Abstract
Preterm infants are at high risk for long-term abnormalities in cardiopulmonary function. Our objectives were to determine the long-term effects of hypoxia or hyperoxia on cardiopulmonary development and function in an immature animal model. Newborn C57BL/6 mice were exposed to air, hypoxia (12% oxygen), or hyperoxia (85% oxygen) from Postnatal Day 2–14, and then returned to air for 10 weeks (n = 2 litters per condition; > 10/group). Echocardiography, blood pressure, lung function, and lung development were evaluated at 12–14 weeks of age. Lungs from hyperoxia- or hypoxia-exposed mice were larger and more compliant (compliance: air, 0.034 ± 0.001 ml/cm H2O; hypoxia, 0.049 ± 0.002 ml/cm H2O; hyperoxia, 0.053 ± 0.002 ml/cm H2O; P < 0.001 air versus others). Increased airway reactivity, reduced bronchial M2 receptor staining, and increased bronchial α-smooth muscle actin content were noted in hyperoxia-exposed mice (maximal total lung resistance with methacholine: air, 1.89 ± 0.17 cm H2O ⋅ s/ml; hypoxia, 1.52 ± 0.34 cm H2O ⋅ s/ml; hyperoxia, 4.19 ± 0.77 cm H2O ⋅ s/ml; P < 0.004 air versus hyperoxia). Hyperoxia- or hypoxia-exposed mice had larger and fewer alveoli (mean linear intercept: air, 40.2 ± 0. 0.8 μm; hypoxia, 76.4 ± 2.4 μm; hyperoxia, 95.6 ± 4.6 μm; P < 0.001 air versus others; radial alveolar count [n]: air, 11.1 ± 0.4; hypoxia, 5.7 ± 0.3; hyperoxia, 5.6 ± 0.3; P < 0.001 air versus others). Hyperoxia-exposed adult mice had left ventricular dysfunction without systemic hypertension. In conclusion, exposure of newborn mice to hyperoxia or hypoxia leads to cardiopulmonary abnormalities in adult life, similar to that described in ex-preterm infants. This animal model may help to identify underlying mechanisms and to develop therapeutic strategies for pulmonary morbidity in former preterm infants.
Keywords: lung function tests, newborn, airway reactivity, lung development, respiratory function tests
Clinical Relevance
Exposure of newborn mice to hyperoxia or hypoxia leads to cardiopulmonary abnormalities in adult life, similar to that described in ex-preterm infants. This animal model may help to identify underlying mechanisms and to develop therapeutic strategies for pulmonary morbidity in former preterm infants.
Approximately 12% of all live births in the United States every year are preterm infants (< 37 completed weeks) with an estimated annual societal economic burden of over $26 billion (1). Prematurity is not only a major contributing factor for neonatal morbidity and mortality, but may also have long-term adverse consequences. Although survival of extremely preterm infants has increased in recent years, survival without major neonatal morbidity, such as bronchopulmonary dysplasia (BPD), has remained static (2). Approximately 30–40% of extremely preterm infants are diagnosed with BPD, defined as receiving oxygen at 36 weeks post menstrual age (2). BPD is characterized by impairment of alveolar development and abnormal vascular remodeling, with varying degrees of fibrosis and inflammation (3). Follow-up studies on infants with mild to moderate BPD who received prolonged oxygen therapy during their neonatal period showed persistent abnormal lung function, including increased risk for airway reactivity later in childhood (4) and adolescence (5, 6).
The pathogenesis of BPD is multifactorial, with contributors of prematurity, respiratory distress syndrome, volutrauma, oxygen toxicity, infection, and inflammatory mediators. In utero organ development and maturation occurs in a relatively hypoxemic environment (arterial oxygen tension/pressure [PaO2], 20–30 mm Hg) and hence even ambient oxygen (air) exposure in premature infants may possibly induce oxidative stress and have detrimental effects on organ development and maturation. In addition, many premature infants in neonatal intensive care units are frequently exposed to supraphysiological levels of oxygen with alternating periods of hypoxemia. Exposure to supraphysiological levels of oxygen arrests lung development in neonatal animals (e.g., rodents) in which alveolar septation occurs postnatally (7). The hyperoxia-induced inhibition of alveolar development is permanent, as recovery of alveolar septation after return to normoxia is incomplete, and long-term abnormalities in cardiopulmonary function may occur (8).
Hypoxia exposure also induces inhibition of alveolar development and abnormal pulmonary vascular remodeling in neonatal animal models (9). However, the long-term effects of neonatal hypoxia on lung development, and how these differ from the effects of neonatal hyperoxia, have not been determined. We hypothesized that both neonatal hypoxia and hyperoxia would permanently impair lung development, and that neonatal hyperoxia, but not hypoxia, would result in increased airway reactivity in adult life, whereas neonatal hypoxia, but not hyperoxia, would result in pulmonary vascular remodeling and adult pulmonary hypertension.
Materials and Methods
All protocols were approved by the Institutional Animal Care and Use Committee of University of Alabama at Birmingham (Birmingham, AL), and were consistent with the Public Health Services policy on humane care and use of laboratory animals and guidelines for the care and use of laboratory animals.
Animal Model
C57BL/6 mice were exposed to normobaric 12% O2 (hypoxia), 85% O2 (hyperoxia), or air (normoxia) from soon after birth (Postnatal Day 2 [P2]) until 14 days of age (P14; period of maximal alveolar development), as described previously (9–13). After completion of exposure, pups were returned to air for 10 additional weeks and measurements were taken at 12–14 weeks of age (adult). Additional mice (six per group) were evaluated at P14 after air, hyperoxia, or hypoxia exposure for lung function and development.
