Abstract
Rationale: Synaptic dopamine (DA) release induced by amphetamine or other experimental manipulations can displace [ 11C]raclopride (RAC*) from dopamine D2-like receptors. We hypothesized that exogenous levodopa might increase dopamine release at striatal synapses under some conditions but not others, allowing a more naturalistic assessment of presynaptic dopaminergic function. Presynaptic dopaminergic abnormalities have been reported in Tourette syndrome (TS).
Objective: Test whether levodopa induces measurable synaptic DA release in healthy people at rest, and gather pilot data in TS.
Methods: This double-blind crossover study used RAC* and positron emission tomography (PET) to measure synaptic dopamine release 4 times in each of 10 carbidopa-pretreated, neuroleptic-naïve adults: before and during an infusion of levodopa on one day and placebo on another (in random order). Five subjects had TS and 5 were matched controls. RAC* binding potential (BP ND) was quantified in predefined anatomical volumes of interest (VOIs). A separate analysis compared BP ND voxel by voxel over the entire brain.
Results: DA release declined between the first and second scan of each day (p=0.012), including on the placebo day. Levodopa did not significantly reduce striatal RAC* binding and striatal binding did not differ significantly between TS and control groups. However, levodopa’s effect on DA release differed significantly in a right midbrain region (p=0.002, corrected), where levodopa displaced RAC* by 59% in control subjects but increased BP ND by 74% in TS subjects.
Discussion: Decreased DA release on the second scan of the day is consistent with the few previous studies with a similar design, and may indicate habituation to study procedures. We hypothesize that mesostriatal DA neurons fire relatively little while subjects rest, possibly explaining the non-significant effect of levodopa on striatal RAC* binding. The modest sample size argues for caution in interpreting the group difference in midbrain DA release with levodopa.
Keywords: dopamine, D2, receptor, raclopride, positron, emission, tomography, PET, levodopa, dopamine, Tourette, syndrome, nucleus, accumbens, substantia, nigra, midbrain
Introduction
Dopamine (DA) release from neurons has often been conceptualized as occurring via two separable mechanisms: tonic, referring to low levels of DA in extrasynaptic spaces that may be more accessible to microdialysis, and phasic, referring to synaptic DA release at synapses following presynaptic action potentials 1. Phasic dopamine release is crucial to dopamine’s role in changing behavior 2, including in learning sequences of movements 3. Normal tonic dopamine release but abnormal phasic dopamine release has been postulated to occur in several disease states, including drug abuse 4 and Tourette syndrome (TS) 5– 8.
The radioligand [ 11C]raclopride (hereinafter RAC*) binds to dopamine D 2-like (D 2, D 3 and D 4) receptors loosely enough to be displaced by physiological increases of dopamine at the synapse. This property has been exploited to detect changes in synaptic DA release induced by experimental manipulations including the administration of amphetamine 9. However, amphetamine also has some disadvantages in this context—primarily, that it does not really produce phasic dopamine release in the usual, temporal, sense of the word. Rather, it causes prolonged, substantial dopamine release regardless of environmental demands. Scientific questions about DA release in the absence of amphetamine might be better tested with a pharmacological stimulus that could potentially increase the magnitude of DA release, but under tighter endogenous control. Additionally, amphetamine can induce symptomatic effects including euphoria 10 and transiently increased tic severity 11; these effects can themselves alter brain activity, complicating interpretation of the results. Ideally, a pharmacological challenge drug to test phasic dopamine release would not produce effects noticed by the subject.
The present study provides preliminary data for a novel approach to testing presynaptic dopamine release using levodopa, the body’s natural synthetic precursor to dopamine. Exogenous levodopa boosts dopamine synthesis almost immediately in both parkinsonian and healthy brains [reviewed in 12]. The extra dopamine is rapidly released at the synapse in people with DA deficiency 13, and there is evidence that this happens also in the non-parkinsonian brain. In people, including in people with tics, levodopa produces dose-dependent yawning, mild sleepiness, and effects on working memory— i.e., CNS-mediated effects 14– 16. Additional evidence for levodopa-induced synaptic DA release in the non-parkinsonian brain is reviewed in 12. When given after an adequate dose of carbidopa, which prevents conversion to dopamine but does not cross the blood-brain barrier, systemic levodopa administration essentially delivers dopamine selectively to the brain, as confirmed by the fact that it does not alter quantitative whole-brain blood flow 17– 19, as dopamine would if it were being delivered systemically or produced outside the brain. In fact, with adequate carbidopa pretreatment, volunteers usually cannot tell whether they are receiving levodopa or a placebo 12, 16.
We used PET and RAC* to measure synaptic dopamine release in response to a standardized levodopa infusion (after carbidopa) in 10 subjects. Since no previous data were available on levodopa effects on RAC* PET, we included before- and during-levodopa RAC* PET scans as well as before- and during-placebo scans. Half of the subjects had a chronic tic disorder and the other half were matched control subjects without tics, to generate preliminary data in each population. The original hypotheses were that levodopa would stimulate striatal dopamine production in the controls, but may affect people with TS differently.
Methods
Participants
This study was approved by the Human Studies Committee of Washington University School of Medicine (IRB, protocol # 03-0347, the WUSM Radioactive Drug Research Committee (protocol # 497F), and the U.S. Food and Drug Administration (Investigator IND #69,745 for i.v. levodopa). All subjects provided written confirmation of informed consent before study participation.
Diagnostic assessment included psychiatric and neurological examination by a movement-disorders-trained neuropsychiatrist (KJB) and a validated semistandardized psychiatric diagnostic interview [SCID-IV; 20]. Tic subjects met DSM-IV-TR criteria for Tourette’s Disorder. Control subjects with no history of tics were matched one-to-one for age, sex and handedness (with one ambidextrous TS subject matched to a right-handed control). Exclusion criteria included any lifetime neurological or Axis I psychiatric disorder (except that TS, ADHD and OCD were allowed in tic subjects, and migraine and specific phobia were allowed in either group), current serious general medical illness, medication history of dopamine antagonists or other drugs likely to affect the dopaminergic system, current use of any neuroactive medication, lactation, possibility of pregnancy, or contraindication to levodopa or MRI.
Clinical features were characterized by the Diagnostic Confidence Index (0=no features of TS; 100=all enumerated features of classic TS; scores in the original clinical validation sample ranged from 5 to 100, mean=61, S.D.=20) 21; the YGTSS, an expert-rated measure of tic severity over the previous week (motor tic scale 0–25, vocal tic scale 0–25, impairment scale 0–50, higher scores indicating a higher symptom burden) 22, 23; the revised Tic Symptom Self-Report (TSSR) scale, a self-report scale including scores of 0–3 for each of 18 motor tics and 16 vocal tics, with 3 indicating tics were “very frequent and very forceful” over the preceding two weeks 24, 25; the ADHD Rating Scale, an expert-rated measure of current severity of Attention-Deficit/Hyperactivity Disorder (ADHD) based on DSM-IV criteria (range 0–54, higher scores indicating a higher symptom burden) 26; and the Y BOCS, an expert-rated measure of current obsessive-compulsive disorder (OCD) severity (range 0–40, higher scores indicating a higher symptom burden) 27, 28.
