Abstract
Background:
Recent years have shown an alarming rise in the incidence of diabetes mellitus (DM) all over the world. The present management of DM it not satisfactory. Hence, alternative systems of medicine are also being explored. Prameha as described in Ayurveda is a disease synonymous with today's DM. The patients of Prameha inherently carry the risk of impaired Agni and depleted Ojas status, that is, hypometabolic and immuno-compromised state. Now the primary goal is not merely to achieve normoglycemia, but also to minimize its complications. In this context, many Ayurvedic drugs are undergoing extensive research.
Aim:
To evaluate the anti-diabetic, immune-enhancer and biofire balancing effects of Naimittika Rasayana drugs viz. Silajatu and Mamajjaka in type-2 DM.
Materials and Methods:
A total of 95 patients of type-2 DM were registered; in which 84 patients turned up for full follow-up. Patients were randomly allocated into three groups; Group-A was treated with Mamajjaka (500mg twice a day) and Group-B with Silajatu (500mg twice a day) and Group-C was treated with modern drug and assessment was done at monthly intervals for three months.
Results:
The selected Rasayana drugs have shown good response on subjective and objective parameters. The Mamajjaka treated patients responded better. However, as regards the reduction of post prandial blood sugar, Silajatu was superior.
Conclusion:
The Ayurveda-inspired holistic approach seems to have a unique response promoting Agni (biofire) and Ojas (immune strength) status leading to good health and wellness.
Keywords: Agni, diabetes mellitus, Madhumeha, Mamajjaka, Ojas, Rasayana, Silajatu
Introduction
Prameha vis-à-vis diabetes mellitus (DM)[1,2] has been described in all classical texts of Ayurveda. The etymology, etiology, pathogenesis, clinical features, complications, prognosis and treatment of this disease are described in an extremely rational perspective. The concept of DM as known in conventional western medicine would reveal that this disease was fairly well-known even in the classical period of Ayurveda.[3,4,5]
The role of genetic and hereditary factors as described in the context of Sahaja Prameha (simulating juvenile type-1 diabetes) and the role of high-calorie diet and sedentary habits have been described as the sheet anchors of Madhumeha diathesis. This idea seems to be amazingly contemporary. Similarly, the description of two types of diabetes under the terms, (1) Krisha Pramehi, that is, thin diabetic and (2) Sthula Pramehi, that is, obese diabetic warranting distinctly different lines of management in Ayurveda again appears quite similar to the classification of diabetes as type-1 and type-2 in modern medicine suggesting the quality treatment modalities.[6,7]
The Ayurvedic texts describe the prodromal features and characteristic symptoms of Prameha/Madhumeha with great clarity and objectivity. The texts also deliberate on the prognosis of this disease putting emphasis on the fact that this disease is difficult to treat. Several complications, including neuropathy, angiopathy, uropathy, retinopathy and proneness to infections, including the formation of carbuncles have been described with their diagnosis and treatment in Ayurvedic classics.[8]
Ayurveda largely involves Nidana Parivarjana, that is, elimination of the cause, dietary restrictions and exercise besides a range of herbal and herbo-mineral formulations for the treatment of different kinds of diabetes. Most of the Ayurvedic texts suggest that diabetic diathesis could be categorized into Kaphaja (10 types), Pittaja (6 types) and Vataja (4 types) Prameha. The Ayurvedic dietetics, lifestyle management including Sadvritta and Achara (code of conduct) are emphasized.
The Ayurvedic texts describe Silajatu as a Naimittika Rasayana for Prameha and hence it is advisable to use Silajatu in prediabetics and/or in diabetic management as an adjuvant therapy for promotive and preventive measure.[9,10] Classically Silajatu is well known for its Naimittika Rasayana effect, Ojovardhaka and Pramehaghna property. Dalhana's commentary on Sushruta considered Silajatu as the best Naimittika Rasayana (Adjuvant therapy) for Prameha.[11]
With this perspective the present study aims to undertake a clinical evaluation of two important Ayurvedic Naimittika Rasayana drugs namely (1) Mamajjaka (Enicostemma littorale Blume.) and (2) Silajatu (Asphaltum punjabinum) in cases of type-2 diabetes mellitus (DM-2).
