Abstract

Allene aziridination generates useful bicyclic methylene aziridine scaffolds that can be flexibly transformed into a range of stereochemically complex and densely functionalized amine-containing stereotriads. The scope of this chemistry has been limited by the poor chemoselectivity that often results when typical dinuclear Rh(II) catalysts are employed with homoallenic carbamates. Herein, Ag(I) catalysts that significantly improve the scope and yield of bicyclic methylene aziridines that can be prepared via allene aziridination are described.
The chemoselective amination of unsaturated compounds can be a challenging endeavor. In the case of alkene amination, the aziridination of a π bond is often favored over competing amination of an allylic C-H bond.1,2 However, our recent studies in allene aziridination have uncovered several examples where the chemoselectivity of the reaction depends more heavily on the structural features of the allene than we had expected.1 As typical Rh-based catalysts did not permit successful tuning of the selectivity of allene amination, we needed an alternative catalyst system that would favor aziridination over C-H insertion (Scheme 1).
Scheme 1.
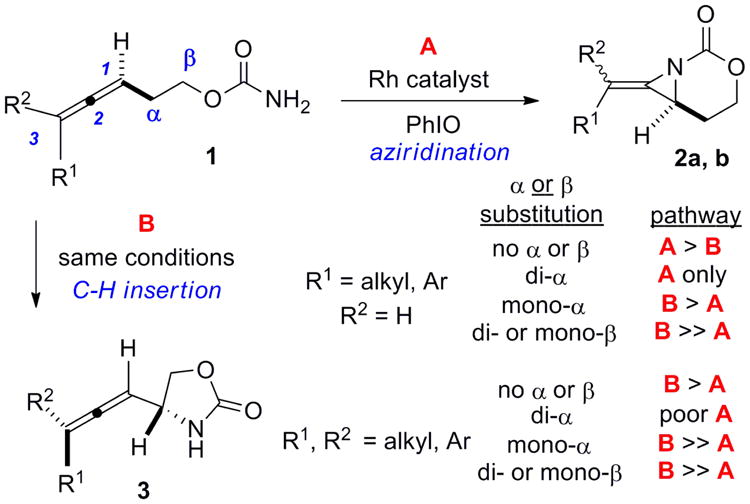
Rh-catalyzed amination of homoallenic carbamates.
Bicyclic methylene aziridines 2a and 2b and allenic amines 3 (Scheme 1, Figure 1) are valuable intermediates for the preparation of complex amine-containing stereotriads, tetrads and heterocycles.1 In our previous studies, we have described the treatment of homoallenic carbamates 1 with tetra-bridged dirhodium(II) carboxylate complexes to promote formation of 2a and 2b as the major products.1a-d However, good chemoselectivity was reliant on the specific substitution of the allene and the presence or absence of branching in the tether.1e Competing C-H insertion to yield 3 was exacerbated when the allene was trisubstituted or branching in the tether between the allene and the nitrogen source was present.1e Efforts to improve chemoselectivity by tuning Rh-based catalysts via the ligand were disappointing. Thus, we needed to identify another catalyst system capable of delivering predictable and superior selectivity for allene aziridination, independent of substrate structure.
Figure 1.
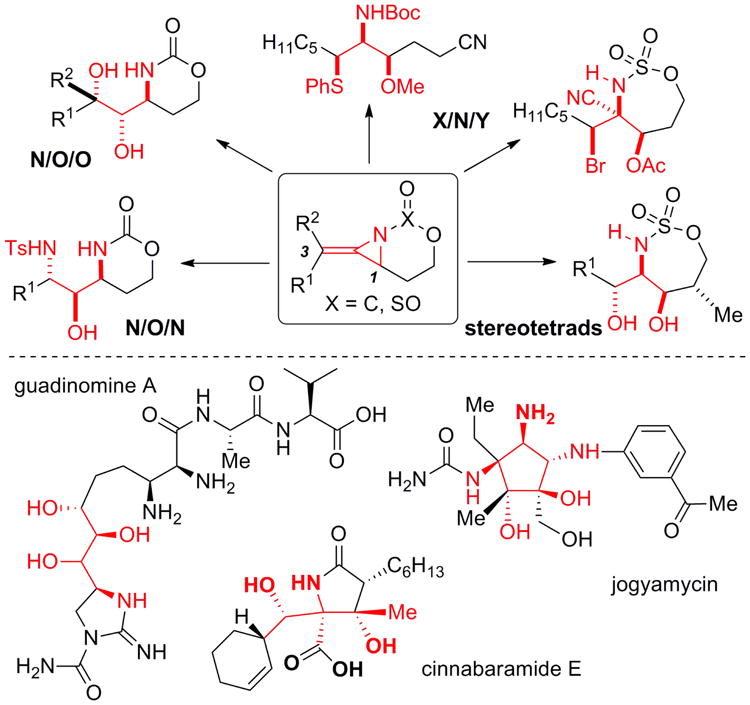
Synthetic utility of bicyclic methylene aziridines and potential natural product targets.