Overview of subject participation
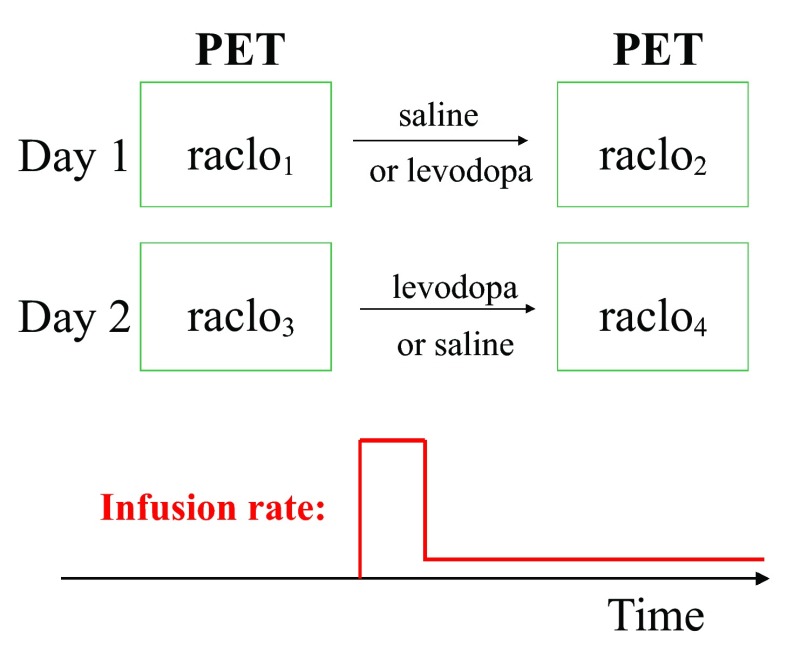
Each subject had 4 RAC* PET scans: two scans on each of two days at least a week apart ( Figure 1). After oral carbidopa and the baseline PET scan, an infusion of levodopa or saline placebo was begun by vein at an individualized dose intended to produce a steady-state levodopa plasma concentration of 600ng/mL. After allowing 30 minutes to approach steady-state levodopa concentration, a second scan was done while the infusion continued. The order (levodopa on day 1 and placebo on day 2, or the reverse) was assigned randomly to each subject, and subjects and PET staff were blind to drug assignment during all scans.
Figure 1. Study overview.
The room was darkened and subjects were instructed to lie quietly in the scanner with eyes closed throughout each scan. Study staff asked subjects every 5 or 10 minutes if they were comfortable and made sure they were awake.
Levodopa infusion
Subjects took 200mg carbidopa by mouth at least 1 hour before levodopa infusion began. A dose of levodopa estimated to fill each subject’s volume of distribution at a target concentration of 600ng/mL was infused over 10 minutes, followed until the second PET scan of the day was completed by a maintenance infusion at a rate estimated to compensate for elimination. In prior work, these infusion rates produced a mean blood level across subjects of ~625ng/mL after 25 minutes of infusion 16. On average, that concentration produces substantial motor benefit in early Parkinson disease 29, 30, yet this infusion method is well enough tolerated that subjects cannot reliably distinguish the levodopa and saline infusions 12, 16.
Levodopa plasma concentration
Levodopa plasma concentration was measured by a validated method 31.
Radiotracer preparation
[ 11C]raclopride was prepared by O-[ 11C]methylation of (S)- O-desmethylraclopride HBr (ABX Advanced Biochemical Compounds, Radeberg, Germany) using a modification of previously reported procedures 32, 33. Carbon-11 was produced as 11CO 2 using the Washington University JSW BC 16/8 cyclotron and the 14N(p,α) 11C nuclear reaction. The 11CO 2 was converted to 11CH 3I using the microprocessor-controlled PETtrace MeI MicroLab (GE Medical Systems, Milwaukee, WI), and immediately used for [ 11C]methylation of (S)- O-desmethylraclopride. Product [ 11C]raclopride was purified via semipreparative HPLC, and reformulated in a 10% ethanol/normal saline solution. The radiochemical purity exceeded 95%, and the specific activity exceeded 500 Ci/mmol, as determined by analytical HPLC. The mass of raclopride was ≤13.9 µg per injected dose.
Image acquisition
RAC* was given i.v. over an interval of 30 seconds (median dose 14.8mCi, interquartile range 11.0–18.9mCi). PET images were acquired on a Siemens ECAT 961 camera beginning with arrival of radiotracer in the head and continuing for 60 minutes using image frames of increasing duration. An MP-RAGE sequence was used to acquire a 3-dimensional T1-weighted image of the brain with acquisition time ~400 sec and voxel dimensions 1.25×1×1mm 3.
Image alignment
The PET images were realigned within each subject and then to the subject’s MRI using a rigid-body alignment method with low measured error, optimized for dynamic PET images 34– 37.
VOI analysis
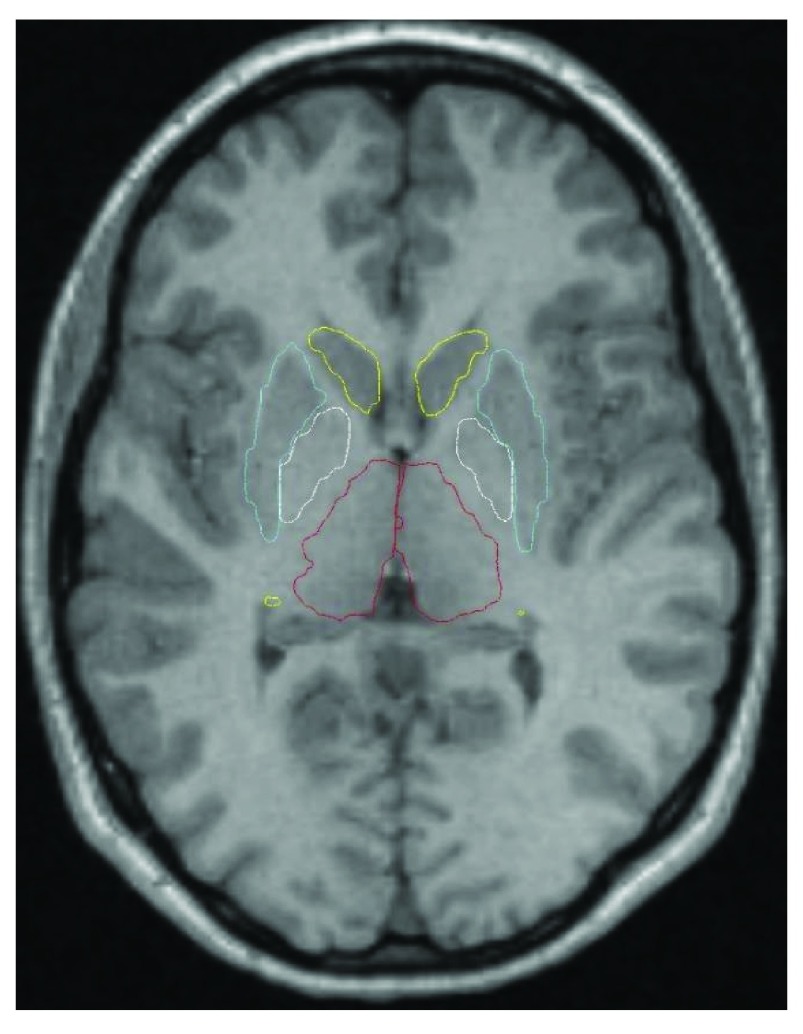
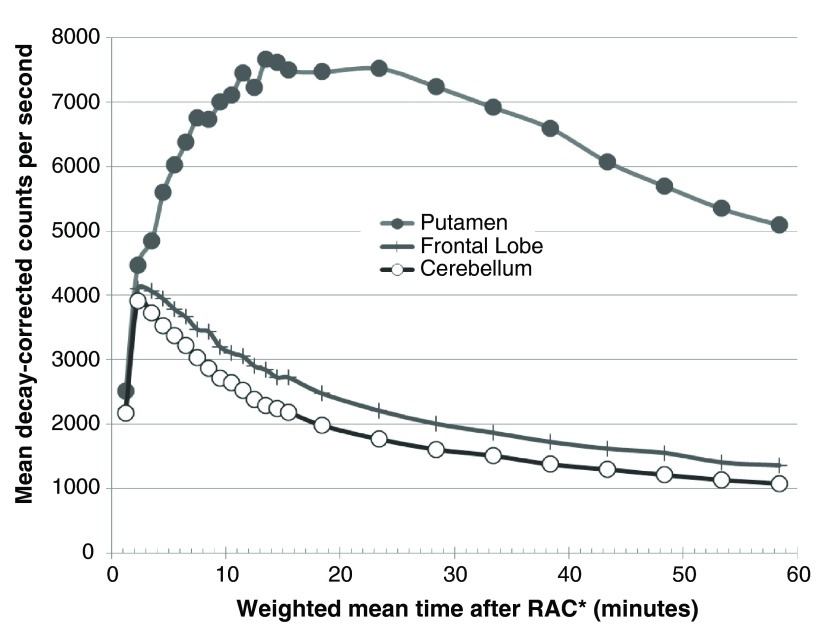
Nine subcortical volumes of interest (VOIs) were defined for each subject from that subject’s MRI by a high-dimensional semi-automated method of known high test-retest reliability 38 ( Figure 2). These VOIs corresponded to the thalamus and the left and right putamen, caudate, nucleus accumbens, and globus pallidus. An additional VOI was created from the average (weighted by region volume) of 22 FreeSurfer-labeled gray matter regions comprising frontal cortex (11 left- and 11 right-hemisphere VOIs). This large frontal VOI produced adequate counting statistics for modest noise in the time-activity curve ( Figure 3). A cerebellum VOI was traced on each subject’s MR image. All VOIs were transferred to each subject’s realigned PET images using the optimized MRI-to-PET transformation matrix computed in the alignment step. The cerebellar VOI was trimmed if needed so that no voxel in the VOI corresponded to any of the inferior-most four slices in any frame of that subject’s original PET images. Thus in each subject each VOI was identical for all four PET scans.