Materials and Methods
Selection of patients
A series of patients of either sex of DM-2 were screened from OPD and IPD of Institute of Medical Sciences, BHU, Varanasi. The study was approved by the institutional ethics committee (IEC). Informed consent was obtained from all patients before their recruitment for this study. Most of the patients were already known diabetics, but few were diagnosed for the first time when they came with some other complaints.
Inclusion criteria
Patients having classical symptoms of diabetes and unequivocal blood sugar elevation checked twice
Patients between age groups of 30 and 65 years
Increased fasting blood sugar (FBS) >126 mg/dl more than two occasions
Increased postprandial blood sugar (PPBS) >200 mg/dl
Cut-off blood sugar: Fasting is 200 mg/dl and PPBS is 300 mg/dl
Non-control of blood sugar in spite of adequate doses of oral hypoglycemic drugs such as any single drug of Sulfonylureas group for the adequate duration on the prescription of a diabetologist.
Exclusion criteria
Patients of DM-1 or juvenile onset diabetes
Patients <30 years and >65 years
Patients of DM-2 on insulin therapy
Diabetes with severe complications such as tuberculosis other pyogenic infection, blindness, stroke, etc
Pregnant and lactating mothers
Patients with recurrent infections
Patients under corticosteroid therapy.
Grouping and pososlogy
A total of 95 patients of DM were registered for the present study. These patients were randomly allocated into three groups based on computer-generated random allocation sequence. Of 95 patients, 84 patients turned up for full follow-up.
Group A: DM-2 patients who were not taking any oral hypoglycemic drug and patients with uncontrolled DM-2 who were already taking oral hypoglycemic drug. Cap. Mamajjaka (500 mg) twice a day with water after meal was administered for three months
Group B: DM-2 patients who were not taking any oral hypoglycemic drug and patients with uncontrolled DM-2 who were already taking oral hypoglycemic drug. Cap. Silajatu (500 mg) twice a day with water after meal was administered for three months
Group C (Control Group): DM-2 patients who were already taking oral hypoglycemic drug on a prescription. The ongoing conventional modern treatment prescribed by Diabetologist allowed to continue witout any other additional intervention.
Procurement of the trial drugs
The total aqueous extract of Mamajjaka (whole plant E. littorale) was procured from Ansar Industries, Surat (Gujarat) and was filled in capsules of 500 mg without any excipient. Standard Suddha Silajatu was procured from Dabur India and was used in 500 mg capsules twice daily. The Dabur Silajatu (having fulvic acid as marker compound 3.45 mg/capsule) is a product purified with Triphala Kwatha standardized as per Ayurvedic Pharmacopoeia of India standard and marketed by Dabur, India.
Dosage, duration and follow-up
The prepared 500 mg Mamajjaka extract capsules and Silajatu 500 mg capsules were administered with water in a dose of one capsule twice daily, after a meal for 3 months. The patients were allowed to continue on the diet, which they were already taking. No additional dietary or life style restrictions were prescribed to eliminate nondrug response, although most of the patients were already on a controlled diet for long time. Diabetic patients who were diagnosed first time were also not enforced any additional dietary restrictions during the trial and were treated with the trial drugs as above and response of treatment was assessed at monthly intervals for 3 months.
Criteria for assessment
The patients were assessed for clinical symptoms and physical findings using a symptom rating scale besides estimation of blood sugar and glycosylated Hb%, Ojas and Agni status in each follow-ups during the trial period with the help of Agni and Ojas rating scales.[12,13]
Clinical parameters
Assessment of drug response was done in terms of grade scores (0–3) of clinical symptoms of Diabetes such as polyuria, polydipsia, weakness, polyphagia, joint pain cramps on walking, loss of libido and sensory neuropathic symptoms such as tingling sensation, hyperesthesia, numbness, hot/cold sensation, burning sensation and pain (especially in lower limbs) and status of Ojas and Agni by using the rating scales designed[14] for this purpose which are prepared on the basis of Ayurvedic classics as described in Sushruta Samhita,[15] and Charaka Samhita.[16] These scales have been designed on the basis of degree of severity of a particular clinical feature grading into 0, 1, 2, 3. For example, Bala (physical strength) is one of the fourteen functions of Ojas which was qualitatively categorized as below:
0 = Can perform excessive work without fatigue
1 = Can carry out routine activity without getting fatigue
2 = Performs routine activity with fatigue
3 = Difficulty to perform or cannot perform routine activity.
