Abstract
B cells play a critical role in the clearance of Pneumocystis (PC). In addition to production of PC-specific antibody, B cells are required during the priming phase for CD4+ T cells to expand normally and generate memory. Clearance of PC was found to be dependent on antigen specific B cells and on the ability of B cells to secrete PC-specific antibody, as mice with B cells defective in these functions or with a restricted B cell receptor were unable to control PC infection. Because PC-specific antiserum was only able to partially protect B cell deficient mice from infection, we hypothesized that optimal T cell priming requires fully functional B cells. Using adoptive transfer and B cell depletion strategies, we determined that optimal priming of CD4+ T cells requires B cells over the first 2–3 days of infection and that this was independent of the production of antibody. T cells that were removed from PC-infected mice during the priming phase were fully functional and able to clear PC infection upon adoptive transfer into Rag1−/− hosts, but this effect was ablated in mice that lacked fully functional B cells. Our results indicate that T cell priming requires a complete environment of antigen presentation and activation signals to become fully functional in this model of PC infection.
Introduction
Pneumocystis jirovecii is an opportunistic fungal pathogen that causes severe disease in immunocompromised individuals. Pneumocystis pneumonia (PCP) is an AIDS-defining illness and a significant contributor to morbidity and mortality in this population (1, 2). As such, the role of CD4+ T lymphocytes in the defense against this organism has been extensively studied, as these cells are essential for the clearance of the pathogen (3, 4). It is presumed that effector T cells that are induced to activation through interactions with APCs in the lymph nodes then migrate to the lungs and activate alveolar macrophages, stimulating them to kill PC organisms (5). Additionally, activated CD4+ T cells interact with B cells, inducing them to produce PC-specific antibody that opsonize the organisms, assisting the alveolar macrophages in phagocytosis (6, 7).
While understudied, the role of B lymphocytes in the defense against PC infection is critically important. Clinically, the increased incidence of PC infection in patients receiving anti-CD20 antibody therapy underscores the significance of the B- lymphocyte population in host defense agains PC (8–10). Although mice deficient in functional B cells are unable to clear PC from the lungs (11, 12), the mechanisms by which B cells promote the clearance of PC are still largely unknown. We previously demonstrated that mice with CD40-deficient B cells can clear PC infection, suggesting that production of class-switched antibody against PC is not required for the clearance of the organism (11). Additionally, mice with mutations targeted to Fcγ and ε receptors are also able to clear PC infections, albeit at a slower rate than wild type (WT) controls (11). Therefore, while class-switched PC-specific antibody enhances clearance of the organism, it does not appear to be required for clearance. This conclusion is consistent with adoptive transfer studies, as CD4+ T cells from PC-infected WT donors will clear the organisms when transferred to PC-infected SCID mice (3, 13). Collectively, these studies suggest that the requirement for B cells in the clearance of PC infection may be independent, at least in part, of their ability to produce class-switched antibody.
Our previous work suggests that the activation of CD4+ T cells in response to PC is altered in mice that lack B cells. The number of activated CD4+ cells present in both the lungs and draining lymph nodes of PC-infected B cell deficient (µMT) mice are reduced as compared to that of normal mice, based on surface marker expression and cytokine production (11). Importantly, we published that T cells that are primed in B cell deficient-mice fail to expand in response to PC infection upon adoptive transfer to SCID mice (14). This suggests that B cells must provide some form of activation or proliferation signal to T cells during priming. The influence that B cells exert on T cells during CD4+ T cell priming has also been demonstrated in other murine models of antigen challenge (15, 16). Although we found that the signals provided by B cells to CD4 T cells during PC infection required interactions through either MHC class II or costimulatory molecules (11, 14), soluble factors including cytokines and secreted antibody may also be important. In support of this hypothesis, we reported recently that B cell-derived TNF is important for driving the T cell response to PC (17). However, we still do not know whether the interactions between B and T cells are critical during the early stages of response, or whether B cells are needed to initiate or maintain PC-specific memory T cells. Therefore, our focus has turned to investigating whether B cell-T cell interactions during PC infection alter the development or maintenance of the T cell compartment.
Here we have addressed whether PC-specific B cells play a role in the activation and survival of CD4+ T cells, thereby governing their ability to clear PC infection. We utilized genetically engineered mouse models and bone marrow (BM) chimeric mice that were unable to produce or secrete PC-specific antibody. We demonstrate that in the absence of secreted antibody, T cell activation and PC clearance is impaired. Furthermore, when T cell priming occurred in an environment devoid of PC-specific antibody, the ability of CD4 cells to confer clearance of PC was ablated and supplementation of antibody only partially rescued the mice from PC pneumonia. B cell interactions with CD4+ T cells were critical early after infection as demonstrated by adoptive transfer and B cell depletion studies. T cells primed in mice that lacked a PC-specific BCR did not expand or produce IFNγ as well as T cells primed in wild type mice upon adoptive transfer into host Rag1−/− mice. T cells primed in mice with B cells that lacked a PC-specific BCR were also unable to control PC infection. These results suggest that B cell-T cell interactions during priming require specificity in order for T cells to reach full functionality.
Materials and Methods
Mice
Adult C57BL/6J, BALB/cJ, B6.129S2-Igh-6tm1Cgn (µMT), C.129S7(B6)-Rag1tm1Mom/J (Rag−/−), and C57BL/6-Tg(IghelMD4)4Ccg/J (MD4) mice were purchased from The Jackson Laboratory. C.129(B6)-IgH-JhDtm1Dhu (Jh), and C.B-Igh-1b/ICR Tac-PrkdcScid (SCID) mice were purchased from Taconic. Mice doubly deficient in activation-induced deaminase (AID) and µS were crossed in the lab of Hidde Ploegh (AID-µS−/−) and fail to secrete antibody but have a polyclonal BCR repertoire (18). All experimental mice were housed in the Veterans Administration (VA) Medical Center veterinary medical unit or University of Kentucky Division of Laboratory Animal Resources units in sanitized cages, and given food and water ad libitum. PC organisms were maintained in a colony of Rag2−/− mice (originally from Jackson Laboratory) as a source for all infections. All procedures were approved by the Lexington VA or University of Kentucky Institutional Animal Care and Use Committees.
Generation of MD4 chimeras
Chimeric mice that were capable of only generating monoclonal B cells specific for hen egg lysozyme, an irrelevant antigen, were created by transplanting MD4 mouse BM into B cell-deficient µMT mice (14, 19). BM chimeric mice were generated by lethally irradiating µMT recipients with 900 Rads from a 137Cs source and reconstituting them with 107 BM cells. Mice either were reconstituted with 75% µMT BM plus 25% C57Bl/6 BM (“WT chimera”), 100% µMT BM (“µMT chimera”), or 75% µMT BM plus 25% MD4 BM (“MD4 chimera”). Mice were then allowed to rest for 8–12 weeks before infection with PC. Chimerism was confirmed by determining the phenotype of peripheral blood lymphocytes by flow cytometry prior to experiments and then confirmed at each time point.
Enumeration of Inoculated PC Organisms
To prepare organisms for inoculation, lungs were removed from PC-infected Rag2−/− or SCID mice, pushed through stainless steel mesh in HBSS, and debris removed by centrifugation at 100 X g for 2 minutes. Aliquots of lung homogenates were spun onto glass slides, fixed with methanol, and stained using DiffQuik (Dade International). The number of PC nuclei present were counted using microscopy. To infect mice, animals were anesthetized lightly with isoflurane gas, and 105 to 107 PC organisms, depending on the experiment, were injected intratracheally (i.t.) in 100µl of HBSS. Organisms were freshly isolated for all of the experiments shown. For detection of PC burden, the right lung lobes of each animal were excised, minced, and digested in RPMI 1640 supplemented with 2% fetal calf serum, 1 mg/ml collagenase A, and 50 U/ml DNase for 1 hour at 37°C. Digested fractions were pushed through mesh screens, and aliquots were spun onto glass slides and stained with DiffQuik for microscopic enumeration. Lung burden is expressed as log10 PC nuclei per right lung lobes, and the limit of detection was 3.23.
Isolation of cells from alveolar space, lungs, and lymph nodes
Mice were killed by exsanguination under deep anesthesia. The lungs were lavaged with 5 × 1ml washes using HBSS containing 3mM EDTA. Bronchial alveolar lavage fluid (BALF) was obtained from the first wash by spinning out cells and retained for subsequent analyses. Cells from the first wash were added to the remaining washes and volumes adjusted for cell enumeration. Lungs were minced and digested in RPMI containing 3% heat inactivated FCS, 50 U/ml DNase (Sigma-Aldrich, St. Louis, MO) and 1mg/ml collagenase A (Sigma) for 1 hr at 37°C prior to pushing through mesh screens to acquire single cell suspensions. After removing an aliquot for enumeration of PC organisms as described, erythrocytes were lysed from the lung digests by exposure to a hypotonic buffer. The remaining single cell suspensions were washed and counted. Tracheobronchial lymph nodes (TBLN) were excised and pushed through mesh screens to create single cell suspensions. Erythrocytes were lysed and cells enumerated.