Measurements
Measurements are discussed in detail in the online supplement.
Systolic blood pressure.
Tail cuff systolic blood pressures (SBPs) were measured (Hatteras Instruments, Cary, NC).
Echocardiography.
Echocardiography was performed using a Visual Sonics Vevo 770 Imaging System (Visual Sonics, Toronto, ON, Canada).
Pulmonary function.
Lung function was evaluated on a flexiVent (SCIREQ, Montreal, PQ, Canada) as previously described (13). Methacholine challenge test was performed using increasing doses of nebulized methacholine (10, 20, 30, 40, and 50 µg/ml) and airway resistance was measured (14).
Lung development assessment.
Mean linear intercepts (MLI) and radial alveolar counts (RAC) were estimated from inflation-fixed lung sections, as described previously (9, 11–13). Parenchymal tethering effect on the airways was assessed by measuring the number and thickness of peribronchial alveolar septa.
Bronchial size assessment.
Bronchial external diameters were measured using hematoxylin and eosin–stained lung sections (n = 6/group).
Pulmonary artery remodeling assessment.
Hematoxylin and eosin–stained lung sections (n = 6/group) were analyzed for pulmonary arterial remodeling, as previously described (9, 15, 16).
Histological analysis of lung collagen, elastin, and bronchial mucin.
Lung sections were analyzed for collagen content using Sirius red F3BA (picric acid Sirius red stain), elastin content using Verhoeff’s elastic tissue stain, and bronchial mucin content using Alcian blue (Sigma-Aldrich, St. Louis, MO) stain.
Immunohistochemical analysis of bronchial α-smooth muscle actin, muscarinic receptors M2 and M3, and β-adrenergic receptor 2.
Immunohistochemistry for α-smooth muscle actin (α-SMA) was performed using a nonenzymatic fluorescent monoclonal anti-actin Cy3 antibody (Sigma-Aldrich). Immunohistochemistry was also performed for muscarinic receptors M2 and M3, and β-adrenergic receptor 2 using horseradish peroxidase–diaminobenzidine staining (13, 16).
Biochemical quantitation of lung collagen.
Collagen in homogenized lung extracts was measured using the Sircol Soluble Collagen Assay (Biocolor Ltd., Newtonabbey, Northern Ireland).
Western blot analysis.
Western blot analyses for M2 (Novus Biologicals, Littleton, CO), M3 (Novus Biologicals), β2 (Sigma-Aldrich) receptors, and β-tubulin (Santa Cruz, Dallas, TX) were performed as previously described (9).
Right ventricular hypertrophy assessment.
Right ventricular (RV) hypertrophy was assessed by measuring the whole heart weight, RV free wall weight, and RV/(LV + S) ratio (Fulton index), where LV is left ventricle and S is interventricular septum (17).
Statistical Analysis
Results are expressed as means (± SE). Data were analyzed by one-way ANOVA, and multiple comparison testing (Student-Newman-Keuls) was performed if statistical significance (P < 0.05) was noted by ANOVA.
Results
Effect of Neonatal Hypoxia or Hyperoxia on Adult SBP
SBP was not significantly different among adult mice exposed to air, hypoxia, or hyperoxia as neonates (SBP [mean ± SE]: air, 136 ± 6 mm Hg; hypoxia, 128 ± 4 mm Hg; hyperoxia, 121 ± 4 mm Hg; P = not significant; n = 3 per group successfully measured of 6 evaluated).
Effects of Neonatal Hypoxia or Hyperoxia Exposure on Adult Cardiac Function
Echocardiographic assessment of LV function of adult mice exposed to neonatal hyperoxia showed increased LV posterior wall thickness during systole (P < 0.02), increased relative wall thickness during systole (P < 0.02), increased interventricular septum to LV posterior wall thickness ratio during systole (P < 0.01), increased interventricular septum to LV posterior wall thickness ratio during diastole (P < 0.01), and shorter aortic valve ejection time (P < 0.02) (Table 1). No differences in LV measurements were noted in hypoxia-exposed mice compared with air-exposed mice (Table 1). No differences in RV function were noted among the groups (Table 2).
Table 1.
Left Ventricular Functions Measured by Echocardiography
| Parameters | Air (21%O2) | Hypoxia (12% O2) | P (21 vs. 12% O2) | Hyperoxia (85% O2) | P (21 vs. 85%O2) | P by ANOVA |
|---|---|---|---|---|---|---|
| LV echo dimensions | ||||||
| LVPW; diastole, mm | 0.6 ± 0.04 | 0.6 ± 0.03 | 0.95 | 0.8 ± 0.03 | 0.06 | 0.02 |
| LVPW; systole, mm | 0.9 ± 0.04 | 0.9 ± 0.02 | 0.82 | 1.1 ± 0.07 | 0.02 | 0.005 |
| RWT; diastole, mm | 0.3 ± 0.02 | 0.3 ± 0.01 | 0.81 | 0.4 ± 0.02 | 0.06 | 0.01 |
| RWT; systole, mm | 0.7 ± 0.05 | 0.6 ± 0.03 | 0.62 | 0.9 ± 0.08 | 0.02 | 0.003 |
| IS/LVPW; diastole | 1.1 ± 0.07 | 1.1 ± 0.06 | 0.7 | 0.9 ± 0.04 | 0.01 | 0.01 |
| IS/LVPW; systole | 1.2 ± 0.05 | 1.1 ± 0.03 | 0.42 | 0.9 ± 0.08 | 0.01 | 0.01 |
| AV | ||||||
| AVAc, m/s2 | 128 ± 18 | 136 ± 20 | 0.77 | 151 ± 19 | 0.4 | 0.72 |
| AVET, ms | 45.5 ± 1.9 | 41.8 ± 1.5 | 0.15 | 40 ± 0.7 | 0.02 | 0.05 |
| AVAc/AVET | 2.9 ± 0.41 | 3.4 ± 0.57 | 0.53 | 3.8 ± 0.5 | 0.2 | 0.51 |
Definition of abbreviations: AV, aortic valve; AVAc, AV acceleration; AVET, AV ejection time; LV, left ventricular; LVPW, LV posterior wall; RWT, relative wall thickness; IS, interventricular septum.