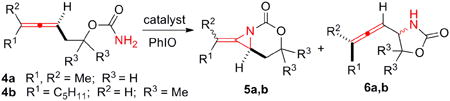
Several transition metals, including Rh, Cu, Ru, Fe, Co, Au and Ag, are known to promote C-N bond formation via presumed nitrene intermediates (Figure 2).2,3,4,5,6,7,8 Recent studies have shown that changing the identity of the metal in the catalyst can control whether predominantly aziridination or C-H amination is observed when both C-H and π-bonds are present.9,10 We focused on two substrates 4a and 4b that gave poor chemoselectivity in Rh-catalyzed aziridination. Treatment of 4a with Rh2(esp)2 (esp = α,α,α′,α′-tetramethyl-1,3-benzene-dipropionic acid, Table 1, entry 1) gave only a 35% yield of 5a and significant C-H insertion to 6a. Rh2(espn)2Cl (entry 2) performed better, yet further attempts to improve the chemoselectivity by changing the nature of the carboxylate ligands on the Rh were unsuccessful.11 A series of Cu catalysts gave poor reactivity and attempts to isolate the iodinane prior to amination were unsuccessful (see Supporting Information for details). However, Cu(MeCN)4PF6 (entry 5) could be induced to give low yields of amination products by pre-mixing 4a with PhIO before the addition of catalyst.12 However, this did not improve the results using Ru and Fe-based catalysts, even when 20 mol % of the metal was employed (entries 6-7).3c,4a,5e While treatment of 4a with AgOTf in the presence of PhIO gave very little 5a (entry 8), the addition of dafone (4,5-diazafluoren-9-one, entry 9) improved the conversion and encouragingly, yielded > 20:1 chemoselectivity for aziridination over C-H insertion. A series of bipyridine (bipy) ligands (entries 10-12) also gave excellent chemoselectivity for aziridination with good yields.8a-d Phen (entry 13) increased the yield to 79%, but the additional bulk in bathophen (entry 14) was not necessary. To our surprise, switching to a terpyridine ligand (terpy, entry 15) reversed the chemoselectivity in favor of 6a.8a Interestingly, the nature of the Ag counteranion also had a significant impact on the reaction. Substitution of AgOTf with AgOAc (entry 16) or AgO2CCF3 (entry 17) gave almost exclusively C-H insertion 6a, although the reactivity of the catalyst was diminished, as these anions can bind tightly to the metal.
Figure 2.