Adoptive transfer of CD4+ T cells
Donor WT (BALB/c and C57BL/6) and B cell-deficient Jh−/− or uMT mice were given i.t. inoculations of 107 PC organisms. Draining lymph nodes were isolated 10–14 days post-infection. CD4+ cells were isolated from the TBLN by employing negative selection columns from R&D Systems according to the manufacturer’s instructions. We routinely obtain >95% pure CD4+ T cell fractions as determined by flow cytometry. Adoptive host Rag−/− mice received retro-orbital injections of 105 purified CD4+ cells and 4 days later were infected i.t. with 105 to 106 PC organisms, depending on the experiment. Note that mice on a C57Bl/6 or BALB/c background were used for these experiments because of the availability of the Rag2−/− and Jh−/− mice bred in our colony. We had performed similar experiments using mice from both backgrounds and obtained similar results (14, 17).
Flow cytometric analysis of lung and LN lymphocytes
Lung lavage, lung digest, and TBLN cells were washed in PBS containing 0.1% BSA and 0.02% sodium azide and stained with appropriate concentrations of fluorochrome-conjugated antibodies specific for murine CD4, CD8, CD19, CD44, CD62L, CD86 and CD80. Antibodies were purchased from eBioscience or BD Biosciences. Expression of these molecules on the surface of lymphocytes was determined by multiparameter flow cytometry using a FACSCalibur cytofluorimeter (BD Biosciences) and analyzed using either WinList (Verity Software House) or FlowJo (Tree Star, Inc.) software.
Generation of and treatment with PC antisera
B cell-deficient µMT mice were infected with 107 PC organisms. Three days later and twice per week thereafter, mice were given intraperitoneal (i.p.) injections of 100µl sera collected and pooled from PC-infected C57Bl/6 or µMT mice. Pooled antisera were stored for up to 2 years at −80°C and were not heat-treated. Alternatively, PC organisms were incubated in PC-immune or µMT sera from PC-infected mice (diluted 1:5) for 30 minutes on ice and then washed prior to i.t. inoculation into recipient mice.
Depletion of B cells
B cells were depleted from BALB/c or C57Bl/6 mice by injecting them i.p. with 10mg/kg of 18B12 anti-mouse CD20 IgG2a antibody (Biogen Idec) either 2 days before, or 2–3 days after being infected i.t. with 107 PC organisms (20). Control groups alternatively received 10mg/kg of 2B8 msIgG2a anti-human CD20 antibody. Mice treated with anti-mouse and anti-human CD20 antibody after infection continued to receive doses of antibody (10mg/kg) every 7 days until sacrifice, while mice receiving the Ab before infection received only the single dose.
Analysis of PC-specific antibody
Blood obtained at euthanasia by severing the abdominal aorta was clotted and centrifuged at 400 xg to obtain sera which were frozen in aliquots at −80°C for subsequent analysis. A sonicate of PC organisms (10µg protein/ml) was coated onto 96-well plates and then plates blocked with 5% dry milk in HBSS containing 0.05% Tween 20 as previously described (21, 22). After extensive washing, sera and BALF samples (first washes) were diluted and incubated on plates overnight. Plates were extensively washed and bound IgG, IgM, or IgA was detected using alkaline phosphatase-conjugated anti-mouse IgG, IgM, or IgA (Sigma) followed by incubation with p-nitrophenylphosphate at 1mg/ml in diethanolamine buffer. Optical density was read at 405nm using a plate reader equipped with KC Junior software (Bio-Tek Instruments, Inc., Winooski, VT).
Statistical analysis
Differences between experimental groups were determined using ANOVA, followed by Dunn’s post hoc test where appropriate. Differences were considered statistically significant when p<0.05. SigmaStat statistical software (SPSS) was used for all analyses.
Results
Antigen-specific BCR is necessary for clearance of PC
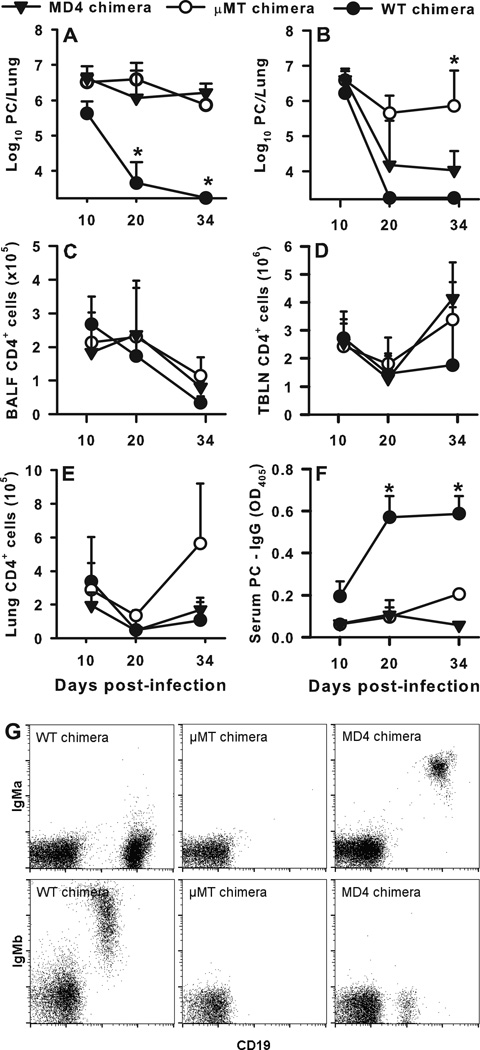
In order to better define the role of B cells in host defense against PC, we sought to determine whether B cells must express an antigen-specific BCR or be able to secrete antibody to clear PC. To address whether B cells specific for PC are required for clearance, we transferred BM from BCR transgenic MD4 mice into irradiated µMT B cell deficient mice to generate chimeras that contain B cells specific for a single irrelevant antigen (hen egg lysozyme or HEL). As controls, we transferred WT BM or µMT BM into the irradiated µMT hosts to generate mice with a complete B cell repertoire (WT chimeras) or mice lacking B cells (µMT chimeras). Two months after reconstitution, we intratracheally infected MD4, WT, and µMT chimeras with 107 PC organisms. In one experiment the MD4 chimeras did not clear PC (Fig. 1A) and had PC burdens that were indistinguishable from the chimeras lacking B cells. In a second experiment, PC lung burden was significantly reduced in the MD4 chimeras compared to the µMT chimeras, however the MD4 chimeras, unlike the WT chimeras, were unable to clear the PC (Fig. 1B). Next, we examined CD4+ cell population dynamics over time post infection in the BALF (Fig. 1C), TBLN (Fig. 1D), and lung digest (Fig. 1E) samples from the chimeras.
FIGURE 1.
Antigen-specific BCR is necessary for clearance of PC. BCR transgenic MD4 mice and B cell-deficient µMT mice were used to generate mixed chimeras whose BCR was specific for hen egg lysozyme. µMT mice were irradiated and then reconstituted with a mix of BM cells from MD4 transgenic and WT (C57Bl/6) animals as described in Methods, to generate WT chimera, µMT chimera, and MD4 chimera groups. After reconstitution, mice were infected with PC organisms and then humanely killed at post-infection timepoints. (A and B) PC lung burden was assessed in lung digest samples and expressed as Log10 PC organisms per lung in 2 replicated experiments. (C–E) T cells were identified by CD4 staining using flow cytometry and enumerated in the BALF (C), TBLN (D), and digested lung (E). (F) PC-specific IgG in the serum was assessed by ELISA. (G) Flow cytometry dot plots for representative samples depicting chimerism of each strain. B cells from C57BL/6 mice express IgMb while MD4 mice express the IgMa allotype. Data are presented as mean ± SD for groups of 4–6 mice per timepoint per group and representative of 2 replicated experiments. Note that in panel A mice died in the µMT chimera and MD4 chimera groups between days 20 and 34 post-infection, mostly likely due to the high organism burden. Statistical significance defined at a p-value of < 0.05 for data compared to WT chimeras at each timepoint (*).
Though there were no significant differences among groups of mice in numbers of CD4+ or CD8+ T cells in the BALF, there was a reproducible trend toward lower numbers of CD4+ T cells in the BAL and lung digest at the earlier time points in the µMT and MD4 chimeras. Serum concentrations of PC-specific IgG antibody are shown over time post-infection in Fig. 1F. Neither the µMT chimeric nor the MD4 chimeric mice mounted antibody responses against PC, as expected, whereas the WT chimeras had detectible specific antibody by day 10 post-infection, with a peak response by day 20. Fig. 1G demonstrates that µMT recipients were appropriately reconstituted with B cells expressing either the IgMa allotype (WT chimeras), IgMb (MD4 chimeras), or no B cells at all (µMT chimeras).
