Abstract
Neonatal immunity exhibits weak Th1 but excessive Th2 responses and the underlying mechanisms remain elusive. Here, we show that neonatal basophils readily produce IL-4, a cytokine that proved to be pivotal in shaping the programs of both lymphocyte subsets. Besides promoting Th2 programs, IL-4 is captured by the IL-4 heteroreceptor (IL-4Rα/IL-13Rα1) expressed on dendritic cells and instigates IL-12 down-regulation. Under these circumstances, differentiating Th1 cells up-regulate IL-13Rα1 leading to an unusual expression of the heteroreceptor which will serve as a death marker for these Th1 cells during re-challenge with antigen (Ag). The resulting Th1/Th2 imbalance impacts childhood immunity culminating in sensitivity to allergic reactions, susceptibility to microbial infection and perhaps poor efficacy of pediatric vaccines.
Introduction
The earliest studies of the newborn’s immune system suggested that neonatal exposure to Ag is more suited for induction of tolerance rather than immunity (1–3). While this notion bodes well with poor childhood immunity and the susceptibility of neonates to microbial infections (4, 5), it faces a dilemma as to the overwhelming sensitivity of newborns to immune-mediated allergic reactions (6). Over the years, we have begun to untangle this puzzle and evidence has arisen linking poor neonatal defenses against microbes to paucity in Th1 cells and the prevalence of pediatric allergies to excess in Th2 lymphocytes (6, 7). These conclusions, however, were drawn from studies that were focused only on secondary neonatal responses as technical limitations did not allow otherwise. Lately, models have been developed which facilitate analysis of the primary neonatal immune response (8–10). We used ovalbumin (OVA)2 323–339 peptide (OVAp)-specific T cell receptor (TCR) transgenic DO11.10 T cells to increase the frequency of responder cells and Ig-OVA, an Ig molecule genetically engineered to carry OVAp, to optimize Ag presentation (10). With these tools, we devised a neonate-to-neonate T cell transfer system that was adapted to trace T cells in vivo and analyze their primary neonatal responses (10). Surprisingly, the findings indicated that both Th1 and Th2 cells develop in the primary neonatal response (11). However, a rechallenge with Ag leads to apoptosis of Th1 cells, hence, the bias of secondary neonatal immunity towards Th2 cells (11). Furthermore, Th1 apoptosis was dependent on IL-4 as neutralization of this cytokine restores Th1 secondary immunity (11). This was intriguing because Th1 cells usually express the conventional IL-4 receptor (IL-4Rα/common γ) through which IL-4 does not signal (12). Subsequently, it was discovered that Th1 cells up-regulate IL-13Rα1 and this chain associates with IL-4Rα to form an IL-4Rα/IL-13Rα1 heteroreceptor (HR) (11, 13). Although the HR has been shown to affect immune responses in a different manner relative to the conventional IL-4 receptor (14), in neonates the HR marks Th1 cells for apoptosis (11, 13) and sustains bias of secondary immunity towards Th2 cells (7, 15). Poor Th1 immunity in neonates stems from the up-regulation of IL-13Rα1 which correlates with a paucity in environmental IL-12, a cytokine produced by neonatal dendritic cells (DCs) during Ag presentation (13, 16). In fact, exogenous IL-12 as well as enrichment with DCs from adult mice prevent IL-13Rα1 up-regulation and HR expression on primary Th1 cells (13, 16). How the function of neonatal DCs and their IL-12 are constrained, resulting in poor neonatal immunity, remains obscure. Because gene disruption of IL-13Rα1 preserves the conventional IL-4R but alters HR expression, we opted to utilize IL-13Rα1-deficient mice (17) to elucidate the physiological mechanism underlying IL-12 cytokine malfunction associated with neonatal DCs. Herein, it is shown that IL-13Rα1-deficient newborn mice dampen Th2 cells yet gain the ability to develop both primary and secondary Th1 immunity. This was due to increased IL-12 production by neonatal DCs but minimal secretion of IL-4 by basophils. Consequently, Th2 differentiation was curtailed whereas Th1 development was potentiated, leading to a Th1 instead of Th2 skewing of newborn immunity. Evidence is provided indicating that the HR on neonatal DCs captures IL-4 from basophils and limits IL-12 production granting IL-13Rα1 up-regulation and HR expression on Th1 cells. This reveals yet another paradigm by which the HR underpins neonatal immunity.
Materials and Methods
Mice
Balb/c mice (H-2d) were purchased from Harlan Sprague Dawley (Indianapolis, IN). DO11.10/Rag2−/− transgenic mice (H-2d) expressing OVA-specific TCR have been previously described (18). IL-13Rα1-deficient mice in which IL-13Rα1 gene expression was disrupted by deletion of exon 7, 8, and 9 were generated in our lab and have been previously described (17). IL-13Rα1−/−DO11.10/Rag2−/− mice have been generated by crossing IL-13Rα1−/− Balb/c mice with DO11.10/Rag2−/− mice. MHC II−/− Balb/c mice (cAN 129 S6 [B6] Ii tm1 Liz −/−H-2d) were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were bred and maintained in our animal care facility for the duration of the experiments. All experimental procedures were performed according to the guidelines of the University of Missouri Animal Care and Use Committee.
Antigens
OVAp (ISQAVHAAHAEINEAGR) encompasses aa residues 323–339 of OVA and is immunogenic in Balb/c (H-2d) mice. Influenza virus hemagglutinin (HA) peptide aa residues 110–120 (SFERFEIFPKE) was used as negative control. Both peptides were purchased from EZBiolab (Carmel, IN). Ig-W, a Balb/c IgG2b Ig molecule generated by transfection of the 91A3 anti-arsonate antibody heavy and light chain genes into the non-Ig-secreting myeloma B cell line SP2/0, has been previously described (11). Ig-OVA, expressing OVAp within the heavy chain variable region of Ig-W, was also previously described (10).
Isolation of CD4 T cells, dendritic cells, basophils, and basophil/mast cell common progenitors (BMCP)
CD4 T cells
Splenic cells were first depleted of DCs using anti-CD11c microbeads (Miltenyi Biotech) and the T cells were purified by positive selection on anti-CD4 microbead columns.
Dendritic cells
DCs were purified from the spleen of neonatal IL-13Rα1−/− and IL-13Rα1+/+ Balb/c mice by positive selection on anti-CD11c microbead columns (Miltenyi Biotec).
Bone marrow derived basophils
Bone marrow cells (1×107 /ml) from neonatal and adult IL-13Rα1−/− and IL-13Rα1+/+ Balb/c mice were incubated with rIL-3 (20ng/mL) for 3 days. The non-adherent cells were collected and re-cultured with rIL-3 for 3 days at a density of 1×106/ml. Re-culture of the non-adherent cells with rIL-3 in fresh media was repeated one more time and the cells were then depleted of CD11c+ and CD11b+ cells by magnetic bead activated cell sorting (MACS) (Miltenyi Biotec). Subsequently, c-kit− FcεR1α+ BAs were sorted on a MoFloTM XDP cell sorter (Beckman Coulter).
Basophil mast cell common progenitor (BMCP)
Bone marrow cells from neonatal IL-13Rα1−/− and IL-13Rα1+/+ Balb/c mice were cultured for 5 days with rIL-3 and then labeled with anti c-kit, anti-FcγRII/III and anti-β7 antibodies. The c-kit−β7hi FcγRII/IIIhi cells were sorted on a MoFloTM XDP cell sorter (Beckman Coulter).
Adoptive transfer of T cells and APCs
Transfer of neonatal T cells
Total splenic cells (3 × 106) from 1-day-old IL-13Rα1−/− or IL-13Rα1+/+ DO11.10/Rag2−/− mice were transferred into 1-day old IL-13Rα1−/− or IL-13Rα1+/+ Balb/c mice by i.v. injection through the facial vein using a 30-gauge needle as described previously (10).
Transfer of neonatal BMCP
Purified BMCP from neonatal IL-13Rα1+/+ or IL-13Rα1−/− mice were transferred (5×104 cells per mouse) into 1 day old MHC-II−/− or IL-13Rα1−/− mice by i.v. injection through the facial vein.
Transfer of neonatal DCs
Purified DCs from neonatal IL-13Rα1+/+ or IL-13Rα1−/− mice were transferred (3×104 cells per mouse) into neonatal MHC-II−/− mice by i.v. injection through the facial vein.
Combined transfer of neonatal DC, Basophils and CD4 T cells
Purified basophil progenitors (BMCP, 5×104 cells/mouse), neonatal DCs (3×104 cells/ mouse) from IL-13Rα1+/+ or IL-13Rα1−/− mice were transferred with purified DO11.10 CD4 T cells (5×104 cells per mouse) into neonatal MHC-II−/− mice by i.v. injection through the facial vein.
In vitro stimulation of DCs and basophils
DCs
Purified neonatal (3×105 cells/well) and adult (5×105 cells /well) DCs were stimulated with LPS (10µg/mL) or LPS+IL-4 (100ng/ml) for 24 hours and IL-12 in culture supernatant was measured by ELISA.
Basophils
The cells (1×105 cells/well) were stimulated with peptidoglycan (PGN) (10 µg/ml) for 24 hours and IL-4 and IL-13 cytokines in the culture supernatant were measured by ELISA.
Flow Cytometry
Antibodies
The antibodies used in this study were purchased from BD Pharmingen or eBioscience and these are: Anti-CD3 (500A2), anti-CD4 (RM4-5), anti-CD8 (53–6.7), F4/80 (BM8), anti-c-Kit (CD117; 2B8), anti-CD11b (M1/70), anti-CD19 (1D3), anti-CD16/32 (2.4G2), anti-CD11c (HL3), anti-IL-4Rα (mIL4R-M1), anti-FcεRII (B3B4), anti-Ly-6G (RB6-8C5), anti-Ly6-C (HK1.4), SiglecF (E50-24440), anti-I-Ab (NIMR-4), anti-CD80 (16-10A1), anti-CD86 (PO3.1), anti-FcεR1α (MAR-1), anti-TCROVA (KJ1-26), anti-β7 integrin (FIB27), anti-IL-4, anti-IFNγ, anti-CD34 (RAM34), anti-CD40 (3/23).