For echocardiography, the 25-MHz, single-crystal, mechanical scan head, Vevo 770 Imaging System was used (n = 10 per group; means ± SE; P versus corresponding air group).
Table 2.
Right Ventricular Function
| Parameters | Air (21% O2) | Hypoxia (12% O2) | P (21 vs. 12%O2) | Hyperoxia(85% O2) | P (21 vs. 85% O2) | P by ANOVA |
|---|---|---|---|---|---|---|
| RV echo dimensions | ||||||
| RVAW; diastole, mm | 0.3 ± 0.03 | 0.3 ± 0.02 | 0.09 | 0.3 ± 0.03 | 0.326 | 0.23 |
| RVAW; systole, mm | 1.2 ± 0.14 | 1 ± 0.16 | 0.47 | 1.2 ± 0.14 | 0.47 | 0.72 |
| RVID; diastole, mm | 1.1 ± 0.14 | 1.1 ± 0.13 | 0.65 | 1.1 ± 0.16 | 0.81 | 0.98 |
| RVID; systole, mm | 0.9 ± 0.1 | 1 ± 0.0 | 0.42 | 1.2 ± 0.1 | 0.06 | 0.1 |
| PV | ||||||
| PVAc, m/s2 | 50.3 ± 3.3 | 54.5 ± 4.7 | 0.47 | 57.4 ± 5.3 | 0.25 | 0.54 |
| PVET, ms | 45.5 ± 1 | 48.7 ± 1.2 | 0.06 | 43.8 ± 0.9 | 0.25 | 0.01* |
| PVAc/PVET | 1.3 ± 0.26 | 1 ± 0.1 | 0.29 | 1 ± 0.1 | 0.82 | 0.37 |
Definition of abbreviations: PV, pulmonary valve; PVAc, PV acceleration; PVET, PV ejection time; RV, right ventricular; RVAW; RV anterior wall; RVID, RV internal diameter.
Values presented are means ± SE; n = 10 per group; P vs. corresponding air group.
P < 0.05 for hypoxia vs. hyperoxia group.
Effects of Neonatal Hypoxia or Hyperoxia Exposure on Adult Lung Functions
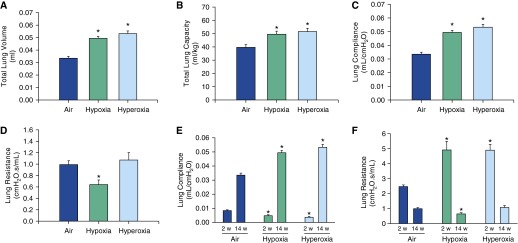
Neonatal hypoxia and hyperoxia exposure resulted in larger total lung volume (Figure 1A), larger total lung capacity (Figure 1B), and higher lung compliance (Figure 1C) in adult mice. Neonatal hypoxia also resulted in decreased total lung resistance in adults (Figure 1D). Compared with air-exposed mice, lung compliance in both hypoxia- and hyperoxia-exposed mice was lower at 2 weeks of age, but higher at 14 weeks of age (Figure 1E).
Figure 1.
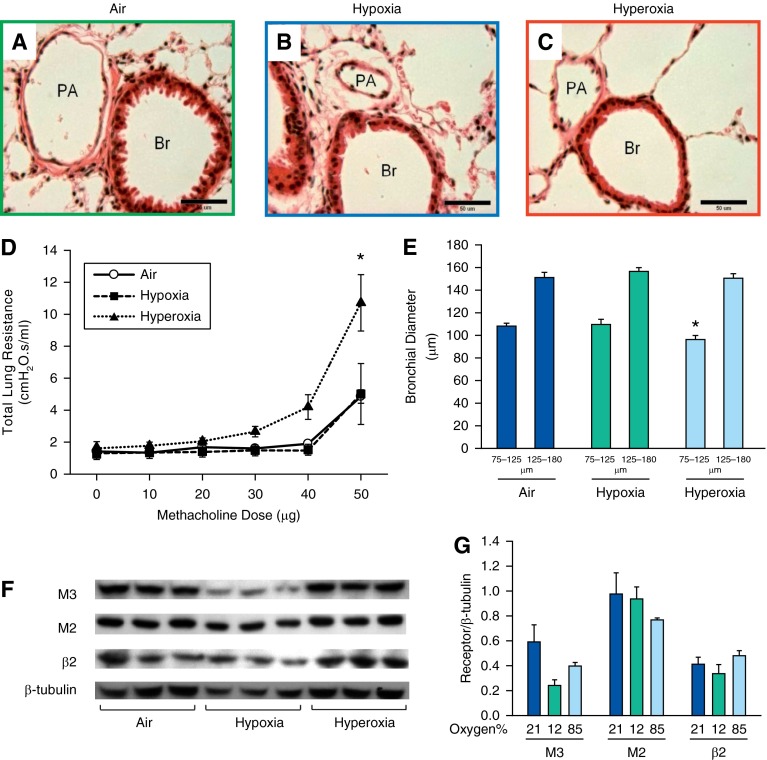
Lung function in adult mice exposed to air, hypoxia, or hyperoxia in the newborn period. Total lung volume (A), total lung capacity (B), and lung compliance (C) were increased in both hypoxia- and hyperoxia-exposed mice. Total lung resistance (D) was decreased in hypoxia-exposed mice. Compliance was lower at 2 weeks of age immediately after the exposure and higher at 14 weeks after the exposure (E) in both hypoxia- and hyperoxia-exposed groups compared with the air controls. Total lung resistance at 2 weeks after the exposure was higher in both hypoxia- and hyperoxia-exposed groups and lower in hypoxia-exposed mice 14 weeks after the exposure (F) (n = 6 per group; means ± SE). *P < 0.05 versus corresponding air group.