PC-specific serum and BALF IgM and IgA were assessed at each timepoint as well. Very low levels of PC-specific IgA or IgM were detected in either serum or BALF samples for the µMT and MD4 chimeric mice, as dilutions of 1:10 caused the signal to be undetectable (Supplemental Figure 1). Together, these data indicate that the lack of PC-specific antibody production decreases the ability of the animal to efficiently clear PC, suggesting that antigen-specific B cells play a role in providing protection following PC infection.
Absence of secreted antibody impairs clearance of PC, T cell activation
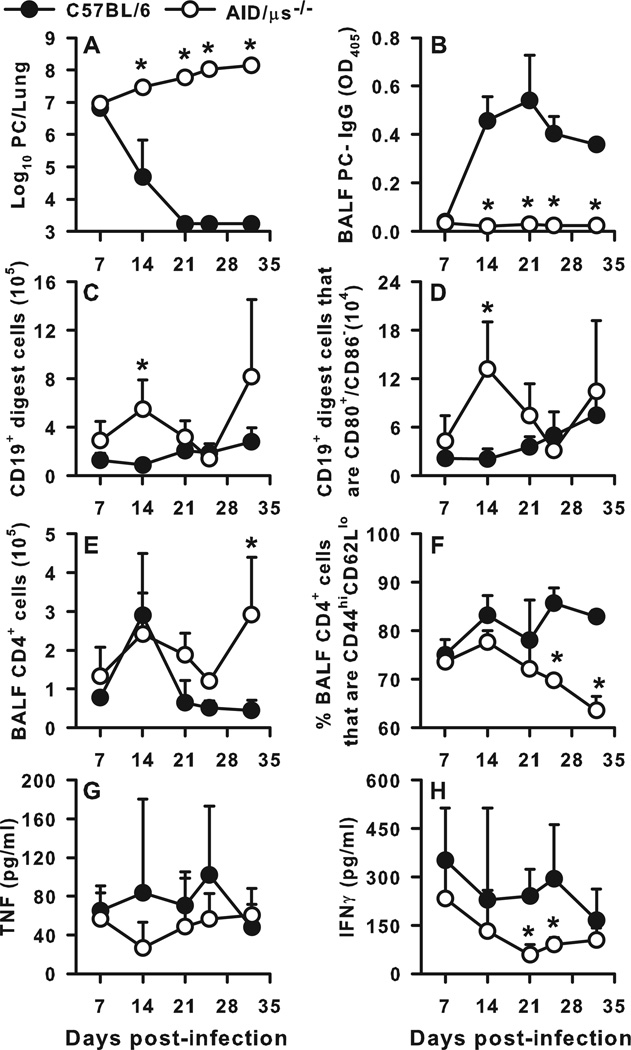
It has long been known that specific antibody can effectively reduce the burden of PC in murine models of infection (23, 24). To eliminate the antibody secreting function of B cells and determine whether B cells can provide protection from PC independently of PC-specific secreted antibody we utilized AID-µS−/− transgenic mice. The AID-µS−/− mice cannot class-switch nor secrete antibody (25) but do exhibit competent BCR surface expression though with a reduced repertoire (18). AID-µS−/− and wild type (C57BL/6) mice were infected with 107 PC organisms intratracheally. As shown in Fig. 2A, AID-µS−/− mice did not clear PC by day 32 post-infection, in contrast to the WT mice which were able to reduce the PC burden to below the limit of detection by day 21 post-infection. To confirm that the AID-µS−/− mice cannot class switch or secrete antibody, we measured IgG titers in the BALF through day 32 post-infection in these mice. As expected, the PC-specific IgG titers remained at background levels throughout the 32 day timecourse (Fig. 2B). Interestingly, the AID-µS−/− mice had increased numbers of B cells in the lung digest as compared to the WT control animals, with the difference on day 14 post-infection reaching statistical significance (Fig. 2C). In addition, a larger proportion of B cells in the lung digest of the knockout animals expressed the activation markers CD80 and CD86 as compared to the WT animals (Fig 2D).
FIGURE 2.
Failure to clear PC infection in the absence of secreted antibody. AID-µS−/− mice, which are unable to secrete or class switch antibody, and WT (C57Bl/6) mice were infected with PC organisms. Mice were sacrificed at post-infection timepoints, and BALF, lung, and TBLN specimens were collected for analysis. (A) PC lung burden clearance kinetics over time post-infection, expressed as Log10 PC organisms per lung. (B) PC-specific IgG titers in the BALF confirming that the AID-µS−/− mice cannot secrete antibody. Numbers of (C) B cells (CD19+) and (D) activated B cells (CD19+CD80+CD86−) depicted in lung digest samples over time post-infection. The number of CD4+ T cells (E) and the percentage of CD4+ T cells that were CD44hiCD62Llo (F) in the BALF over time post-PC infection. The concentrations of TNFα (G) and IFNγ (H) in BALF over time post-infection. Data are presented as mean ± SD for groups of 4 mice per timepoint per group. Statistical significance defined at a p-value of < 0.05 for data compared to WT mice at each timepoint (*).
Because our earlier data suggested that class switched B cells and antibody are not required for PC clearance and that CD4+ T cells primed in a normal host are sufficient to induce clearance of PC when adoptively transferred into SCID mice, we hypothesized that the inability of the AID-µS−/− mice to clear PC may be due to a role for antibody on CD4+ cell activation. We therefore examined the characteristics of the CD4+ T cell infiltration into the infected lungs of these mice. At day 7, alveolar infiltration by CD4+ T cells was evident in both mouse strains, but by day 21 the AID-µS−/− mice had higher CD4+ cell infiltration that persisted at higher levels through day 32 post-infection (Fig. 2E). Despite this, the percentage of CD4+ T cells in the alveolar space that were activated (CD44hiCD62Llo) was reduced over time post-infection as compared to the WT strain, with the difference becoming more pronounced late in the response (Fig. 2F). In addition, the concentrations of TNFα and IFNγ in the lavage fluid were lower from day 14 through 25 post-infection (Fig. 2G and H) in the AID-µS−/− mice. Together these data suggest that secreted antibody may be required for T cells to reach normal levels of activation in response to PC infection and facilitate efficient organism elimination from the lungs of the infected host.
Antibody can partially rescue B cell deficient mice and facilitate control of PC
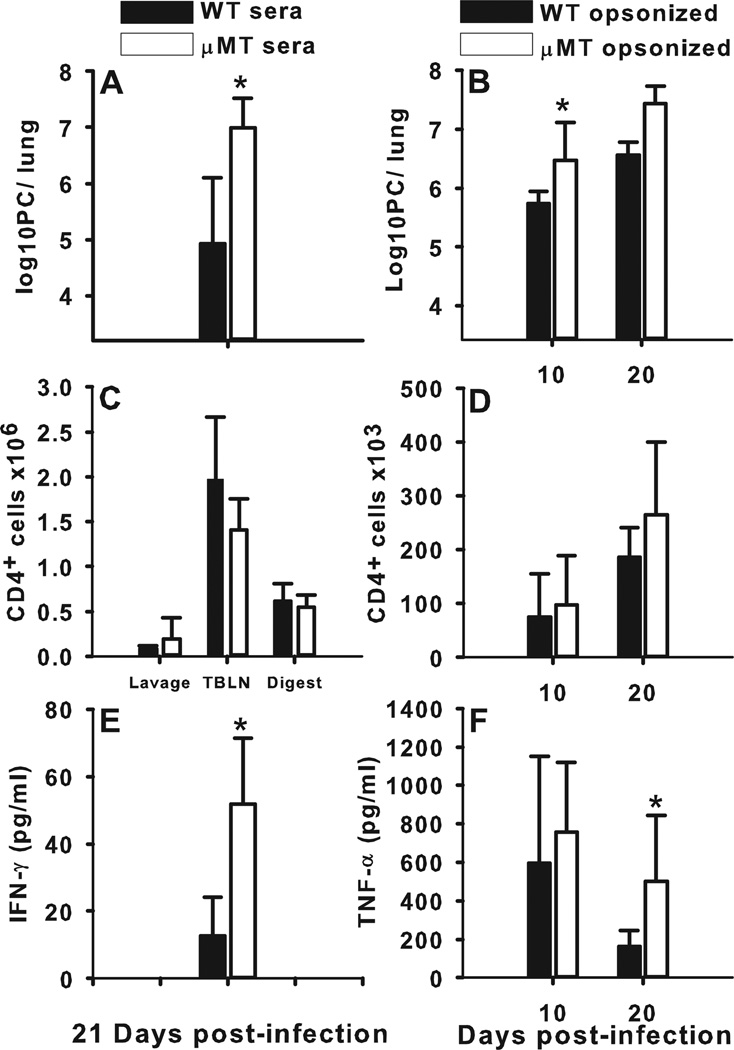
Since we found that specific B cells and secreted antibody are necessary for clearance of PC, we asked whether antibody can control PC infection in the absence of B cells. µMT mice were infected with PC and then given twice-weekly injections of serum either from infected C57BL/6 donors or from infected µMT donors. As shown in Fig. 3A, B cell deficient mice given passive immunization with serum from PC-infected wild type donors had a significantly lower PC burden at day 21 post-infection than did B cell deficient mice given sera from PC-infected µMT mice. Immunization using serum from naïve mice did not affect the clearance of PC from µMT mice through day 28 post-infection (data not shown). We used a second approach of incubating PC organisms with either immune serum from PC-infected C57BL/6 mice or from infected µMT mice prior to instillation in the lungs. Mice that received antibody opsonized organisms had a lower lung burden over the day 10 and day 20 time points than did the mice that received PC incubated with serum from B cell deficient µMT mice (Fig. 3B). PC-specific antibody isotypes were assessed from the serum of infected mice, and the majority was shown to be IgG, with only trace amounts of PC-specific IgM present (Supplemental Figure 2). Importantly, despite lowering PC burden in the lung, the presence of antibody, either injected or through pre-opsonization, did not result in clearance of organisms within 3 weeks as we routinely see in wild type mice (Fig. 1 and Fig. 2). The infiltration of CD4+ T cells into the alveolar spaces was not altered by either the treatment with PC immune serum or infection with opsonized PC (Fig. 3C, D) nor were the activation levels different (data not shown). However, the levels of IFNγ in the BALF of mice given wild type immune serum (Fig. 3E) or TNF in the BALF of mice infected with PC-serum opsonized organisms (Fig. 3F) was significantly lower at three weeks post-infection than in mice without specific antibody.