Pain was measured by the VAS (grade score 0–10).[17] Tingling sensation, hyperesthesia, numbness, hot/cold sensation and burning sensation were measured by Likert scale (grade scores 0–2).[18]
Laboratory parameters
Laboratory assessment in terms of fasting and PPBS at 1-month interval and glycosylated Hb% (HbA1c %) before and after 3 months treatment were done.
Statistical analysis
At the end of trial period, the data were analyzed on SPSS software version-16. (SPSS Inc. Released 2007. SPSS for Windows, Version 16.0. Chicago, SPSS Inc.).
Observation and Results
The observations made in this study are displayed in Tables 1–4.
Table 1.
Symptom grade score shift index indicating overall symptomatic improvement
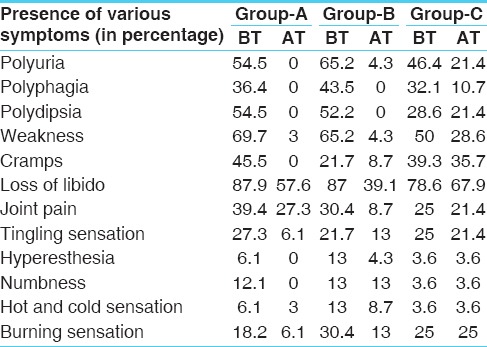
Table 4.
Pattern of percentage fall in fasting and PPBS after treatment in different trial groups
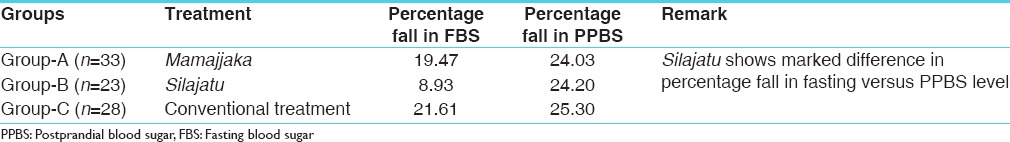
Clinical symptoms of diabetes mellitus
Therapeutically it was observed that the trial treatment could produce statistically significant favorable shift of grade scores (P < 0.01) in most of the symptoms (polyuria, polyphagia, polydipsia, weight loss, weakness, loss of libido, joint pains etc.) associated with DMs in group A, B and C. While comparing the symptoms between the groups, the trial response were statistically significant (P < 0.01) in all the Groups in follow-up-3, except in joints pain and loss of libido (P > 0.05). It was also observed that clinically the patients of Group A improved better than other groups [Table 1].
Clinical symptoms of diabetic neuropathy
Clinically it was observed that the shift of grades of tingling sensation within the group comparison in different trial groups was statistically not significant. Similar was the trend in the case of hyperesthesia, numbness and hot/cold sensation. Thus, the 3 months treatment with the trial drugs did not produce any neurological impact.