Figure 2. Automated striatal VOIs.
Atlas-based VOI outlines are shown on an axial section from one subject (Cd yellow, Pu light blue, Pl white, Th red; NA does not appear on this section).
Figure 3. Time-activity curves.
Decay-corrected time-activity curves are shown for the right putamen (filled circles), the frontal lobe VOI (+’s), and the cerebellar reference region (empty circles) from one subject’s pre-levodopa PET scan.
The binding potential BP ND 39, 40, an estimate of the quotient B max/K D, was computed as one less than the distribution volume ratio (DVR), which was derived for each of the nine subcortical VOIs and the frontal lobe VOI using the cerebellar reference region 41. As we had no a priori hypothesis about laterality of results in any of the paired basal ganglia nuclei, we averaged corresponding left and right BP NDs (weighted by VOI volume) to produce for each PET scan six final BP ND values, one each for frontal lobe cortex (FL), thalamus (Th), putamen (Pu), caudate (Cd), nucleus accumbens (NA), and globus pallidus (Pl).
The primary statistical analysis used a repeated-measures analysis of variance (rmANOVA) with BP ND as dependent variable, diagnosis (tic or control) as a between-group variable, time (before or during the infusion) and day (placebo or levodopa) as within-subject variables, and region (the six VOI-based BP NDs) as a repeated measure. Exploratory analyses used an ANOVA for each region.
Whole-brain analysis
For each subject, a DVR image was computed using at each voxel in the brain the Logan graphical method with the cerebellar VOI described in the preceding section as reference region 41. As a methods check, the mean across striatal VOIs of the voxelwise DVR value was essentially identical to the regional DVR computed using the standard methods described above. Analysis was limited to voxels in atlas space at which every subject contributed data from all frames of the dynamic PET acquisition.
Whole-brain comparisons used voxelwise t tests corrected for multiple comparisons in SPM 8, as follows. A t test compared DVR images between the TS and the control group, and clusters of contiguous voxels with t exceeding the threshold corresponding to p<0.001 were accepted as significantly different between groups if cluster volume exceeded the threshold required to control False Discovery Rate (FDR) for the entire dataset at p<0.05.
Two comparisons were made, one based on mean baseline DVR images and the other based on levodopa effect ΔDVR images. Each subject’s two pre-infusion RAC* PET scans, one from each scan day, were averaged to create that subject’s mean baseline DVR image. The difference of the during-levodopa DVR image and the during-placebo DVR image in a subject was used to create that subject’s levodopa effect ΔDVR image.
Results
Subjects
Subject characteristics and adequacy of matching are reported in Table 1, and clinical characteristics of the TS group are reported in Table 2.
Table 1. Subject characteristics and adequacy of matching.
| Measure | Tic Subjects
(N=5) |
Controls
(N=5) |
|---|---|---|
| Age (years; mean ± S.D.) | 33.8 ± 12.9 | 32.8 ± 11.1 |
| Sex, male (N) | 4 | 4 |
| Race, Caucasian (N) | 4 | 4 |
| Handedness, right (N) | 4 | 3 |
| OCD diagnosis (N) | 1 | 0 |
| ADHD diagnosis (N) | 2 | 0 |
Abbreviations: OCD=Obsessive-compulsive disorder, ADHD=Attention Deficit Hyperactivity Disorder.
Table 2. Clinical characteristics of the Tourette syndrome group.
The Y BOCS was completed for only 1 tic subject; the score was 9 on day 1 and 14 on day 2
| Scale | Scores
(mean ± S.D.) |
|
|---|---|---|
| DCI score | 36.8 ± 22.0 | |
| YGTSS | Motor tic score | 10.6 ± 3.4 |
| Vocal tic score | 7.8 ± 4.0 | |
| Impairment score | 9.4 ± 9.8 | |
| TSSR score | Motor | 9.3 ± 5.9 |
| Vocal | 3.2 ± 2.3 | |
| Total | 12.5 ± 7.9 | |
| ADHD Rating Scale | 11.6 ± 10.7 | |
Abbreviations: DCI=Tourette Syndrome Diagnostic Confidence Index, YGTSS=Yale Global Tic Severity Scale, Y-BOCS=Yale-Brown Obsessive Compulsive Scale, ADHD=Attention Deficit Hyperactivity Disorder, TSSR=Tic Symptom Self Report.
Levodopa levels
Levodopa plasma concentrations were ~800–1000ng/ml before the RAC* scan and ~500–700ng/ml after the RAC* scan, and did not differ significantly between groups ( Table 3).
Table 3. Levodopa plasma concentrations in ng/ml, mean ± SD.
| Time | Controls | Tic subjects | p ( t test) |
|---|---|---|---|
| Peak (10' into
infusion) |
1591.5 ± 232.5 | 1938.8 ± 726.3 | 0.36 |
| Just before
RAC* scan |
788.0 ± 152.4 | 992.4 ± 322.9 | 0.26 |
| Just after
RAC* scan |
529.5 ± 149.2 | 662.8 ± 136.1 | 0.21 |
Counting statistics in VOIs at baseline
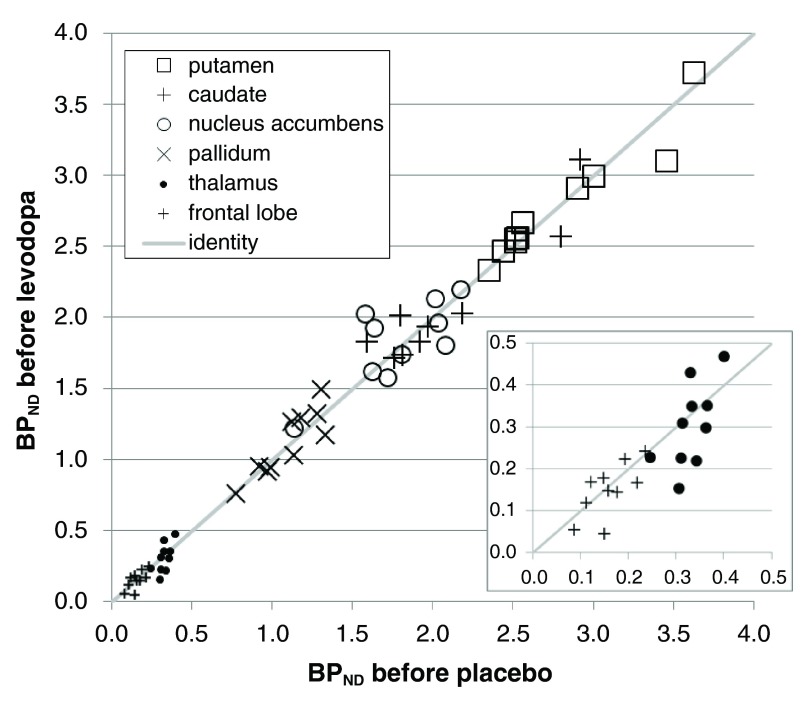
The a priori VOIs showed higher and more reliable binding in striatum and pallidum, as expected. Nevertheless, the thalamus, GP and frontal cortex VOIs also produced good counting statistics ( Figure 3). For every one of the VOIs the baseline BP ND estimates were positive in all 120 scans, and were very similar between the two scan days ( Table 4, Figure 4).
Figure 4. Stability of baseline binding between scan days in the a priori VOIs.