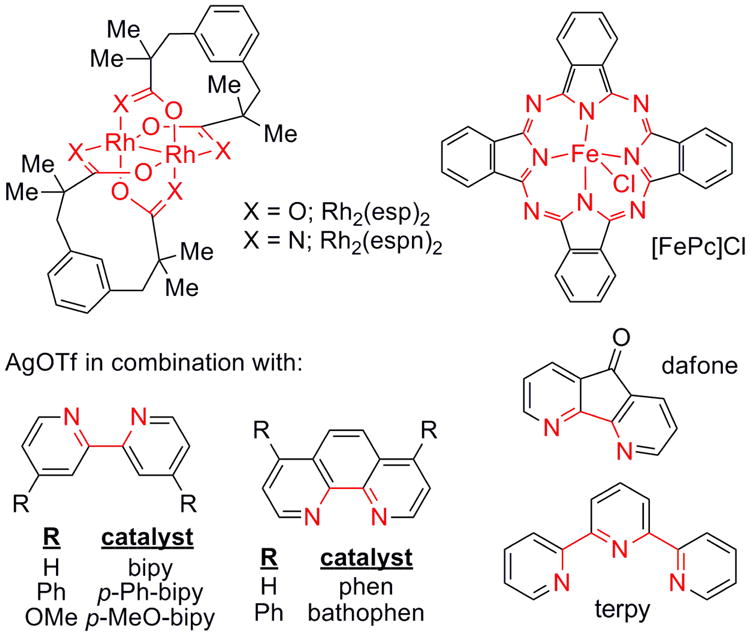
Catalysts for chemoselective allene amination.
Table 1.
Investigation of catalysts for chemo-selective allene amination reactions.
| ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| entry | catalysta | A:lb | % 5a(6a)c | entry | catalysta | E:Z | A:lb | %5b(6b)c |
| 1 | Rh2(esp)2 | 2:1 | 35 (17) | |||||
| 2 | Rh2(espn)2CI | 2.7:1 | 40 (15) | 18 | Rh2(esp)2 | 2:1 | 1:17 | 5 (80) |
| 3 | Cu(MeCN)4PF6/ph en | --- | 2 (0) | 19 | Rh2(espn)2CI | 3:1 | 1:4.7 | 9 (42) |
| 4 | Cu(OTf)2/phen | --- | 0 (0)d | 20 | Cu(MeCN)4PF6 | --- | --- | 0 |
| 5 | Cu(MeCN)4PF6 | 3.3:1 | 13 (4)d | |||||
| 6 | Ru2(hp)4CI | --- | 0 (4) | 21 | [Ru2(hp)4CI] | --- | --- | 0 |
| 7 | [FePc]CI | --- | 0 (0) | 22 | [FePc]CI | --- | --- | 0 |
| 8 | AgOTf | >20:1 | 6 (O)e | 23 | AgOTf | nd | --- | 2 (0)d |
| 9 | AgOTf/dafone | >20:1 | 32 | 24 | AgOTf/dafone | 2.3:1 | 2.7:1 | 59 (22) |
| 10 | AgOTf/bipy | >20:1 | 60 | 25 | AgOTf/bipy | 2.4:1 | 6.4:1 | 68 (11) |
| 11 | AgOTf/p-Ph-bipy | >20:1 | 66 | |||||
| 12 | AgOTf/p-MeObipy | >20:1 | 72 | 26 | AgOTf/p-Ph-bipy | 2.3:1 | 3.1:1 | 62 (20) |
| 13 | AgOTf/phen | >20:1 | 79 | 27 | AgOTf/p-MeObipy | 2.2:1 | 6.4:1 | 73 (11) |
| 14 | AgOTf/bathophen | 20:1 | 57 | 28 | AgOTf/phen | 2.2:1 | 5.9:1 | 80 (14) |
| 15 | AgOTf/terpy | 1:1.3 | 27 (35) | 29 | AgOTf/bathophen | 1.9:1 | 7:1 | 84 (12) |
| 16 | AgOAc/phen | 1:14.6 | 0 (29)f | 30 | AgOTf/terpy | 2.3:1 | 1:6.6 | 9 (61) |
| 17 | AgO2CCF3/phen | 1:20 | 0 (40)g | |||||
Rh: 5 mol % catalyst, 2.0 equiv PhIO, CH2CI2, rt. Cu, Ru, Fe: 20 mol % catalyst, 2.0 equiv PhIO, rt. Ag: 20 mol % AgOTf, 25 mol % ligand, 4 Å MS, 2.0 equiv PhIO, CH2CI2.
A = aziridination 5a,5b; I = insertion 6a,b.
NMR yields using mesitylene as the internal standard.