Fasting blood sugar
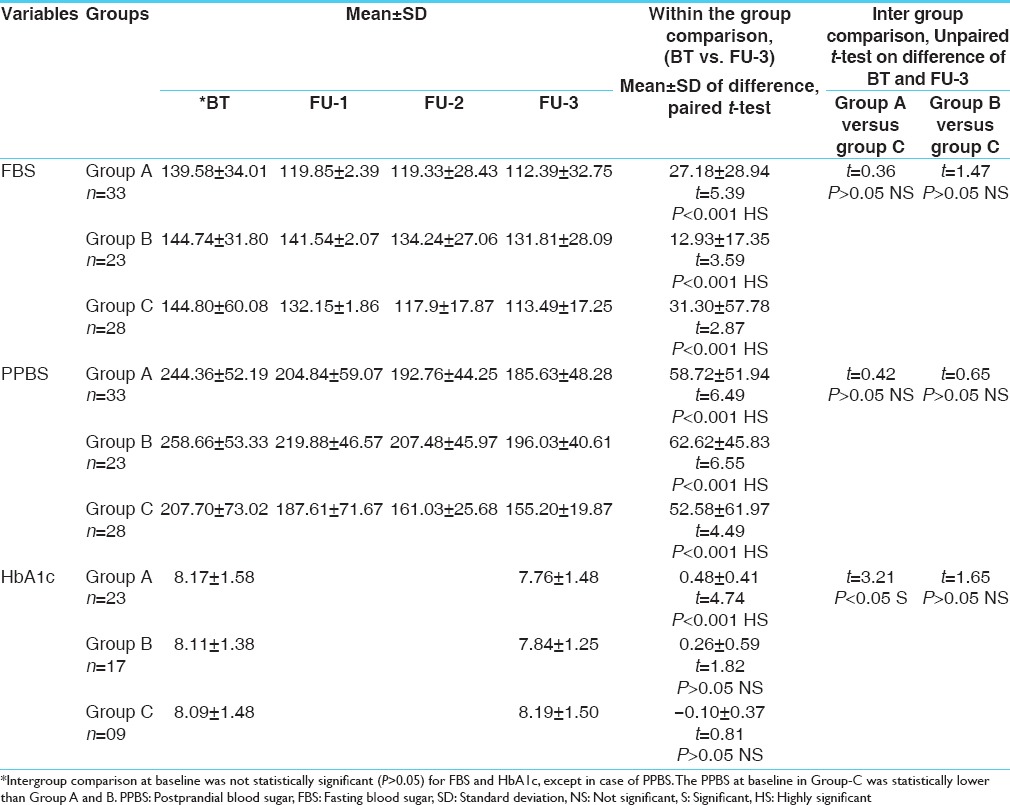
The initial mean and standard deviation (SD) FBS for Group-A was 139.58 ± 34.01, after 3 months of treatment it reduced to 112.39 ± 32.75. The improvement was statistically significant (P < 0.001). In Group-B, the initial mean and SD was144.74 ± 31.80, after 3 months of treatment it reduced to 131.81 ± 28.09. The improvement in blood sugar fasting was statistically significant (P < 0.001). In Group-C, the initial mean and SD was 144.80 ± 60.08 after 3 months of treatment, it reduced to 113.49 ± 17.25. The improvement in blood sugar fasting was statistically significant (P < 0.001). The intergroup comparison of FBS changes was statistically not significant (P > 0.05) in Group A versus Group-C and Group-B versus Group-C.
Postprandial blood sugar
The initial mean and SD of PPBS for Group-A was 244.36 ± 52.19, after 3 months of treatment it reduced to 185.63 ± 48.28. The improvement was statistically significant (P < 0.001). In Group-B, the initial mean and SD was 258.66 ± 53.33, after 3 months of treatment it reduced to196.03 ± 40.61. The improvement in PPBS was statistically significant (P < 0.00). In Group-C, the initial mean and SD was 207.79 ± 73.02 after 3 months of treatment, it reduced to 155.20 ± 19.87. The improvement in PPBS was statistically significant (P < 0.001). The intergroup comparison there was no statistically significant (P > 0.05) change in PPBS in Group-A versus Group-C and Group-B versus Group-C [Tables 2 and 4].
Table 2.
The pattern of changes in blood sugar (F, PP), HbA1c before and after treatment
Glycosylated Hb
The initial mean and SD of HbA1c for Group-A was 8.17 ± 1.58, after 3 months of treatment it reduced to 7.76 ± 1.48. The improvement was statistically highly significant (P < 0.001). In Group-B the initial mean and SD was 8.11 ± 1.38, after 3 months of treatment it reduced to 7.84 ± 1.25. The improvement in glycosylated Hb was statistically not significant (P > 0.05). In Group-C, the initial mean and SD was 8.09 ± 1.48, after 3 months of treatment, it increased to 8.19 ± 1.50 and the change in HbA1c as statistically not significant (P > 0.05).
