Significance
The brain is never at rest, and patterns of ongoing correlated activity have been found to resemble patterns during active behavior. A fundamental problem in neuroscience concerns the relationship between spontaneous and task-driven activity. During a demanding task that requires selective attention to sensory stimuli, correlated patterns of spontaneous (rest) activity are generally preserved. However, specific changes in synchronization occur within and between networks that correlate with behavioral performance. These results indicate that attention modifies spontaneous activity patterns in support of task performance.
Keywords: functional connectivity, directional connectivity, resting-state networks, attention networks, task-evoked activity
Abstract
Fundamental problems in neuroscience today are understanding how patterns of ongoing spontaneous activity are modified by task performance and whether/how these intrinsic patterns influence task-evoked activation and behavior. We examined these questions by comparing instantaneous functional connectivity (IFC) and directed functional connectivity (DFC) changes in two networks that are strongly correlated and segregated at rest: the visual (VIS) network and the dorsal attention network (DAN). We measured how IFC and DFC during a visuospatial attention task, which requires dynamic selective rerouting of visual information across hemispheres, changed with respect to rest. During the attention task, the two networks remained relatively segregated, and their general pattern of within-network correlation was maintained. However, attention induced a decrease of correlation in the VIS network and an increase of the DAN→VIS IFC and DFC, especially in a top-down direction. In contrast, within the DAN, IFC was not modified by attention, whereas DFC was enhanced. Importantly, IFC modulations were behaviorally relevant. We conclude that a stable backbone of within-network functional connectivity topography remains in place when transitioning between resting wakefulness and attention selection. However, relative decrease of correlation of ongoing “idling” activity in visual cortex and synchronization between frontoparietal and visual cortex were behaviorally relevant, indicating that modulations of resting activity patterns are important for task performance. Higher order resting connectivity in the DAN was relatively unaffected during attention, potentially indicating a role for simultaneous ongoing activity as a “prior” for attention selection.
The function of the brain has been traditionally studied in response to controlled stimuli at the level of single neurons, cortical circuits, or systems, and spontaneous activity has been modeled as stochastic noise, with its variability randomly affecting the threshold of postsynaptic firing (hence, the forward transmission of information through cortical circuits) (1). However, in the last two decades, it has become apparent that spontaneous activity is far from random but organized in space and time at the level of micro- and macrocircuitries (2) as well as at the level of large-scale distributed neuroanatomical systems (3). The large-scale organization of spontaneous activity has been most effectively studied by computing the temporal correlation of the blood oxygenation level-dependent (BOLD) signal (or functional connectivity) measured at rest with functional magnetic resonance imaging (fMRI) in the absence of any task or stimulus. The whole cerebral cortex has been subdivided in a relatively small number of networks formed by regions that show correlated activity over long periods of time [resting-state networks (RSNs)] (4, 5). The relatively small number of RSNs raises the question of how these networks can support the presumably very large number of sensory–motor–cognitive states that form our behavior, which undoubtedly must require the dynamic and flexible coordination of brain regions.
One leading idea is that RSNs represent spatiotemporal “priors” for task networks and that their modulation contributes to task-evoked responses (6, 7). According to this view, the connectivity at rest reflects experience-dependent plasticity that constrains subsequent activity during stimulus processing and maintains predictions about forthcoming stimuli. Another hypothesis considers RSNs as reflecting a state of “idling” (or inactivity) of the brain that must be reorganized for task-dependent interactions to emerge (8). The former view is supported by the stability of RSNs topography across behavioral states (9, 10) and the similarity of RSNs to task networks recruited by common cognitive tasks (7, 11). The latter view is, instead, supported by studies showing that task execution reconfigures resting connectivity to allow task-dependent interactions (12, 13).
To address this fundamental question, we examined how resting functional connectivity is modulated during the execution of a spatial attention task with underlying circuitry that is well-understood (14–19). The task involves either maintaining attention to a stream of sensory stimuli or shifting attention to a different stream simultaneously presented in the opposite visual field. After each attention shift, visual information must be then dynamically rerouted from one visual field/hemisphere to the other. Task activation studies have shown that this task recruits both frontal and parietal control regions of the dorsal attention network (DAN) and occipital visual (VIS) network regions involved in sensory processing, but their dynamic interaction has never been studied. Critically, in a state of idle wakefulness (visual fixation), regions in the DAN and VIS are largely segregated (i.e., their within-network temporal correlation is stronger than their between-network correlation) (4, 5). Therefore, these networks represent an ideal system for examining the questions of how RSNs are modified by task performance and specifically, how functional connections are dynamically modulated when transitioning from a resting to an attentive state. If RSNs represent priors of task networks, then performing the attention task should maintain and even strengthen RSNs interaction. If, however, RSNs represent idling cortical rhythms, then task performance should induce a reorganization of functional connectivity patterns in a task- and behavior-dependent manner.
Results
Paradigm and Behavioral Results.