BP NDs from the first scan of each day are plotted for all 10 subjects, with the BP ND from the pre-placebo scan on the horizontal axis and from the pre-levodopa scan on the vertical axis. For the paired VOIs the mean of the left and right BP ND is used. The diagonal line is the line of identity. The inset shows an enlarged view of the data from the frontal lobe and thalamus VOIs.
Table 4. RAC* binding in a priori VOIs at baseline.
| VOI | FL | Th | Pl | NA | Cd | Pu |
| BP ND (mean) | 0.15 | 0.32 | 1.11 | 1.80 | 2.13 | 2.79 |
| BP
ND (standard
deviation) |
0.05 | 0.08 | 0.20 | 0.30 | 0.45 | 0.42 |
| BP
ND values > 0
(of 20 scans) |
20 | 20 | 20 | 20 | 20 | 20 |
|
p for mean > 0
(one-sample t test) |
.0000 | .0000 | .0000 | .0000 | .0000 | .0000 |
| Correlation
r
between days, across subjects |
.70 | .63 | .88 | .76 | .94 | .96 |
|
p for correlation
(8 df, 1 tail) |
.012 | .025 | .0003 | .005 | .0000 | .0000 |
Abbreviations: FL, frontal lobes; Th, thalamus; Pl, pallidum; NA, nucleus accumbens; Cd, caudate; Pu, putamen.
Stability of RAC* binding between days and with time
This study includes a before- and after-infusion scan on each of two days. On one day the infusion contains levodopa, and on the other day it is a saline placebo. Thus each subject has three non-levodopa scans (the first scan of each day plus the scan during the placebo infusion). As expected, BP ND was quite reproducible in the two pre-levodopa scans (correlated at r = 0.99 across VOI and subject).
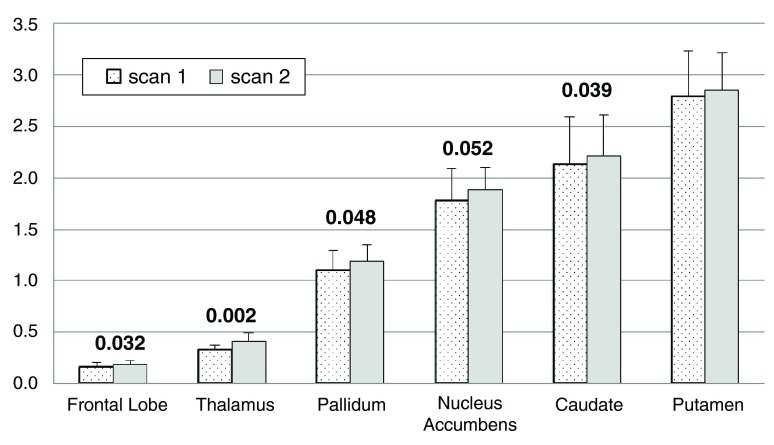
To our surprise, BP ND increased between the 1 st and 2 nd scan of the day (main effect of time, F=10.605, df=1,8, p=0.012), and this change did not differ significantly between the levodopa and placebo days (time × day interaction, F=0.014, df=5,4, p=0.909). In other words, the two scans on the placebo day were not identical. Mean BP ND was 2.7% to 24.0% higher during the placebo infusion, indicating decreased dopamine release compared to earlier on the same day. The change from the first to the second scan of each day was significant in most individual region analyses: main effect of time, thalamus p=0.002, frontal lobe p=0.032, caudate p=0.039, pallidum p=0.048, and nucleus accumbens p=0.052 (multivariate time × region interaction F=4.173, df=5,4, p=0.096). Figure 5 shows the BP ND for each VOI from both scans on the placebo day only.
Figure 5. Change in BP ND on the placebo day.
For each of the a priori VOIs, mean BP ND across all 10 subjects is shown before and during the infusion on the placebo day only. Error bars show SD. Numeric labels are p values for the main effect of time in the individual region ANOVAs (putamen p=.115).
Effect of levodopa on RAC* binding
Since the pre- and on-placebo scans differed, the appropriate comparison for the on-levodopa RAC* scan is the on-placebo scan. Therefore we assessed the effect of levodopa by comparing the BP ND in the on-LD and on-placebo scans. In the VOI analysis, there was no significant effect of LD (day × time interaction, F=0.014, df=1,8, p=0.909).
Comparison of RAC* binding between TS and control groups
TS vs control at baseline. For the ANCOVA across all regions, RAC* binding did not differ significantly between tic and control subjects (main effect of diagnosis, F=0.744, df=1,8, p=0.413; tic vs control). Nevertheless, baseline RAC* binding was numerically higher in TS by 13–17% in the three striatal VOIs and by 5–7% in the frontal lobe and thalamus VOIs. The whole-brain analysis identified no significant regional differences in baseline RAC* binding between TS and control subjects.
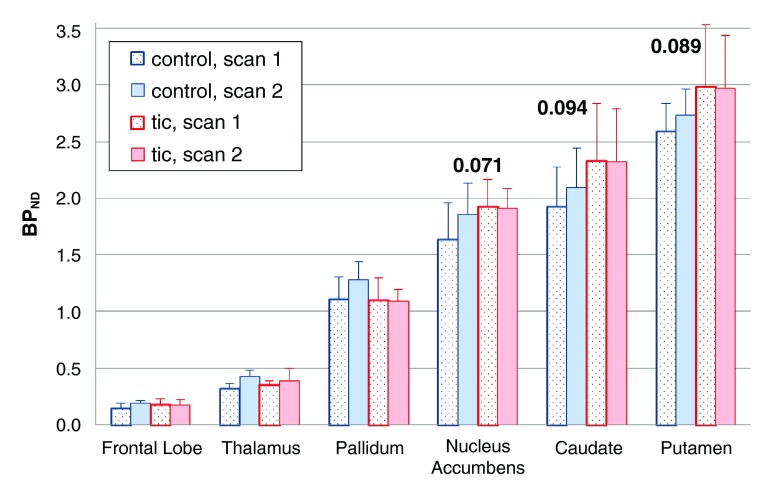
TS vs control: time effect (change from first to second scan). There was a trend for the change in BP ND during the infusion to be smaller in tic subjects (time × diagnosis interaction F=4.211, df=1,8, p=0.074). Each of the three striatal regions showed a similar effect when analyzed individually (0.05 < p < 0.10). Figure 6 shows the VOI BP ND values before and during the placebo infusion, by diagnosis.
Figure 6. Change in [ 11C]raclopride binding on placebo day, by diagnosis.
Mean BP NDs from the a priori VOIs, before and during the infusion on the placebo day only. Error bars show SD. The p values shown are for the time × diagnosis interaction in the individual region ANOVAs.
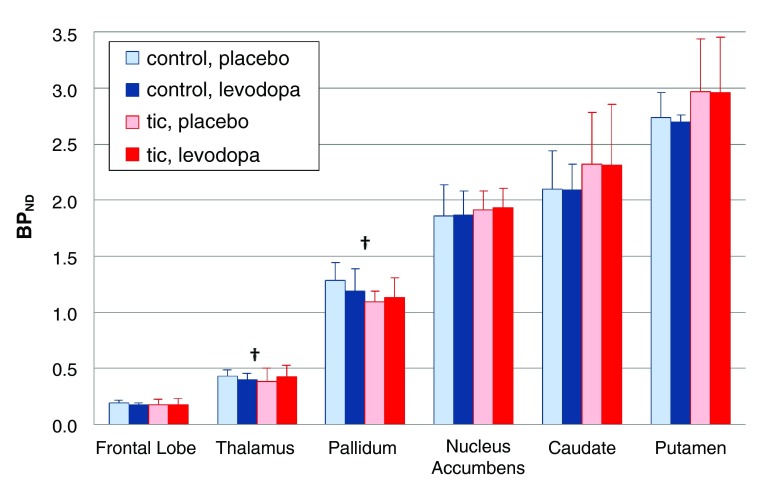
TS vs control: effect of levodopa on RAC* binding. In the a priori VOIs, the effect of LD did not differ overall in tic subjects (day × time × diagnosis interaction, F=1.308, df=1,8, p=0.286), and the 4-way interaction (day × time × diagnosis × region) was not significant (F=1.577, df=5,4, p=0.340). Although not statistically significant, pallidal and thalamic BP ND tended to decrease in control subjects but increase in the tic subjects ( Figure 7).