FIGURE 3.
Exogenous PC-specific antibody in B cell-deficient mice partially protects against infection. B cell deficient mice (µMT) were infected with PC and then given twice-weekly injections of serum either from infected C57BL/6 donors or from infected µMT donors (A, C, E) or were infected with PC pre-opsonized with antiserum from infected C57BL/6 or µMT donors (B, D, F). (A, B) PC lung burden was assessed in lung digest samples and expressed as Log10 PC organisms per lung. (C, D) T cells were identified by CD4 staining using flow cytometry and enumerated in the BALF, digested lung, and TBLN. (E, F), Concentrations of IFNγ and TNFα in the BALF were assessed by ELISA. Data are presented as mean ± SD for groups of 5 mice per timepoint per group. Statistical significance defined at a p-value of < 0.05 for data compared to WT mice at each timepoint (*).
B cell-T cell interactions are important in the priming phase
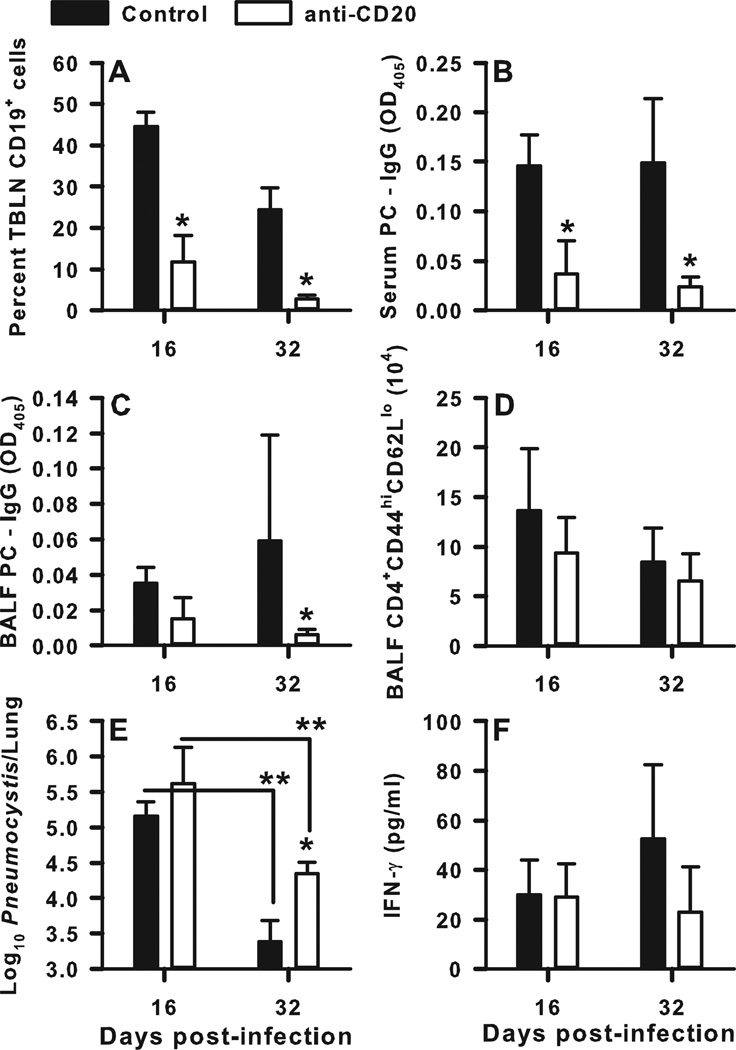
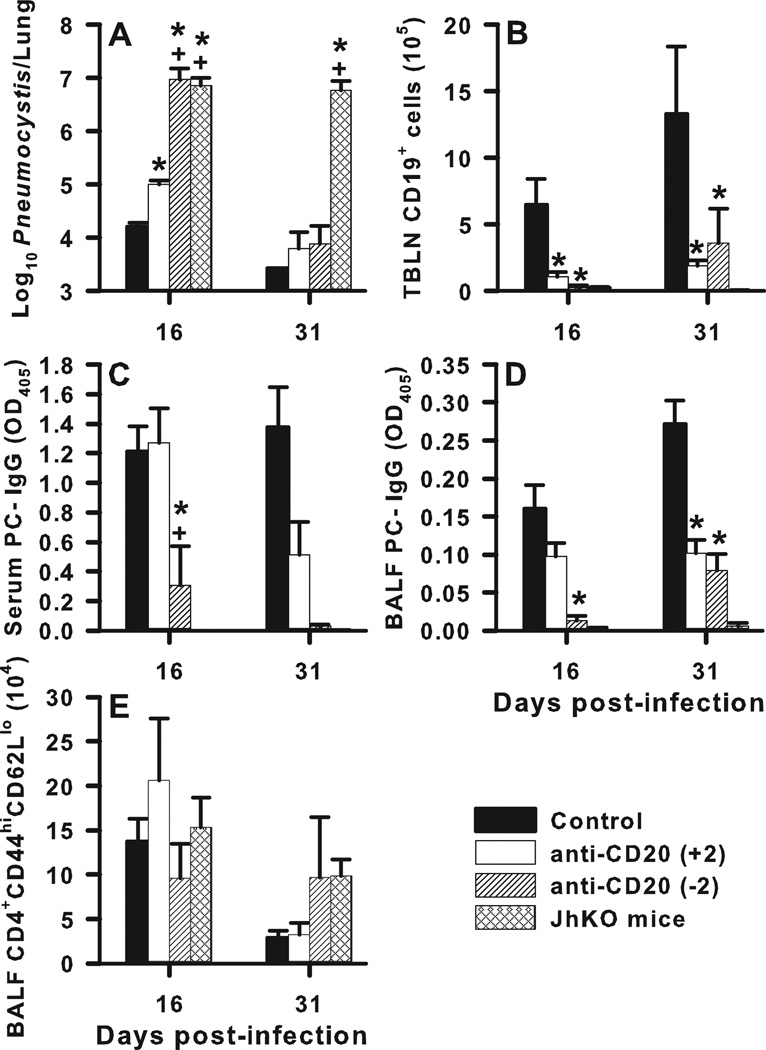
We previously found that the presence of B cells during the first two weeks of infection with PC was sufficient for expansion of CD4+ T cells in adoptive hosts (14, 17), so we sought to determine whether B cells are critical early during infection at the time CD4+ T cells are primed. To address this, B cells were depleted in wild type mice using anti-CD20 antibody (Fig. 4). Anti-mouse CD20 antibody (18B12) was injected i.p. 3 days after intratracheal infection of BALB/c mice with 107 PC organisms, and every 7 days thereafter. Administration of this antibody was compared with exposure to an anti-human CD20 antibody as a control. The anti-mouse CD20 antibody was effective at depleting nearly all CD19+ lymphocytes (B cells) in the draining lymph nodes on days 16 (p<0.001) and 32 (p=0.029) post-infection (Fig. 4A). Additionally, treatment with anti-mouse CD20 significantly reduced PC-specific IgG concentrations in both the BALF and in the serum post-infection as compared to the anti-human CD20 control treatment (Fig. 4B and C). While there was a small decrease in the number of CD4+ T cells infiltrating the alveolar space on day 16 post-infection in the B cell-depleted group, the difference did not reach statistical significance (p=0.285) (Fig 4D). We also observed a non-significant decrease in IFNγ concentration on day 32 post-infection in the B cell depleted animals (Fig. 4F), despite a significantly higher PC burden (p=0.018) at that time point (Fig. 4E). Although the PC burden was higher on day 32 post-infection in the group that received anti-mouse CD20 antibody, both groups reduced PC significantly as compared to the burdens on day 16 post-infection.