On the other hand, compared with the air-exposed group, total lung resistance (Figure 1F) in both hypoxia- and hyperoxia-exposed mice was significantly higher at 2 weeks of age and reduced in hypoxia-exposed (but not hyperoxia-exposed) mice at 14 weeks of age.
Effect of Neonatal Hypoxia or Hyperoxia Exposure on Adult Airway Reactivity
Adult mice exposed to neonatal hyperoxia had higher total lung resistance when challenged with increasing doses of methacholine (Figure 2D). No difference in airway reactivity was noticed between hypoxia-exposed and air-exposed mice.
Figure 2.
Airway reactivity was increased in adult mice exposed to neonatal hyperoxia. (A–C) Representative photomicrographs of hematoxylin and eosin (H&E)–stained lung sections of 14-week-old mice exposed to air (A), hypoxia (B), or hyperoxia (C) in the newborn period (400×; calibration bars, 50 μm). Administration of increasing doses of nebulized methacholine (10, 20, 30, 40, and 50 μg) showed an increased airway reactivity only in hyperoxia-exposed mice at the 50-μg dose (D) (n = 21, 7 per group; means ± SE). *P < 0.05 versus corresponding air group. Average diameter of bronchi in the size range of 75–125 μm was decreased in 14-week-old mice exposed to neonatal hyperoxia compared with air control mice (E) (n = 18, 6 per group; means ± SE). *P < 0.05 versus corresponding air group. (F and G) Representative Western blots showing no significant difference in M3, M2, and β2 receptors of lung homogenates of adult mice exposed to air, hypoxia, or hyperoxia in the newborn period (n = 6 per group). Br, bronchiole; PA, pulmonary artery.
Effects of Neonatal Hypoxia or Hyperoxia on Adult Bronchial Size
The average diameter of smaller bronchi (75–125 μm) in adult mice exposed to neonatal hyperoxia was less than in air- or hypoxia-exposed mice (Figure 2E).
Effect of neonatal hypoxia or hyperoxia exposure on lung M2, M3, and β receptors.
Immunohistochemical (IHC) staining for M2 receptors indicated that both hypoxia- and hyperoxia-exposed mice had reduced bronchial M2 staining compared with air-exposed mice (see Figure E1 in the online supplement). IHC for M3 receptor was not satisfactory. IHC of β2 in the airways did not show any differences among the groups (% of thresholded β2 receptor stained area per bronchial tissue area [mean ± SE]: air, 15 ± 1%; hypoxia, 13.9 ± 0.5%; hyperoxia, 13.3 ± 0.9%; P = not significant; n = 3). Western blot analysis of lung homogenates for β2, M2, and M3 receptors did not show any quantitative difference among the groups (Figures 2F and 2G).
Effect of Neonatal Hypoxia or Hyperoxia Exposure on Alveolar Development
Both neonatal hypoxia and hyperoxia exposure resulted in reduced RAC (Figure 3D) and increased alveolar size (MLI) (Figure 3E) at 14 weeks of age. This reduction in number and increase in size of alveoli was also noted in both hypoxia- and hyperoxia-exposed groups at the 2-week time point (Figures 3F and 3G). The number and thickness of the septa attached per bronchus were decreased in both hypoxia- and hyperoxia-exposed groups (Figures 4D and 4E).
Figure 3.
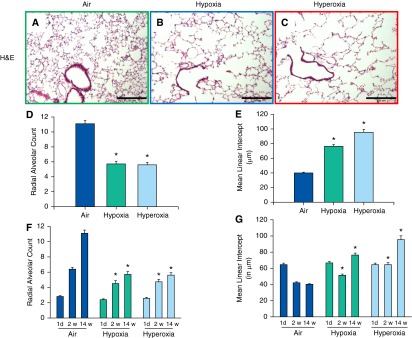
Alveolar development of adult mice exposed to air, hypoxia, or hyperoxia in the newborn period. (A–C) Representative photomicrographs of H&E–stained lung sections of 14-week-old mice exposed to air (A), hypoxia (B), or hyperoxia (C) in the newborn period (100×; calibration bars, 250 μm). Radial alveolar count, an index of alveolar number, was reduced in 14-week-old mice exposed to neonatal hypoxia or hyperoxia (D). The reduction in number of alveoli persisted from 2 to 14 weeks of age (F). Mean linear intercept, a measure of alveolar diameter, was increased in adult mice exposed to neonatal hypoxia or hyperoxia (E). The increase in the size of the alveoli persisted from 2 to 14 weeks of age (G) (n = 18 at each time point with 6 per group; means ± SE). *P < 0.05 versus corresponding air group.
Figure 4.
