, black bars) as assessed by standardized cognitive tests (see Methods section for details). ELISA OD values for the subjects (Mean ± SEM, n = 90) were interpolated from standard curves constructed from qualified lots of anti-phospholipid antisera as positive controls. Intrinsic antibody activity (interpolated OD values of the same serum sample prepared in the absence of hemin and analyzed identically) is subtracted from the hemin-treated interpolated OD values to give the R-RAA aPL OD units for the serum samples reported in the figure.
A goal of the pilot study was to determine if an in-sample discriminant model using subsets of R-RAA aPL data could construct a reasonable model for use in the follow-up study to assess the strength and utility of a predictive relationship of serum R-RAA aPL with AD-related cognitive decline. The machine learning software known as Classification and Regression Trees, CART, implemented using the WEKA Data Mining Software [41] and based on Breiman’s original algorithm [42], was used to create an inductive decision tree to classify the sample subjects. An inductive decision tree is a set of rules represented by decisional nodes and leaves (i.e. terminal nodes) that are assigned to a class. The learning process consists of selecting the most discriminative variable according to an impurity function to partition the data, and repeating this partition recursively until the nodes are considered pure enough to be terminal and then pruning the resultant complete tree to avoid over fitting.
Mini-mental state examination (MMSE) scores were used by the ADNI to assign study subjects. Following completion of the ELISA data analysis, baseline MMSE scores of the 90 participating subjects (obtained from the ADNI database) were statistically analyzed for significance using the Student–Newman–Keuls Multiple Comparisons Test. Significance between MMSE between scores at baseline and last visit of each group was assessed using Student’s two-tailed t-test.
Results
Pilot study
By far the most robust differences between R-RAA aPL were observed within the IgG isotype. Trends in differences in R-RAA aPL between AD and HC were apparent for both IgA and IgM ELISA data, but, because the OD values for the IgA and IgM data were very low, only the IgG R-RAA aPL data are presented in this study.
The R-RAA aPEi OD values for the AD group were significantly reduced compared to both the MCI and HC groups (Figure 1, panel A). This confirms the observation from our previous study of HC and AD, wherein the IgG R-RAA aPEi was significantly reduced in the AD group compared to the healthy controls [31], and reflects a similar reduction in R-RAA aPL observed in the CSF of autopsy confirmed AD subjects compared to HC [33].
In this pilot study robust increases in R-RAA aCLd, aPEd and aPCi were observed in the MCI diagnostic group compared to the HC and AD diagnostic groups (Figure 1, panels B–D). The CART algorithms were used to attempt classification between HC and MCI rather than including the group with advanced disease (AD). This justified, as the R-RAA aPL response was biphasic with respect to disease progression, and as such classifying three phases of AD progression unnecessarily complicated classification statistical analysis. We therefore view R-RAA aPL data from patients with AD as integral to understanding the physiological relationship between these analytes and the patho-physiology of neurodegeneration, but uninformative in terms of diagnostic utility for advanced AD.
CART analysis of the pilot study by using R-RAA IgG aPL data sets from HC and MCI subjects to probe for aPL discriminators correctly classified all six subjects in each group using a single analyte (aPEd OD ≤ 0.154 = six HC subjects, and aPEd OD>0.154 = six MCI subjects) with 100% sensitivity and specificity. When the aPEd data set was removed and an independent CART analysis was performed on the remaining IgG aPL classes, aPCi emerged as a discriminator with 87.5% sensitivity and 83.5% specificity (OD ≤ 0.895 = five HC subjects and OD>0.895 = six MCI subjects with one HC subject misclassified). One further round of CART analysis yielded IgG aCLd with OD ≤ 0.078 defined five HC subjects, and OD>0.078 included seven subjects (6 MCI, 1 HC) (sensitivity = 87.5% and specificity = 83.5%). As a first approximation, this pilot study provided a model for discrimination of MCI subjects from HC.
The validity of the predictor model built with the results from the pilot study used as a training set was tested with a separate set of samples used in a follow-up study. The hypothesis is that subjects with MCI have elevated R-RAA aPEd, aCLd and aPCi compared to HC.
Follow-up study
Demographics for the 90 subjects included in the follow-up study are shown in Table 1. In this follow-up study using serum samples of 30 subjects from each of the three diagnostic groups (n = 30), three of the eight IgG R-RAA aPL ELISA tests were significantly different between at least two diagnostic groups. The results in Figure 2 show R-RAA aPSd, aPEd and aPCi demonstrated significant elevation in the MCI group compared to HC, while R-RAA aPCi were significantly elevated in the MCI group compared to AD. In the follow-up study, quantitative elevation in the R-RAA aCLd, in the MCI group compared to HC and AD did not reach statistical significance (Figure 2, panel B).