Detection of basophils and their progenitors
For detection of basophils and their progenitors in the BM, PB, and SP, single cell suspensions were prepared from these organs and stained with a cocktail of antibodies against common lineage (CD4, CD8, CD19, CD11b and CD11c) and cell specific markers. Basophils are identified as Lin−c-kit− FcεR1α+basophil progenitors (BAP) Lin−CD34+FcεRIα+c-kit−BMCP as Lin−c-kit− β7hiFcγRII/IIIhicommon myeloid progenitors (CMP) as Lin−IL-7Rα1−Sca1−c-kit+FcγRII/IIILo and granulocyte macrophage progenitors (GMP) as Lin−IL-7Rα1−Sca1−c-kit+FcγRII/IIIhi.
Basophil expansion in neonates
Newborn mice were given one injection of rIL-3 (400 ng/mouse) on day 1 alongside Ig-OVA. Another injection of rIL-3 alone was given on day 2 and 3. Basophil frequency in SP and blood was analyzed by flow cytometry.
Depletion of basophils in neonates
Newborn mice were given i.p. 0.5µg of MAR-1 anti-FcεR1α antibody on day 1, 2 and 3 after birth and basophil frequency in SP and PB were analyzed by flow cytometry on day 5.
Analysis of T cell responses
Ex vivo responses
Two weeks after T cell transfer and exposure to Ig-OVA (100 µg/mouse) the SP cells containing both T cells and APCs were stimulated for a short period of time (4 hours) with 10 µM OVAp to enhance cytokine accumulation in the cytoplasm and facilitate intracellular detection. The CD4 T cells were labeled for surface CD4 and TCROVA by staining with anti-CD4 and KJ1-26 antibody, respectively. Subsequently, the cells were permeabelized with 0.2% saponin and stained for intracellular IFNγ and IL-4 with cytokine specific antibodies. Cytokine production was analyzed by flow cytometry.
Recall Responses
Two weeks after T cell transfer and exposure to Ig-OVA (100 µg/mouse), the splenic cells containing both T cells and APCs were stimulated with 10µM OVAp for 24 hours and the frequency of cytokine producing cells was determined by ELISPOT while the quantity of cytokine secreted in the supernatant was determined by ELISA. In some experiments involving MHC II−/− mice, the splenic culture was supplemented with OVAp-loaded MHC II+/+ Balb/c APCs (2×105 cells/well, for 24 hours) to carry out peptide presentation as the residual APCs (from the transfer) would not be sufficient for the task. In other experiments the culture is stimulated with phorbol myristic acid (PMA) (50ng/mL) and ionomycin (500ng/mL) for 4 hours.
Secondary responses
Two months after T cell transfer and exposure to Ig-OVA (100 µg/mouse) the mice were challenged with 125 µg OVAp in CFA. Ten days later, the SP (1×106 cells/well) and LN (5×105 cells/well) containing both T cells and APCs were stimulated with 10 µM OVAp for 24 h and the frequency of cytokine producing cells was determined by ELISPOT while the quantity of cytokine secreted in the supernatant was determined by ELISA.
In vitro T cell responses
Splenocytes from IL-13Rα1+/+ and or IL-13Rα1−/− DO11.10/Rag2−/− mice were depleted of CD11c+ and CD11b+ cells and OVAp-specific CD4+T cells were purified by positive selection on anti-CD4 antibody coupled microbeads (Miltenyi Biotech). The CD4+T cells (3×105 cell per well) were stimulated with purified basophils (3×104 cells/well) or DCs (3×104 cells/well) in presence of 10µM OVA or HA peptide for 24 hours. The CD4 T cells were labeled for surface CD4 and TCROVA by staining with anti-CD4 and KJ1-26 antibody, respectively. Subsequently, the cells were permeabilized with 0.2% Saponin and stained for intracellular IFNγ and IL-4 with cytokine specific antibodies. Cytokine production was analyzed by flow cytometry and ELISA.
Cytokine detection assays
ELISA
IL-4, IL-12, IL-13, and IFN-γ were detected in culture supernatant using standard protocol as described (11, 13). The OD450 was read on a SpectraMax 190 counter (Molecular Devices, Sunnyvale, CA) and analyzed using SOFTmax PRO 3.1.1 software. Graded amounts of recombinant cytokine were included for construction of a standard curve. Cytokine concentrations were extrapolated from the linear portion of the standard curve.
ELISPOT
Detection of IFNγ and IL-4 by ELISPOT was carried out as described (11, 13, 16). Briefly, HA multiscreen plates (Millipore, Bedford, MA) were coated with capture antibody and free sites were saturated with DMEM culture media containing 10% fetal calf serum. Subsequently, 1×106 SP cells per well were added and the culture was stimulated with 10µM OVAp. Biotinylated anti-IFN-γ antibody (1µg/mL) and anti-IL-4 (2µg/mL) was added and bound antibody was revealed with avidin-peroxidase. Spots were counted using Immunospot software (Cellular Technology Ltd, Cleveland, OH).
Antigen presentation assay
Splenocytes from IL-13Rα1+/+ and or IL-13Rα1−/− DO11.10/Rag2−/− mice were depleted of CD11c+ and CD11b+ cells and OVAp-specific CD4+T cells were purified by positive selection on anti-CD4 antibody coupled microbeads (Miltenyi Biotech). The CD4+T cells (3×105 cell per well) were then labeled with CFSE and stimulated with purified basophils (3×104 cells/well) in presence of 10µM OVA or HA peptide or 1 µM Ig-OVA or Ig-W chimera. After 72 hours, T cell proliferation was determined by measurement of CFSE dilution as described (19).
Statistical Analysis
Unpaired two-tailed Student t test was used to analyze data in all figures except panel 8a which utilized ANOVA with a Bonferroni post-test to compare more than 2 groups. Prism Software v4.0c (Graphpad) was used in all statistical analyses. Data significance is denoted by one asterisk for p<0.05 and two asterisks for p<0.01.
Online supplemental material
Fig. S1 shows in vivo restoration of Th2 responses by exogenous IL-4 in IL-13Rα1-deficent mice. Fig. S2 shows a comparison of the frequency of various innate and lymphoid cells in IL-13Rα1-deficient versus IL-13Rα1-sufficient mice.
Results
Newborns lacking functional IL-13Rα1 develop Th1 skewed secondary immune responses
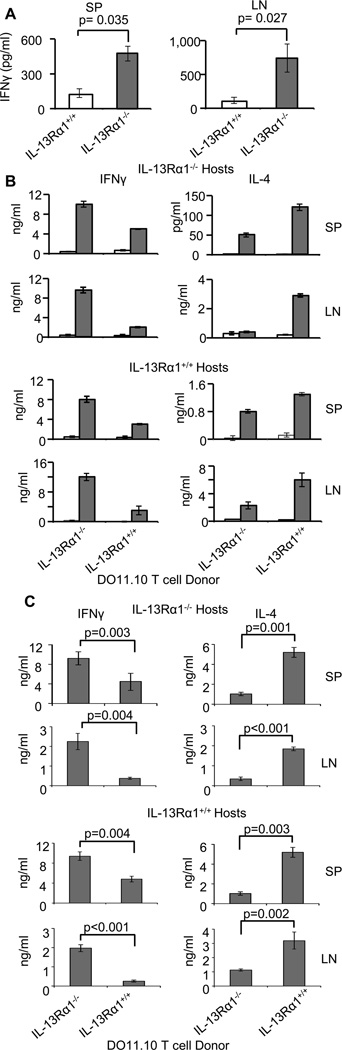
Neonatal exposure to Ag usually gives rise to poor secondary Th1 immunity but an excess of Th2 cells (3, 9, 20). However, neutralization of IL-13Rα1 restores neonatal Th1 secondary responses (11). Similarly, Th1 secondary responses develop in newborn mice in which the IL-13Rα1 gene is disrupted and HR expression is compromised (Fig. 1). Indeed, IL-13Rα1−/− mice that were given Ig-OVA on the day of birth and challenged with OVAp in CFA (OVAp/CFA) at 7 weeks of age mounted strong IFNγ (Th1) responses both in the spleen (SP) and lymph nodes (LN) as compared to IL-13Rα1+/+ mice (Fig. 1A). To gain insight on the mechanism by which non-functional HR restores Th1 secondary responses, we adapted the IL-13Rα1-deficiency to the neonate-to-neonate TCR transgenic transfer system (10, 11, 13, 16) and performed fine analysis of the responses. Accordingly, when neonatal IL-13Rα1−/− DO11.10 T cells were transferred to either IL-13Rα1−/− or IL-13Rα1+/+ newborn Balb/c mice and the hosts were given Ig-OVA and later challenged with OVAp/CFA, the DO11.10 T cell responses were skewed towards Th1 cells (Fig. 1B). Indeed, when the T cell donors had intact IL-13Rα1, the SP and LN IFNγ responses were minimal while IL-4 responses were normal in the mice exposed to Ig-OVA relative to Ig-W whether the hosts were IL-13Rα1-deficient or sufficient (Fig. 1B). In contrast, when the T cell donors had non-functional IL-13Rα1, the SP and LN IFNγ responses were restored while IL-4 responses were minimal in the mice exposed to Ig-OVA relative to Ig-W, whether the hosts were IL-13Rα1-deficient or sufficient (Fig. 1B). In fact, compiled results from different experiments confirm the data from the representative experiments and again show that when the donor T cells are deficient for IL-13Rα1, exposure to Ig-OVA and challenge with OVA/CFA yields SP and LN secondary responses that display greater Th1 but lower Th2 cells in a significant manner relative to donor T cells from IL-13Rα1+/+ mice whether the host is IL-13Rα1-sufficient or deficient (Fig. 1C). Overall, HR deficiency restores Th1 secondary immunity but nullifies Th2 responses.
Figure 1.