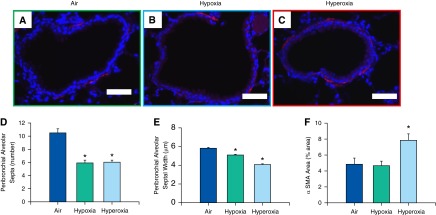
Analysis of bronchial α-smooth muscle actin (α-SMA) content and peribronchial alveolar septa assessment in adult mice exposed to air, hypoxia, or hyperoxia in the newborn period. (A–C) Representative photomicrographs of nonenzymatic fluorescent Cy-3 α-SMA–stained lung sections of adult mice exposed to air (A), hypoxia (B), or hyperoxia (C) in the newborn period (400×; calibration bars, 50 μm). Number of septa attached per bronchus (D) and their thickness (E) were reduced in both hypoxia- and hyperoxia-exposed mice (n = 18, 6 per group; means ± SE). *P < 0.05 versus corresponding air group. Percentage of total area occupied by α-SMA was increased in adult mice exposed to neonatal hyperoxia (F) (n = 6 per group; means ± SE). *P < 0.05 versus corresponding air group.
Effect of Neonatal Hypoxia or Hyperoxia Exposure on Adult Bronchial α-SMA Content
The percentage of area occupied by bronchial smooth muscle was increased in the hyperoxia-exposed group as compared with the other two groups (Figure 4F).
Effect of Neonatal Hypoxia or Hyperoxia Exposure on Adult Lung Collagen, Elastin, and Bronchial Mucin
Histologic analysis of lung sections of air-, hypoxia-, and hyperoxia-exposed mice did not show any difference among the groups in the percentage of area occupied by collagen and elastin (Figures 5D and 5E). Overall, lungs exposed to hyperoxia had reduction in total tissue area (Figures 5D and 5E). However, hyperoxia-exposed mice had higher total collagen by biochemical quantitation after adjustment for total protein compared with air- or hypoxia-exposed mice (Figure 5F). Alcian blue–stained sections did not demonstrate qualitative differences in mucin content, with minimal staining noted in airways in all three groups (data not shown).
Figure 5.
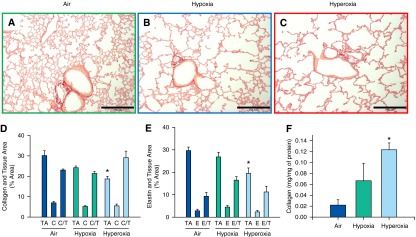
Analysis of lung collagen and elastin content of adult mice exposed to air, hypoxia, or hyperoxia in the newborn period. (A–C) Representative photomicrographs of picrosirius red–stained lung sections of adult mice exposed to air (A), hypoxia (B), or hyperoxia (C) in the newborn period (100×; calibration bars, 250 μm). (D and E) C, collagen-stained area as percentage of total image area; C/T, percentage of collagen area/percentage of tissue area; E, elastin-stained area as percentage of total image area; E/T, percentage of elastin area/percentage of tissue area; TA, tissue area as a percentage of total image area (n = 6 per group; means ± SE). *P < 0.05 versus corresponding air group. Histological quantitation did not show differences in collagen (D) or elastin content (E) among groups. Measurement of soluble collagen in lung homogenates by Sircol soluble collagen assay (F) showed increased collagen in hyperoxia-exposed mice compared with air controls (n = 6 per group). *P < 0.05 versus corresponding air group.
Effect of Neonatal Hypoxia or Hyperoxia on Adult RV Mass
Neither neonatal hypoxia nor hyperoxia exposure affected heart weight per body weight (Figure 6A), RV to LV with septum weight ratio (Figure 6B), or RV weight per body weight (Figure 6C).
Figure 6.
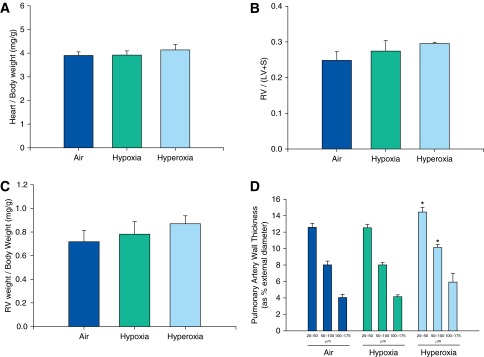
Analysis of cardiac and pulmonary arterial remodeling of adult mice exposed to air, hypoxia, or hyperoxia in the newborn period. Ratios of heart to body weight (A), RV weight per LV plus septum (S) weight (B), and RV weight per body weight (C) were not significantly different among adult mice exposed to air, hypoxia, or hyperoxia in the newborn period. Pulmonary arterial wall thickness in small arteries (20–50 μm and 50–100 μm) was increased in adult mice exposed to neonatal hyperoxia compared with air controls (D) (n = 6 per group). Data presented are means ± SE. *P < 0.05 versus corresponding air group.
Effects of Neonatal Hypoxia and Hyperoxia Exposure on Adult Pulmonary Arterial Muscularization
Neonatal hypoxia did not affect adult pulmonary arterial wall thickness compared with air-exposed mice. However, neonatal hyperoxia resulted in increased small pulmonary arterial wall thickness (arterial size, 20–50 μm and 50–100 μm) compared with air-exposed mice at 14 weeks of age (Figure 6D).
Discussion
The major findings of this study were that both neonatal hypoxia and hyperoxia exposure resulted in an emphysema phenotype in adult mice, and neonatal hyperoxia (but not hypoxia) exposure increased adult airway reactivity, and was associated with LV dysfunction. Increase in adult airway reactivity with neonatal hyperoxia exposure was also accompanied by a decrease in the size of small-caliber airways, increase in the quantity of bronchial smooth muscle, and a decrease in thickness and number of septa attached per bronchi.