FIGURE 4.
Depletion of B cells after infection hinders specific IgG production and clearance of PC. BALB/c mice were infected with PC organisms. Anti-mouse CD20 antibody was injected i.p. at a dose of 10mg/kg starting on day 3 post-infection and every 7 days thereafter. Control mice received anti-human CD20 antibody at the same dose. Mice were sacrificed on days 16 and 32 post-infection, and BALF, lung, and TBLN specimens were collected for analysis. Lungs were homogenized and digested, and TBLN were pushed though mesh to create single-cell suspensions. B cell populations were defined by CD19 expression, and T cells were identified by CD4, CD44, and CD62L staining using flow cytometry. PC-specific IgG and IFNγ concentrations were assessed by ELISA, and PC lung burden was assessed in lung digest samples. (A) Percentage of TBLN cells that were CD19+ over time. PC-specific IgG titers in the serum (B) and BALF (C) over time post-infection. D) Percentage of CD4+ T cells in the BALF that were activated, defined as CD44hiCD62Llo. (E) The PC lung burden over time post-infection, expressed as Log10 PC organisms per lung. (F) IFNγ concentrations in the BALF samples post-infection. Data are presented as mean ± SD for groups of 4 mice per timepoint per group. Statistical significance defined at a p-value of < 0.05 using ANOVA and Dunn’s post hoc test for data compared to control group at the same timepoint (*) and for data compared between timepoints within the same treatment group (**).
Next, we compared the effects of B cell depletion either 2 days before or two days after infection to confirm the timing of when B cells are required for clearance of PC. Normal BALB/c mice were treated with anti-mouse CD20 either 2 days before infection, or every 7 days starting 2 days after infection with 107 PC organisms. Depleting B cells 2 days before infection impaired the clearance of PC to a more significant degree as compared to mice whose B cells were depleted after inoculation (Fig. 5A). PC burden in the mice receiving anti-mouse CD20 prior to infection was as high on day 16 post-infection as in Jh−/− mice that lack B cells altogether. By day 31 post-infection both sets of anti-mouse CD20-treated mice were able to clear the organisms to levels of that of the control group (Fig. 5A). Depletion either before or after PC infection resulted in very low numbers of B cells in the draining lymph nodes (Fig. 5B). PC-specific IgG was decreased to a greater degree on day 16 post-infection in the mice receiving B cell depletion before infection, although mice with depleted B cells after infection also had a significantly lower PC-specific IgG concentration in the serum and BALF on day 31 (Fig. 5C and 5D). Additionally, there were very low levels of PC-specific IgM present in the mice that received anti-CD20 antibody at day 16 post-infection, with a small amount of recovery at the later timepoint (Supplemental Figure 3). Consistent with the results presented in the previous figure, mice depleted of B cells with anti-CD20 had similar numbers of activated CD4+ T cells, as defined by CD44 and CD62L expression, in the BALF compared to mice with an intact B cell compartment at the time of infection (Fig. 5E).
FIGURE 5.
Depletion of B cells by anti-CD20 antibody prior to infection impedes the early clearance of PC as well as antibody responses. BALB/c mice were infected with 107 PC organisms. To deplete B cells, mice received either a one-time i.p. injection of 10mg/kg anti-mouse CD20 antibody 2 days before infection (noted as “−2”), or a series of injections every 7 days starting at day 2 after infection (“+2”). Control groups consisted both of BALB/c mice that received anti-human CD20 antibody, and Jh−/− mice that lack B cells. (A)The PC lung burden at each timepoint post-infection, expressed as Log10 PC organisms per lung. (B) Depletion of B cells (CD19+) in the TBLN depicted over time post-infection. Serum (C) and BALF (D) PC-specific IgG levels at postinfection time points assayed at a 1:10 dilution and neat, respectively, are shown. (E) Number of CD4+ T cells that were activated (CD44hiCD62Llo) in the BALF after infection. Data are presented as mean ± SD for groups of 4 mice per timepoint per group. Statistical significance defined at a p-value of < 0.05 using ANOVA and Dunn’s post hoc test for data compared to control group (*) and to the +2 anti-CD20 treatment group (+).
T cells primed in the absence of B cells fail to expand upon adoptive transfer
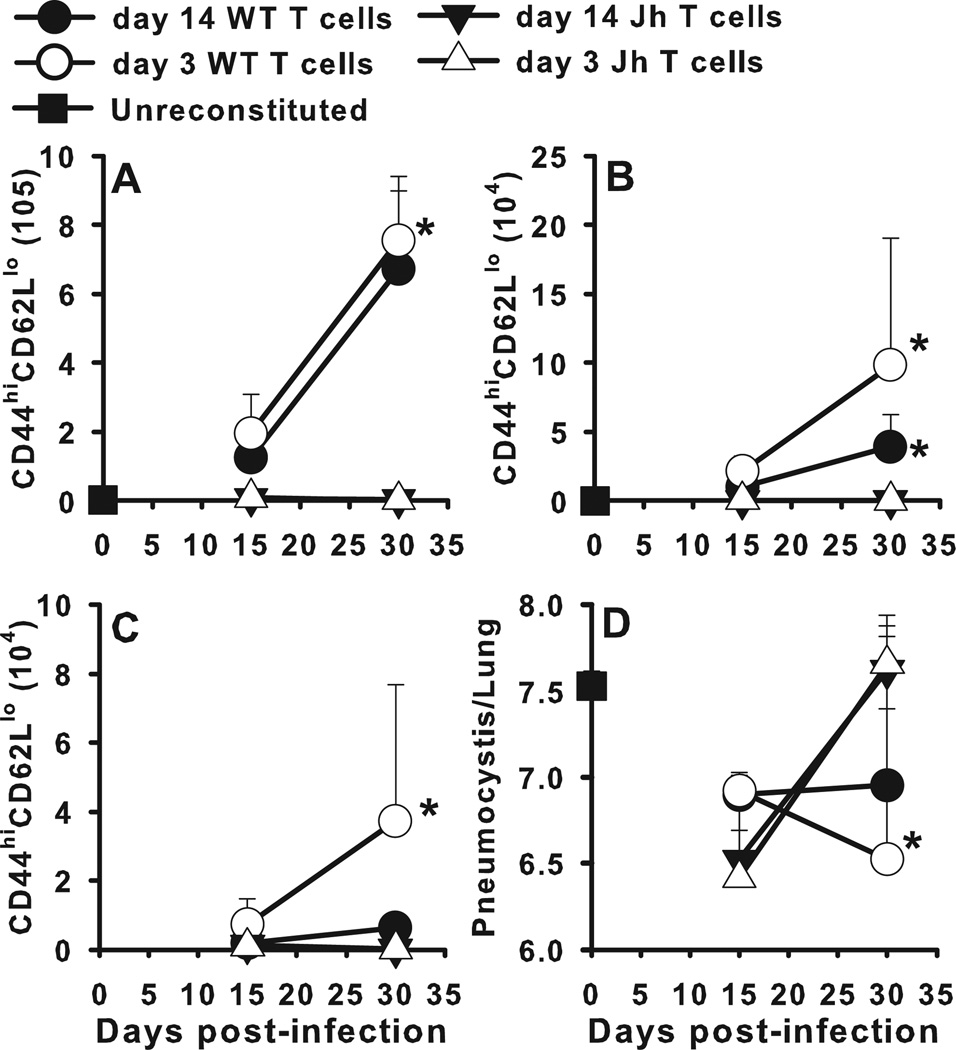
Data to this point confirm that antigen specific B cells are required for host defense against PC and PC-specific antibody does not completely compensate for the absence of B cells. We have previously demonstrated that CD4+ T cells primed for 14 days in the absence of B cells fail to expand and control PC infection upon adoptive transfer to immunodeficient SCID recipients (14). Since DCs are thought to be the most important cells for presenting antigen to naïve T cells, we asked the question of whether B cells are required early after infection to prime CD4+ T cells. To address this we isolated CD4+ T cells during the first 3 days after infection to determine if they would clear PC in adoptive hosts. Donor wild type BALB/c or B cell-deficient Jh−/− mice were infected with PC and CD4+ T cells were isolated from the draining TBLN at 3 or 14 days post-infection. T cells were injected into Rag−/− recipients followed by infection 4 days later. As shown in Fig. 6, CD4+ T cells from Jh−/− mice failed to expand in the TBLN, lungs, or alveolar spaces of adoptive hosts and failed to control lung PC burden. In contrast CD4+ T cells isolated from wild type mice did control infection (Fig. 6D). Importantly, CD4+ cells from mice infected for 3 days were as good as or better than those from mice infected for 2 weeks in terms of expansion and migration to the lungs. These data suggest B cells at the initial priming stage confer some sort of survival or proliferation signal to CD4+ T cells that is required for expansion upon secondary challenge.