Ablation of IL-13Rα1 leads to skewing of neonatal secondary immunity towards Th1 cells. (A) Newborn IL-13Rα1−/− or IL-13Rα1+/+ Balb/c mice were given Ig-OVA in saline, and 2 months later challenged with OVAp/CFA. The SP and LN cells pooled from 3 to 6 mice were stimulated with OVAp and IFNγ was measured by ELISA 24 hours later. Each bar represents the mean ± SD of triplicate wells from a representative of 3 experiments. (B) Newborn IL-13Rα1−/− or IL-13Rα1+/+ Balb/c hosts recipient of neonatal T cells from IL-13Rα1−/− or IL-13Rα1+/+ DO11.10 donors were given Ig-OVA (closed bars) or Ig-W (open bars) and later challenged with OVAp/CFA. The SP and LN cells were harvested on day 10 after challenge and stimulated with OVAp in vitro. IFNγ (left column) and IL-4 (right column) production by SP and LN cells were analyzed by ELISA. Each bar represents the mean ± SD of triplicate wells. This is representative of 5 experiments. (C) Shows compiled results from the 5 experiments carried out as described in (B). The bars represent the mean ± SE of the 5 experiments. The statistical analysis used student’s t test to compare results between donor T cells from IL-13Rα1−/− versus IL-13Rα1+/+ mice within both IL-13Rα1−/− and IL-13Rα1+/+ hosts.
Ablation of IL-13Rα1 unbalances primary neonatal responses towards Th1 cells
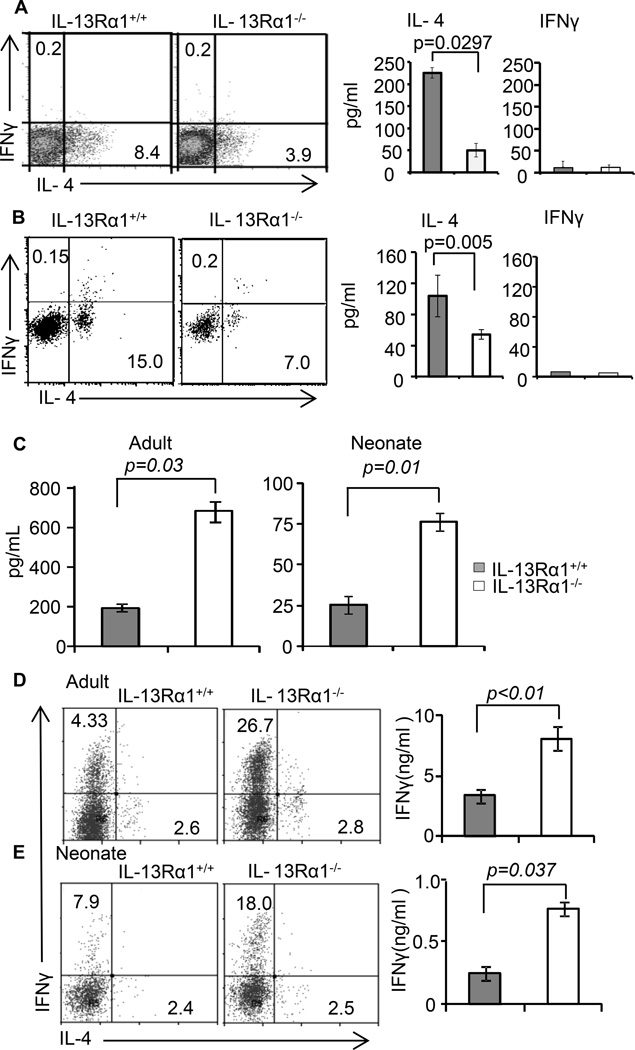
In the newborn mouse both Th1 and Th2 cells develop in the primary response (11, 13, 16). However, the former express IL-13Rα1 which associates with IL-4Rα to form a HR that marks the cells for death during re-challenge with Ag, hence the bias of secondary neonatal immunity towards Th2 cells (11). When the expression of IL-13Rα1 is nullified by gene deletion one would expect to preserve Th2 alongside the restored Th1 cells in the secondary response. This, however, was not the case, and the findings indicate that while Th1 cells arise in the secondary response of IL-13Rα1−/− mice, the Th2 counterparts were significantly diminished. This prompted us to delineate the mechanism underlying the loss of secondary neonatal Th2 responses in the IL-13Rα1-deficient mice. To this end, we began by determining whether the Th2 cells develop in the primary responses and if so whether they respond to a recall with Ag. The findings were even more intriguing as the IL-13Rα1−/− newborns could not develop primary Th2 cells and the responses were biased toward Th1 cells (Fig. 2). Indeed, IL-13Rα1+/+ DO11.10 T cells developed balanced Th1 and Th2 ex vivo primary responses when exposed to Ag in either IL-13Rα1+/+ or IL-13Rα1−/− hosts (Fig. 2A). However, the IL-13Rα1−/− DO11.10 T cells yielded responses that were skewed towards Th1 cells (14.0%) with very little Th2 cells (3.3%) in the IL-13Rα1−/− hosts but balanced Th1 and Th2 cells in the IL-13Rα1+/+ hosts (Fig. 2A). Compiled results from 3 experiments confirm the observation that ablation of IL-13Rα1 enables naïve DO11.10 T cells to differentiate into Th1 cells but hinders generation of primary Th2 cells when the host mice also lack functional IL-13Rα1 (Fig. 2B). Analysis of the recall responses of the primary cells upon stimulation in vitro with OVAp again indicates that the balanced Th1/Th2 primary immunity shifts into Th2-biased recall responses when the donor T cells and/or the host mice are sufficient for IL-13Rα1 whether the cytokines are measured by ELISA or ELISPOT (Fig. 2C). However, when both the donor T cells and the host mice were deficient for IL-13Rα1 there was greater Th1 but diminished Th2 recall responses by either cytokine measurement assay (Fig. 2C). These results are Ag-specific because none of the responses developed when the exposure was carried out with the backbone control Ig-W instead of Ig-OVA. Compiled results from 3 to 5 different experiments confirm the observation that when both the donor T cells and the host mice are deficient for IL-13Rα1, the recall responses are significantly biased towards Th1 cells (Fig. 2D). Overall, the results indicate that shifting of the primary responses towards Th1 with minimal Th2 cells requires deficiency in IL-13Rα1 in host APCs, in addition to donor T cells.
Figure 2.
Ablation of IL-13Rα1 unbalances primary neonatal immunity and skews the responses towards Th1 cells. (A–D) IL-13Rα1+/+ and IL-13Rα1−/− neonatal Balb/c mice recipient of IL-13Rα1−/− or IL-13Rα1+/+ T cells from 1 day old DO11.10 donors were given Ig-OVA or the control Ig-W in saline and 14 days later their splenic primary ex vivo and recall responses were analyzed. (A) Shows a representative ex vivo intracellular IFNγ and IL-4 production by CD4+KJ1-26+ DO11.10 T cells. (B) Shows compiled ex vivo intracellular IFNγ and IL-4 production by CD4+KJ1-26+ DO11.10 T cells from 3 experiments. Each bar represents the mean ± SE. (C) Shows recall IFNγ and IL-4 responses after in vitro stimulation with OVAp as measured by ELISA (top panels) and ELISPOT (bottom panels). Each bar represents the mean ± SD of triplicate wells. (D) Shows compiled results from 5 experiments carried out as described in (C). The bars represent the mean ± SE of the 5 experiments. The statistical analysis used student’s t test to compare results between donor T cells from IL-13Rα1−/− versus IL-13Rα1+/+ mice within both IL-13Rα1−/− and IL-13Rα1+/+ hosts.
Th1-biased primary neonatal immunity in IL-13Rα1-deficient mice parallels with reduced frequency of basophils and basophil progenitors
Given that Th2 cells fail to develop in IL-13Rα1−/− neonatal mice, we envisioned that during priming with Ag, there is paucity in environmental IL-4, which is essential for differentiation of naïve T cells towards the Th2 phenotype (21). To test this hypothesis, exogenous IL-4 was provided during neonatal exposure to Ag and primary, ex vivoas well as recall Th2 responses were analyzed. Accordingly, 1-day-old IL-13Rα1−/− Balb/c mice recipient of IL-13Rα1−/− neonatal DO11.10 T cells were given Ig-OVA and one injection of IL-4 per day for 3 consecutive days. Th2 cells were then analyzed on day 14 after exposure to Ig-OVA. The results clearly indicate that the DO11.10 T cells become able to differentiate into Th2 cells when Ag exposure is carried out in the presence of exogenous IL-4 (Fig. S1). In fact, the frequency of IL-4-producing Th2 cells increased in a cytokine dose-dependent manner as the percentage of IL-4-positive cells went up from a 2.3% background level in the absence of IL-4 to 8.5% and 23.6% with 200 and 400 ng exogenous IL-4, respectively (Fig. S1A). Furthermore, when the cells were stimulated in vitro with Ag there was no IL-4 production in the culture from mice which did not receive exogenous IL-4 during priming whether the stimulation was carried out with OVAp or the negative control, HA peptide (Fig. S1B). Though, IFNγ secretion was observed with OVAp but not with HA peptide stimulation. In contrast, there was IL-4 production in the cultures from mice recipient of exogenous IL-4 upon stimulation with OVAp but not HA peptide and this was similar to IFNγ indicating that both Th1 and Th2 responses develop during priming with Ag in the presence of exogenous IL-4. Overall, these observations indicate that there is paucity in environmental IL-4 in IL-13Rα1−/− neonates which limits Th2 differentiation unless exogenous cytokine is provided.