Other investigators have also noted similar alveolar simplification and abnormal lung function in mice at 8 weeks of age after a shorter period (P1–P4) of oxygen exposure (18). This alveolar simplification, increased compliance, and increased resistance was associated with a loss of type II alveolar epithelial cells and minimal changes in endothelial cells, and no changes in surfactant activity (18, 19). In aged (67 wk) mice exposed to neonatal hyperoxia, abnormalities in lung compliance persisted, but alveolar simplification and increased airway resistance were no longer seen (8). However, changes in adult airway reactivity after neonatal hyperoxia have not been previously described. We found that neonatal hyperoxia increased adult airway reactivity with overall increase in baseline total lung resistance. This increase in airway reactivity was associated with an increase in bronchial α-SMA and decrease in bronchial M2 staining (but without any change in M2 content in whole-lung homogenate, perhaps because alveolar staining was not different). In addition, hyperoxia-exposed mice may also have abnormal airway stability, as evidenced by reduction in number and thickness of peribronchial alveolar septa. The reduction in average size of small airways suggests that early hyperoxia exposure may impair bronchial development. We speculate that abnormal bronchial development, increase in SMA, reduction in M2 receptors (20, 21), and poor airway stability (22) might lead to the development of adult airway hyperreactivity in our neonatal hyperoxia-exposed mice. It has been shown that hyperoxia exposure may increase airway reactivity by alterations in bronchial smooth muscle contractility induced by increases in substance P (23), impaired relaxation resulting from altered smooth muscle myosin phosphatase phosphorylation (24), cAMP-dependent mechanisms (25), or mast cell accumulation (26). These observations may be clinically relevant, as survivors of BPD demonstrate airflow reductions and bronchial hyperresponsiveness during adolescence and young adulthood (6, 27, 28). Interestingly, we noted that alveolar size continues to increase at 14 weeks in both hypoxia- and hyperoxia-exposed groups despite the increase (but at a lower rate) in RAC over time. Increase in the alveolar size in both hypoxia- and hyperoxia-exposed mice is probably because of the markedly increased lung compliance. As all lungs were inflated to the same inflating pressure, the hypoxia- and hyperoxia-exposed mouse lungs distend to a greater extent, increasing alveolar size and thereby producing a greater measured MLI in adult life as compared with at 2 weeks, despite the fact that there are more alveoli, as indicated by RAC.
We did not find any change by collagen or elastin staining, although we identified an increase in collagen quantity by the Sircol assay after adjustment for total protein in the hyperoxia-exposed mice. Infants with BPD have been noted to have larger saccules with more collagen, with thickening, tortuosity, and disorganization of collagen fibers in the saccular wall as compared with controls (29). Young adult survivors of moderate to severe BPD also have pulmonary abnormalities characteristic of emphysema (30). The histology of our mouse model is similar, with reduced alveolar septation, and less collagen in the alveolar septal tips and more condensed collagen in the saccular wall.
Neonatal hyperoxia exposure increases systemic blood pressure and impairs vasoreactivity in adult rats (31), possibly due to developmental programming of endothelial nitric oxide synthase uncoupling and enhanced vascular oxidative stress (32). Our study in mice indicates that neonatal hyperoxia results in LV dysfunction without alterations in systemic blood pressure at the 12- to 14-week time point. It is possible that this dissimilarity in response may be due to differences between species or in methods. It is possible that a longer-term follow up may indicate development of systemic hypertension in the mouse model as well. This finding is clinically important, as infants born prematurely are at higher risk of developing systemic hypertension in childhood (33), adolescence (34), and adult life (35), which may be a result of early exposure to various postnatal factors, including early hyperoxia exposure or as a complication of prematurity itself.
We have previously shown that exposure of newborn mice to hypoxia induces pulmonary arterial remodeling and RV hypertrophy at 2 weeks of age (9, 10). However, these findings were no longer evident at the 12- to 14-week time point, indicating that the early effects of hypoxia on the pulmonary vasculature are reversible. Neonatal hyperoxia exposure increased wall thickness of small pulmonary arteries in adult mice, but without any corresponding changes in RV/(LV + S) ratio, or echocardiographic evidence of pulmonary hypertension. It is possible that the thicker small arteries and the possible loss of progenitor cell capability (36) associated with alveolar simplification may lead to the development of pulmonary hypertension, in the presence, or a lower threshold, of a “second hit” (e.g., infection or hypoxia).
There are certain strengths to our study, which compared and contrasted the effects of neonatal exposures to both hypoxia and hyperoxia. Lung structure (histology) and function (including airway reactivity) were evaluated, in addition to bronchial smooth muscle, receptor quantity, and estimation of lung collagen, elastin, and α-SMA. However, our study also has certain limitations. Although newborn mouse lungs are structurally similar (saccular stage) to preterm infant lungs, there are interspecies differences. For example, mouse lungs at birth are able to provide sufficient gas exchange and have adequate surfactant activity compared with a preterm human lung at a similar developmental stage, which usually demonstrates respiratory distress syndrome. In the clinical setting, low- and high-oxygen exposures are intermittent and not a constant exposure, as in our animal model. However, fetal lung development in utero takes place in a relatively hypoxemic (low PaO2) environment with no direct alveolar oxygen exposure. Therefore, postnatal lung development in preterm infants occurs in the setting of a constant alveolar hyperoxic (high PaO2) and hyperoxemic (high PaO2) environment compared with that in utero, even in infants who are maintained in ambient air without supplemental oxygen exposure. To achieve targeted oxygen saturations, preterm infants often require fractional inspired oxygen concentration higher than room air. In addition, regions of developing premature lung may experience alveolar hypoxia (resulting from inadequate ventilation secondary to either lung disease or prematurity) with or without regional hypoxemia (secondary to intrapulmonary shunting within poorly ventilated lung). This relative hyperoxia or hypoxia in preterm infants may persist throughout infancy, especially in infants with moderate to severe BPD who are often exposed to prolonged supplemental oxygen and have episodes of hypoxemia. Our mouse model with exposure of mice until P14 corresponds to exposure in human infants until 1–2 years of age (37). Although alterations in oxygen concentration (hypoxia or hyperoxia) are often used as good and reproducible animal models for BPD, they do not simulate BPD pathogenesis accurately, as there is no additional insult, such as infection or ventilation-induced lung injury.