FIGURE 6.
CD4+ T cells primed for 3 days in B cell sufficient donors are capable of affecting clearance of PC in adoptive Rag−/− hosts. Donor wild type BALB/c or B cell-deficient Jh−/− mice were infected with PC and CD4+ T cells were isolated from the draining TBLN at 3 or 14 days post-infection. T cells were adoptively transferred into Rag−/− recipients followed by infection with PC nuclei 4 days later. T cells were identified by CD4, CD44, and CD62L staining using flow cytometry, with the number of activated CD4+ T cells (CD44hiCD62Llo) depicted over time in the (A) lung digest, (B) BALF, and (C) TBLN. (D) PC lung burden was assessed in lung digest samples and expressed as Log10 PC organisms per lung. Data are presented as mean ± SD for groups of 4 mice per timepoint per group and representative of 3 replicated experiments. Statistical significance defined at a p-value of < 0.05 for data generated in the Jh−/− recipients compared to recipients of T cells from WT mice at each timepoint (*).
Antigen-specific BCR is required for adequate priming of T cells
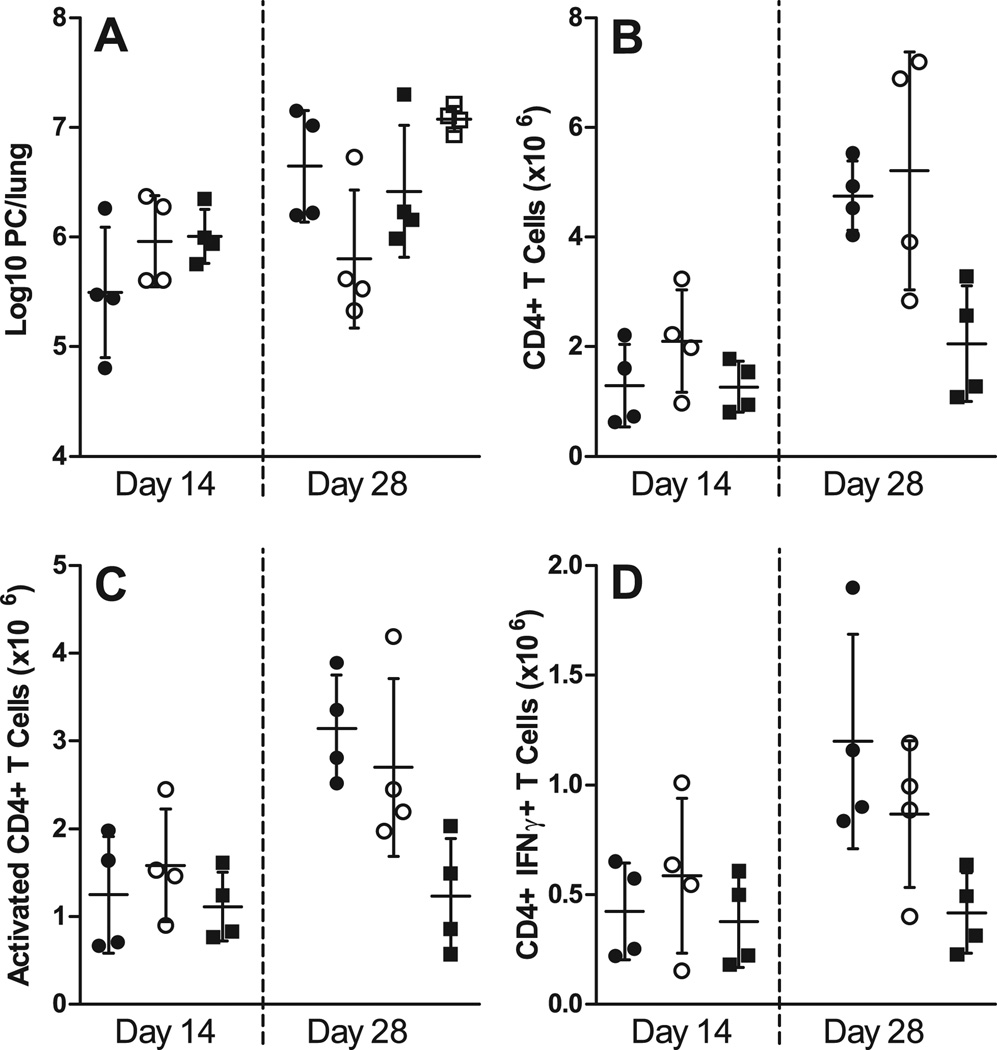
We demonstrated in Fig. 2 that an antigen-specific BCR is required for complete clearance of PC. The next experiments were designed to determine whether T cells primed in mice that lacked PC-specific BCR would be able to confer PC clearance upon adoptive transfer into Rag1−/− hosts. WT, µMT, and MD4 chimeras were infected with PC, and after 3 days T cells were isolated and adoptively transferred into Rag1−/− mice, which were infected with PC 4 days later. By day 28 post-infection, the mice receiving transferred cells from the WT chimeras began to clear the infection (Fig. 7A). Figure 7B shows that the number of CD4+ T cells as well as the number of activated CD4+ T cells, based on surface protein expression and IFNγ expression, tended to be lower when primed in MD4 chimeras as compared to WT and µMT chimeras. Interestingly, the mice that received cells primed in the µMT chimeric animals did show expansion of T cells, and these T cells did show signs of activation (Fig. 7B). However, transfer of these cells could not confer control of PC (Fig. 7A). These results are similar to those that we published previously that showed T cells from µMT mice can expand and become activated, but are also unable to clear the pathogen (17). These results demonstrate that the mere presence of B cells is not sufficient for efficient priming of T cells. Rather, PC-specific B cells are required for the early priming of T cells against PC.
FIGURE 7.
Ag-specific BCR is required for proper CD4+ T cell priming. WT, µMT, and MD4 chimeras were infected with PC, and after 3 days T cells were adoptively transferred into Rag1−/− mice, which were then infected with PC 4 days later. Mice receiving T cells from µMT chimeras (closed circles), WT (B6+µMT) chimeras (open circles), MD4 chimeras (closed squares), and mice that were unreconstituted (open squares) were then humanely killed at post-infection timepoints. (A) PC lung burden was assessed in lung digest samples and expressed as Log10 PC organisms per lung on days 14 and 28 post-infection. The numbers of (B) CD4+ T cells, (C) activated CD4+ T cells (based on high CD44 and low CD62L expression), and (D) CD4+ T cells producing IFNγ (assessed via intracellular cytokine staining and flow cytometry) are depicted at the corresponding timepoints. Data are presented as mean ± SD along with symbols representing each individual mouse. No statistically significant differences were observed defined at a p-value of < 0.05 using ANOVA and Dunn’s post hoc test for data compared to the WT chimera (B6+µMT) group at each timepoint.
Discussion
There are increasing reports that PC colonizes the lungs of individuals with immunosuppressing conditions such as HIV infection and chronic obstructive pulmonary disease (COPD) corresponding with worsening pathology (26, 27). Though much is known about the elements of the immune response necessary for host defense against PC, there is still a lack of understanding of how these immune factors interact with each other. We have previously reported that B cells provide expansion and/or survival signals to CD4+ T cells that contribute significantly to their function (11, 14). In the data presented here, we demonstrate that B cells need to be present during the first few days after infection to provide signals to T cells and for an optimal antibody response. We further confirmed that B cells need to have a BCR with specificity to PC and be able to secrete antibody in order to clear the infection. Together these data indicate that B cells are multifunctional in the response to PC. Specific antibody production is important for control of PC lung burden. Consistent with our previously published data showing that mice with B cells that don't express MHCII don't clear PC (14), we found here that B cells are required early during the response to PC, suggesting a role in the initial priming of T cells.