We recorded BOLD activity in 21 healthy young subjects during simple fixation (rest) and a continuous visuospatial attention task (17, 18). In this task (Materials and Methods and Fig. 1A), participants maintained central fixation while covertly directing attention to one of two peripheral moving gratings to discriminate occasional targets (i.e., a brief clockwise or counterclockwise orientation change). The attended location was randomly indicated by a color change of one grating, indicating to either maintain attention to the same location or shift attention to the location in the opposite field. To control for differences related to sensory stimulation, both peripheral gratings changed color briefly, with the relevant color instructed at the beginning of each block (Fig. 1A). As expected, target discrimination accuracy was higher at the attended vs. unattended locations (Fig. S1A). This difference was confirmed by a two-way ANOVA with target validity (valid and invalid) and location (left and right) as factors that revealed a significant effect of target validity (F1,20 = 42.74; P < 0.0001) but no effect of target location (F1,20 = 0.86; P = n.s.) or interaction between the two factors (F2,40 = 0.49; P = n.s.). Eye movement analyses confirmed that subjects were accurate in maintaining fixation on the central cross (SI Materials and Methods).
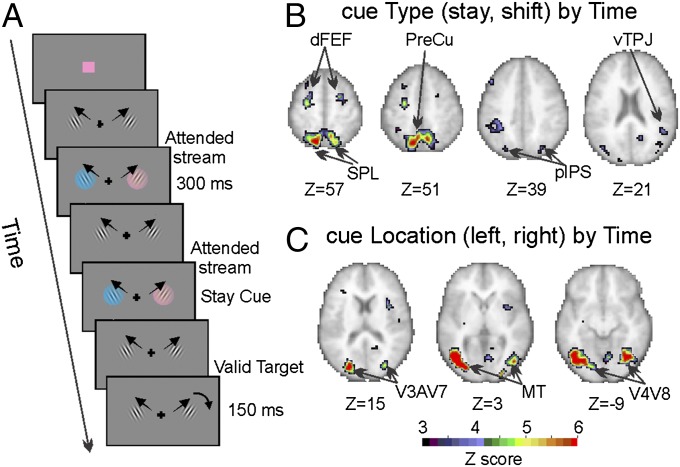
Fig. 1.
Paradigm and ROIs selection. (A) Example of the display sequence in the visuospatial attention task. (B) Voxels showing significantly groupwise different fMRI activation after shift vs. stay cues (cue type by time map) and (C) regions exhibiting significantly different fMRI activation after left vs. right cues (cue location by time map).
Selection of the Regions of Interest for Connectivity Analyses.
Frontoparietal (DAN) and occipital visual (VIS) regions were selected based on their response profile during the attention task by means of a whole-brain voxelwise repeated measures ANOVA with cue type (shift and stay), cue location (right and left), and time [seven magnetic resonance frames (MR frames)] as factors (16). Stronger cue-related responses for shift vs. stay cues, regardless of cue location, were identified in core regions of the DAN (20): the superior parietal lobule (SPL), the dorsal aspect of the human frontal eye fields (dFEF), and the posterior intraparietal sulcus (pIPS) (Fig. 1B). A representative BOLD signal time course from right- (R-) SPL (with stronger response to shift than stay cues and no visual field differences) is shown in Fig. S1B. Importantly, this response reflects a control signal to shift or maintain attention as the sensory change induced by the cue stimuli was matched across the two locations. Robust shift-related activity was also observed in the precuneus (PreCu) and as expected (16, 21) in the right ventral temporoparietal junction (vTPJ), a node of the so-called ventral attention network (VAN) involved in attention reorienting (20).
The same ANOVA also identified regions that responded in a spatially selective manner independent of cue type. A strong preference for cue stimuli presented in the contralateral hemifield was identified in extrastriate visual cortex and additional frontoparietal regions (Fig. 1C). Importantly, this response reflects not only the response to the cue but also, the spatially selective modulation to the stream of moving gratings, which were matched sensorialy across the visual fields (Fig. 1A). Peaks of spatially selective activity in occipital visual cortex were identified bilaterally in the ventral (corresponding to visual V4–V8), dorsal (corresponding to V3a–V7), and lateral occipital cortex (corresponding to the human middle temporal visual area, MT). A representative example of the spatially selective (contra > ipsi) BOLD response is shown from left- (L-) V4–V8 (Fig. S1C). In addition, as expected from previous studies (16), the same contrast highlighted spatially selective regions of the DAN (SI Results and Fig. S2).
DAN regions showing greater responses to shift than stay cues and visual regions showing robust spatially selective responses were selected for the assessment of task-induced modulations of instantaneous functional connectivity (IFC) and directed functional connectivity (DFC). To avoid spurious correlations induced by event-related activity, modulations were examined after removing the mean BOLD response induced by cue and target stimuli, including the associated motor response (22), using linear regression (SI Materials and Methods and Fig. S3). Finally, because spatially selective visual responses were obtained primarily in intermediate regions of the visual hierarchy (V3a–V7, V4–V8, and MT), we also evaluated connectivity modulations in early visual areas (V1–V3) using a functional atlas of human visuotopic regions (SI Results and Fig. S4).