Table 1.
Participant demographics.
| Features | HC | MCI | AD |
|---|---|---|---|
| Number of subjects | 30 | 30 | 30 |
| Age, years (SD) | 76.90 ± 4.67§ | 77.24 ± 6.77§ | 77.41 ± 8.18§ |
| Gender, male % | 50.00 | 66.67 | 53.33 |
| APOE-4 (% with at least 1 allele) | 36.67 | 46.67 | 60.00 |
| Baseline MMSE | 29.33 ± 0.84 | 27.10 ± 2.17 | 23.5 ± 2.01 |
| Last visit MMSE | 29.00 ± 1.55§ | 24.43 ± 6.19* | 19.40 ± 6.07*** |
Abbreviations: HC, healthy control; MCI, mild cognitive impairment; AD, Alzheimer’s disease; MMSE, Mini-Mental State Examination.
Analysis of variance of MMSE scores of the three groups at either baseline or at last visit were all significantly different from each other (p<0.001, Student–Newman–Keuls multiple comparisons test). Two-tailed t-test of MMSE between scores at baseline and last visit of each group are appended to the table.
Not significant.
p<0.05.
p<0.001.
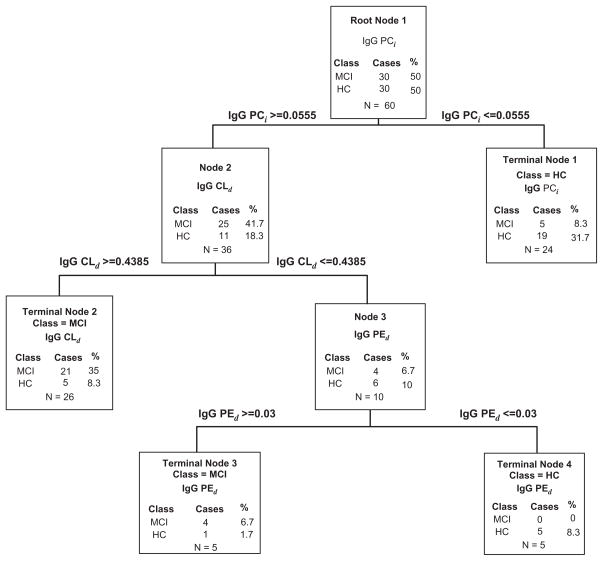
Due to the biphasic distribution of R-RAA aPL ELISA values among HC and MCI and AD, CART analysis across all IgG R-RAA was performed only between the HC and MCI groups. The results of CART analysis of 30 HC and 30 MCI are shown in block diagram in Figure 3. Data obtained from three of the eight R-RAA aPL IgG analytes classified subjects as MCI with a sensitivity of 80.0% and specificity of 83.3%. The discriminators described in the pilot study by CART classification statistics, aPCi, aCLd and aPEd, are the three analytes identified in the follow-up study.
Figure 3.
Classification and regression tree (CART) analysis of R-RAA aPL from 60 serum samples from the ADNI [30 healthy control subjects (HC) and 30 subjects diagnosed with Mild Cognitive Impairment (MCI)].
Follow-up study diagnostic outcomes
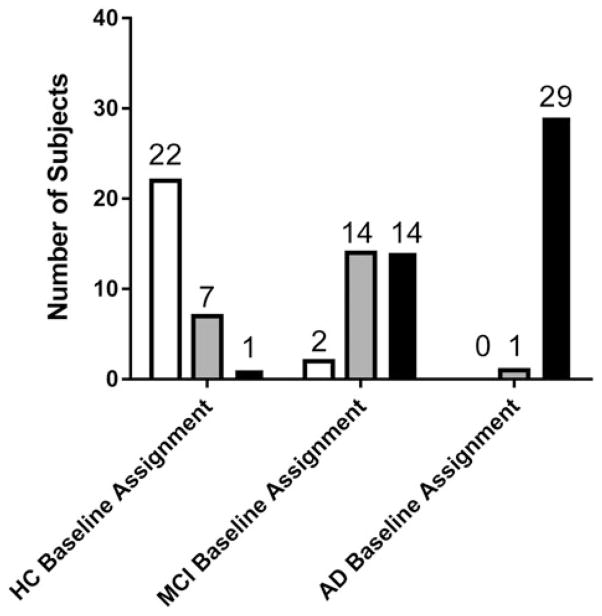
ADNI study subjects whose serum samples were tested in the R-RAA aPL assay were routinely evaluated using the standard ADNI protocols for an additional 2–3 years after serum collection. The MMSE score classifications for baseline and the most recent visit are shown in Figure 4. One subject assigned as AD at baseline was reassigned to MCI at the most recent visit. Inspection of the diagnostic data revealed that this subject had the highest baseline MMSE (28) within the AD diagnostic group and at 4 year follow-up, the subject’s MMSE score dropped to 26.