While it is important to define these basic immunologic functions, the importance of the interaction between B cells and T cells is underscored by the clinical observation that B cell depletion with anti-CD20 antibody therapies impairs T cell activation (28, 29). This is associated with an increased incidence of PC pneumonia (8–10), in addition to other T-cell mediated infections including hepatitis B (30, 31). Stroopinsky et al examined this phenomenon and in 2012 reported that activation of T cells isolated from lymphoma patients treated with rituximab was reduced as defined by ex vivo cytokine secretion (32). Interestingly, this study also demonstrated direct inhibitory effects upon T cells with rituximab, indicating that clarity is needed as to the mechanism of this interaction (32).
We have previously reported that bone marrow chimeric mice with B cells lacking MHC class II were unable to clear PC infection confirming that antigen presentation by B cells is important for production of class switched antibody as well as activation of helper T cells (14). Here we determined that antigen-specific B cells are required for clearance of PC. Transgenic mice expressing the irrelevant MD4 BCR specific for hen egg lysozyme were unable to clear infection indicating that specific responses, and not merely a populated B cell compartment, is required for host defense against PC. A similar result was reported in Salmonella-infected MD4 transgenic mice, though it was reported that the specific BCR was required for memory Th1 cell development (33). To determine whether specific antibody secretion is required for clearance of PC, we utilized the AID-sµ−/− mice that have B cells unable to class switch or secrete antibody. These mice also failed to clear PC which is consistent with a previous report in which sIgM−/− mice, able to class switch but not secrete IgM antibody, had a defective ability to control PC infection (34). IgM in the absence of IgG is not sufficient for clearance of PC, suggesting that complete B cell responses including secreted IgG and IgM work together to control PC burden (11, 14). In the absence of class-switched IgG, as well as in the absence of IgM, PC clearance does not occur, suggesting that both isotypes are important in this process. Importantly, the AID-µs−/− mice have a relatively normal repertoire of B cell receptors (18) and so these data confirm that secretion of specific antibody is critical for controlling PC infection. Interestingly, the AID-µs−/− mice had reduced proportions of activated CD44hiCD62Llo CD4+ T cells in alveolar spaces which corresponded to reduced BALF IFNγ levels compared to wildtype mice.
The numbers of total and activated B cells in the lungs of AID-µs−/− mice were increased, suggesting that in the absence of secreted antibody and in the face of increasing lung PC burden, T and B cells migrate to the lungs in increasing numbers to try to deal with the infection. Alternatively, it may be that AID-µs−/− mice cannot adequately control immune responses due to a lack of antibody available to bind to inhibitory Fc receptors (35). Multiple groups, including us, have reported that passive immunization of immunodeficient mice with PC-specific antibody reduces organism lung burden (3, 24, 36). We now show here that injection of antiserum or infection with pre-opsonized PC organisms significantly reduced the PC lung burden in B cell-deficient µMT mice, but did not result in complete clearance of organisms by the end of the experiment. These data are consistent with the conclusion that while specific antibody plays an important role in reducing lung PC burden, B cells also contribute to clearance of PC via antibody independent mechanisms.
We hypothesized that if B cells are important antigen presenting cells, then the timing of the exposure of T cells to B cells would be important in activating CD4+ T cells to effector cells. Adoptive transfer of CD4+ T cells from wild type or B cell deficient mice at 3 days post-infection had little effect on clearance of PC compared to the 14 days at which we usually take lymph node T cells for transfer (14). We had previously found that T cells isolated from mice that lack B cells (Jh−/−) at day 14 post-infection failed to expand in adoptive immunodeficient hosts. Interestingly, 3 days after infection was enough time for T cells from wild type mice to become activated and to expand and migrate to the lungs to the same extent as T cells isolated at day 14 post-infection. We did find that the number of CD4+ T cells in the draining lymph nodes of the adoptive hosts that received cells from wild type mice infected for 3 days was near the limit of detection which was similar to what we saw in mice that received T cells from Jh−/− donors. It is possible that had we taken our time points out past 30 days post-infection, the cells from wild type mice infected for 3 days would have become exhausted. This would be consistent with the possibility that B cells provide survival signals to CD4+ T cells. We have preliminary data showing that a high proportion of CD4+ T cells in PC-infected B cell deficient mice undergo apoptosis (data not shown) and we are working at determining whether this contributes to failure of these mice to clear infection.
Our adoptive transfer approach has the advantage of examining CD4+ T cell function after priming with or without B cells in the absence of antibody, but is not a physiological model. Utilizing anti-CD20, we were able to deplete the naïve pool of B cells 2 days before infection, or alternatively 2 days after infection which allows for some specific B cell expansion. We found that depletion of B cells prior to infection had more serious consequences for clearance of PC than depletion 2 or 3 days after infection. However, depletion of B cells prior to infection had only minor effects on CD4+ T cell numbers in the BALF and a more significant effect on PC-specific IgG than depletion of B cells 2 days after infection. This may imply that in the presence of DCs, naïve CD4+ T cells are not optimally primed and in the absence of specific antibody are thereby unable to effect clearance of PC. We did not find a difference in the TNF or IFNγ levels in the BALF between the groups suggesting that B cell depletion had no effect on local cytokine responses. TNF can be made by multiple cell types in response to PC, including T cells. IFNγ was likely produced mostly by T cells which suggests that the T cell effector responses are intact in the anti-CD20-treated mice. A recent report shows that B cells are necessary for the generation of an optimal Th2-type of response to the intestinal nematode Heligmosomoides polygyrus (37). In mice treated with anti-CD20, T cells and DCs failed to co-localize, thereby affecting the development of a normal T cell response. Interestingly, the investigators used influenza as a control and found that in the primary Th1 response to the virus, B cells were dispensable, suggesting that the role of B cells in the priming phase may be specific to Th2-type responses that require antibody for clearance, much like PC (6, 37).
One caveat to the B cell depletion experiments is that we did not assess whether B1 cells were depleted by the anti-CD20 treatment. Based upon other models in the literature, it is likely that B1 cells were still present. In a study involving lupus-prone mice, anti-CD20 antibody treatment showed depletion of B1 cells (CD11b+IgM+) to be incomplete (38). The production of natural antibodies from B1 cells could be contributing to the clearance of PC in our mice. Rapaka et al demonstrated that mice lacking natural antibodies specific for carbohydrate moieties present on fungi including PC, had defects in antibody isotype switching and T cell subset differentiation resulting in a reduced ability to control infection (34). Our IgM and IgG isotype assessments at both timepoints by PC-specific ELISA show very low levels of IgM present in the serum of mice on day 16 post-infection, with some recovery in both anti-CD20 treated groups on day 31. In spite of the possibility of natural antibody being present, the mice undergoing anti-CD20 antibody depletion of B cells had a decreased ability to clear PC. Our results are also corroborated by a recently published work that shows treatment with anti-CD20 antibody impaired the clearance of PC infection, and in addition made CD4+ T cells defective upon later transfer into Rag1−/− mice (39).
DCs have long been considered the professional APC required for optimal stimulation of naïve T cells. This has been confirmed in other infection models using the CD11c–DTR mice. Depletion of DCs during the priming stage of infection with Schistosoma mansoni resulted in a significant reduction in Th2 cells and skewing toward a Th1 response (40). Depletion of DCs has also been shown to abrogate priming of CD8+ cells in response to Listeria monocytogenes or Plasmodium yoelii (41) as well as compromise the recall response to viral and bacterial pathogens (42). We have preliminary data suggesting that depletion of DCs just prior to PC infection has no negative impact on expansion and activation of CD4+ T cells. Our ongoing work will address whether B cells can compensate for the absence of DCs in this model.
The results presented here confirm that B cells play a significant role in host defense against PC. In addition to secreting specific antibody, B cells are involved in the expansion and survival of CD4+ T cells, a function that can provide protection, potentially in the absence of dendritic cells. This may infer that B cells have a non-redundant role in promoting presentation of PC antigen to CD4+ T cells in the process of responding to infection with PC.
Supplementary Material
Acknowledgements
We thank Biogen Idec for providing the 18B12 anti-mouse CD20 antibody.
This work was supported by a grant from the National Institutes of Health, National Heart Lung and Blood Institute (HL088989) awarded to B.A.G.