4a and PhIO were mixed for 1 h prior to adding the catalyst
significant decomposition of the carbamate occurred
45% conversiong
60% conversion
The allenic carbamate 4b was also a challenging substrate for aziridination. When Rh2(esp)2 was employed as the catalyst, an 80% yield of 6b was obtained (entry 18),1e highlighting the impact of substrate structure on the chemoselectivity of Rh-catalyzed C-N bond formation. Switching to Rh2(espn)2 (entry 19) did not improve the outcome, nor did Cu, Fe or Ru-based catalysts (entries 20-22). However, employing a dafone ligand in the presence of AgOTf (entry 24) reversed the chemoselectivity to 2.7:1 in favor of aziridination. Bipyridine ligands (entries 25-27) improved the chemoselectivity further, resulting in good yields of the aziridine 5b and A:I selectivities ranging from 3.1:1 to 6.4:1. Phen and bathophen (entries 28-29) gave comparable yields, in contrast to the aziridination of the more sterically demanding 4a (entries 13-14). As in the case of 4a, a 2,2′:6′,2″-terpyridine ligand (entry 30) resulted in a reversal of the chemoselectivity, providing a 5b:6b ratio of 1:6.6. To our knowledge, these results represent the first examples of reagent-controlled amination of unsaturated substrates with Ag(I) catalysis.
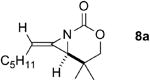
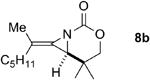
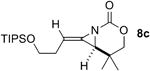
A series of allenes containing α,α-dimethyl groups (Table 2) were investigated with Ag(I) catalysts as an attractive alternative to Rh catalysts. Gratifyingly, treatment of 7a with AgOTf/phen gave an excellent yield of 8a (entry 1), which compared well with our previous results using Rh2TPA4 (TPA = triphenylacetate). Interestingly, the E:Z ratio was not greatly affected by the nature of the catalyst. AgOTf/phen also performed on par with Rh when a 1,3,3-trisubstituted homoallenic carbamate was employed (entry 2) and other 1,3-disubstituted allene carbamates (entries 3-4, 8) gave good to excellent yields of the methylene aziridines 8c, 8d and 8h. The presence of a polar carboxyethyl group in 7h (entry 8) gave very different behavior depending on whether a Rh- or Ag-based catalyst was employed. While Ag gave the E methylene aziridine as the expected stereoisomer, Rh2(esp)2 unexpectedly yielded the Z isomer as the major product. In addition to the 1,3,3-trisubstituted homoallenic carbamate 7b, other highly substituted allenes bearing α,α-dimethyl branching (entries 5-7) gave good yields of methylene aziridines 8e-g.
Table 2.
Aziridination of α,α-disubstituted allene carbamates.
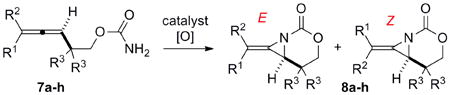
| ||||
|---|---|---|---|---|
|
| ||||
| entry | desired product | catalysta,b | E:Z | yield |
| 1 |
|
Rh2TPA4 | 4:1 | 92% |
| AgOTf/phen | 4:1 | 88% | ||
| 2 |
|
Rh2(esp)2 | 2.3:1 | 88% |
| AgOTf/phen | 2.3:1 | 81% | ||
| 3 |
|
AgOTf/phen | 3:1 | 87% |
| 4 |
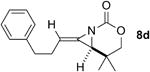
|
AgOTf/phen | 2.6:1 | 83% |
| 5 |
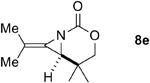
|
AgOTf/phen | -- | 98% |
| 6 |
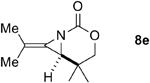
|
AgOTf/phen | 3:1 | 90% |
| 7 |
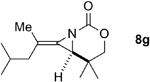
|
AgOTf/phen | 2.3:1 | 97% |
| 8 |
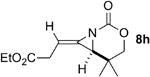
|
Rh2(esp)2 | 1:2.8 | 86% |
| AgOTf/phen | 2.2:1 | 85% | ||
Rh conditions: 3 mol % catalyst, 2 equiv PhIO, 4 A MS, CH2CI2, rt
Reaction conditions: 20 mol % AgOTf, 25 mol % ligand, 2 equiv PhIO, 4 Å MS, CH2CI2, rt.