Figure 4.
Diagnostic group assignments at last follow-up (white bars – HC, grey bars – MCI and black bars – AD) of a total of 30 subjects grouped by diagnostic group assignment at baseline. Of the two subjects assigned as MCI at baseline and HC at last follow-up, the first subject (baseline MMSE = 26) had a last follow-up at 6 months (MMSE score = 30). The second subject had a consistent MMSE score of 30 at 7, 13 and 19 and last follow-up at 25 months, and may have been incorrectly assigned in the database. The single subject assigned AD at baseline (MMSE score = 28) and subsequently reassigned MCI was followed up at 9, 18, 19, 38 and 49 months with MMSE scores of 26, 29, 28, 28 and 26, respectively, indicating uncertainty in interpreting the subject’s assigned diagnostic groups from the MMSE scores alone.
With a few exceptions, the AD group demonstrated cognitive decline over time. From the 90 subjects evaluated in our study, seven HC converted to MCI, one HC converted to AD and 14 MCI subjects converted to AD (Figure 4). Two subjects within the original MCI diagnostic groups were reclassified as HC upon follow-up as the subject’s MMSE scores appeared stable over time. The limitations in the MMSE tool for distinguishing HC from MCI in borderline cases are apparent in these two subjects.
Discussion and conclusions
The increase of R-RAA aPL in MCI patients was demonstrated by exposure of serum to redox-reactive iron in the form of hemin. It is possible that hemin accumulation at sites of oxidative stress in the early phase of AD promotes formation of post-translationally modified autoantibodies to cellular components and these R-RAA aPL decline with disease progression. To support this hypothesis, hemin has been shown to bind immunoglobulins and to dramatically broaden their antigen binding repertoire [43].
Although little is known about the origin of R-RAA aPL, there is increasing evidence that in neurodegenerative diseases dysregulation of the cell membranes of dystrophic neurites can result in altered phospholipid profiles in the blood, particularly for PC and PE [44]. Externalization of the aminophospholipids (PS and PE) to the outer leaflet of the plasma membrane [45] and exposure of mitochondrial CL during neuronal apoptosis, initiated long before overt cognitive symptomatology is apparent may elicit an immune response to membrane phospholipids. The recent lipidomic mass spectrometry discovery of robust serum biomarker lipid profiles in AD subjects [46–48] reinforces the concept that alterations in phospholipid metabolism occur early in the prodromal phase of AD.
The concept of using autoantibodies in blood as biomarkers for neurological disturbances in the CNS is gaining acceptance. For example, antibodies against both physiological [26] and pathological proteins within the CNS are detectable in the blood [49,50]. The persistence and abundance of specific biomarker autoantibodies in the blood of neurological disease patients may be more disease specific and stable when compared to inflammatory molecule bio-markers in the same patients. Our approach to detect specific R-RAA aPL serum autoantibodies for biomarker selection may offer a unique opportunity to uncover very early pathological events in AD and other neurodegenerative diseases. Further studies are needed to explore the relationship between disease progression and R-RAA aPL titers.
The data presented in this study strongly support R-RAA aPL measurement as a promising serum biomarker for detection of early AD. Elevated serum levels of R-RAA aPL decline with disease progression to AD, as previously noted [31], and reflect a similar reduction in R-RAA aPL observed in the CSF of autopsy confirmed AD subjects compared to HC [33].
The biphasic response of serum R-RAA aPL biomarkers in studies with a small number of subjects progressing from pre-symptomatic through MCI to AD would be expected to result in some inter-study variation across diagnostic groups between the pilot and follow-up study. A second contributor to inter-study variability is that several subjects diagnosed with MCI at baseline did not progress to a definitive clinical diagnosis of AD by conclusion of the follow-up study (Figure 4). It is therefore possible that some of these subjects were suffering from neurodegenerative disease(s) other than AD.