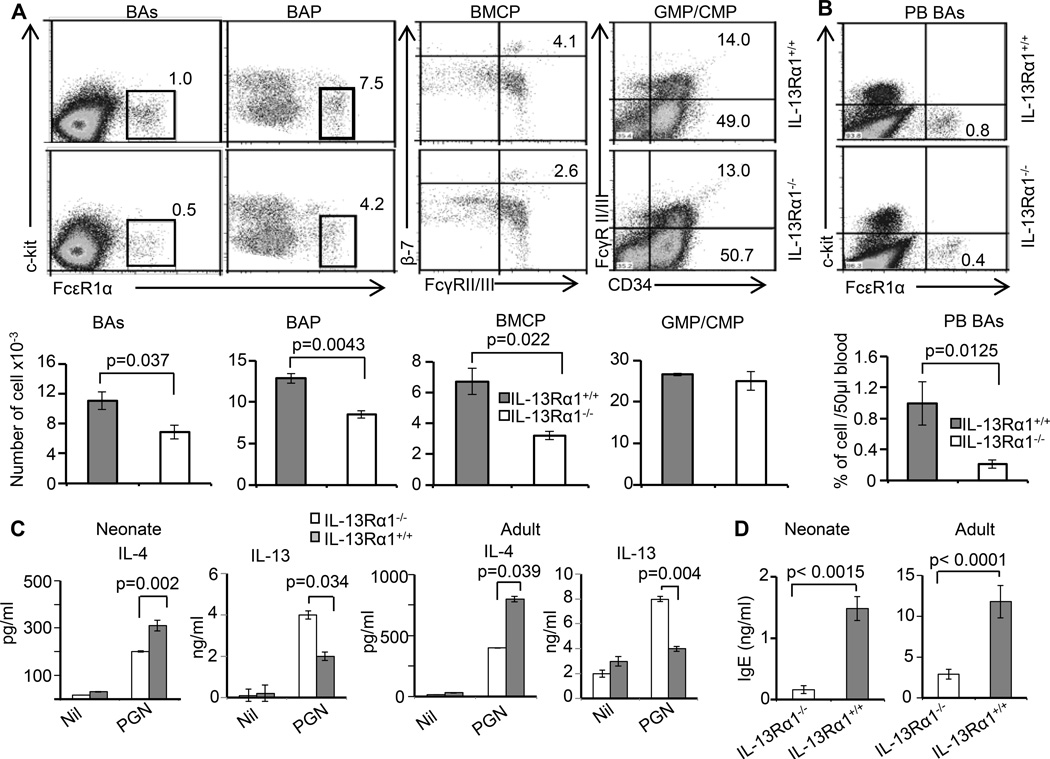
Since IL-4 is critical for Th2 differentiation and restores Th2 responses in IL-13Rα1−/− neonates, it is possible that IL-13Rα1 plays a role in the development and/or function of IL-4-producing cells of the innate immune system. To address this issue we began by determining the frequency of cells known to serve as a source of IL-4 in IL-13Rα1−/− in comparison to IL-13Rα1+/+ neonates. The findings indicate that IL-13Rα1+/+ and IL-13Rα1−/− neonates have comparable frequencies of T cells (CD4 and CD8), B cells (B220), macrophages (CD11b), conventional DCs (CD11c), eosinophils (Siglec F), and neutrophils (Gr-1) (Fig. S2A–S2F). However, there was a significant decrease in the frequency of bone marrow (BM) basophils in IL-13Rα1−/− relative to IL-13Rα1+/+ neonates (Fig. 3A). Furthermore, there was also a diminished frequency of basophil, and basophil/mast cell common progenitors (BAP and BMCP, respectively) that was not observed with the common myeloid (CMP) and granulocyte macrophage (GMP) progenitors (Fig. 3A). Compiled results from 5 experiments indicate that the decrease in the frequency of basophils and their progenitors in IL-13Rα1−/− neonates are statistically significant relative to IL-13Rα1+/+ newborns (Fig. 3A). This likely resulted in the reduced frequency of basophils in peripheral blood (PB BAs) (Fig. 3B). Since mast cells (c-kit+FcεR1α+) were not detectable in either strain (Fig. 3B) and basophils are known to serve as a source of IL-4 (22, 23), the diminished basophil frequency in IL-13Rα1−/− mice may be responsible for the paucity of the cytokine in these animals. Further investigation of this issue indicated that BM-derived basophils from both neonatal and adult IL-13Rα1−/− mice produced significantly less IL-4 but more IL-13 cytokine than their IL-13Rα1+/+ counterparts upon in vitro stimulation with the TLR-2 ligand, peptidoglycan (PGN) (Fig. 3C). Moreover, both neonatal and adult IL-13Rα1−/− mice had lower levels of serum IgE relative to IL-13Rα1+/+ mice (Fig. 3D). Since B cells require IL-4 to undergo switching to IgE, it is likely that the diminished production of this isotype in IL-13Rα1−/− mice is related to the environmental paucity of IL-4 in these animals.
Figure 3.
IL-13Rα1 ablation reduces the frequency of neonatal basophils and interferes with the production of IL-4 cytokine and serum IgE. (A) The top panel shows the frequency of basophils (BAs), basophil progenitor (BAP), basophil/mast cell common progenitors (BMCP), common myeloid progenitors (CMP), and granulocyte monocyte progenitors (GMP) in the bone marrow of IL-13Rα1−/− and IL-13Rα1+/+ neonates. The bar graphs in the bottom panel show the mean ± SD of compiled cell frequencies from 3–5 neonates. (B) The top panel shows the frequency of basophils in peripheral blood (PB BAs) and the bottom panel shows the mean ± SD of compiled cell frequencies from 3–5 neonates. (C) Shows IL-4 and IL-13 production by basophils from neonate (left panel) and adult (right panel) IL-13Rα1−/− and IL-13Rα1+/+ mice upon stimulation with peptidoglycan, a TLR2 ligand. The bars represent the mean ± SD of triplicate wells. The results are representative of 4 experiments with 2–4 mice per group. (D) Shows serum IgE levels in neonatal and adult IL-13Rα1−/− and IL-13Rα1+/+ Balb/c mice as measured by ELISA. Each bar represents the mean ± SD of triplicate wells.
Basophils regulate Th2 immunity in neonates
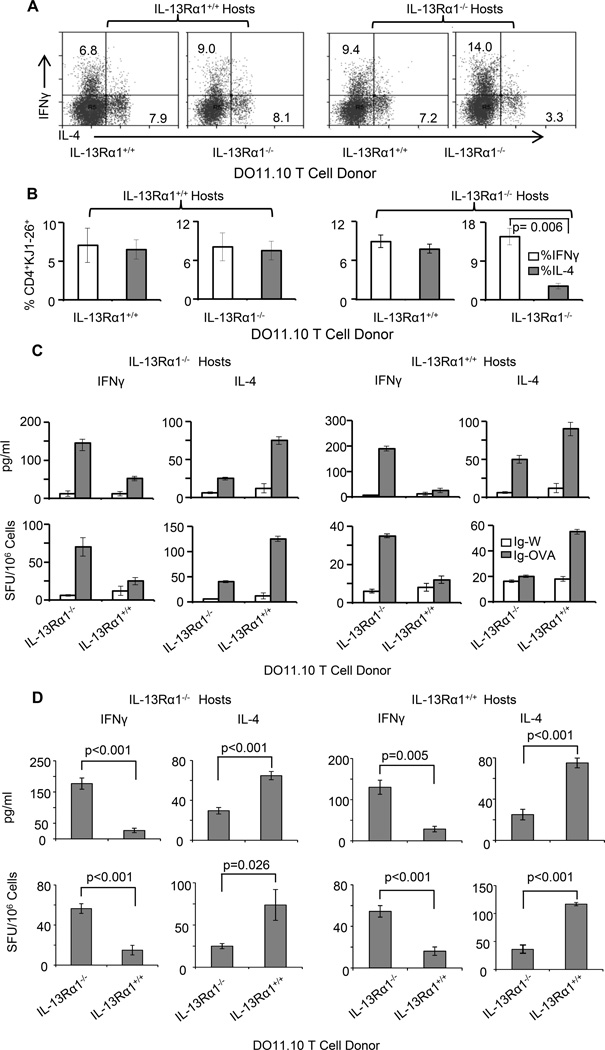
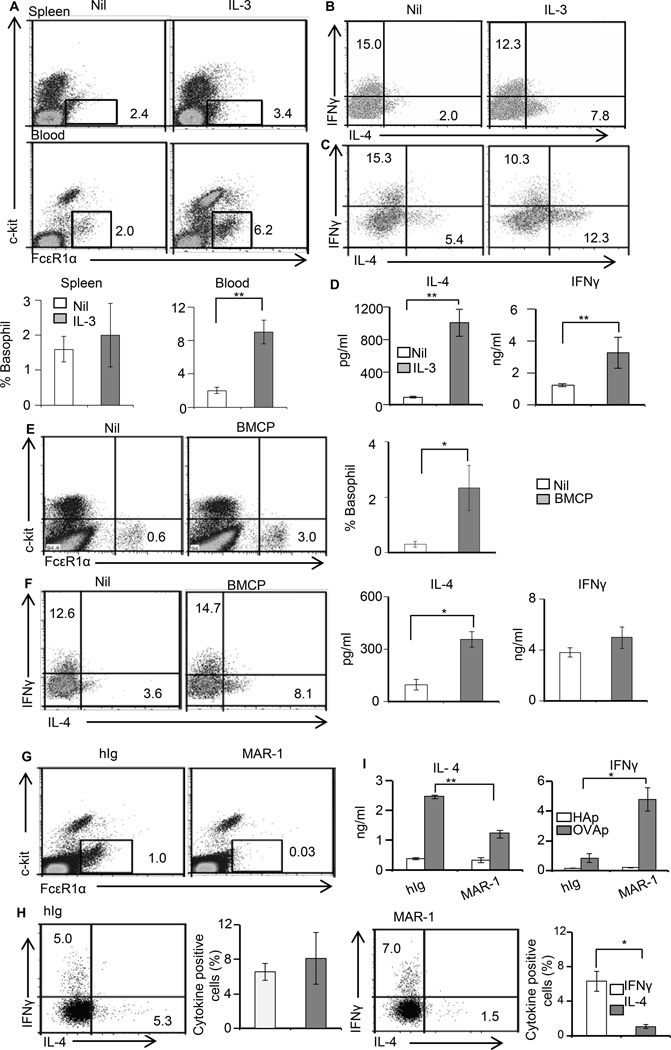
IL-13Rα1−/− mice have a lower frequency of mature basophils and BAP (Fig. 3). Given that basophils serve as a source of IL-4 for Th2 differentiation (22, 24) the diminished number of basophils may be responsible for paucity of environmental IL-4 and the consequent poor Th2 differentiation in the IL-13Rα1−/− mice. To test this premise we sought to stimulate the generation of basophils in vivo and test for restoration of Th2 differentiation. Initial analysis for stimulation of basophil differentiation by administration of rIL-3 into IL-13Rα1−/− mice indicated an increase of basophil frequency in the blood from 2.1% ± 0.56% (or absolute cell number of 33,000 ± 2,500 per ml) in untreated mice to 9.09% ± 1.26% (or absolute cell number of 157,000 ± 24,000 per ml) in mice given rIL-3 (Fig. 4A). Compiled results from 3 experiments indicated that such an increase is significant and parallels with previous reports on in vivo expansion of basophils (25). Furthermore, when IL-13Rα1−/− newborns recipient of neonatal T cells from IL-13Rα1+/+ DO11.10 donor mice were given Ig-OVA accompanied with rIL-3, primary ex vivo and recall Th2 responses were restored (Fig. 4B–4D). Indeed, the percentage of ex vivo Th2 cells went up from 2% in the mice without rIL-3 to 7.8% in those recipients of rIL-3 (Fig. 4B). The percentages of Th1 cells were similar in both groups of mice. Furthermore, upon in vitro stimulation with OVAp the recall Th2 cytokine responses of rIL-3 recipient mice increased significantly as measured by both intracellular staining and ELISA (Fig. 4C and D). We then attempted to enrich with mature IL-13Rα1+/+ basophils to restore IL-4 production and neonatal differentiation of Th2 cells but this caused instant death of the newborn mice perhaps due to degranulation of basophils and excess serotonin and histamine release. The alternative then was to enrich with BMCP from IL-13Rα1+/+ mice instead of mature basophils and test for restoration of neonatal Th2 differentiation. The results indicated an increase of the frequency of basophils in the blood, which was higher than in mice without BMCP transfer (Fig. 4E). Compiled results from 3 experiments indicated that such an increase is statistically significant. When IL-13Rα1−/− newborns recipient of neonatal T cells from IL-13Rα1−/− DO11.10 donors were also given a transfer of BMCP and exposed to Ig-OVA both ex vivo and recall Th2 responses were restored (Fig. 4F). Indeed, the percentage of ex vivo Th2 cells went up from 3.6% in the mice without BMCP transfer to 8.1% in those recipients of BMCP transfer (Fig. 4F). The percentages of Th1 cells were similar in both groups of mice. Furthermore, upon in vitro stimulation with OVAp the recall Th2 cytokine responses of mice recipient of BMCP transfer increased significantly (Fig. 4F). Overall, basophil expansion driven by IL-3 or IL-13Rα1+/+ BMCP restores Th2 differentiation in IL-13Rα1−/− mice.