While comparing in different groups there was statistically significant (P < 0.05) change in HbA1c in Group-A versus Group-C. The change in HbA1c was statistically not significant (P > 0.05) in Group-B versus Group-C [Table 2].
Ojas status scores
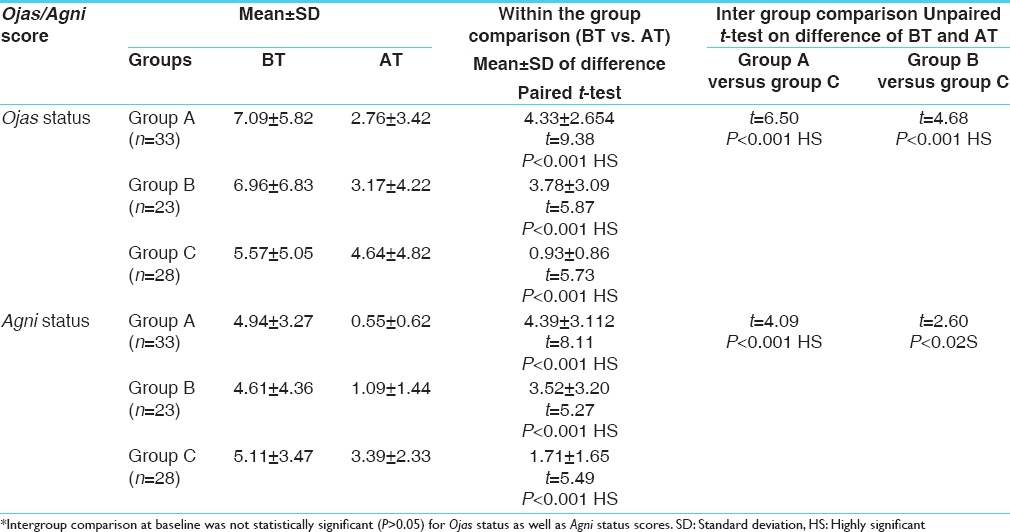
The initial mean and SD of negative Ojas status score for Group-A was 7.09 ± 5.82, after 3 months of treatment it reduced to 2.76 ± 3.42. The improvement was statistically significant (P < 0.001). In Group-B the initial mean and SD was 6.96 ± 6.83, after 3 months of treatment it reduced to 3.17 ± 4.22. The improvement in Ojas status score was statistically significant (P < 0.001). In Group-C, the initial mean and SD was 5.57 ± 5.05, after 3 months of treatment, it reduced to 4.64 ± 4.82. The improvement in Ojas status score was statistically significant (P < 0.001). Maximum improvement was seen in Group-A (Mamajjaka treated), then in Group-B (Silajatu treated) whereas in Group-C minimal change was seen. The intergroup comparison showed. Statistically significant change in Ojas status in Group-A versus Group-C (P < 0.001) and Group-B versus Group-C (P < 0.001) [Table 3].
Table 3.
The pattern of changes in, Agni and Ojas scores before and after treatment in different groups
Agni status scores
The initial mean and SD of Agni status score for Group-A was 4.94 ± 3.27, after 3 months of treatment it reduced to 0.55 ± 0.62. The change was statistically significant (P < 0.001)). In Group-B the initial mean and SD was 4.61 ± 4.36, after 3 months of treatment it reduced to 1.09 ± 1.44. The change in Agni status scores was statistically significant (P < 0.001). In Group-C the initial mean and SD was 5.11 ± 3.47, after 3 months of treatment, it reduced to 3.39 ± 2.33. The change in Agni status scores was statistically significant (P < 0.001). While comparing in different groups there is statistically significant change in Agni status in Group-A versus Group-C (P < 0.001) and Group-B versus Group-C (P < 0.02). Maximum improvement was seen in Group-A (Mamajjaka treated) then in Group-B (Silajatu treated) while in Group-C minimal change was seen [Table 3].