Studies to assess fluctuations of serum biomarker levels from a larger population of subjects are anticipated to assess predictability of the R-RAA aPL biomarker for staging AD by analyzing measurements from serial serum samples. These data will determine whether the sensitivity for detection of early AD at the stage of MCI is enhanced by following the changes in the R-RAA aPL biomarker over time in a given individual.
Further collaborative studies are anticipated to assess selectivity of the biomarker for AD versus other neurological diseases, thereby establishing the feasibility of developing the R-RAA aPL diagnostic as a routine screening technology for individuals at risk for developing AD. As with any new biomarker diagnostic that has the potential for identifying individuals at risk for developing a life-threatening and disabling disease, acceptance will only come after the diagnostic yields are proven accurate and predictive when used in a large population of individuals over time.
Acknowledgments
We are indebted to Drs. Leslie Shaw and John Trojanowski (ADNI Biomarker Core Group) for selection of the samples for this study, and to Dr. Shannon Risacher for assistance with updated ADNI clinical data.
Appendix
The ADNI was launched in 2003 by the National Institute on Aging (NIA), the National Institute of Biomedical Imaging and Bioengineering (NIBIB), the Food and Drug Administration (FDA), private pharmaceutical companies and non-profit organizations, as a $60 million, 5-year public–private partnership. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). Determination of sensitive and specific markers of very early AD progression is intended to aid researchers and clinicians to develop new treatments and monitor their effectiveness, as well as lessen the time and cost of clinical trials. The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California – San Francisco. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the US and Canada. The initial goal of ADNI was to recruit 800 subjects but ADNI has been followed by ADNI-GO (RC2-AG036535) and ADNI-2. To date these three protocols have recruited over 1500 adults, aged 55–90, to participate in the research, consisting of cognitively normal older individuals, people with early or late MCI, and people with early AD. The follow up duration of each group is specified in the protocols for ADNI-1, ADNI-2 and ADNI-GO (RC2 AG036535). Subjects originally recruited for ADNI-1 and ADNI-GO had the option to be followed in ADNI-2. Additional support for data analysis was provided by NIH grants P30 AG10133 and RO1 AG19771.
Footnotes
Declaration of interest
This work was supported by funding from Franciscan St. Francis Health, Indianapolis, IN and IPCo, Arlington, VA. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904 and RC2 AG036535) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec, Inc.; Bristol-Myers Squibb Company; Eisai, Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc. and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. Additional support to AJS was provided by NIH grants RC2 AG036535, R01 AG19771, P30 AG10133, and R01 LM011360.
The authors declare no conflict of interest.
References
- 1.Alzheimer’s disease facts and figures. Alzheimers Dement. 2014;10:1–76. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Alkalay A, Rabinovici GD, Zimmerman G, et al. Plasma acetylcholinesterase activity correlates with intracerebral beta-amyloid load. Curr Alzheimer Res. 2013;10:48–56. [PMC free article] [PubMed] [Google Scholar]
- 3.Alsadany MA, Shehata HH, Mohamad MI, Mahfouz RG. Histone deacetylases enzyme, copper, and IL-8 levels in patients with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2013;28:54–61. doi: 10.1177/1533317512467680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai L, Song N, Yu J, et al. Elevated plasma levels of soluble TNFRs and TACE activity in Alzheimer’s disease patients of Northern Han Chinese descent. Curr Alzheimer Res. 2013;10:57–62. [PubMed] [Google Scholar]
- 5.Honma T, Hatta K, Hitomi Y, et al. Increased systemic inflammatory interleukin-1ss and interleukin-6 during agitation as predictors of Alzheimer’s disease. Int J Geriatr Psychiatry. 2013;28:233–241. doi: 10.1002/gps.3816. [DOI] [PubMed] [Google Scholar]