Abbreviations used in this paper
- PC
Pneumocystis murina
- LN
lymph node
- WT
wild type
- PCP
PC pneumonia
- ZDV
zidovudine
- SMX/TMP
sulfamethoxazole/trimethoprim
- BALF
bronchial alveolar lavage fluid
- TBLN
tracheobronchial lymph node
References
- 1.Morris A, Lundgren JD, Masur H, Walzer PD, Hanson DL, Frederick T, Huang L, Beard CB, Kaplan JE. Current epidemiology of Pneumocystis pneumonia. Emerg Infect Dis. 2004;10:1713–1720. doi: 10.3201/eid1010.030985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masur H, Michelis MA, Greene JB, Onorato I, Stouwe RA, Holzman RS, Wormser G, Brettman L, Lange M, Murray HW, Cunningham-Rundles S. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981;305:1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- 3.Roths JB, Sidman CL. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J Clin Invest. 1992;90:673–678. doi: 10.1172/JCI115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harmsen AG, Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990;172:937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limper AH, Hoyte JS, Standing JE. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J Clin Invest. 1997;99:2110–2117. doi: 10.1172/JCI119384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect. Immun. 1997;65:5052–5056. doi: 10.1128/iai.65.12.5052-5056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steele C, Marrero L, Swain S, Harmsen AG, Zheng M, Brown GD, Gordon S, Shellito JE, Kolls JK. Alveolar macrophage-mediated killing of Pneumocystis carinii f.sp. muris involves molecular recognition by the Dectin-1 b-glucan receptor. J. Exp. Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamel S, O'Connor S, Lee N, Filshie R, Nandurkar H, Tam CS. High incidence of Pneumocystis jirovecii pneumonia in patients receiving biweekly rituximab and cyclophosphamide, adriamycin, vincristine, and prednisone. Leuk Lymphoma. 2010;51:797–801. doi: 10.3109/10428191003699860. [DOI] [PubMed] [Google Scholar]
- 9.Kolstad A, Holte H, Fossa A, Lauritzsen GF, Gaustad P, Torfoss D. Pneumocystis jirovecii pneumonia in B-cell lymphoma patients treated with the rituximab-CHOEP-14 regimen. Haematologica. 2007;92:139–140. doi: 10.3324/haematol.10564. [DOI] [PubMed] [Google Scholar]
- 10.Venhuizen AC, Hustinx WN, van Houte AJ, Veth G, van der Griend R. Three cases of Pneumocystis jirovecii pneumonia (PCP) during first-line treatment with rituximab in combination with CHOP-14 for aggressive B-cell non-Hodgkin's lymphoma. Eur J Haematol. 2008;80:275–276. doi: 10.1111/j.1600-0609.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 11.Lund FE, Schuer K, Hollifield M, Randall TD, Garvy BA. Clearance of Pneumocystis carinii in Mice Is Dependent on B Cells But Not on P. carinii-Specific Antibody. J Immunol. 2003;171:1423–1430. doi: 10.4049/jimmunol.171.3.1423. [DOI] [PubMed] [Google Scholar]
- 12.Marcotte H, Levesque D, Delanay K, Bourgeault A, de la Durantaye R, Brochu S, Lavoie MC. Pneumocystis carinii infection in transgenic B cell-deficient mice. J Infect Dis. 1996;173:1034–1037. doi: 10.1093/infdis/173.4.1034. [DOI] [PubMed] [Google Scholar]
- 13.Wiley JA, Harmsen AG. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J. Immunol. 1995;155:3525–3529. [PubMed] [Google Scholar]
- 14.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B Cells Are Required for Generation of Protective Effector and Memory CD4 Cells in Response to Pneumocystis Lung Infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 15.Constant SL. B Lymphocytes as Antigen-Presenting Cells for CD4+ T Cell Priming In Vivo. J Immunol. 1999;162:5695–5703. [PubMed] [Google Scholar]
- 16.Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role int he initiation of T cell responses to Chlamydia trachomatis (mouse pneumonitis) lung infection. J. Immunol. 1998;161:1439–1446. [PubMed] [Google Scholar]
- 17.Opata MM, Ye Z, Hollifield M, Garvy BA. B cell production of tumor necrosis factor in response to Pneumocystis murina infection in mice. Infect Immun. 2013;81:4252–4260. doi: 10.1128/IAI.00744-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumazaki K, Tirosh B, Maehr R, Boes M, Honjo T, Ploegh HL. AID−/−{micro}s−/− Mice Are Agammaglobulinemic and Fail to Maintain B220-CD138+ Plasma Cells. J Immunol. 2007;178:2192–2203. doi: 10.4049/jimmunol.178.4.2192. [DOI] [PubMed] [Google Scholar]
- 19.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 20.Hamel K, Doodes P, Cao Y, Wang Y, Martinson J, Dunn R, Kehry MR, Farkas B, Finnegan A. Suppression of proteoglycan-induced arthritis by anti-CD20 B Cell depletion therapy is mediated by reduction in autoantibodies and CD4+ T cell reactivity. J Immunol. 2008;180:4994–5003. doi: 10.4049/jimmunol.180.7.4994. [DOI] [PubMed] [Google Scholar]
- 21.Garvy BA, Harmsen AG. Susceptibility to Pneumocystis carinii infection: host responses of neonatal mice from immune or naive mothers and of immune or naive adults. Infect Immun. 1996;64:3987–3992. doi: 10.1128/iai.64.10.3987-3992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurkjian C, Hollifield M, Lines JL, Rogosky A, Empey KM, Qureshi M, Brown SA, Garvy BA. Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection. Infect Immun. 2012;80:2835–2846. doi: 10.1128/IAI.05707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roths JB, Sidman CL. Single and combined humoral and cell-mediated immunotherapy of Pneumocystis carinii pneumonia in immunodeficient scid mice. Infect Immun. 1993;61:1641–1649. doi: 10.1128/iai.61.5.1641-1649.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Empey KM, Hollifield M, Schuer K, Gigliotti F, Garvy BA. Passive immunization of neonatal mice against Pneumocystis carinii f.sp. muris enhances control of infection without stimulating inflammation. Infect Immun. 2004;72:6211–6220. doi: 10.1128/IAI.72.11.6211-6220.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 26.Morris A, Sciurba C, Lebedeva IP, Githaiga A, Elliott WM, Hogg JC, Huang L, Norris KA. Association of chronic obstructive pulmonary disease severity and Pneumocystis colonization. Am. J. Respir. Crit. Care Med. 2004;178:408–413. doi: 10.1164/rccm.200401-094OC. [DOI] [PubMed] [Google Scholar]
- 27.Morris A, Sciurba FC, Norris KA. Pneumocystis: A Novel Pathogen in Chronic Obstructive Pulmonary Disease? COPD: Journal of Chronic Obstructive Pulmonary Disease. 2008;5:43–51. doi: 10.1080/1541255070181756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liossis SN, Sfikakis PP. Rituximab-induced B cell depletion in autoimmune diseases: potential effects on T cells. Clin Immunol. 2008;127:280–285. doi: 10.1016/j.clim.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo YX, Tan DS, Tan IB, Tao M, Lim ST. Hepatitis B virus reactivation in a patient with resolved hepatitis B virus infection receiving maintenance rituximab for malignant B-cell lymphoma. Ann Intern Med. 2009;150:655–656. doi: 10.7326/0003-4819-150-9-200905050-00024. [DOI] [PubMed] [Google Scholar]
- 31.Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, Dai MS, Chiu BC, Fintel B, Cheng Y, Chuang SS, Lee MY, Chen TY, Lin SF, Kuo CY. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011;22:1170–1180. doi: 10.1093/annonc/mdq583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stroopinsky D, Katz T, Rowe JM, Melamed D, Avivi I. Rituximab-induced direct inhibition of T-cell activation. Cancer Immunol Immunother. 2012;61:1233–1241. doi: 10.1007/s00262-011-1168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengten E, Kolls JK. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med. 2010;207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maglione PJ, Xu J, Casadevall A, Chan J. Fc gamma receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J Immunol. 2008;180:3329–3338. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- 36.Gigliotti F, McCool T. Glycoprotein A is the immunodominant antigen of Pneumocystis carinii in mice following immunization. Parasitol. Res. 1996;82:90–91. doi: 10.1007/s004360050075. [DOI] [PubMed] [Google Scholar]
- 37.Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5(+) dendritic cells and lymphotoxin-expressing B cells. Nat Immunol. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekar KW, Owen T, Dunn R, Ichikawa T, Wang W, Wang R, Barnard J, Brady S, Nevarez S, Goldman BI, Kehry M, Anolik JH. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum. 2010;62:2443–2457. doi: 10.1002/art.27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elsegeiny W, Eddens T, Chen K, Kolls JK. Anti-CD20 and susceptibility to Pneumocystis Pneumonia. Infect Immun. 2015 doi: 10.1128/IAI.03099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hammerling GJ, Maizels RM, MacDonald AS. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung S, Unutmaz D, Wong P, Sano G-I, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F. In Vivo Depletion of CD11c+ Dendritic Cells Abrogates Priming of CD8+ T Cells by Exogenous Cell-Associated Antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zammit DJ, Cauley LS, Pham Q-M, Lefrancois L. Dendritic Cells Maximize the Memory CD8 T Cell Response to Infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.