The next challenge for Ag(I)-based catalysts was to explore the chemoselectivity of amination in substrates where C-H insertion was a competing process (Table 3). When 1,3-disubstituted allenes 7i and 7j were employed (entries 1-2), AgOTf in the presence of bipyridine and phenanthroline ligands gave comparable to slightly improved yields of bicyclic methylene aziridines. The benefit of substituting Ag catalysts for Rh became apparent when substitution was present in the tether between the allene and the carbamate (entries 3-4). AgOTf/phen increased the aziridination:insertion (A:I) ratio from 1:1 to 9:1 in the amination of 7k to 8k (entry 3). This effect was even more dramatic in the case of 4b, with a change in the A:I ratio of 1:17 to 5.9:1 (entry 4). Surprisingly, the 1,3,3-trisubstituted allene carbamates 5a and 8l-m (entries 5-7) exhibited much greater chemoselectivity for aziridination using AgOTf/phen catalysts compared to conventional Rh catalysts. This was counterintuitive, as we expected the increased steric congestion around the allene to favor more of the C-H insertion product. The ability to prepare such highly substituted bicyclic methylene aziridines represents a valuable step forward in our ability to prepare densely functionalized and complex nitrogen-containing stereotriads using these reactive scaffolds (Figure 1).
Table 3.
Chemoselective aziridination of homoallenic carbamates using Ag(I) catalysis.
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | desired product | Catalysta,b | E:Z | A:lc | yield |
| 1 |
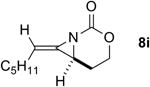
|
Rh2TPA4 | 4:1 | 4:1 | 80% |
| Rh2(esp)2 | 3:1 | 4:1 | 66% | ||
| AgOTf/bipy | 3:1 | 4:1 | 72% | ||
| 2 |
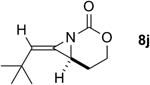
|
Rh2(esp)2 | >99:1 | 2:1 | 49% |
| AgOTf/phen | >99:1 | 1:1 | 40%d | ||
| AgOTf/bipy | >99:1 | 3.7:1 | 67% | ||
| 3 |
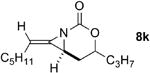
|
Rh2(esp)2 | --- | 1:1 | 34% |
| AgOTf/phen | 4.8:1 | 9:1 | 80%e | ||
| 4 |
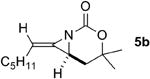
|
Rh2(esp)2 | 2:1 | 1:17 | 5% |
| AgOTf/phen | 1.9:1 | 5.9:1 | 70% | ||
| 5 |
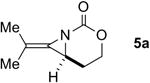
|
Rh2(esp)2 | --- | 2:1 | 35% |
| AgOTf/phen | --- | >20:1 | 79% | ||
| 6 |
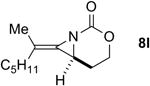
|
Rh2(esp)2 | 2.3:1 | 1:1.3 | 34% |
| AgOTf/phen | 2.6:1 | 11.5:1 | 87% | ||
| 7 |
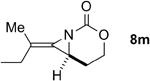
|
Rh2(esp)2 | --- | 1.1:1 | 32% |
| AgOTf/phen | 2.3:1 | 19:1 | 70% | ||
Rh conditions: 3 mol % catalyst, 2 equiv PhIO, 4 Å MS, CH2CI2, rt.
Reaction conditions: 20 mol % AgOTf, 25 mol % ligand, 2 equiv PhIO, 4 Å MS, CH2CI2, rt.
A = aziridination; I = insertion.
73% conversion.
dr E = 1:1; dr Z = 3:1.
In conclusion, we have demonstrated that the scope and utility of allene aziridination can be greatly increased by employing readily available and tunable Ag(I)-based catalysts. These complexes promote aziridination with superior chemoselectivity over conventional Rh-based catalysts, and the resultant bicyclic methylene aziridines are being studied as scaffolds for the synthesis of complex amines. However, the reasons for the high chemoselectivity exhibited by Ag(I) catalysts compared to Rh2(L)n are not yet clear.13 One major difference between the two systems is that Rh2(L)n complexes adopt only paddlewheel or “lantern-type” geometries, while Ag(I) complexes can adopt a variety of coordination geometries depending on the ligand, solvent, counteranion and concentration of the reaction.3a-c,14,15 Studies to address our hypothesis that the coordination geometry of the Ag catalyst impacts chemoselectivity are underway and will be reported in due course.