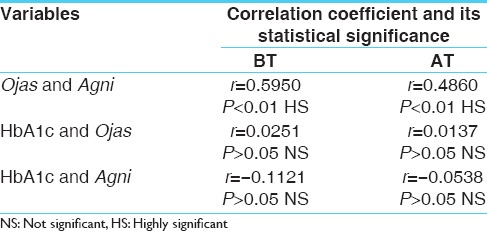
Correlation between Ojas and Agni status
To measure the degree of linear relationship between Ojas and Agni, HbA1c and Ojas, HbA1c and Agni coefficient of correlation was determined. Correlation between Ojas and Agni was found positive and statistically significant at before and after treatment points. Correlation between HbA1c and Ojas was positive and lower but not statistically significant. Whereas correlation between HbA1c and Agni was negative and smaller and was not statistically significant. The negative value of this correlation shows that HbA1c and Agni are inversely related, that is, if one increases the other decreases and vice versa. The trends show that Ojas and Agni status correspond each other. HbA1c and Ojas status also influence each other. On the other hand HbA1c shows declining trend with improving Agni status [Table 5].
Table 5.
The correlation between the following variables
Discussion
Diabetes mellitus is a disease known since the beginning of human civilization.[19] Now it is the fifth leading cause of death in most developed countries and epidemic in many developing nations. The recent years have shown a significantly rising trend in the incidence of this disease all over the world especially in India. The Ayurvedic scholars since antiquity had the knowledge of Madhumeha, a disease synonymous with DM. Ayurveda considers diabetes as Kaphaja disease in which Medas (adipose factor) and Ojas (bio-strength cum immune strength) have been described as the main factors, which are affected in this disease. Agni (GI and cellular biofire) and Ama, that is, autotoxins; play a major role in disease diathesis and complications. These ancient observations are outstanding information regarding the nature of the disease. It is now well-known that DM results due to the metabolic derangement. Similarly, it is being now gradually conceived that there is a strong evidence of immune disorder and immunodeficiency in all diabetics and its related complications. Possibly, because of such morbid factors, the propounders of Ayurveda considered Ojas as an important Dusya (morbid factor) of Madhumeha, hence it is also termed as Ojomeha. Medas or the bodily lipids have strong association with DM and obesity, which may lead to a variety of other disorders.[20,21,22] Medas with or without Ama is predicted to play a major role in the development of DM and its complications.
Conventional modern medicine is not always successful to control diabetes in all cases. Insulin is not always indicated due to the development of insulin resistance and generation of Insulin antagonists in the body, whereas the oral hypoglycemic drugs are found to be of limited use in many cases due to the major side-effects. Therefore, search for better remedies from Ayurvedic resources continues. The present study identifies a treatment strategy that not only corrects the metabolic derangements, but also helps in maintaining the Agni and Ojas status, that is, metabolic stability and immune strength in diabetic patients.
This study is based on investigations of 95 diagnosed cases of Madhumeha (DM-2), out of which 11 patients dropped out mostly because of change of place and only 84 patients could be studied under clinical trial of the two selected drugs along with a group of patients under conventional treatment for comparative purpose. In this study, Mamajjaka shows better results as compared to other groups. It is suggested that a combined treatment of DM with Mamajjaka and Silajatu could give still better results. Silajatu[23] being a Naimittika Rasayana and Mamajjaka[24,25] being a therapeutic agent for this purpose could jointly form the full treatment modality for DM.
This study also suggests that the selected drugs help in preventing or delaying the complications in DM. The selected Rasayana drugs (Silajatu and Mamajjaka) by virtue of their Rasayana properties act at the level of Agni, Srotasa and Rasa, providing nourishment to the Sapta Dhatu [Table 5].[26,27]
The Mamajjaka treated patients have shown better percentage of fall in FBS (19.47%), in comparison to Silajatu treated patients (8.93%) while in case of PPBS the percentage of fall was almost equal in both the groups, that is, 24.03%, but the patients on conventional treatment showed more or less equal percentage of fall in both fasting and PPBS level. The special pattern of blood sugar response to Silajatu treatment (in relation to fasting and PP) deserves special consideration [Table 4].