There are limitations in using the mouse model of airway reactivity (38, 39). There are dissimilarities between mouse and human airway anatomy, such as fewer airway generations, lack of airway submucosal glands, paucity of smooth muscle beyond the first bronchial generation, and different airway smooth muscle phenotype in mice (39, 40). These species differences may limit the generalizability of our results to humans. The specific role of oxidative stress in modulating airway reactivity was not assessed in our study. However, we have previously demonstrated increased oxidative stress in lung homogenates after neonatal hyperoxia exposure (41).
In addition, although we evaluated mice using echocardiography, tail cuff pressures, and pulmonary vascular morphometry, invasive pressure monitoring was not performed, as it is technically challenging and subject to many sources of variation. Additional longer-term follow-up and histological analysis of the heart and major vessels is required to confirm our findings of LV dysfunction after hyperoxia exposure.
In conclusion, neonatal exposure to hypoxia and hyperoxia induce alveolar simplification and abnormal lung function that persists to adult life in mice. In addition, exposure to neonatal hyperoxia (but not hypoxia) also leads to increased airway reactivity, LV dysfunction, and thickening of small pulmonary arteries of a magnitude not sufficient to result in pulmonary hypertension. Some of these findings simulate aspects of longer-term sequelae of extremely premature infants. The mouse model of neonatal hyperoxia exposure may be useful to determine the underlying mechanisms of these effects and to test potential therapeutic strategies.
Footnotes
This work was supported by a Dixon Fellowship, an Ikaria fellowship grant, National Institutes of Health grants R01 HL092906 and R01 HD66982, and the Translational Research in Normal and Disordered Development Program.
Author Contributions: conception and design—M.R. and N.A.; analysis and interpretation—M.R., N.A., W.E.B., and L.J.D.’I.; drafting the manuscript for important intellectual content—M.R., N.A., W.E.B., and L.J.D.’I.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0491OC on September 25, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Behrman RE, Butler AS.Preterm birth: causes, consequences, and prevention Washington, DC: National Academies Press2007 [PubMed] [Google Scholar]
- 2.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants Am J Obstet Gynecol 2007196147.e1–8 [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Carlo WA. Bronchopulmonary dysplasia: new insights. Clin Perinatol. 2004;31:613–628. doi: 10.1016/j.clp.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the epicure study. Am J Respir Crit Care Med. 2010;182:237–245. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 6.Halvorsen T, Skadberg BT, Eide GE, Roksund OD, Carlsen KH, Bakke P. Pulmonary outcome in adolescents of extreme preterm birth: a regional cohort study. Acta Paediatr. 2004;93:1294–1300. [PubMed] [Google Scholar]
- 7.Massaro D, Massaro GD. Invited review: pulmonary alveoli: formation, the “call for oxygen,” and other regulators. Am J Physiol Lung Cell Mol Physiol. 2002;282:L345–L358. doi: 10.1152/ajplung.00374.2001. [DOI] [PubMed] [Google Scholar]
- 8.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O'Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178:2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambalavanan N, Nicola T, Hagood J, Bulger A, Serra R, Murphy-Ullrich J, Oparil S, Chen YF. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L86–L95. doi: 10.1152/ajplung.00534.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res. 2005;57:631–636. doi: 10.1203/01.PDR.0000159512.55862.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambalavanan N, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-1 mediates hypoxia-induced increases in vascular collagen in the newborn mouse lung. Pediatr Res. 2007;61:559–564. doi: 10.1203/pdr.0b013e318045beae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James ML, Ross AC, Bulger A, Philips JB, III, Ambalavanan N. Vitamin A and retinoic acid act synergistically to increase lung retinyl esters during normoxia and reduce hyperoxic lung injury in newborn mice. Pediatr Res. 2010;67:591–597. doi: 10.1203/PDR.0b013e3181dbac3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicola T, Hagood JS, James ML, Macewen MW, Williams TA, Hewitt MM, Schwiebert L, Bulger A, Oparil S, Chen YF, et al. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol. 2009;296:L738–L750. doi: 10.1152/ajplung.90603.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGovern TK, Robichaud A, Fereydoonzad L, Schuessler TF, Martin JG. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp. 2013;(75):e50172. doi: 10.3791/50172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang BH, Maruyama J, Yokochi A, Iwasaki M, Amano H, Mitani Y, Maruyama K. Prolonged nitric oxide inhalation fails to regress hypoxic vascular remodeling in rat lung. Chest. 2004;125:2247–2252. doi: 10.1378/chest.125.6.2247. [DOI] [PubMed] [Google Scholar]