Figure 7. Levodopa-induced change in BP ND, by diagnosis.
Mean BP ND for the a priori VOIs is shown during the levodopa and placebo infusions; the error bar indicates SD. The day × time × diagnosis interaction and the day × time × diagnosis × region interaction were not significant. The daggers indicate a trend in the thalamic and pallidal VOIs for BP ND to decrease with levodopa in the control group but increase with levodopa in the tic group (regional ANOVA, day × time × diagnosis interaction, pallidum p=0.050, thalamus p=0.098).
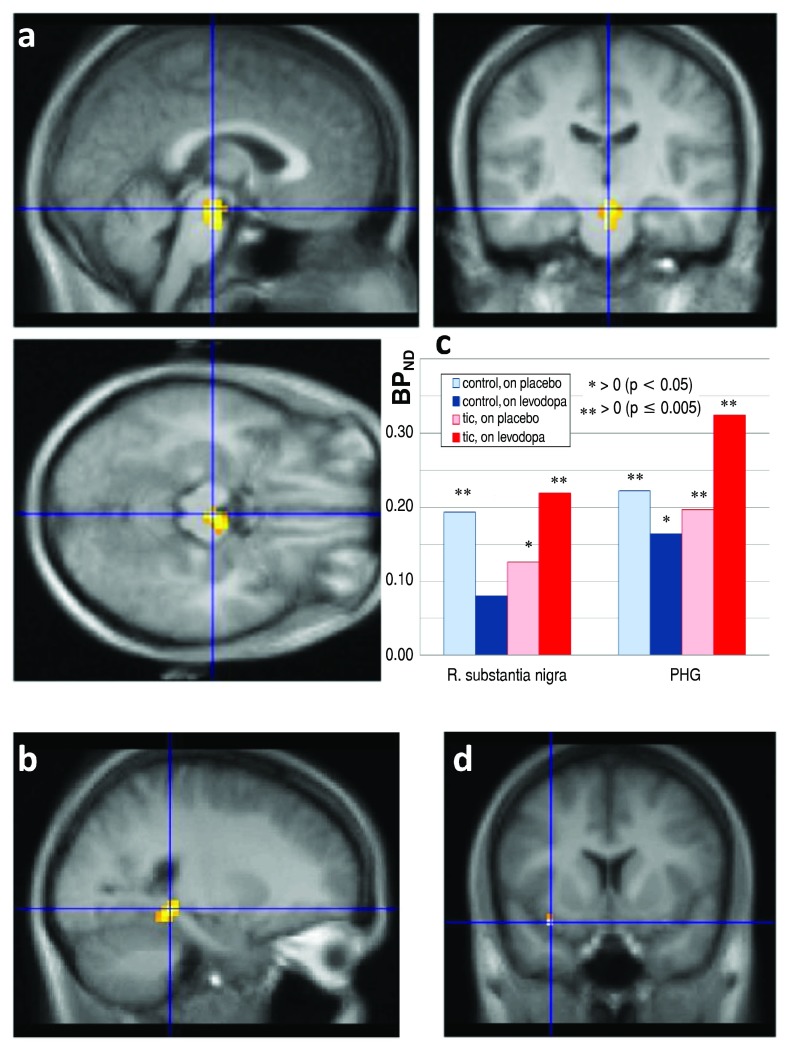
The whole-brain analysis identified a similar but statistically significant effect in two clusters, where RAC* binding decreased with levodopa in controls, consistent with increased dopamine release during the levodopa infusion, but RAC* binding increased in the TS group. The first cluster included 38 voxels in midbrain (1.0 ml, FDR corrected p=0.002), with a peak t value of 9.0 (8 df) at atlas coordinate (1.5, −21, −15) and extending laterally in approximately the right substantia nigra/ventral tegmental area ( Figure 8a). A second significant cluster of 19 voxels (0.5 ml, corrected p=0.023) occurred in parahippocampal gyrus, with peak t=7.92 at (22.5, −39, −6) ( Figure 8b). The mean change in BP ND with levodopa in these regions is shown in Figure 8c. In both these clusters, the BP ND on placebo was positive in all subjects ( p < 0.001, binomial distribution), consistent with nontrivial RAC* binding. The highest t value in the whole-brain comparison, 11.62, occurred in Brodmann’s area 13, but the cluster volume was only 0.1 ml, not significant by FDR correction ( Figure 8d).
Figure 8. RAC* binding on levodopa vs. placebo, by diagnosis.
Differences in the RAC* binding response to levodopa between TS and control subjects, thresholded at uncorrected p = 0.001, in color, laid over the MRI template image in grayscale. a, b: Significant clusters, with blue lines crossing at the peak t value in midbrain ( a, three views) and in parahippocampal gyrus ( b). A third statistically significant cluster was centered at the posterior edge of the occipital lobe, but both the location and the observation that in this cluster the BP ND on placebo was negative in half the subjects suggests that this cluster likely does not reflect specific binding. c: Levodopa-induced change in BP ND, TS vs. control, in the clusters shown in A and B. R., Right; PHG, parahippocampal gyrus. Asterisks indicate that mean BP ND differs significantly from zero. d: The blue lines cross at the voxel with the highest t value in the whole-brain SPM analysis of levodopa effect ΔDVR images ( t=11.62, 8 df).
Discussion
Change in striatal BP ND on the placebo day
BP ND increased from before to during the placebo infusion in the striatum, thalamus and frontal lobe VOIs, especially in control subjects ( Figure 5, Figure 6). Surprisingly little information describes within-day stability of RAC* binding, though several studies compare binding across time intervals of days to months 42– 45. Mawlawi et al. 46 scanned 10 subjects twice each on the same day using a bolus-plus-constant-infusion method, and found no significant mean change from the first to the second scan. However, Alakurtti and colleagues 47 found that mean BP ND increased from the first to the second scan of the day in striatal and thalamic regions, with the change (about +5%) reaching statistical significance in medial and lateral thalamus.
The observation in the present study that BP ND increased from the first to second scan of the day is consistent with this background, and is relevant to RAC* challenge PET studies in general, because essentially all such studies use a before- vs. after-intervention design. Slifstein et al. [ 48, p. 357] argue that the existence of placebo-induced DA responses make the before-after model more appropriate for amphetamine challenge studies. However, our results and those of Alakurtti et al. 47 suggest that BP ND increases from the first to the second scan even without active intervention. This does not invalidate the results of most before-after RAC* studies, since amphetamine challenge decreases striatal RAC* BP ND by a large fraction, and to a lesser extent so do many cognitive and behavioral interventions in such studies, including studies of the placebo effect. However, the present results suggest that before-after RAC* studies may be less sensitive to manipulations that would decrease dopamine release.
Possible pathophysiological interpretation. The increase in BP ND during the placebo infusion is most likely associated with passage of time rather than a placebo effect per se, especially as placebo administration is more likely to increase dopamine release 48– 50. The presumed decrease in dopamine release during the placebo infusion could indicate that control subjects accommodate to the scanner environment over the course of the study day.
Effect of levodopa infusion on RAC* binding
Levodopa effect on RAC* binding in striatum. Striatal RAC* binding was not substantially changed by levodopa. Initially this result came as a surprise to the authors, because levodopa was given expressly with the expectation that it would increase synaptic dopamine levels. Briefly, support for this expectation includes the following. First, in Parkinson disease there is overwhelming evidence both by clinical observations and by RAC* PET imaging that exogenous levodopa substantially increases striatal dopamine release 51– 53. But there is also evidence in subjects without dopamine deficiency: intravenous levodopa is rapidly taken up from the bloodstream into the brain and converted into dopamine, and several studies show that it then boosts synaptic dopamine release [reviewed in 12]. For instance, exogenous levodopa produces clear sedative and cognitive effects in healthy people 54– 56. Thus the authors originally expected that exogenous levodopa would decrease striatal RAC* binding.