Task-Induced Modulation of IFC.
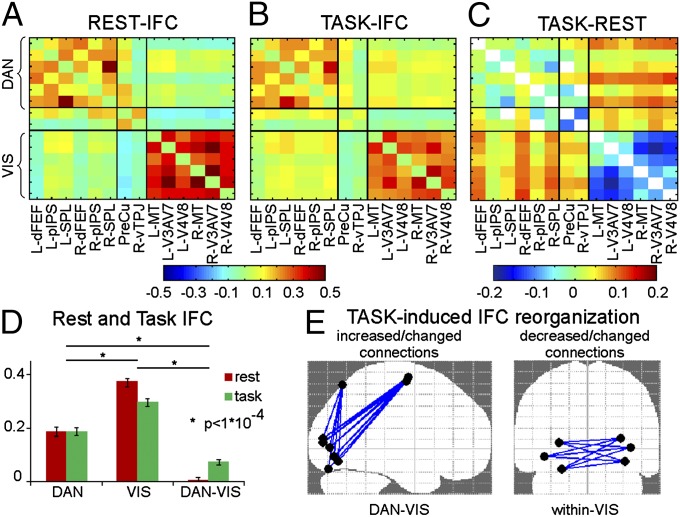
Previous studies have shown that DAN and VIS networks are segregated in the resting state (4, 5) and that their topology is maintained during task (11). Here, we first tested whether network segregation is maintained during task execution by computing a region of interest (ROI) to ROI instantaneous correlation (z Fisher-transformed, Materials and Methods) during rest (Fig. 2A) and task (Fig. 2B, while Fig. 2C shows pairwise task-induced modulations of IFC, and SI Results shows pairwise statistical comparisons). We next grouped the correlation coefficients according to three submatrices DAN, VIS, and between DAN–VIS (based on their anatomical and functional profile, vTPJ and PreCu were treated as part of a different network, VAN) and applied a two-way ANOVA with condition (rest and task) and network (within DAN, within VIS, and between DAN–VIS) as factors. The results indicate a significant main effect of network (F2,40 = 152.2; P < 5 × 10−8), no significant main effect of condition (F1,20 = 0.1; P = n.s.), and a significant network by condition interaction (F2,40 = 30.8; P < 5 × 10−8). The interaction was explained by a task-induced decrease of IFC within the VIS network (P < 1 × 10−4) and an increase of the DAN–VIS between-network IFC (P < 1 × 10−4). Crucially, despite these task-induced modulations, posthoc tests showed greater within- than between-network correlation both at rest and during task (all P values < 1 × 10−4) (Fig. 2D), hence confirming the resilience of network segregation.
Fig. 2.
IFC results. IFC for each ROI pair during (A) rest and (B) task execution as obtained from the average across voxel pairs and subjects of the z Fisher-transformed Pearson correlation coefficients. (C) Pairwise task-induced modulation of IFC, in which the diagonal and the elements that do not change significantly during task with respect to fixation are represented in white. (D) The average IFCs within and between networks during rest and task are shown as a bar plot together with the ANOVA results. (E) Significant task-induced changes in graph components are displayed on sagittal and coronal planes.
We next tested whether task execution induced a change in the topology of the within-network correlation patterns by examining the similarity of the correlation matrices using the Mantel test (SI Materials and Methods). Results indicated a significant maintenance of the within-network spatial pattern (19 and 18 of 21 subjects for DAN and VIS, respectively; P values < 5 × 10−2, Bonferroni corrected), suggesting that the overall topology of the within-network connectivity was preserved.
We next tested whether the network by condition ANOVA interaction reflected a network reorganization using an approach based on graph theory. Specifically, the individual correlation matrices at rest and during task were first converted into graphs and then analyzed by applying the Network-Based Statistics toolbox (23) (Materials and Methods). The results indicated a significant task-induced increase of connectivity between DAN and VIS (including dFEF, R-SPL, and all regions of the VIS independent of hemisphere) and a significant decrease of interhemispheric connectivity within the VIS (e.g., left and right V3a–V7) (Fig. 2E). Interestingly, the task did not affect any of the graph components representing within DAN connectivity. Finally, the Brain Connectivity Toolbox (24) (Materials and Methods) was used to compare graph modularity across conditions. Despite significant graph modularity was present in each condition (tests against associated random graphs, P < 0.01), the results indicate a significant reduction of modularity from rest to task (P < 1 × 10−6).
In summary, although network segregation and topology of within-network IFC were largely maintained during spatial attention, task execution induced a reduction of graph modularity and a reorganization of the IFC patterns, with a decrease of the within VIS internal correlation and an increase of the VIS–DAN between-network correlation. Interestingly, the strength and topology of DAN IFC did not change.
Behavioral Relevance of Task-Induced Modulation.