Supplementary Material
Acknowledgments
Funding was provided by the University of Wisconsin, Madison. We thank Professor John Berry of the University of Wisconsin-Madison for a sample of Rh2(espn)2. The NMR facilities at UW-Madison are funded by the NSF (CHE-9208463, CHE-9629688) and NIH (RR08389-01).
Footnotes
Supporting Information Available. Experimental procedures and full characterizations are available for all new compounds.
References
- 1.(a) Adams CS, Boralsky LA, Guzei IA, Schomaker JM. J Am Chem Soc. 2012;134:10807. doi: 10.1021/ja304859w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Boralsky LA, Marston D, Grigg RD, Hershberger JC, Schomaker JM. Org Lett. 2011;13:1924. doi: 10.1021/ol2002418. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Weatherly CD, Rigoli JW, Schomaker JM. Org Lett. 2012;14:1704. doi: 10.1021/ol300269u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Rigoli JW, Boralsky LA, Hershberger JC, Marston D, Meis AR, Guzei IA, Schomaker JM. J Org Chem. 2012;77:2446. doi: 10.1021/jo3000282. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Grigg RD, Schomaker JM, Timokhin V. Tetrahedron. 2011;67:4318. [Google Scholar]
- 2.For selected references on Rh-catalyzed amination, see: Espino CG, Du Bois J. Angew Chem Int Ed. 2001;40:598. doi: 10.1002/1521-3773(20010202)40:3<598::AID-ANIE598>3.0.CO;2-9.Espino CG, Wehn PM, Chow J, Du Bois J. J Am Chem Soc. 2001;123:6935.Padwa A, Flick AC, Leverett CA, Stengel T. J Org Chem. 2004;69:6377. doi: 10.1021/jo048990k.Espino CG, Fiori KW, Kim M, Du Bois J. J Am Chem Soc. 2004;126:15378. doi: 10.1021/ja0446294.Fiori KW, Du Bois J. J Am Chem Soc. 2007;129:562. doi: 10.1021/ja0650450.
- 3.For selected references on Cu-catalyzed amination, see: Nakanishi M, Salit A, Bolm C. Adv Synth Catal. 2008;350:1835.Han H, Park SB, Kim SK, Chang S. J Org Chem. 2008;73:2862. doi: 10.1021/jo800134j.Duran F, Leman L, Ghini A, Burton G, Dauban P, Dodd RH. Org Lett. 2002;4:2481. doi: 10.1021/ol0200899.Lebel H, Lectard S, Parmentier M. Org Lett. 2007;9:4797. doi: 10.1021/ol702152e.Liu RM, Herron SR, Fleming SA. J Org Chem. 2007;72:5587. doi: 10.1021/jo0705014.Gephart RT, Warren TH. Organometallics. 2012;31:7728.
- 4.Harvey ME, Musaev DG, Du Bois J. J Am Chem Soc. 2011;133:17207. doi: 10.1021/ja203576p. and references therein. [DOI] [PubMed] [Google Scholar]
- 5.For selected references on Fe-catalyzed amination, see: Jung N, Bräse S. Angew Chem Int Ed. 2012;51:5538. doi: 10.1002/anie.201200966.Cramer SA, Jenkins DM. J Am Chem Soc. 2011;133:19342. doi: 10.1021/ja2090965.Klotz KL, Slominski LM, Riemer ME, Phillips JA, Halfen JA. Inorg Chem. 2009;48:801. doi: 10.1021/ic8020244.Mahy J, Battioni P, Mansuy D. J Am Chem Soc. 1986;108:1079.Paradine SM, White MC. J Am Chem Soc. 2012;134:2036. doi: 10.1021/ja211600g.