Clinically, it was also observed that Mamajjaka treated patients showed a noticeable response in overall neurological symptoms. Silajatu treated patients showed significant and modern medicine treated patients showed an insignificant response in neurological symptoms in intergroup comparison.
The probable mode of action of the trial drugs in terms of conventional bio-sciences, cannot be explained at this stage. This study is largely an efficacy-validating investigation. It is not a mechanism testing study. Silajatu has been described as Naimittika Rasayana for Prameha by Susruta and Dalhana and Mamajjaka is in practice and being used by many Ayurveda physicians in India.
Conclusion
The present clinical study shows that the selected Rasayana drugs may help to control blood sugar levels in mild to moderate diabetic patients and may retard the complications of DM. However, further longitudinal studies are warranted to explore the mode of action of these drugs in preventing neurological complications of DM. Although it is difficult to presume the possible mechanism of action of these drugs at this stage of study, but the authors are inclined to suggest that in view of their Naimittika Rasayana impact these drugs are likely to promote insulin production. Beside this, these drugs have capacity to improve overall health status of diabetic subjects. It was also observed that these Rasayana remedies not only correct the hyperglycemic episodes, but also produce their effect by enhancing the Agni and Ojas status in these patients, meaning thereby an improved metabolic and immune status. The Rasayana drugs used in this study seem to be safe, because no side-effects were noted in trial period in the present study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Resnick HE, Harris MI, Brock DB, Harris TB. American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: Results from the Third National Health and Nutrition Examination Survey. Diabetes Care. 2000;23:176–80. doi: 10.2337/diacare.23.2.176. [DOI] [PubMed] [Google Scholar]
- 2.Boon NA, Colledge NR, Walker BR. 20th ed. London: Churchill Livingstone Elsevier, Elsevier; 2007. Davidson's Principles of Practice of Medicine; pp. 805–47. [Google Scholar]
- 3.Udupa KN, Singh RH. 1st ed. Nagpur: Sri Baidyanath Ayurveda Bhawan; 1990. Science and Philosophy of Indian Medicine. [Google Scholar]
- 4.Valiathan MS. 1st ed. New Delhi: National Academy of Sciences; 2007. Towards Ayurvedic Biology. [Google Scholar]
- 5.Agnivesha, Charaka, Dridhabal, Charaka Samhitta, Chikitsa Sthana, Prameha Chikitsa Adhyaya. In: 6. Vaidya Jadavji Trikamji Achrya., editor. Varanasi: Chaukhambha Orientalia; 2002. p. 444. [Google Scholar]
- 6.Vagbhatta, Astang Sangrah, Sutra Sthana, Prameha Nidana Adhyaya. In: 10/15. 1st ed. Shivprasad Sharma., editor. Chaukhamba Sanskrit Pratisthan; 2006. p. 393. [Google Scholar]
- 7.Vagbhatta, Ashtanga Hridyam, Nidana Sthana, Prameha Nidana Adhyaya. In: 10/18-19. Vaidya Yadunandana Upadhyaya., editor. Varanasi: Chowkhamba Sanskrit Sansthan; 2005. p. 254. [Google Scholar]
- 8.Singh RH. Varanasi: Choukhamba Amarbharti Prakasan; 1985. Ayurvediya Nidan Cikitsa Ke Siddhanta. [Google Scholar]