Figure 4.
IL-13Rα1 on basophils controls the development of neonatal Th2 immunity. IL-13Rα1−/− newborns were given rIL-3 (A–D), basophil progenitors (E–F), or MAR-1 anti-FcεR1α antibody (G–I). The mice were then transferred with DO11.10 T cells from IL-13Rα1+/+ newborn mice and injected with Ig-OVA. (A) Shows the frequency of SP and peripheral blood basophils after injection of rIL-3 but before T cell transfer. The dot plots show a representative experiment and the bar graphs show compiled frequencies from 3 experiments. The bars represent the mean ± SE. (B) Shows ex vivo intracellular IL-4 and IFNγ responses of DO11.10 T cells. (C–D) Show recall cytokine responses as measured by intracellular staining (C) and ELISA (D). (E) Shows SP and PB basophil frequency after transfer of BMCP but before T cell transfer. The dot plots show a representative experiment and the bar graphs show compiled frequency results from 3 experiments. (F) Shows ex vivo intracellular (dot plots) and recall (bar graphs) cytokine responses. (G) Shows PB basophil frequency after MAR-1 or hamster Ig (hIg) control injection but before T cell transfer. (H) Shows ex vivo intracellular cytokine responses of DO11.10 T cells after exposure to Ig-OVA. The left panel shows representative cytokine results and the right panels show compiled results from 3 experiments. The bars represent the mean ± SD. (I) Shows recall cytokine responses as measured by ELISA after in vitro stimulation of the splenocytes with OVAp or the control HA peptide. The bars represent the mean ± SD of triplicate wells. This experiment was repeated 3 times.
Since IL-13Rα1 seems to regulate Th2 differentiation in neonatal mice by controlling the frequency of basophils and their ability to produce IL-4, one would envision that depletion of these cells in IL-13Rα1+/+ mice would limit the development of Th2 cells and skew neonatal immunity towards Th1 cells. To test this premise, basophils were depleted by intra-peritoneal injection of anti-FcεR1α antibody (clone MAR-1) and the neonates were tested for ex vivo and recall Th2 responses. The findings show that the depletion regimen reduces the frequency of basophils from 1.0% in isotype control treated (hIg) mice to 0.03% in the mice recipient of MAR-1 antibody (Fig. 4G). The consequence of this effective basophil depletion translates into diminished ex vivo and recall primary Th2 responses (Fig. 4H). Indeed, while the percentage of Th2 cells was nearly equal to that of Th1 cells in the mice given the isotype control hIg, the frequency of Th2 cells was significantly reduced relative to that of Th1 cells in the mice recipient of MAR-1 antibody (Fig. 4H). In addition, while the recall response was dominated by IL-4 in the mice recipient of the isotype control, a skewing towards IFNγ (Th1) was observed in the basophil-depleted mice (Fig. 4I). Although, MAR-1 antibody is known to deplete mast cells alongside basophils (26), this would not affect the outcome because mast cells are not known to present Ag and would not have been triggered to produce IL-4 in this system. This indicates that basophil depletion interferes with Th2 differentiation and skews neonatal immunity towards Th1 cells.
Overall, the findings indicate that IL-13Rα1 utilizes basophils and their IL-4 to control Th2 neonatal immunity.
Neonatal basophils are able to present Ag and support Th2 differentiation in an IL-13Rα1-dependent manner
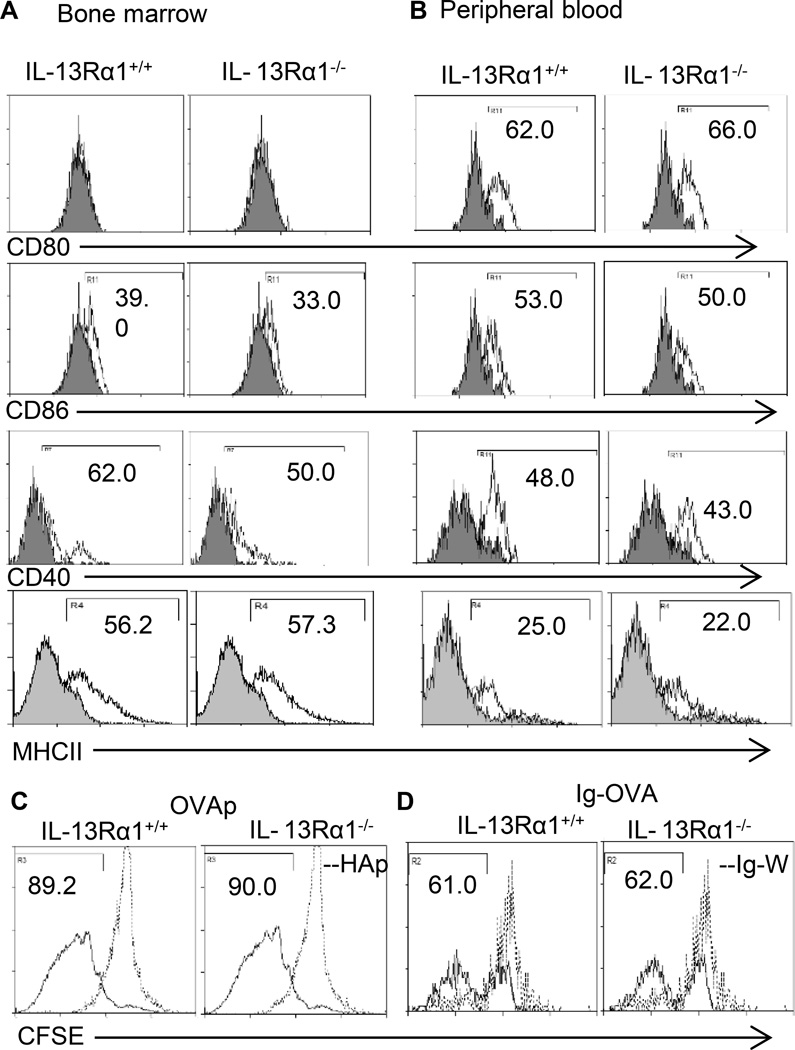
The data presented so far indicates that basophils are required to produce IL-4 in order for neonatal Th2 cells to develop. However, it is not clear whether such a contribution requires the basophils to present Ag, especially that in adult mice this feature remains debatable (27–31). To address this question in the neonatal system, we examined the basophils for expression of MHC and co-stimulatory molecules, and then, for Ag presentation to T cells of both OVAp and Ig-OVA. The results show that basophils derived from BM or peripheral blood cells of IL-13Rα1+/+ and IL-13Rα1−/− mice express comparable levels of CD80, CD86, CD40 and MHC class II molecules (Fig. 5A and B).
Figure 5. Both IL-13Rα1−/− and IL-13Rα1+/+ basophils function as antigen presenting cells.
BM-derived (A) and peripheral blood (B) basophils were stained with anti-CD80, anti-CD86, anti-CD40 and anti-MHC-II antibodies (open histogram) or isotype control (filled histogram) and marker expression was analyzed by flow cytometry on Lin−c-kit+FcεR1α+ gated basophils. Data is representative of 4 experiments. In (C and D) CFSE-labeled DO11.10 CD4+ T cells (1×106/well) were incubated with bone marrow-derived basophils (1×104/well) from IL-13Rα1+/+ or IL-13Rα1−/− in presence of OVAp (C) or Ig-OVA (D) and antigen presentation was measured by CFSE dilution as described in Materials and Methods. This is representative of 3 independent experiments.