- 6.Gao G, Harden JD, Zhang XP. Org Lett. 2005;7:3191. doi: 10.1021/ol050896i. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Ding X, He C. J Org Chem. 2006;71:5876. doi: 10.1021/jo060016t. [DOI] [PubMed] [Google Scholar]
- 8.For selected references on Ag-catalyzed amination, see: Cui Y, He C. J Am Chem Soc. 2003;125:16202. doi: 10.1021/ja038668b.Cui Y, He C. Angew Chem Int Ed. 2004;43:4210. doi: 10.1002/anie.200454243.Li Z, Capretto DA, Rahaman RH, He C. Angew Chem Int Ed. 2007;46:5184. doi: 10.1002/anie.200700760.Li Z, He C. Eur J Org Chem. 2006;19:4313.Kumar KA, Rai LKM, Umesha KB. Tetrahedron Lett. 2001;57:6993.Minakaa S, Kano D, Fukuoka R, Oderaotoshi Y, Komatsu M. Heterocycles. 2003;60:289.Gomez-Emeterio BP, Urbano J, Diaz-Requejo MM, Perez PJ. Organometallics. 2008;27:4126.Llaveria J, Beltrán A, Mar Díaz-Requejo M, Matheu MI, Castillón S, Pérez PJ. Angew Chem Int Ed. 2010;122:7246. doi: 10.1002/anie.201003167.
- 9.For selected examples of tuning of chemoselectivity of amination using Rh and Ru catalysis, see: Hayes CJ, Beavis PW, Humphries LA. Chem Commun. 2006:4501. doi: 10.1039/b611662k.Fiori KW, Du Bois J. J Am Chem Soc. 2007;129:562. doi: 10.1021/ja0650450.Kornecki KP, Berry JF. Eur J Inorg Chem. 2012;3:562.
- 10.Control of chemoselectivity in carbene chemistry is well-developed. Davies HML, Morton D. Chem Soc Rev. 2011;40:1857. doi: 10.1039/c0cs00217h.Nadeau E, Ventura DL, Brekan JA, Davies HML. J Org Chem. 2010;75:1927. doi: 10.1021/jo902644f.
- 11.Kornecki KP, Berry JF. Chem Commun. 2012;48:12097. doi: 10.1039/c2cc36614b. [DOI] [PubMed] [Google Scholar]
- 12.We thank a reviewer for this suggestion.
- 13.Ag(I) catalysts have been shown to cyclopropanate sterically congested olefins in contrast to Rh(II) catalysts: Thompson JL, Davies HML. J Am Chem Soc. 2007;129:6090. doi: 10.1021/ja069314y.
- 14.For selected references illustrating the coordination geometries that can be adopted by Ag(I) complexes, see: Hung-Low F, Renz A, Klausmeyer KK. Polyhedron. 2009;28:407.Hung-Low F, Renz A, Klausmeyer KK. J Chem Crystallogr. 2011;41:1174.Du J, Hu T, Zhang S, Zeng Y, Bu X. CrystEngComm. 2008;10:1866.Zhang H, Chen L, Song H, Zi G. Inorg Chim Acta. 2011;366:320.Xiong K, Wu M, Zhang Q, Wei W, Yang M, Jiang F, Hong M. Chem Commun. 2009:1840. doi: 10.1039/b821961c.Hung-Low F, Renz A, Klausmeyer KK. J Chem Cryst. 2009;39:438.Schultheiss N, Powell DR, Bosch E. Inorg Chem. 2003;42:5304. doi: 10.1021/ic034277m.Levason W, Spicer MD. Coord Chem Rev. 1987;75:45.Harmata M, editor. Silver in Organic Chemistry. John Wiley & Sons; Hoboken, NJ: 2010. Ma Z, Xing Y, Yang M, Hu M, Liu B, Guedes de Silva MFC, Pombeiro AJL. Inorg Chim Acta. 2009;362:2921.Hou L, Dan L. Inorg Chem Commun. 2005;8:128.
- 15.Evidence for a dinuclear Cu complex containing a bridging nitrene: Badiei YM, Krishnaswamy A, Melzer MM, Warren TH. J Am Chem Soc. 2006;128:15056. doi: 10.1021/ja065299l.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