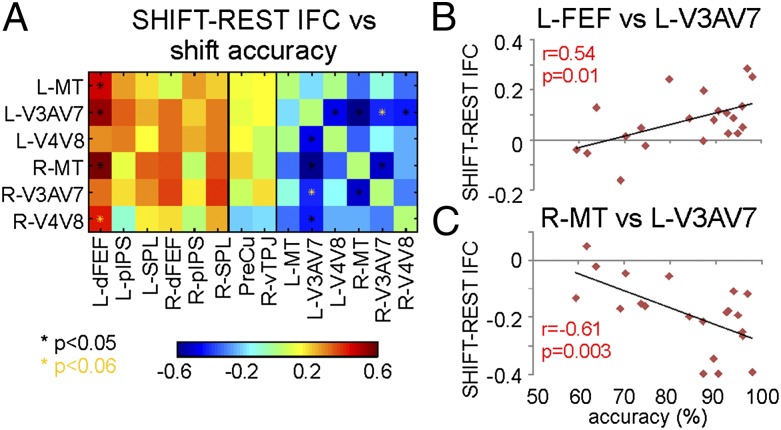
If task performance requires a dynamic reorganization of functional connections between task-relevant regions, then IFC modulations may be related to behavioral performance. This relationship was examined by correlating changes of within-/between-network coupling with measures of target discrimination accuracy. The analysis was performed either within or between networks and on ROI pairs (Fig. S5) (e.g., L-dFEF vs. R-MT) that were modulated by the attention task. A positive correlation [mean r = 0.5; P < 0.05, corrected via false discovery rate (FDR)] was found between discrimination accuracy and DAN–VIS between-network connectivity, such that stronger coupling was associated with better performance across subjects. This result was strongest for L-dFEF whose connectivity with nearly all visual ROIs showed a significant relationship with behavior. Decrements of IFC (task–rest) between specific visual ROIs (e.g., L-V3a–V7 and R-MT) were also correlated with accuracy (Fig. S5).
The presence of multiple trials in which the same cue stimulus was presented (e.g., consecutive shift cues) further allowed for a nonstationary analysis of connectivity (Materials and Methods), in which we examined the association between performance and modulation of IFC for specific attention processes (stay, shift). Overall, we found a strong correlation between connectivity modulations and discrimination accuracy during extended periods of shift (Fig. 3A). This association was true both for positive (DAN–VIS: mean r = 0.5; P < 0.05, FDR corrected) and negative (within VIS: mean r = −0.5; P < 0.05, FDR corrected) correlations between IFC changes and discrimination accuracy. Again, IFC increments between left dFEF and multiple visual areas were associated with improved accuracy during shift cues (Fig. 3 A and B), and decreased IFC between left V3a–V7 and multiple left and right visual field areas (R-V4–V8, R-V3a–V7, R-MT, and L-V4–V8) also correlated with improved accuracy (Fig. 3 A and C). Finally, no significant correlation was found between connectivity changes in PreCu and vTPJ and discrimination accuracy.
Fig. 3.
Behavioral relevance of IFC modulations. (A) Correlation between discrimination accuracy and IFC changes during shift of attention. Because no significant task-induced within-network modulations were observed, correlation is not shown for the DAN. *Significant correlations. Scatter plot of shift-specific changes of connectivity as a function of discrimination accuracy for (B) L-dFEF vs. L-V3a–V7 and (C) R-MT vs. L-V3a–V7.
In summary, both task-induced IFC increases of between networks (DAN–VIS), especially between left dFEF and multiple visual areas, and IFC decreases within the VIS, especially to/from left V3a–V7, were relevant to behavior when the task called for a shift of attention from one to the other stream of stimuli across hemifields. This result is consistent with the idea that changes of functional connectivity are related to rerouting of visual information from one visual field/hemisphere to the other.
Task-Induced Increase of Top-Down Directional Influence.
Previous studies have shown that activity in DAN regions (IPS/FEF) predicts activity in visual cortex during a spatial cue preparatory period (15) and that interference with IPS and FEF preparatory activity has a disruptive effect on synchronization of α-rhythms in visual occipital regions (25). Hence, it is of interest to consider changes of resting-state directional interactions between DAN and VIS regions induced by the attention task.
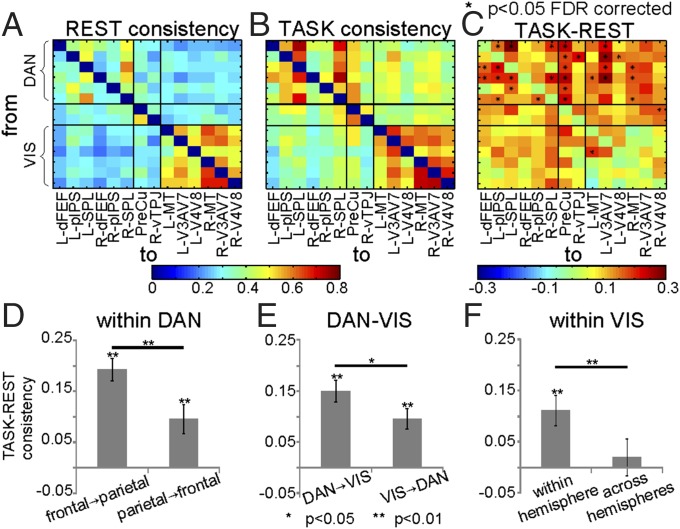
DFC was assessed using Granger causality analysis (Materials and Methods and Fig. S3). For each ROI pair, the degree of directional influence was measured in both directions by the portion of voxel pairs with significant F statistics (15). The mean across subjects of this quantity is shown in Fig. 4 A and B for rest and task periods, respectively. At rest, a modest degree of directed interaction was observed within each network, particularly within the visual network, but not between the networks. This result is consistent with the segregation highlighted by IFC.