However, further reflection and reading have motivated a different view whereby the results support the original goal of choosing a pharmacological challenge agent that would stimulate phasic dopamine release, but under endogenous control. Recall that the concern with stimulants as challenge agents was that they cause a substantial release of dopamine at the striatal synapse regardless of current environmental demands; this approach may produce a ceiling effect for dopamine release that does not reflect typical endogenous control. A sensible hypothesis to explain the results of the present study would be that a research subject lying awake in a quiet, darkened room without specific cognitive demands has no need for substantial phasic release of dopamine, and thus even if exogenous levodopa has added dopamine to presynaptic vesicles, they are not released at a substantial rate at the synapse. A levodopa-raclopride study of a motor task in healthy individuals provides direct experimental support of this hypothesis 57. That study was properly designed with two sessions, placebo on one day and levodopa on another, with randomized order. Levodopa increased striatal dopamine release during performance of a motor task, but not at rest. Since in the present study all subjects were at rest during all scans, the results are consistent with those of Flöel and colleagues 57.
TS and control group comparisons
The tic and control subgroups have only five subjects each, and differences between the tic and control groups in the a priori VOIs were not statistically significant, so there is little need to comment further on these results. Previous drafts of this report included such discussion 58.
The whole-brain analysis comparing RAC* binding with levodopa vs. placebo did identify statistically significant differences ( Figure 8a–c). In midbrain (approximately substantia nigra/VTA) and in parahippocampal gyrus, levodopa stimulated dopamine release in controls but reduced it in TS subjects in. A similar pattern, though not statistically significant, was observed in orbital cortex (Brodmann’s area 13), thalamus and globus pallidus ( Figure 7 and Figure 8d).
One expects exogenous levodopa to increase dopamine release in the substantia nigra, as occurred in the control subjects. D 2 and D 3 dopamine receptors are present in the substantia nigra and their activation inhibits spike firing, dopamine synthesis and dopamine release by nigral dopaminergic cells 59. We hypothesize that levodopa increased dopamine stimulation of these inhibitory D2-like receptors in control subjects, and this may have prevented levodopa from stimulating nigrostriatal dopamine release into the striatum.
Subjects with TS, however, showed an increase in substantia nigra RAC* binding with levodopa, consistent with a decrease in nigral dopamine release. Nigral dopamine release has been related to reward and novelty in humans. Healthy adults with higher novelty seeking scores had lower D2-like binding ([ 18F]fallypride) in SN, consistent with greater dopamine release 60. Functional MRI studies have also demonstrated substantia nigra signal related to stimulus novelty or to the Novelty Seeking trait 61– 63. Healthy adults receiving a sweet vs salty taste had BOLD activation in this region 64. Despite this information, it is not clear how to relate a decrease in levodopa-stimulated dopamine release in substantia nigra to the pathophysiology of TS. Explaining the similar difference in nigral levodopa response in TS in parahippocampal gyrus and orbital cortex is no easier, though dopaminergic effects on D2-like binding in hippocampus have been documented in Parkinson disease 65 and dopamine agonists evoke changes in orbital cortex activity 66. The trend for a similar effect in thalamus is consistent with a [ 11C]FLB-457 PET study in which amphetamine provoked thalamic dopamine release in control subjects but not in TS 67. Overall, these results are consistent with an abnormality of presynaptic dopaminergic pharmacology in TS, but the limitations of this comparison must be acknowledged.
Limitations
Higher-affinity radioligands, such as [ 18F]fallypride or [ 11C]FLB 457, have advantages for measuring cortical D2Rs, e.g. in the frontal lobe where D2Rs appear at much lower concentrations than in the striatum. There are two primary concerns with RAC* outside the striatum [reviewed thoroughly in 9]. The first concern is a reliability issue: since the concentration of D2-like receptors is low in cortex compared to striatum, the counting statistics are poor for cortical VOIs of similar volume, and this renders the computed BP NDs suspect. For instance, some regional RAC* BP NDs are negative or close enough to zero that displacement studies produce results that are hard to interpret. In the present study, FreeSurfer-defined cortical regions allowed the creation of a large, reliably defined frontal lobe VOI, in which PET time-activity curves were low in noise ( Figure 3), allowing statistically reliable estimates of BP ND that were uniformly positive ( Table 4, Figure 4). Similarly RAC* displacement in thalamus has shown adequate counting statistics and reliability in previous studies 47, 68.
The second concern with RAC* in extrastriatal regions is one of validity or interpretation. RAC* binding in cortex includes some nonspecific binding 33, so a fair question is to what extent specific binding in cortex represents dopamine D2-like receptors. D2 and D4 receptors are expressed in human prefrontal cortex, though at relatively low concentrations compared to striatum 69. On the other hand, at least one study’s results suggest that raclopride may have superior sensitivity to fallypride for measuring dopamine release in some cortical regions 70. The validity concern is less worrisome in human thalamus, which contains predominantly D 3 rather than D 2 receptors 71, and in substantia nigra, where D 2 and D 3 receptors are well characterized. Other authors have interpreted substantia nigra RAC* displacement as indicating synaptic dopamine release 9.
Finally, comparing TS and control subgroups of only five subjects each provides insufficient power to identify some true group differences (type II error). More importantly, the small sample size lowers confidence in how representative the statistically significant differences are of the overall population of adults with TS.
Future directions
These results suggest that a natural next step for research in TS is to test whether dopamine release in TS differs during a dopamine-releasing cognitive (or other) task. Levodopa may augment the task-evoked release or interact with it differently in people with versus without tics. Along these lines, a cognitive-pharmacological interaction fMRI study in TS found that LD changed the BOLD responses to a working memory task 72. A newer levodopa infusion method produced roughly twice as high a levodopa plasma concentration as the infusion used in this study 12, and may produce greater dopamine release.
The spreadsheet in OpenDocument file format provides the clinical data and links each PET scan to the subject scanned and the condition (i.e., before or during the placebo or levodopa infusion). Also 40 PET files are provided with the filename extension .v, one for each dynamic PET scan. These files are in ECAT file format; users of other imaging file formats will find useful information at the following web site: http://www.turkupetcentre.net/petanalysis/format_image_ecat.html
Data availability
F1000Research: Dataset 1. PET images and clinical data, 10.5256/f1000research.5672.d42172 74
Consent
All subjects provided written confirmation of informed consent before study participation.
Acknowledgments
The authors gratefully acknowledge recruitment assistance from the Tourette Syndrome Association, editorial suggestions from Tamara Hershey, Ph.D., and technical assistance from Johanna M. Hartlein, R.N., M.S.N., Stephen Moerlein, Ph.D., BCNP, Susan Loftin, Kathryn I. Alpert, B.A., Meghan C. Campbell, Ph.D., Kathryn Vehe, Pharm.D., Michael P. McEvilly. These data were presented in part at the 14th International Congress of Parkinson Disease and Movement Disorders, Buenos Aires, 16 June 2010 73, and earlier drafts were circulated as preprints 58.