- 6.Liang F, Jia J, Wang S, et al. Decreased plasma levels of soluble low density lipoprotein receptor-related protein-1 (sLRP) and the soluble form of the receptor for advanced glycation end products (sRAGE) in the clinical diagnosis of Alzheimer’s disease. J Clin Neurosci. 2013;20:357–361. doi: 10.1016/j.jocn.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Lopez N, Tormo C, De Blas I, et al. Oxidative stress in Alzheimer’s disease and mild cognitive impairment with high sensitivity and specificity. J Alzheimers Dis. 2013;33:823–829. doi: 10.3233/JAD-2012-121528. [DOI] [PubMed] [Google Scholar]
- 8.Huang CW, Wang SJ, Wu SJ, et al. Potential blood biomarker for disease severity in the taiwanese population with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2013;28:75–83. doi: 10.1177/1533317512467674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.May JE, Pemberton RM, Hart JP, et al. Use of whole blood for analysis of disease-associated biomarkers. Anal Biochem. 2013;437:59–61. doi: 10.1016/j.ab.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 10.Leung R, Proitsi P, Simmons A, et al. Inflammatory proteins in plasma are associated with severity of Alzheimer’s disease. PLoS One. 2013;8:e64971. doi: 10.1371/journal.pone.0064971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laske C, Schmohl M, Leyhe T, et al. Immune profiling in blood identifies sTNF-R1 performing comparably well as bio-marker panels for classification of Alzheimer’s disease patients. J Alzheimers Dis. 2013;34:367–375. doi: 10.3233/JAD-121558. [DOI] [PubMed] [Google Scholar]
- 12.Ray S, Britschgi M, Herbert C, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 13.O’Bryant SE, Xiao G, Barber R, et al. A blood-based algorithm for the detection of Alzheimer’s disease. Dement Geriatr Cogn Disord. 2011;32:55–62. doi: 10.1159/000330750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soares HD, Potter WZ, Pickering E, et al. for the Biomarkers Consortium Alzheimer’s Disease Plasma Proteomics. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer Disease. Arch Neurol. 2012;69:1–8. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doecke JD, Laws SM, Faux NG, et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69:1318–1325. doi: 10.1001/archneurol.2012.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray L, Khemka VK, Behera P, et al. Serum homocysteine, dehydroepiandrosterone sulphate and lipoprotein (a) in Alzheimer’s disease and vascular dementia. Aging Dis. 2013;4:57–64. [PMC free article] [PubMed] [Google Scholar]
- 17.Hye A, Riddoch-Contreras J, Baird AL, et al. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimers Dement. 2014;6:799–807. doi: 10.1016/j.jalz.2014.05.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekris LM, Lutz F, Montine TJ, et al. MicroRNA in Alzheimer’s disease: an exploratory study in brain, cerebrospinal fluid and plasma. Biomarkers: Biochem Indicat Exposure Response Suscept Chem. 2013;18:455–466. doi: 10.3109/1354750X.2013.814073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiandaca MS, Kapogiannis D, Mapstone M, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapogiannis D, Boxer A, Schwartz JB, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2014 doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruce-Keller AJ, Gupta S, Parrino TE, et al. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12:1371–1382. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MA, Zhu X, Tabaton M, et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 2010;19:363–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Padurariu M, Ciobica A, Hritcu L, et al. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Lavados M, Guillon M, Mujica MC, et al. Mild cognitive impairment and Alzheimer patients display different levels of redox-active CSF iron. J Alzheimers Dis. 2008;13:225–232. doi: 10.3233/jad-2008-13211. [DOI] [PubMed] [Google Scholar]
- 26.Levin EC, Acharya NK, Han M, et al. Brain-reactive autoantibodies are nearly ubiquitous in human sera and may be linked to pathology in the context of blood–brain barrier breakdown. Brain Res. 2010;1345:221–232. doi: 10.1016/j.brainres.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 27.Koehler NK, Stransky E, Shing M, et al. Altered serum IgG levels to alpha-synuclein in dementia with lewy bodies and Alzheimer’s disease. PLoS One. 2013;8:e64649. doi: 10.1371/journal.pone.0064649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gnanapavan S, Grant D, Pryce G, et al. Neurofilament a biomarker of neurodegeneration in autoimmune encephalomyelitis. Autoimmunity. 2012;45:298–303. doi: 10.3109/08916934.2012.654865. [DOI] [PubMed] [Google Scholar]