Fig. 4.
DFC results. Average consistencies during (A) rest and (B) task execution. (C) Task-induced modulations of Granger consistencies, in which significant values, evaluated with two-sample t tests, are indicated with an asterisk. For each ROI pair, color represents portions of voxel pairs with significant F statistic, and row causes column. Task-induced modulations of average consistency (D) within DAN, (E) between DAN–VIS, and (F) within VIS are shown as bar plots, in which error bars represent the SE. Significant differences are evaluated by means of paired two-sample t tests.
Task execution partially preserved the within-network DFC topology [Mantel test applied to each subject (17 of 21 in the DAN and to a lesser extent in the VIS, 9 of 21); P values < 5 × 10−2, Bonferroni corrected]. Task execution, however, was also associated with a general increase of DFC in both networks and between the networks (Fig. 4B). To identify those ROI pairs showing a significant task-induced modulation of DFC, we directly compared measures of directionality between rest and task using two-sample t tests across subjects (P = 0.05, FDR corrected) (Fig. 4C). DFC increases were observed between DAN regions and between DAN and VIS regions. Bilateral dFEF and SPL were the main sources of directional influence, whereas V3a–V7 in visual cortex was the main receiver of directional influences from DAN regions. The PreCu region was also a strong receiver.
To quantify the strength of DFC interaction in each direction, we performed separate statistical analyses on the DAN, DAN–VIS, and VIS regions (Fig. 4 D–F) on the task–rest consistency matrix (Fig. 4C). For instance, in the DAN, consistency changes were averaged over pairs of ROIs separately for each direction (frontal→parietal and parietal→frontal) and then, compared with t tests. The results revealed a significant DFC consistency in both directions during task compared with rest (t test vs. 0; P < 0.01), although it was stronger from frontal to parietal regions (paired two-sample t test; P < 0.01) (Fig. 4D). Next, we assessed the amount of directed influences between frontoparietal (DAN) and visual regions. During tasks, DFC consistency increased in both directions (P < 0.05) but was significantly stronger in the DAN→VIS direction (Fig. 4E). Finally, DFC between visual occipital regions of the same hemisphere was significantly increased (P < 0.01) and was stronger than DFC across hemispheres (paired two-sample t test; P < 1 × 10−5), which in turn, was not different from rest (Fig. 4F).
In summary, task execution did not substantially modify the within-network DFC topology but produced a general increase of DFC within the DAN (especially in a frontal to parietal direction), from DAN to VIS (especially from dFEF and SPL to V3a–V7), and between intrahemispheric regions of the VIS network.
Relationship Between Task-Induced IFC and DFC Modulations.
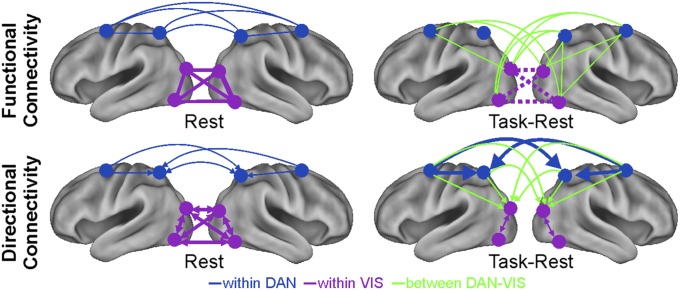
Fig. 5 provides an illustration of the main resting-state connections (Fig. 5, Left) and the significant task-induced changes of IFC and DFC (Fig. 5, Right). The main feature of IFC/DFC at rest is the presence of interactions between regions that belong to the same network (either DAN or VIS). The two networks are clearly segregated in line with previous work (4, 5). The most striking task-induced changes are the uncoupling of the visual network, with a decrement of the interhemispheric IFC, and a stronger coupling between networks, which was indexed by an increase of both IFC and top-down (DAN to VIS) DFC. Notably, the functional organization of the DAN, which was indexed by IFC and DFC, is modulated but not disrupted by the attention task. The only significant modulation is an increase in the strength of directional influences between frontal to parietal (especially the SPL hub) regions, which are, however, already present at rest. The overall impression from inspection of Fig. 5 is that VIS and DAN networks show profoundly different modulations of connectivity between rest and task states.