Funding Statement
Data collection was supported by the Tourette Syndrome Association and manuscript preparation was supported in part by NIH grants K24 MH087913 and R21 MH098670.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v1; ref status: indexed
References
- 1. Grace AA: Neuropsychopharmacology: The Fifth Generation of Progress. Dopamine. In: Davis KL, Charney D, Coyle JT, Nemeroff C, eds. Philadelphia, PA: Lippincott, Williams, & Wilkins,2002;119–132. Reference Source [Google Scholar]
- 2. Breitenstein C, Korsukewitz C, Flöel A, et al. : Tonic dopaminergic stimulation impairs associative learning in healthy subjects. Neuropsychopharmacology. 2006;31(11):2552–2564. 10.1038/sj.npp.1301167 [DOI] [PubMed] [Google Scholar]
- 3. Badgaiyan RD, Fischman AJ, Alpert NM: Striatal dopamine release in sequential learning. Neuroimage. 2007;38(3):549–556. 10.1016/j.neuroimage.2007.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wanat MJ, Willuhn I, Clark JJ, et al. : Phasic dopamine release in appetitive behaviors and drug addiction. Curr Drug Abuse Rev. 2009;2(2):195–213. 10.2174/1874473710902020195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer HS, Szymanski S, Giuliano J, et al. : Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am J Psychiatry. 2002;159(8):1329–1336. 10.1176/appi.ajp.159.8.1329 [DOI] [PubMed] [Google Scholar]
- 6. Yeh CB, Lee CS, Ma KH, et al. : Phasic dysfunction of dopamine transmission in Tourette’s syndrome evaluated with 99mTc TRODAT-1 imaging. Psychiatry Res. 2007;156(1):75–82. 10.1016/j.pscychresns.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 7. Wong DF, Brasic JR, Singer HS, et al. : Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacol. 2008;33(6):1239–1251. 10.1038/sj.npp.1301528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singer HS: The neurochemistry of Tourette syndrome. In: Martino D, Leckman JF, eds. Tourette Syndrome. New York: Oxford University Press.2013;276–300. [Google Scholar]
- 9. Egerton A, Mehta MA, Montgomery AJ, et al. : The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neurosci Biobehav Rev. 2009;33(7):1109–1132. 10.1016/j.neubiorev.2009.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drevets WC, Gautier C, Price JC, et al. : Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49(2):81–96. 10.1016/S0006-3223(00)01038-6 [DOI] [PubMed] [Google Scholar]
- 11. Denys D, de Vries F, Cath D, et al. : Dopaminergic activity in Tourette syndrome and obsessive-compulsive disorder. Eur Neuropsychopharmacol. 2013;23(11):1423–31. 10.1016/j.euroneuro.2013.05.012 [DOI] [PubMed] [Google Scholar]
- 12. Gordon M, Markham J, Hartlein JM, et al. : Intravenous levodopa administration in humans based on a two-compartment kinetic model. J Neurosci Methods. 2007;159(2):300–307. 10.1016/j.jneumeth.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 13. Tedroff J, Pedersen M, Aquilonius SM, et al. : Levodopa-induced changes in synaptic dopamine in patients with Parkinson’s disease as measured by [ 11C]raclopride displacement and PET. Neurology. 1996;46(5):1430–1436. 10.1212/WNL.46.5.1430 [DOI] [PubMed] [Google Scholar]
- 14. Newman RP, Weingartner H, Smallberg SA, et al. : Effortful and automatic memory: effects of dopamine. Neurology. 1984;34(6):805–807. 10.1212/WNL.34.6.805 [DOI] [PubMed] [Google Scholar]
- 15. Black KJ, Mink JW: Response to levodopa challenge in Tourette syndrome. Mov Disord. 2000;15(6):1194–1198. [DOI] [PubMed] [Google Scholar]
- 16. Black KJ, Carl JL, Hartlein JM, et al. : Rapid intravenous loading of levodopa for human research: clinical results. J Neurosci Methods. 2003;127(1):19–29. 10.1016/S0165-0270(03)00096-7 [DOI] [PubMed] [Google Scholar]
- 17. Hershey T, Black KJ, Stambuk MK, et al. : Altered thalamic response to levodopa in Parkinson’s patients with dopa-induced dyskinesias. Proc Natl Acad Sci U S A. 1998;95(20):12016–12021. 10.1073/pnas.95.20.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hershey T, Black KJ, Carl JL, et al. : Dopa-induced blood flow responses in nonhuman primates. Exp Neurol. 2000;166(2):342–349. 10.1006/exnr.2000.7522 [DOI] [PubMed] [Google Scholar]
- 19. Hershey T, Black KJ, Carl JL, et al. : Long term treatment and disease severity change brain responses to levodopa in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74(7):844–851. 10.1136/jnnp.74.7.844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. First MB, Spitzer RL, Gibbon M, et al. : Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York: Biometrics Research, New York State Psychiatric Institute,2002. Reference Source [Google Scholar]
- 21. Robertson MM, Banerjee S, Kurlan R, et al. : The Tourette syndrome diagnostic confidence index: development and clinical associations. Neurology. 1999;53(9):2108–2112. 10.1212/WNL.53.9.2108 [DOI] [PubMed] [Google Scholar]
- 22. Leckman JF, Riddle MA, Hardin MT, et al. : The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573. 10.1097/00004583-198907000-00015 [DOI] [PubMed] [Google Scholar]
- 23. Walkup JT, Rosenberg LA, Brown J, et al. : The validity of instruments measuring tic severity in Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry. 1992;31(3):472–477. 10.1097/00004583-199205000-00013 [DOI] [PubMed] [Google Scholar]
- 24. Cohen DJ, Leckman JF, Shaywitz BA: The Tourette’s syndrome and other tics. In: Shaffer D, Ehrhardt AA, Greenhill L, eds. Diagnosis and Treatment in Pediatric Psychiatry. New York: MacMillan Free Press,1984:3–28. [Google Scholar]
- 25. Scahill L, King RA, Schultz RT, et al. : Selection and use of diagnostic and clinical rating instruments. In: Leckman JF, Cohen DJ, eds. Tourette’s syndrome -- tics, obsessions, compulsions: Developmental psychopathology and clinical care. New York: John Wiley & Sons, Inc.,1999:310–324. [Google Scholar]
- 26. DuPaul GJ, Power TJ, Anastopoulos AD, et al. : ADHD Rating Scale-IV: Checklists, Norms, and Clinical Interpretation. New York: Guilford Publications,1998. Reference Source [Google Scholar]
- 27. Goodman WK, Price LH, Rasmussen SA, et al. : The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. 10.1001/archpsyc.1989.01810110048007 [DOI] [PubMed] [Google Scholar]
- 28. Goodman WK, Price LH, Rasmussen SA, et al. : The Yale-Brown Obsessive Compulsive Scale. II. validity. Arch Gen Psychiatry. 1989;46(11):1012–1016. 10.1001/archpsyc.1989.01810110054008 [DOI] [PubMed] [Google Scholar]
- 29. Harder S, Baas H: Concentration-response relationship of levodopa in patients at different stages of Parkinson’s disease. Clin Pharmacol Ther. 1998;64(2):183–191. 10.1016/S0009-9236(98)90152-7 [DOI] [PubMed] [Google Scholar]
- 30. Contin M, Riva R, Martinelli P, et al. : Levodopa therapy monitoring in patients with Parkinson disease: a kinetic-dynamic approach. Ther Drug Monit. 2001;23(6):621–629. 10.1097/00007691-200112000-00005 [DOI] [PubMed] [Google Scholar]
- 31. Karimi M, Carl JL, Loftin S, et al. : Modified high-performance liquid chromatography with electrochemical detection method for plasma measurement of levodopa, 3-O-methyldopa, dopamine, carbidopa and 3,4-dihydroxyphenyl acetic acid. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;836(1–2):120–123. 10.1016/j.jchromb.2006.03.027 [DOI] [PubMed] [Google Scholar]
- 32. Ehrin E, Gawell L, Högberg T, et al. : Synthesis of (methoxy- 3H)- and (methoxy-C-11)-labeled raclopride, specific dopamine D-2 receptor ligands. J Labelled Comp Radiopharm. 1986;24(8):931–940. 10.1002/jlcr.2580240808 [DOI] [Google Scholar]
- 33. Farde L, Pauli S, Hall H, et al. : Stereoselective binding of 11C-raclopride in living human brain--a search for extrastriatal central D2-dopamine receptors by PET. Psychopharmacology (Berl). 1988;94(4):471–478. 10.1007/BF00212840 [DOI] [PubMed] [Google Scholar]
- 34. Perlmutter JS, Snyder AZ, Tolia VN, et al. : Does the spatial distribution of putaminal D 2 receptors differ in patients with blepharospasm vs. hand cramp? Abstracts of the Society for Neuroscience. 1998;24:1475 Reference Source [Google Scholar]
- 35. Black KJ, Snyder AZ, Koller JM, et al. : Template images for nonhuman primate neuroimaging: 1. Baboon. Neuroimage. 2001;14(3):736–743. 10.1006/nimg.2001.0752 [DOI] [PubMed] [Google Scholar]