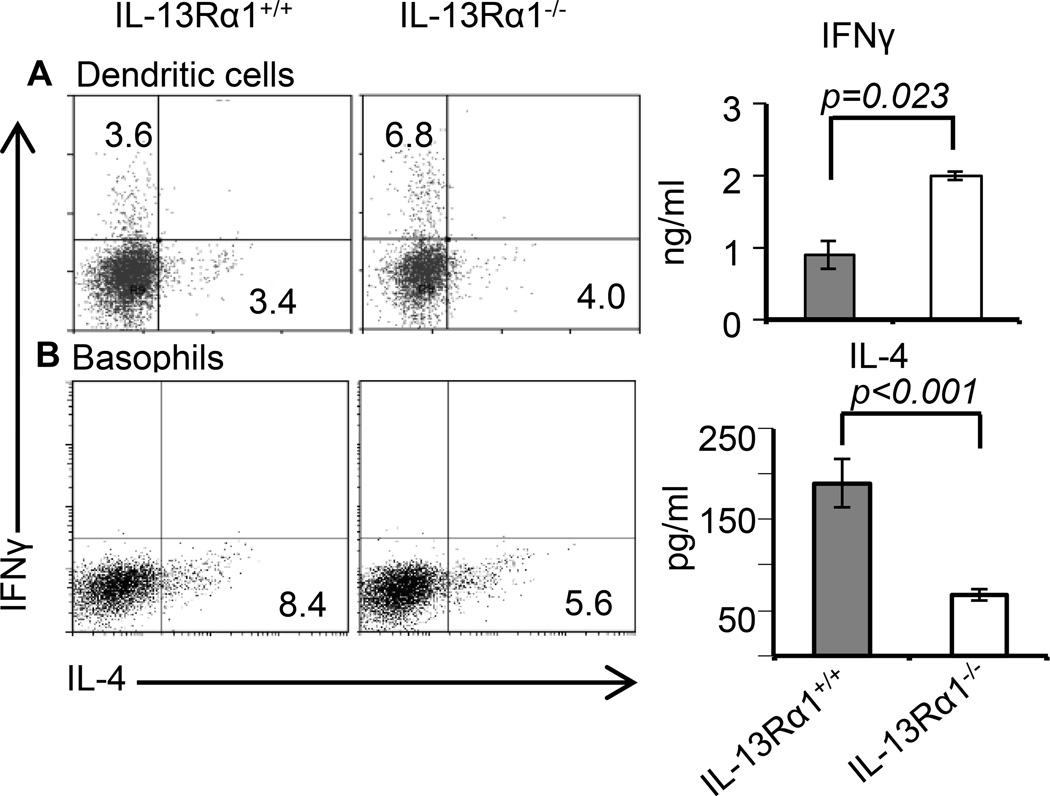
Moreover, when BM-derived basophils were loaded with OVAp or Ig-OVA and incubated with CFSE-labeled naïve DO11.10 CD4 T cells, they were able to induce division of the T cells (Fig. 5C and D). Furthermore, while CFSE-dilution was similar whether the basophils were from IL-13Rα1+/+ or IL-13Rα1−/− mice, more T cells have undergone division with OVAp relative to Ig-OVA stimulation. This likely reflects the fact that free peptide does not require internalization and processing while Ig-OVA requires binding and internalization through Fcγ receptor and processing within endosomes (32). Nevertheless, the findings indicate that basophils from neonatal mice, like those from adult animals (27–30), are equipped with functional processing and presentation machinery as are professional APCs such as DCs. Moreover, when basophils were loaded with OVAp and incubated with naïve DO11.10 T cells, there was IL-4 but not IFNγ production by the T cells which suggests that the basophils support Th2 but not Th1 differentiation as they are not able to produce IL-12 (Fig. 6A). Note that basophils from IL-13Rα1−/− mice were much less efficient in driving Th2 differentiation perhaps because they produce less IL-4 (Fig. 3C). The presentation machinery of basophils is also functional in vivo as transfer of MHC-sufficient basophils alongside neonatal DO11.10 T cells into MHC-deficient newborn mice restores antigen presentation and the animals were able to develop primary Th2 responses upon exposure to Ig-OVA (Fig. 6B). More importantly, IL-13Rα1−/− basophils were less efficient than IL-13Rα1+/+ basophils in inducing Th2 responses because IL-13Rα1-deficiency interferes with the ability of the cells to produce the IL-4 required to support Th2 differentiation. The findings are significant because compiled results from 3 experiments confirm the lack of IFNγ responses and the diminished efficacy of IL-13Rα1−/− basophils in driving Th2 differentiation. Overall, neonatal basophils are able to present Ig-OVA in vivo which is likely facilitated through Fcγ receptor (29), produce IL-4, and support differentiation of naïve T cells into Th2 cells, and IL-13Rα1 is crucially involved in this function.
Figure 6.
IL-13Rα1 controls antigen presentation and T cell programing function of neonatal basophils and DCs. (A) Shows in vitro Ag presentation and the consequent T cell differentiation driven by OVAp-loaded IL-13Rα1+/+ or IL-13Rα1−/− BM-derived basophils as measured by cytokine production. The dot plots show intracellular cytokines while the bar graphs show cytokine secretion in culture supernatant. (B) MHC-II deficient adult Balb/c mice recipient of neonatal DO11.10 CD4+ T cells were given bone marrow-derived basophils from IL-13Rα1+/+ or IL-13Rα1−/− neonatal mice and the hosts were immunized with Ig-OVA. On day 14 the SP cells were stimulated with PMA and Ionomycin and cytokine production was measured by intracellular staining (left panel) and ELISA (right panel). The dot plots show intracellular cytokine production by DO11.10 T cells. The bar graphs show cytokine secretion in culture supernatant from mice recipient of IL-13Rα1+/+ (closed bars) or IL-13Rα1−/− (open bars) bone marrow-derived basophils. (C) Shows IL-12 production by DCs from IL-13Rα1−/− and IL-13Rα1+/+ neonatal and adult mice upon in vitro stimulation with LPS. (D and E) Show differentiation of naïve DO11.10 T cells into Th1 (IFNγ) and Th2 (IL-4) upon stimulation with OVAp-loaded DCs from adult (D) and neonatal (E) IL-13Rα1−/− and IL-13Rα1+/+ mice as measured by intracellular staining (dot plots) and ELISA (bar graphs). Each bar represents the mean ± SD of triplicate wells. Data are representative of 2 experiments.
IL-13Rα1 controls IL-12 production by DCs and plays a critical role in Th1 differentiation
The results presented in Figure 1 and 2 indicate that the primary neonatal response is skewed towards Th1 instead of Th2 when IL-13Rα1 is non-functional in APCs. Since differentiation of naïve T cells into Th1 cells requires IL-12 production by APCs, we envisioned that IL-13Rα1 may play a role in IL-12 production in order to control T cell differentiation. To test this premise we began by comparing the production of IL-12 by DCs from IL-13Rα1−/− versus IL-13Rα1+/+ mice. The results indicate that upon stimulation with LPS, a TLR-4-ligand, the DCs from IL-13Rα1−/− mice produce much higher IL-12 relative to those from IL-13Rα1+/+ mice and this is true for DCs from neonatal and adult mice (Fig. 6C). Moreover, when the DCs were loaded with OVAp and used for activation and differentiation of naïve DO11.10 T cells, the DCs from IL-13Rα1−/− mice yielded a greater Th1 response relative to DCs from IL-13Rα1+/+ mice whether the animals were adults or neonates (Fig. 6D and E). This led to an increase of IFNγ in the cultures stimulated with IL-13Rα1−/− relative to IL-13Rα1+/+ DCs (Fig. 6D and E). Taken together, these results indicate that DCs in which IL-13Rα1 is non-functional produce higher amounts of IL-12 and drive superior Th1 differentiation in comparison to DCs with intact IL-13Rα1.
Upon transfer into MHCII−/− mice IL-13Rα1−/− DCs shift primary neonatal immunity towards Th1 while IL-13Rα1−/− basophils nullify development of Th2 responses
In vitroIL-13Rα1−/− neonatal basophils were able to process and present Ag to the same extent as IL-13Rα1+/+ basophils, but due to diminished ability in IL-4 production, they were much less efficient in driving Th2 differentiation (Fig. 3 and Fig. 6). However, IL-13Rα1−/− neonatal DCs produce higher amounts of IL-12 than IL-13Rα1+/+ DCs and sustain better Th1 differentiation (Fig. 6C–E). We then sought to investigate the effects of IL-13Rα1 on the function of these non-lymphoid cells in vivo and determine the type of neonatal immunity that ensues when IL-13Rα1−/− DCs and basophils are serving as APCs in neonatal MHCII−/− mice. The results show that newborn mice recipient of IL-13Rα1+/+ neonatal DCs alongside IL-13Rα1+/+ DO11.10 T cells and given Ig-OVA develop similar frequencies of Th1 and Th2 cells ex vivo (3.6% versus 3.4%) but upon stimulation with OVAp, the recall responses become biased towards Th2 cells because their IL-4 drives apoptosis of IL-13Rα1-positive Th1 cells (Fig. 7A). In contrast, transfer of IL-13Rα1−/− neonatal DCs yielded a higher frequency of Th1 relative to Th2 cells ex vivo (6.8% versus 4%) and the recall response was skewed towards Th1 cells (Fig. 7A). The basophils, however, had more effects on Th2 cells and did not support the development of Th1 cells as they do not produce IL-12 (Fig. 7B). Indeed, IL-13Rα1−/− basophils, which produce diminished levels of IL-4, yielded a lower frequency of Th2 cells ex vivo that responded poorly to OVAp stimulation relative to IL-13Rα1+/+ basophils (Fig. 7B). Overall, IL-13Rα1 shapes neonatal immunity by controlling the production of IL-4 by basophils and IL-12 by DCs.
Figure 7.
IL-13Rα1 exercises differential control of DC and basophil mediated T cell programing in vivo. MHC-II −/− IL-13Rα1+/+ Balb/c neonatal mice recipient of DCs or basophils from 1-day-old IL-13Rα1+/+ or IL-13Rα1−/− mice alongside neonatal DO11.10 T cells and the hosts were given Ig-OVA. On day 14, the SP cells from the MHC-II −/− IL-13Rα1+/+ hosts were harvested, supplemented with MHC-II-sufficient DCs from MHC-II+/+ IL-13Rα1+/+ mice (to serve as APCs) and the culture was stimulated with OVA peptide. Ex-vivo (dot plots) and recall (bar graphs) cytokines responses by CD4 T cells were then analyzed by intracellular staining and ELISA, respectively. (A and B) shows cytokine production by T cells from hosts recipient of (A) DCs or (B) basophils. Each bar represents the mean ± SD of triplicate wells. Data is representative of 2 experiments.