Fig. 5.
Summary results of IFC and DFC analyses. Main resting-state (Upper Left) IFC and (Lower Left) DFC and significant (Upper Right) IFC and (Lower Right) DFC task-induced modulations are represented as lines and arrows on standard brains with two levels of thickness (e.g., at rest, within VIS was higher than within DAN IFC). Different colors are used to represent within DAN, within VIS, and between DAN–VIS connectivity, and dotted lines are used to distinguish decreases from connectivity increases.
Discussion
We investigated the adjustments in IFC and DFC within and between the DAN and the VIS networks when going from rest to performing a demanding visuospatial attention task. The attention task was designed to induce dynamic rerouting of selected visual information across hemispheres. Our results show that, although dorsal attention and visual networks remain segregated, fundamental aspects of their functional organization and interaction are profoundly altered by the attention task. Task-induced modulations of connectivity are quite different for the two networks, indicating that patterns of ongoing activity contribute differently to task-evoked activity patterns.
From Rest to Attention: Maintenance of RSN Topography.
Our results indicate that the functional architecture of the VIS and DAN (i.e., segregation and topology) was relatively preserved in the transition between rest and attention. These results are consistent with the relative invariance of RSNs to behavioral state (9, 10), anesthesia (26, 27), and sleep (28); the common topography of RSN and task networks across many behaviors (7, 11); and neurophysiological evidence indicating that stimulus contributes modestly to the overall level of activity within visual cortex, which instead, is strongly modulated by internal fluctuations of activity (29–31). These findings have been taken to indicate that RSNs represent either spatial or temporal priors for task-evoked activity (6). By spatial prior, it is meant that connections that are synchronized during task performance maintain, even at rest, a high level of coherence. This assumption is based on the principle that RSN topography is shaped and determined by the history of regional coactivation in the course of development and experience (32). By temporal prior, slow fluctuations of spontaneous activity may time the excitability of cortical circuitries during task performance (33, 34).
However, when considering the significant and behaviorally relevant alterations in IFC and DFC generated by the attention task, an alternative interpretation is that the relative preservation of RSN structure reflects the relative paucity of connections recruited by any given task. If neural correlations at rest represent overall brain homeostasis, including connectional memory of prior patterns of activity, it is not surprising that this long-term memory functional architecture, representing not just the environment but also, the body and cognition, would not be altered much by performing a specific, even highly demanding task. In fact, any task represents by itself just a small fraction of the possible behavioral states that the brain can represent and the problem gets even worse when one considers the putatively different cognitive tasks that can be performed in a limited set of behavioral conditions (i.e., lying flat and pushing buttons to stimuli presented on a monitor).
From Rest to Attention: Changes in Functional Connectivity in DAN and VIS Networks.
Previous work has shown that regions of the DAN are involved in establishing and maintaining preparatory signal for spatial attention (14, 20), which causally modulates activity in visual regions (15, 25, 35). The relative importance of prefrontal vs. posterior parietal sites for top-down attention control is debated (20, 21). Here, we show that performing a visuospatial attention task (requiring either maintenance of the focus of processing on a stream of visual stimuli or a shift of attention to a competing visual stream) induces increases of temporal correlation between frontoparietal regions of the DAN and visual regions bilaterally, with stronger top-down influence from prefrontal (dFEF) to posterior parietal (IPS/SPL) and both prefrontal–parietal to visual regions bilaterally, especially V3a–V7 (Fig. 5). These functional interactions co-occurred with the uncoupling of resting temporal correlation, especially interhemispherically, and the increase of intrahemispheric directed interactions between visual areas. Functional connectivity modulations were behaviorally significant, because they correlated with accuracy.
The observed alterations in IFC/DFC indicate that rest and attention selection reflect fundamentally different brain states, at least for those connections that are actively engaged by the task. This conclusion is strengthened by the observation that IFC/DFC modulations were more pronounced after shift cues, which require visual information to be flexibly selected from one to another hemifield or hemisphere, than stay cues, in which the current visual processing status quo is maintained. Importantly, these changes in functional connectivity do not occur on a trial by trial basis but reflect adjustments of connectivity that occur during a task block or a series of trials, such as in our shift/stay cue analysis. Finally, from an attention standpoint, our results also clearly indicate the importance of dFEF as a source of top-down signals in line with neurophysiological evidence (36) and the preeminence of prefrontal over parietal regions in top-down control.
RSN: Idling vs. Priors.
This study points to key differences between networks in the relationship between rest and task patterns. Specifically, although the correlation structure in visual cortex and its relationship with the DAN were strongly modified by task execution, higher order functional connections in posterior parietal and prefrontal cortex in the DAN were relatively unaffected by task execution.