- 36. Eisenstein SA, Koller JM, Piccirillo M, et al. : Characterization of extrastriatal D2 in vivo specific binding of [ 18F](N-methyl)benperidol using PET. Synapse. 2012;66(9):770–780. 10.1002/syn.21566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Black KJ, Snyder AZ, Mink JW, et al. : Spatial reorganization of putaminal dopamine D 2-like receptors in cranial and hand dystonia. PLoS One 2014;9(2):e88121. 10.1371/journal.pone.0088121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang L, Lee DY, Bailey E, et al. : Validity of large-deformation high dimensional brain mapping of the basal ganglia in adults with Tourette syndrome. Psychiatry Res. 2007;154(2):181–190. 10.1016/j.pscychresns.2006.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mintun MA, Raichle ME, Kilbourn MR, et al. : A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15(3):217–227. 10.1002/ana.410150302 [DOI] [PubMed] [Google Scholar]
- 40. Innis RB, Cunningham VJ, Delforge J, et al. : Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. 10.1038/sj.jcbfm.9600493 [DOI] [PubMed] [Google Scholar]
- 41. Logan J, Fowler JS, Volkow ND, et al. : Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 1996;16(5):834–840. 10.1097/00004647-199609000-00008 [DOI] [PubMed] [Google Scholar]
- 42. Volkow ND, Fowler JS, Wang GJ, et al. : Reproducibility of repeated measures of carbon-11-raclopride binding in the human brain. J Nucl Med. 1993;34(4):609–613. [PubMed] [Google Scholar]
- 43. Volkow ND, Wang GJ, Fowler JS, et al. : Imaging endogenous dopamine competition with [ 11C]raclopride in the human brain. Synapse. 1994;16(4):255–262. 10.1002/syn.890160402 [DOI] [PubMed] [Google Scholar]
- 44. Hietala J, Nagren K, Lehikoinen P, et al. : Measurement of striatal D2 dopamine receptor density and affinity with [ 11C]-raclopride in vivo: a test-retest analysis. J Cereb Blood Flow Metab. 1999;19(2):210–217. 10.1097/00004647-199902000-00012 [DOI] [PubMed] [Google Scholar]
- 45. Yoder KK, Albrecht DS, Kareken DA, et al. : Test-retest variability of [ 11C]raclopride-binding potential in nontreatment-seeking alcoholics. Synapse. 2011;65(7):553–561. 10.1002/syn.20874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mawlawi O, Martinez D, Slifstein M, et al. : Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. 10.1097/00004647-200109000-00002 [DOI] [PubMed] [Google Scholar]
- 47. Alakurtti K, Aalto S, Johansson JJ, et al. : Reproducibility of striatal and thalamic dopamine D2 receptor binding using [ 11C]raclopride with high-resolution positron emission tomography. J Cereb Blood Flow Metab. 2011;31(1):155–165. 10.1038/jcbfm.2010.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Slifstein M, Kegeles LS, Xu X, et al. : Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64(5):350–362. 10.1002/syn.20734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de la Fuente-Fernandez R, Ruth TJ, Sossi V, et al. : Expectation and dopamine release: mechanism of the placebo effect in Parkinson’s disease. Science. 2001;293(5532):1164–1166. 10.1126/science.1060937 [DOI] [PubMed] [Google Scholar]
- 50. de la Fuente-Fernandez R, Stoessl AJ: The placebo effect in Parkinson’s disease. Trends Neurosci. 2002;25(6):302–306. 10.1016/S0166-2236(02)02181-1 [DOI] [PubMed] [Google Scholar]
- 51. Antonini A, Leenders KL, Vontobel P, et al. : Complementary PET studies of striatal neuronal function in the differential diagnosis between multiple system atrophy and Parkinson’s disease. Brain. 1997;120(Pt 12):2187–2195. 10.1093/brain/120.12.2187 [DOI] [PubMed] [Google Scholar]
- 52. de la Fuente-Fernandez R, Lu JQ, Sossi V, et al. : Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson’s disease: PET evidence of increased dopamine turnover. Ann Neurol. 2001;49(3):298–303. 10.1002/ana.65 [DOI] [PubMed] [Google Scholar]
- 53. Pavese N, Evans AH, Tai YF, et al. : Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology. 2006;67(9):1612–1617. 10.1212/01.wnl.0000242888.30755.5d [DOI] [PubMed] [Google Scholar]
- 54. Andreu N, Chale JJ, Senard JM, et al. : L-Dopa-induced sedation: a double-blind cross-over controlled study versus triazolam and placebo in healthy volunteers. Clin Neuropharmacol. 1999;22(1):15–23. 10.1097/00002826-199901000-00004 [DOI] [PubMed] [Google Scholar]
- 55. Kelly C, de Zubicaray G, Di Martino A, et al. : L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29(22):7364–7378. 10.1523/JNEUROSCI.0810-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Weis T, Puschmann S, Brechmann A, et al. : Effects of L-dopa during auditory instrumental learning in humans. PLoS One. 2012;7(12):e52504. 10.1371/journal.pone.0052504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Flöel A, Garraux G, Xu B, et al. : Levodopa increases memory encoding and dopamine release in the striatum in the elderly. Neurobiol Aging. 2008;29(2):267–279. 10.1016/j.neurobiolaging.2006.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Black KJ, Piccirillo ML, Koller JM, et al. : Levodopa-stimulated dopamine release in Tourette syndrome. PeerJ PrePrints. 2013;1:e30 10.7287/peerj.preprints.30 [DOI] [Google Scholar]
- 59. Grace AA: Dopamine. In; Davis KL, Charney D, Coyle JT, Nemeroff C, eds. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia, PA: Lippincott Williams & Wilkins,2002:2080 Reference Source [Google Scholar]
- 60. Zald DH, Cowan RL, Riccardi P, et al. : Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci. 2008;28(53):14372–14378. 10.1523/JNEUROSCI.2423-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bunzeck N, Duzel E: Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51(3):369–379. 10.1016/j.neuron.2006.06.021 [DOI] [PubMed] [Google Scholar]
- 62. Krebs RM, Schott BH, Duzel E: Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biol Psychiatry. 2009;65(2):103–110. 10.1016/j.biopsych.2008.08.019 [DOI] [PubMed] [Google Scholar]
- 63. Krebs RM, Heipertz D, Schuetze H, et al. : Novelty increases the mesolimbic functional connectivity of the substantia nigra/ventral tegmental area (SN/VTA) during reward anticipation: Evidence from high-resolution fMRI. Neuroimage. 2011;58(2):647–655. 10.1016/j.neuroimage.2011.06.038 [DOI] [PubMed] [Google Scholar]
- 64. O’Doherty JP, Deichmann R, Critchley HD, et al. : Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–826. 10.1016/S0896-6273(02)00603-7 [DOI] [PubMed] [Google Scholar]
- 65. Bohnen NI, Gedela S, Herath P, et al. : Selective hyposmia in Parkinson disease: association with hippocampal dopamine activity. Neurosci Lett. 2008;447(1):12–16. 10.1016/j.neulet.2008.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Black KJ, Hershey T, Koller JM, et al. : A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci U S A. 2002;99(26):17113–17118. 10.1073/pnas.012260599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Steeves TD, Ko JH, Kideckel DM, et al. : Extrastriatal dopaminergic dysfunction in tourette syndrome. Ann Neurol. 2010;67(2):170–181. 10.1002/ana.21809 [DOI] [PubMed] [Google Scholar]
- 68. Hirvonen J, Aalto S, Lumme V, et al. : Measurement of striatal and thalamic dopamine D2 receptor binding with 11C-raclopride. Nucl Med Commun. 2003;24(12):1207–1214. [DOI] [PubMed] [Google Scholar]
- 69. Meador-Woodruff JH, Damask SP, Wang J, et al. : Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology. 1996;15(1):17–29. 10.1016/0893-133X(95)00150-C [DOI] [PubMed] [Google Scholar]
- 70. Slifstein M, Kegeles LS, Xu X, et al. : Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse. 2010;64(5):350–362. 10.1002/syn.20734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sun J, Xu J, Cairns NJ, et al. : Dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) densities in aged human brain. PLoS One. 2012;7(11):e49483. 10.1371/journal.pone.0049483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hershey T, Black KJ, Hartlein JM, et al. : Cognitive-pharmacologic functional magnetic resonance imaging in Tourette syndrome: a pilot study. Biol Psychiatry. 2004;55(9):916–925. 10.1016/j.biopsych.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 73. Black KJ, Koller JM, Campbell MC, et al. : Levodopa-stimulated dopamine release in Tourette syndrome. Movement Disorders. 2010;25:S373 Reference Source [Google Scholar]
- 74. Black KJ, Piccirillo ML, Koller JM, et al. : PET images and clinical data. F1000Research. 2015. Data Source [Google Scholar]