- 29.McIntyre JA, Wagenknecht DR, Faulk WP. Redox-reactive autoantibodies: detection and physiological relevance. Autoimmun Rev. 2006;5:76–83. doi: 10.1016/j.autrev.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 30.McIntyre JA, Wagenknecht DR, Faulk WP. Autoantibodies unmasked by redox reactions. J Autoimmun. 2005;24:311–317. doi: 10.1016/j.jaut.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 31.McIntyre JA, Wagenknecht DR, Ramsey CJ. Redox-reactive antiphospholipid antibody differences between serum from Alzheimer’s patients and age-matched controls. Autoimmunity. 2009;42:646–652. doi: 10.3109/08916930903074833. [DOI] [PubMed] [Google Scholar]
- 32.McIntyre JA, Chapman J, Shavit E, Hamilton RL, Dekosky ST. Redox-reactive autoantibodies in Alzheimer’s patients’ cerebrospinal fluids: preliminary studies. Autoimmunity. 2007;40:390–396. doi: 10.1080/08916930701421020. [DOI] [PubMed] [Google Scholar]
- 33.McIntyre JA, Hamilton RL, DeKosky ST. Redox-reactive autoantibodies in cerebrospinal fluids. Ann N Y Acad Sci. 2007;1109:296–302. doi: 10.1196/annals.1398.035. [DOI] [PubMed] [Google Scholar]
- 34.Li SD, Su YD, Li M, Zou CG. Hemin-mediated hemolysis in erythrocytes: effects of ascorbic acid and glutathione. Acta Biochim Biophys Sin. 2006;38:63–69. doi: 10.1111/j.1745-7270.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre JA, Wagenknecht DR, Faulk WP. Antiphospholipid antibodies: discovery, definitions, detection and disease. Prog Lipid Res. 2003;42:176–237. doi: 10.1016/s0163-7827(02)00048-6. [DOI] [PubMed] [Google Scholar]
- 36.Nishida T, Kummerow FA. Interaction of low-density lipoproteins of serum with hemin. J Lipid Res. 1962;3:448–455. [Google Scholar]
- 37.Agar C, van Os GM, Morgelin M, et al. Beta2-glycoprotein I can exist in 2 conformations: implications for our understanding of the antiphospholipid syndrome. Blood. 2010;116:1336–1343. doi: 10.1182/blood-2009-12-260976. [DOI] [PubMed] [Google Scholar]
- 38.Sugi T, McIntyre JA. Autoantibodies to phosphati-dylethanolamine (PE) recognize a kininogen-PE complex. Blood. 1995;86:3083–3089. [PubMed] [Google Scholar]
- 39.McIntyre JA, Wagenknecht DR, Sugi T. Phospholipid binding plasma proteins required for antiphospholipid antibody detection – an overview. Am J Reprod Immunol. 1997;37:101–110. doi: 10.1111/j.1600-0897.1997.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 40.Zar JH. Biostatistical Analysis. Prentice-Hall, Inc; Upper Saddle River, New Jersey: 1999. [Google Scholar]
- 41.Hall M, Eibe F, Holmes B, et al. The WEKA data mining software: an update. SIGKDD Explor. 2009;11:10–18. [Google Scholar]
- 42.Breiman L, Friedman J, Olshen R, Stone C. Classification and Regression Trees. Wadsworth Publishing Co; Pacific Grove, CA: 1984. [Google Scholar]
- 43.Dimitrov JD, Roumenina LT, Doltchinkova VR, et al. Antibodies use heme as a cofactor to extend their pathogen elimination activity and to acquire new effector functions. J Biol Chem. 2007;282:26696–26706. doi: 10.1074/jbc.M702751200. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Dominguez R, Garcia-Barrera T, Gomez-Ariza JL. Combination of metabolomic and phospholipid-profiling approaches for the study of Alzheimer’s disease. J Proteomics. 2014;104:37–47. doi: 10.1016/j.jprot.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 45.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mapstone M, Cheema AK, Fiandaca MS, et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat Med. 2014;20:415–418. doi: 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-Domínguez R, García-Barrera T, Gómez-Ariza JL. Metabolomic study of lipids in serum for biomarker discovery in Alzheimer’s disease using direct infusion mass spectrometry. J Pharm Biomed Anal. 2014;98:321–326. doi: 10.1016/j.jpba.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez-Dominguez R, Garcia-Barrera T, Gomez-Ariza JL. Using direct infusion mass spectrometry for serum metabolomics in Alzheimer’s disease. Anal Bioanal Chem. 2014;406:7137–7148. doi: 10.1007/s00216-014-8102-3. [DOI] [PubMed] [Google Scholar]
- 49.Nagele E, Han M, Demarshall C, et al. Diagnosis of Alzheimer’s disease based on disease-specific autoantibody profiles in human sera. PLoS One. 2011;6:e23112. doi: 10.1371/journal.pone.0023112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maftei M, Thurm F, Schnack C, et al. Increased levels of antigen-bound beta-amyloid autoantibodies in serum and cere-brospinal fluid of Alzheimer’s disease patients. PLoS One. 2013;8:e68996. doi: 10.1371/journal.pone.0068996. [DOI] [PMC free article] [PubMed] [Google Scholar]