From an electrophysiological perspective, there is an emerging consensus that BOLD signal fluctuations correlate with band-limited power fluctuations (37). Accordingly, decrements of temporal correlation in visual cortex during visuospatial attention match the desynchronization of α-rhythms observed during anticipation, spatial attention, or visual processing (8, 38, 39). Moreover, notwithstanding differences in the spatial scale, reduction of neuronal noise in the low-frequency range (<10 Hz) has been identified as a powerful correlate of spatial attention (40). These results are consistent with the interpretation that, in a state of rest, visual regions synchronize to a common idling rhythm (mainly α) in large bilateral spatiotemporal clusters identified with the visual network in fMRI. This network must desynchronize, especially between hemispheres, and develop smaller, more local clusters during visual processing, consistent with increases of directional influence intrahemispherically and interactions with the DAN. These findings closely resemble the patterns found in fMRI/magnetoencephalography (MEG) studies performed at rest and during movie watching, in which it was observed a preservation of α/β-band–limited power topography, the main correlation of RSN in MEG, coupled with a decrement of correlation within the visual networks and an increase in correlation between networks (8).
Changes of synchronization in visual cortex were associated with stronger coupling and top-down directional influence from DAN regions. Not only the number of active connections increased during attention, mainly through dFEF, but also, the directional influences from DAN to visual cortex became focused onto specific regions at the intermediate level of the visual hierarchy (V3a–V7 and MT) (SI Discussion). We have provided direct evidence that transcranial magnetic stimulation over the IPS/FEF is associated with both an impairment of performance and a α-desynchronization in occipitoparietal cortex during the allocation of spatial attention (25). An important contrast, however, is that functional connections between prefrontal and posterior parietal areas were modulated in strength and directionality but not in topology by the visuospatial attention task. One interpretation is that the stability of functional connections within the DAN simply reflects the structure of the underlying anatomical circuitry. However, it is not clear why connections between dFEF and pIPS/SPL would be less prone to modulation than connections between dFEF or pIPS/SPL and V3a–V7, which are localized to the same white matter tracts (superior longitudinal fasciculus) and instead, profoundly affected by the task.
Rather, we interpret the relative stability of DAN functional connections by proposing that they are tuned (even at rest) in such a way to be anticipatory of an attention stance, possibly consistent with a role of DAN as a prior for incoming information. The relative stability of the connection pattern in the DAN might reflect the centrality of this network, far away from the influence of sensory stimuli (41, 42). In contrast, we interpreted the ongoing resting activity in visual cortex as reflecting a state of idling that must be interrupted for active vision to develop. This interpretation may be related to the relative proximity of visual cortex to the sensory periphery with subcortical gating of visual information (43, 44).
Conclusions
Using a visuospatial attention task, we show that resting connectivity profoundly decreases in visual cortex and increases between visual cortex and dorsal attention regions. These results are consistent with a dynamic reorganization of active visual connections from task to rest and the idling hypothesis of RSNs. Connections in the more central dorsal attention network were enhanced but not qualitatively changed. These results suggest that patterns of correlation in this network play a possible role as priors for attention-related responses.
Materials and Methods
Subjects and Stimuli.
Twenty-one right-handed healthy volunteers participated in the study after providing written informed consent according to the Ethics Committee at the University of Chieti. The stimuli consisted of two drifting Gabor patches presented symmetrically on the horizontal line at 5.5° of eccentricity for the whole experiment. Subjects were instructed to maintain central fixation, covertly direct attention to the cued stimulus, and report target discrimination with a manual response. Every ∼6 s on average, the cue (i.e., a color change of the patches) instructed subjects to either maintain (stay) attention to the same stimulus or redirect attention (shift) to the contralateral stimulus. Cues indicated with high probability (80%) the location of the target, which consisted of a brief change of one grating orientation, but provided no temporal information about target onset (SI Materials and Methods).
fMRI Procedure, Preprocessing, and ROI Creation.
Subjects, selected through a preliminary behavioral session, performed an fMRI session, including 15 min of resting-state scans followed by ∼45 min of task execution. Eye movements were recorded during the whole experiment (Fig. S6). The BOLD activity during task period was analyzed using a general linear model that made no assumption of the shape of the hemodynamic response with finite impulse response basis functions. ROIs were identified through a whole-brain voxelwise ANOVA with cue type (shift and stay), cue location (right and left), and time (seven MR frames) as factors (SI Materials and Methods).
Functional and Directional Connectivity Analysis.
Task-induced modulations of IFC were examined after removal of the mean evoked responses (residual fluctuations). For both resting- and task-state datasets, IFC was computed as the Pearson correlation coefficient (z Fisher-transformed) between the time series extracted from the ROIs. Graph theory measures were then used to evaluate topology changes across conditions. Specifically, the Network-Based Statistics toolbox (23) and the Brain Connectivity Toolbox (24) were used to asses changes in graph components and modularity, respectively. Moreover, to evaluate modulations of IFC corresponding to different attentional operations (stay vs. shift), a time course of the nonstationary correlation (nine MR frames in a moving window) was computed. The average correlation coefficient during periods of continuous stay and shift cues was then calculated.