IL-4 from neonatal basophils utilizes the HR to regulate IL-12 production by DCs and controls primary Th1 cells
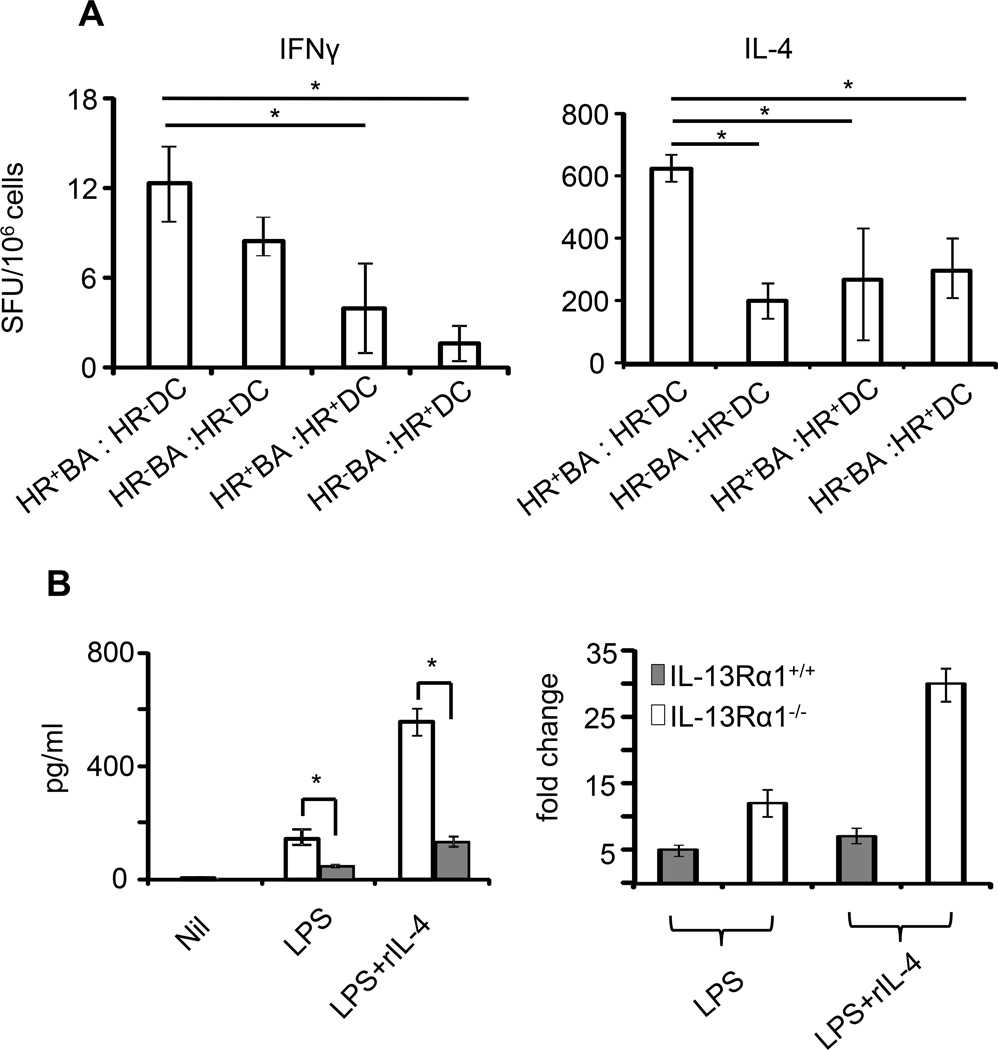
The results in Fig. 7 indicate that basophils produce IL-4 to support differentiation of Th2 cells while DCs secrete IL-12 to sustain Th1 programs. These observations explain the balance between Th1 and Th2 cells in the primary response of the newborn but do not resolve developmental expression of the HR on the Th1 cells. We have previously shown that neonatal DCs produce IL-12 sufficient to drive Th1 differentiation but not abundant enough to counter HR up-regulation on these cells (13, 16). Because neonatal DCs express the HR (33) and basophils produce IL-4 it is thus logical to envision that the diminished IL-12 production by DCs is the outcome of a negative regulation by IL-4 from basophils. To test this postulate we performed DC and basophil co-transfer experiments and found that when neonatal DCs do not express the HR (HR−DC), significant (as analyzed by ANOVA with Bonferroni post-test) primary Th1 responses develop regardless of the amount of IL-4 produced by HR-negative (HR−BA) or HR-positive (HR+BA) basophils (Fig. 8A, left panel). In contrast, when the DCs are HR-positive (HR+DC), even a low amount of IL-4 produced by HR−BA has a profound effect on the development of Th1 responses similar to HR+BA, which produce higher amounts of IL-4 (Fig. 8A, left panel). Thus, IL-4 from basophils efficiently utilizes the HR to counter DC function and IL-12 production. On the other hand the primary neonatal Th2 responses have shown direct correlation with the amount of IL-4 produced by basophils when the DCs were HR-negative (HR−DC). Indeed, the Th2 primary response was higher when the basophils were HR-positive (compare HR+ BA:HR−DC versus HR− BA:HR−DC) (Fig. 8A, right panel). However, when the DCs had the HR, the primary Th2 response was similar whether the basophils were HR-positive or HR-negative (compare HR+ BA:HR+DC to HR− BA:HR+DC) despite the fact that HR+BA produce higher IL-4. This is perhaps related to absorption of IL-4 by HR+DC, otherwise the response should be at levels comparable to HR+BA:HR−DC. This suggests that HR expression on DCs may limit IL-4 availability for Th2 programing. Overall, IL-4 from basophils utilizes the HR on DCs to control programing of primary Th1 cells. This statement is further supported by the findings that addition of IL-4 during stimulation of DCs with LPS (TLR-4 ligand) diminishes IL-12 production by HR-positive DCs relative to HR-negative DCs (Fig. 8B, left panel). In fact, there was about 5-fold decrease in IL-12 production when the HR is functional. Thus, it seems that when the DCs are lacking the HR they produce more IL-12 in the presence of IL-4 perhaps due to signaling through the conventional IL-4R. However, when the HR is present such increase in IL-12 production is nullified indicating that IL-4 utilizes the HR to counter the function of the conventional IL-4R. In all, the HR stages a stepwise negative regulation against Th1 programs by inciting basophils against the function of DCs during early life and then taking over as a death marker for primary Th1 cells upon a later encounter with Ag.
Figure 8.
IL-4 from basophils modulates IL-12 production by DCs to sustain control of primary Th1 immunity in the newborn. (A) Neonatal MHC-II−/− IL-13Rα1+/+ mice (3 per group) recipient of basophils and DCs from 1-day-old IL-13Rα1+/+ (HR+BA: HR+DC) or IL-13Rα1−/− (HR−BA:HR−DC) mice were given neonatal DO11.10 CD4+ T cells and injected with Ig-OVA. On day 14, the SP cells were harvested and incubated with OVAp-loaded MHC-II+/+ DCs (to serve as APCs). IFNγ and IL-4 production by KJ1-26+CD4+ DO11.10 T cells were measured by ELISPOT. The bars represent the mean ± SD spot forming units (SFU) of triplicate wells. The results are representative of 3 independent experiments. (B) shows IL-12 production by IL-13Rα1−/− and IL-13Rα1+/+ DCs upon stimulation with control media (NIL), LPS alone, or in combination with rIL-4 cytokine (left panel). Each bar represents the mean ± SD of triplicate wells. The right panel shows the fold change in IL-12 production by IL-13Rα1+/+ and IL-13Rα1−/− DCs induced by exogenous IL-4. Each bar represents the mean ± SE of cytokine production ratio obtained upon stimulation in the presence versus absence of IL-4. The data is derived from 3 experiments.