- 16.Ambalavanan N, Nicola T, Li P, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res. 2008;63:26–32. doi: 10.1203/PDR.0b013e31815b690d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. Br Heart J. 1952;14:413–420. doi: 10.1136/hrt.14.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yee M, Chess PR, McGrath-Morrow SA, Wang Z, Gelein R, Zhou R, Dean DA, Notter RH, O'Reilly MA. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. Am J Physiol Lung Cell Mol Physiol. 2009;297:L641–L649. doi: 10.1152/ajplung.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1101–L1111. doi: 10.1152/ajplung.00126.2006. [DOI] [PubMed] [Google Scholar]
- 20.Fryer AD, Jacoby DB. Muscarinic receptors and control of airway smooth muscle. Am J Respir Crit Care Med. 1998;158:S154–S160. doi: 10.1164/ajrccm.158.supplement_2.13tac120. [DOI] [PubMed] [Google Scholar]
- 21.Coulson FR, Fryer AD. Muscarinic acetylcholine receptors and airway diseases. Pharmacol Ther. 2003;98:59–69. doi: 10.1016/s0163-7258(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 22.Bates JH, Rincon M, Irvin CG. Animal models of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;297:L401–L410. doi: 10.1152/ajplung.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agani FH, Kuo NT, Chang CH, Dreshaj IA, Farver CF, Krause JE, Ernsberger P, Haxhiu MA, Martin RJ. Effect of hyperoxia on substance p expression and airway reactivity in the developing lung. Am J Physiol. 1997;273:L40–L45. doi: 10.1152/ajplung.1997.273.1.L40. [DOI] [PubMed] [Google Scholar]
- 24.Smith PG, Dreshaj A, Chaudhuri S, Onder BM, Mhanna MJ, Martin RJ. Hyperoxic conditions inhibit airway smooth muscle myosin phosphatase in rat pups. Am J Physiol Lung Cell Mol Physiol. 2007;292:L68–L73. doi: 10.1152/ajplung.00460.2005. [DOI] [PubMed] [Google Scholar]
- 25.Mhanna MJ, Haxhiu MA, Jaber MA, Walenga RW, Chang CH, Liu S, Martin RJ. Hyperoxia impairs airway relaxation in immature rats via a cAMP-mediated mechanism. J Appl Physiol. 2004;96:1854–1860. doi: 10.1152/japplphysiol.01178.2002. [DOI] [PubMed] [Google Scholar]
- 26.Schultz ED, Potts EN, Mason SN, Foster WM, Auten RL. Mast cells mediate hyperoxia-induced airway hyper-reactivity in newborn rats. Pediatr Res. 2010;68:70–74. doi: 10.1203/PDR.0b013e3181e0cd97. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118:108–113. doi: 10.1542/peds.2005-2522. [DOI] [PubMed] [Google Scholar]
- 28.Gough A, Linden M, Spence D, Patterson CC, Halliday HL, McGarvey LP. Impaired lung function and health status in adult survivors of bronchopulmonary dysplasia. Eur Respir J. 2013;43:808–816. doi: 10.1183/09031936.00039513. [DOI] [PubMed] [Google Scholar]
- 29.Thibeault DW, Mabry SM, Ekekezie II, Zhang X, Truog WE. Collagen scaffolding during development and its deformation with chronic lung disease. Pediatrics. 2003;111:766–776. doi: 10.1542/peds.111.4.766. [DOI] [PubMed] [Google Scholar]
- 30.Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32:321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 31.Yzydorczyk C, Comte B, Cambonie G, Lavoie JC, Germain N, Ting Shun Y, Wolff J, Deschepper C, Touyz RM, Lelievre-Pegorier M, et al. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension. 2008;52:889–895. doi: 10.1161/HYPERTENSIONAHA.108.116251. [DOI] [PubMed] [Google Scholar]
- 32.Yzydorczyk C, Comte B, Huyard F, Cloutier A, Germain N, Bertagnolli M, Nuyt AM. Developmental programming of eNOS uncoupling and enhanced vascular oxidative stress in adult rats after transient neonatal oxygen exposure. J Cardiovasc Pharmacol. 2013;61:8–16. doi: 10.1097/FJC.0b013e318274d1c4. [DOI] [PubMed] [Google Scholar]
- 33.Bonamy AK, Kallen K, Norman M. High blood pressure in 2.5-year-old children born extremely preterm. Pediatrics. 2012;129:e1199–e1204. doi: 10.1542/peds.2011-3177. [DOI] [PubMed] [Google Scholar]
- 34.Doyle LW, Faber B, Callanan C, Morley R. Blood pressure in late adolescence and very low birth weight. Pediatrics. 2003;111:252–257. doi: 10.1542/peds.111.2.252. [DOI] [PubMed] [Google Scholar]
- 35.Hack M, Schluchter M, Cartar L, Rahman M. Blood pressure among very low birth weight (<1.5 kg) young adults. Pediatr Res. 2005;58:677–684. doi: 10.1203/01.PDR.0000180551.93470.56. [DOI] [PubMed] [Google Scholar]
- 36.Yee M, Buczynski BW, O'Reilly MA. Neonatal hyperoxia stimulates the expansion of alveolar epithelial type II cells. Am J Respir Cell Mol Biol. 2014;50:757–766. doi: 10.1165/rcmb.2013-0207OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thurlbeck WM. Postnatal growth and development of the lung. Am Rev Respir Dis. 1975;111:803–844. doi: 10.1164/arrd.1975.111.6.803. [DOI] [PubMed] [Google Scholar]
- 38.Kumar RK, Foster PS. Modeling allergic asthma in mice: pitfalls and opportunities. Am J Respir Cell Mol Biol. 2002;27:267–272. doi: 10.1165/rcmb.F248. [DOI] [PubMed] [Google Scholar]
- 39.Wenzel S, Holgate ST. The mouse trap: it still yields few answers in asthma. Am J Respir Crit Care Med. 2006;174:1173–1176. doi: 10.1164/rccm.2609002. discussion 1176–1178. [DOI] [PubMed] [Google Scholar]
- 40.Wagers S, Lundblad LK, Ekman M, Irvin CG, Bates JH. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol. 2004;96:2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 41.James ML, Ross AC, Nicola T, Steele C, Ambalavanan N. VARA attenuates hyperoxia-induced impaired alveolar development and lung function in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2013;304:L803–L812. doi: 10.1152/ajplung.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]