DFC was assessed using the Granger causality analysis, a method that estimates the influence of signal X in predicting signal Y (unrestricted model) compared with the prediction offered by the past of signal Y itself (restricted model) (45). The degree of directional influence is measured by an F statistics that compares the reduction of variance of the restricted model provided by the unrestricted model. For each ROI pair, F statistics were computed in both directions (A to B and vice versa) using a bivariate autoregressive model between BOLD residuals of each pairwise combination of voxels. The portion of voxel pairs with significant F statistics was computed (Granger consistency). Pairwise statistical differences between rest and task IFC and DFC were evaluated through paired two-sample t tests (P = 0.05, corrected for multiple comparisons). Details can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
This research was funded by the European Community’s Seventh Framework Programme Grant Agreement HEALTH-F2-2008-200728 (BrainSynch). M.C. was supported by National Institute of Mental Health Grant R01MH096482-01 and National Institute of Child Health and Human Development Grant 5R01HD061117-08.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415439112/-/DCSupplemental.
References
- 1.Shadlen MN, Newsome WT. The variable discharge of cortical neurons: Implications for connectivity, computation, and information coding. J Neurosci. 1998;18(10):3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273(5283):1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 3.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 4.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hacker CD, et al. Resting state network estimation in individual subjects. Neuroimage. 2013;82:616–633. doi: 10.1016/j.neuroimage.2013.05.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raichle ME. The restless brain. Brain Connect. 2011;1(1):3–12. doi: 10.1089/brain.2011.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith SM, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betti V, et al. Natural scenes viewing alters the dynamics of functional connectivity in the human brain. Neuron. 2013;79(4):782–797. doi: 10.1016/j.neuron.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arfanakis K, et al. Combining independent component analysis and correlation analysis to probe interregional connectivity in fMRI task activation datasets. Magn Reson Imaging. 2000;18(8):921–930. doi: 10.1016/s0730-725x(00)00190-9. [DOI] [PubMed] [Google Scholar]
- 10.Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao W, Gilmore JH, Alcauter S, Lin W. The dynamic reorganization of the default-mode network during a visual classification task. Front Syst Neurosci. 2013;7:34. doi: 10.3389/fnsys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci USA. 2012;109(31):12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282(5386):108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 15.Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28(40):10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shulman GL, et al. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29(14):4392–4407. doi: 10.1523/JNEUROSCI.5609-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capotosto P, et al. Anatomical segregation of visual selection mechanisms in human parietal cortex. J Neurosci. 2013;33(14):6225–6229. doi: 10.1523/JNEUROSCI.4983-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capotosto P, et al. Dynamics of EEG rhythms support distinct visual selection mechanisms in parietal cortex: A simultaneous transcranial magnetic stimulation and EEG study. J Neurosci. 2015;35(2):721–730. doi: 10.1523/JNEUROSCI.2066-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tosoni A, Shulman GL, Pope AL, McAvoy MP, Corbetta M. Distinct representations for shifts of spatial attention and changes of reward contingencies in the human brain. Cortex. 2013;49(6):1733–1749. doi: 10.1016/j.cortex.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 21.Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10(1):38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Al-Aidroos N, Said CP, Turk-Browne NB. Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proc Natl Acad Sci USA. 2012;109(36):14675–14680. doi: 10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: Identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 24.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Capotosto P, Babiloni C, Romani GL, Corbetta M. Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci. 2009;29(18):5863–5872. doi: 10.1523/JNEUROSCI.0539-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greicius MD, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29(7):839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent JL, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 28.Larson-Prior LJ, et al. Cortical network functional connectivity in the descent to sleep. Proc Natl Acad Sci USA. 2009;106(11):4489–4494. doi: 10.1073/pnas.0900924106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berkes P, Orbán G, Lengyel M, Fiser J. Spontaneous cortical activity reveals hallmarks of an optimal internal model of the environment. Science. 2011;331(6013):83–87. doi: 10.1126/science.1195870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohn A, Zandvakili A, Smith MA. Correlations and brain states: From electrophysiology to functional imaging. Curr Opin Neurobiol. 2009;19(4):434–438. doi: 10.1016/j.conb.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiser J, Chiu C, Weliky M. Small modulation of ongoing cortical dynamics by sensory input during natural vision. Nature. 2004;431(7008):573–578. doi: 10.1038/nature02907. [DOI] [PubMed] [Google Scholar]
- 32.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci USA. 2009;106(41):17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32(1):9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruff CC, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16(15):1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 36.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421(6921):370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 37.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc Natl Acad Sci USA. 2008;105(41):16039–16044. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci. 2006;26(37):9494–9502. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60(4):709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12(12):1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76(5):2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- 42.Corbetta M, Miezin FM, Shulman GL, Petersen SE. A PET study of visuospatial attention. J Neurosci. 1993;13(3):1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Briggs F, Mangun GR, Usrey WM. Attention enhances synaptic efficacy and the signal-to-noise ratio in neural circuits. Nature. 2013;499(7459):476–480. doi: 10.1038/nature12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37(3):424–438. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.