Discussion
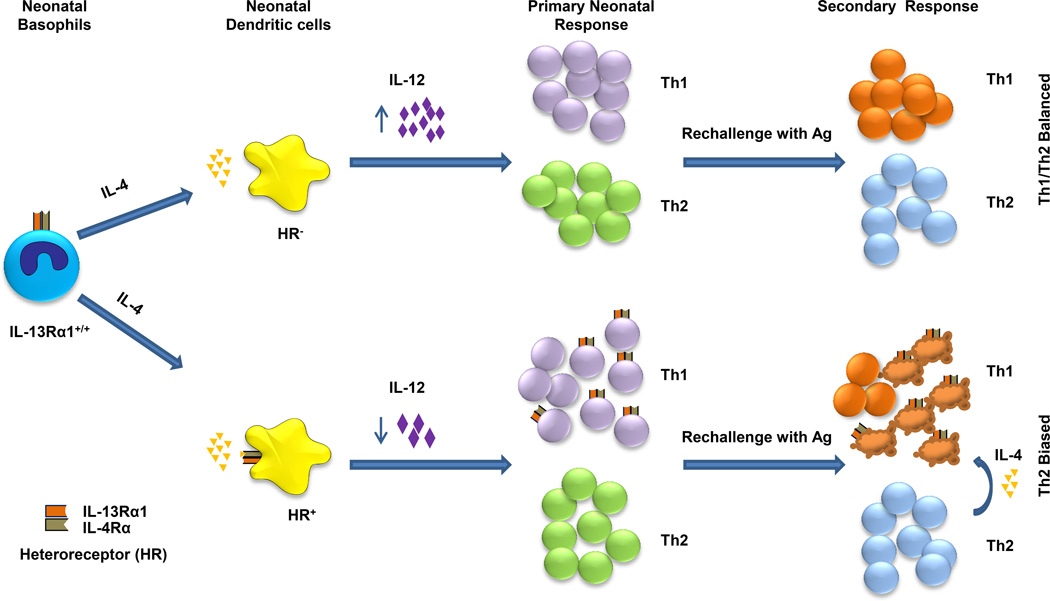
Th1 cells usually express the conventional IL-4R (IL-Rα/common γ) which does not support signaling by IL-4 cytokine (12, 34). In earlier studies, while interrogating IL-4 for death of neonatal Th1 cells, we discovered that IL-13Rα1 is developmentally up-regulated during Ag-induced cell differentiation and associates with IL-4Rα providing a HR that sustains signaling by IL-4 and drives apoptosis of neonatal Th1 cells upon re-encounter with Ag (11, 13, 16). Thus, IL-13Rα1 serves as a death marker for primary neonatal Th1 cells (7, 15). While these findings explained the Th2 bias of secondary neonatal immunity, the developmental up-regulation of IL-13Rα1 remained obscure. Further studies indicated that delayed maturation of dendritic cells in the newborn and the consequent Ag presentation under limited environmental IL-12 is responsible for IL-13Rα1 up-regulation and HR expression on neonatal Th1 cells (13). In fact, IL-12 which was shown to restore neonatal Th1 responsiveness (11), was found not only to support differentiation of Th1 cells but also to counter the up-regulation of IL-13Rα1 (16). In this report, we uncovered yet another facet whereby IL-13Rα1 instigates basophils to impede the function of DCs setting the stage for HR expression on Th1 cells (Fig. 9). Indeed, it is shown that early life basophils function as APCs, produce IL-4 cytokine, and promote the programs of Th2 cells as was reported for adult basophils (22, 27–29). In the neonatal setting though, IL-4 is further tasked to engage the HR on DCs and dampen their function and IL-12 production (Fig. 9). This is supported by the findings that DCs sufficient for IL-13Rα1, where IL-4 can carry out signaling through the HR, support primary responses devoid of Th1 cells which is in sharp contrast with the significant Th1 cells observed with IL-13Rα1-deficient DCs. Moreover, the HR appears to intrinsically maintain a safeguard mechanism to preserve this inverse regulation of neonatal Th1 and Th2 programs. Indeed, when the HR is functional, DCs produce less IL-12 while basophils secrete more IL-4. Furthermore, given that the HR has high affinity to IL-4 (35, 36) even modest amounts of the cytokine produced by HR-negative basophils sustain diminished Th1 responses as long as the DCs express the HR. While the findings support the functional diversity attributed to the HR (14, 37) and explain the mechanism underlying early-life paucity in Th1 cells, the resolve of the HR to undermine inflammatory T cells in neonates may have been established during early gestation. In fact, it has been suggested that in order to preserve fetal engraftment in uterothe maternal-fetal interface has to be conditioned to restrict the presence of pro-inflammatory lymphocytes (38). Indeed, macrophages and trophoblasts within the decidua produce indoleamine 2,3-dioxygenase (IDO) that selectively induces tryptophan starvation of inflammatory Th1 cells (39). Moreover, trophoblasts produce IL-13 which induces IL-13Rα1 expression on neighboring maternal APCs and promotes the development of anti-inflammatory Th2 cells but restricts Th1 responses (40). While it is not clear how IL-13Rα1 sustains this lymphocyte imbalance at the fetal interface, on a speculative basis it is possible that the chain associates with IL-4Rα leading to HR expression on APCs. Thus, one would envision that IL-4 of the Th2 cells may then utilize the HR to limit IL-12 production by fetal APCs and restrict differentiation of Th1 cells. Given that neonatal APCs express IL-13Rα1 on the day of birth (33), it is likely that trophoblast-driven IL-13Rα1 expression spills over to fetal APCs. The latter transit to the neonatal immune system during birth and uphold the Th1/Th2 imbalance during early life through IL-4 from basophils. This is probably supported by the gradual process of neonatal colonization with commensals in two ways. On one hand, there is delayed maturation of DCs and thus limited accumulation of environmental IL-12 (13, 16). On the other hand, marginal commensal colonization results in elevated serum Ig-E which in turn activates basophils to produce IL-4 (41) and further promotes down-regulation of IL-12 production by DCs. Also, it remains to be determined whether innate lymphoid cells (ILCs) (42) such as nuocytes (43), innate helper 2 cells (44), and natural helper cells (45), which have been shown to produce Th2 cytokines such IL-13, contribute to the maintenance of neonatal Th1/Th2 imbalance through a similar process.
Figure 9.
Stepwise regulation of neonatal immunity by IL-4α/IL-13Rα1 HR. During neonatal exposure to Ag, basophils function as APCs and produce IL-4. This cytokine binds to the HR on neighboring Ag presenting DCs and signals malfunction and diminished IL-12 production. Limited IL-12 in the neonatal environment, although sufficient to drive differentiation of naïve T cells into Th1 cells, cannot counter up-regulation of IL-13Rα1. Consequently, HR formation on neonatal Th1 cells ensues and serves as a death marker for the cells. During rechallenge with Ag, Th2 cells produce IL-4 which signals apoptosis of Th1 cells leading to bias of secondary immunity towards Th2 cells. In fact, if the DCs are devoid of the HR, IL-4 from basophils will not be able to limit IL-12 production by DCs. In this case, Th1 differentiation will occur but without HR expression yielding Th1/Th2 balanced neonatal immunity.
Overall, IL-13Rα1 seems to instigate basophils to stifle the function of DCs and impacts childhood immunity leading to sensitivity to allergic reactions and susceptibility to microbial infection and perhaps poor efficacy of pediatric vaccines.
Supplementary Material
Acknowledgments
Funding. This work was supported by grants RO1AI048541, R21HD060089 (to H.Z.) from the National Institutes of Health. M.M.M. was supported by 5T32GM008396-22 training grant from National Institute of General Medical Sciences.
Footnotes
Abbreviations: BAP, basophil progenitors; BMCP, basophil/mast cell common progenitors; HA, hemagglutinin; OVAp, OVA 323-339 peptide; PB, peripheral blood ; PGN, peptidoglycan; SP, spleen. LN, lymph nodes; HR, IL-4Rα/IL-13Rα1 heteroreceptor.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Gammon G, Dunn K, Shastri N, Oki A, Wilbur S, Sercarz EE. Neonatal T-cell tolerance to minimal immunogenic peptides is caused by clonal inactivation. Nature. 1986;319:413–415. doi: 10.1038/319413a0. [DOI] [PubMed] [Google Scholar]
- 3.Min B, Legge KL, Pack C, Zaghouani H. Neonatal exposure to a self-peptide-immunoglobulin chimera circumvents the use of adjuvant and confers resistance to autoimmune disease by a novel mechanism involving interleukin 4 lymph node deviation and interferon gamma-mediated splenic anergy. J Exp Med. 1998;188:2007–2017. doi: 10.1084/jem.188.11.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 5.Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- 6.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 7.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice. Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- 9.Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160:4217–4224. [PubMed] [Google Scholar]
- 10.Li L, Legge KL, Min B, Bell JJ, Gregg R, Caprio J, Zaghouani H. Neonatal immunity develops in a transgenic TCR transfer model and reveals a requirement for elevated cell input to achieve organ-specific responses. J Immunol. 2001;167:2585–2594. doi: 10.4049/jimmunol.167.5.2585. [DOI] [PubMed] [Google Scholar]
- 11.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 12.Huang H, Paul WE. Impaired interleukin 4 signaling in T helper type 1 cells. J Exp Med. 1998;187:1305–1313. doi: 10.1084/jem.187.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, Divekar R, Tartar DM, Haymaker CL, Zaghouani H. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205:2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Jr, Donnelly RP, Wynn TA. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeman C, Dhakal M, Zaghouani H. Overcoming dendritic cell tardiness to triumph over IL-13 receptor: a strategy for the development of effective pediatric vaccines. Discov Med. 2010;9:554–559. [PubMed] [Google Scholar]
- 16.Hoeman CM, Dhakal M, Zaghouani AA, Cascio JA, Wan X, Khairallah MT, Chen W, Zaghouani H. Developmental Expression of IL-12Rbeta2 on Murine Naive Neonatal T Cells Counters the Upregulation of IL-13Ralpha1 on Primary Th1 Cells and Balances Immunity in the Newborn. J Immunol. 2013;190:6155–6163. doi: 10.4049/jimmunol.1202207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haymaker CL, Guloglu FB, Cascio JA, Hardaway JC, Dhakal M, Wan X, Hoeman CM, Zaghouani S, Rowland LM, Tartar DM, VanMorlan AM, Zaghouani H. Bone marrow-derived IL-13Ralpha1-positive thymic progenitors are restricted to the myeloid lineage. J Immunol. 2012;188:3208–3216. doi: 10.4049/jimmunol.1103316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 19.Bell JJ, Ellis JS, Guloglu FB, Tartar DM, Lee HH, Divekar RD, Jain R, Yu P, Hoeman CM, Zaghouani H. Early effector T cells producing significant IFN-gamma develop into memory. J Immunol. 2008;180:179–187. doi: 10.4049/jimmunol.180.1.179. [DOI] [PubMed] [Google Scholar]
- 20.Powell TJ, Jr, Streilein JW. Neonatal tolerance induction by class II alloantigens activates IL-4-secreting, tolerogen-responsive T cells. J Immunol. 1990;144:854–859. [PubMed] [Google Scholar]
- 21.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min B, Prout M, Hu-Li J, Zhu J, Jankovic D, Morgan ES, Urban JF, Jr, Dvorak AM, Finkelman FD, LeGros G, Paul WE. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, Kuroki Y, Ohara O, Koyasu S, Kubo M. Basophil-Derived Interleukin-4 Controls the Function of Natural Helper Cells, a Member of ILC2s, in Lung Inflammation. Immunity. 2014;40:758–771. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- 25.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 26.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, Kawano Y, Minegishi Y, Shimizu T, Karasuyama H. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 30.Oh K, Shen T, Le Gros G, Min B. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood. 2007;109:2921–2927. doi: 10.1182/blood-2006-07-037739. [DOI] [PubMed] [Google Scholar]
- 31.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Legge KL, Min B, Potter NT, Zaghouani H. Presentation of a T cell receptor antagonist peptide by immunoglobulins ablates activation of T cells by a synthetic peptide or proteins requiring endocytic processing. J Exp Med. 1997;185:1043–1053. doi: 10.1084/jem.185.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhakal M, Hardaway JC, Guloglu FB, Miller MM, Hoeman CM, Zaghouani AA, Wan X, Rowland LM, Cascio JA, Sherman MP, Zaghouani H. IL-13Ralpha1 is a surface marker for M2 macrophages influencing their differentiation and function. Eur J Immunol. 2014;44:842–855. doi: 10.1002/eji.201343755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 35.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 36.LaPorte SL, Juo ZS, Vaclavikova J, Colf LA, Qi X, Heller NM, Keegan AD, Garcia KC. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell. 2008;132:259–272. doi: 10.1016/j.cell.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor alpha1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 39.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 40.Dealtry GB, Clark DE, Sharkey A, Charnock-Jones DS, Smith SK. Expression and localization of the Th2-type cytokine interleukin-13 and its receptor in the placenta during human pregnancy. Am J Reprod Immunol. 1998;40:283–290. doi: 10.1111/j.1600-0897.1998.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 41.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, Kambayashi T, Larosa DF, Renner ED, Orange JS, Bushman FD, Artis D. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18:538–546. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 43.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.