- 9.Kaplan LA, Pesce AJ, editors. Clinical Chemistry: Theory, Analysis and Co-realation. Toronto: C.V. Mosby; 1984. Carbohydrate and metabolite; pp. 1024–32. [Google Scholar]
- 10.Badesha GS, Singh, RH Psychophysiological and Immunological Studies on Diabetes Mellitus and its Treatment with E. Jambolana Lam. M.D.(Ay.) Kayachikitsa, Thesis, B.H.U., Varanasi. 1982 [Google Scholar]
- 11.9th ed. Varanasi: Chaukhambha Orientalia; 2007. Dalhana commentator. Susruta Samhita, Chikitsa Sthana, Sarvopaghata Shamaniya Adhyaya, 27/2, edited by Vaidya Jadavji Trikamji Acharya; p. 498. [Google Scholar]
- 12.Singh RH. 1st ed. II. Delhi: Chaukhamba Sanskrit Pratisthan; 2001. Kayachikitsa; pp. 572–87. [Google Scholar]
- 13.Bharati, Singh RH, Chansouria JPN. Hypoglycaemic property of shilajeet and yashada bhasma. Anc Sci Life. 1996;16(2):118–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Mehra PS, Singh RH. Clinical evaluation of effect of Amrita-pippali-nimba yoga in diabetes mellitus with special reference to the role of Agni and Ojas. J Res Ayurveda Siddha. 2001;21:183–97. [Google Scholar]
- 15.Chaukhambha Orientalia, Varanasi. 9th ed. 2007. Sushruta. Sushruta Samhita, Sutra Sthana, Dosha-dhatu-Mala Vriddhi-Kshaya Vijnaniya Adhyaya, 15/25, edited by Vaidya Jadavji Trikamji Acharya; p. 72. [Google Scholar]
- 16.Agnivaesha, Charaka, Dridhabala, Charaka Sanhita, Vimana Sthana, Rogabhishagjitiya Vimana Adhyaya. In: 8/89. 9th ed. Vaidya Jadavji Trikamji Acharya., editor. Varanasi: Chaukhambha Orientalia; 2007. p. 275. [Google Scholar]
- 17.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–36. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 18.Wuensch KL. What is a Likert Scale? and How Do You Pronounce ‘Likert?’. East Carolina University. [Revised on 2015 Jan 25]. Available from: http://core.ecu.edu/psyc/wuenschk/stathelp/Likert.htm .
- 19.Bharti, Singh RH. A Clinical Study of Diabetes Mellitus Vis-a-Vis Madhumeha and its Treatment with Bilwa. M.D.(Ay.) Kayachikitsa Thesis, IMS, BHU, Varanasi. 1990 [Google Scholar]
- 20.Bharti, Singh RH. Constitutional study of patients of diabetes mellitus vis-à-vis Madhumeha. Anc Sci Life. 1995;15(1):35–42. [PMC free article] [PubMed] [Google Scholar]
- 21.Harish A. A Clinical Study on the Efficacy of Virecana Therapy and Medoghna Rasayana in the Management of Madhumeha with special reference to Diabetes Mellitus M.D. (Ayu) Kayachikitsa Thesis, IPGT and RA, Gujarat Ayurved University, Jamnagar. 2004 [Google Scholar]
- 22.Kohli KR, Singh RH. A study on the Kriyakala of Diabetes Mellitus and its Treatment with Some Indigenous Drugs. M.D.(Ay.) Kayachikitsa, Thesis, B.H.U., Varanasi. 1983 [Google Scholar]
- 23.Dole VA, editor. Chowkhamba Sanskrit Series Office, Varanasi. 1rd ed. 2006. Vagbhata, Rasa Ratna Samuchaya, Adhyaya 2/109-22; pp. 63–70. [Google Scholar]
- 24.Vaishya Shaligram, Shaligrama Nighantu. In: 1st ed. Khemaraj Srikrishnadas., editor. Bombay: Shree Venkateswer Press; 1953. [Google Scholar]
- 25.Sharma PV. 1st ed. Vol. 2. Varanasi: Chaukhambha Bharati Academy; 2003. Dravyaguna Vijnana; p. 704. [Google Scholar]
- 26.Sodhala, Sodhala Nighantu. In: 1st ed. Sharma PV, editor. Baroda: Oriental Institute; 1978. [Google Scholar]
- 27.Ghosal S, Lal J, Srivastava RS, Bhattacharya SK, Upadhyay SN, Jaiswal AK, et al. Immuno modulatory and CNS effects of sitoindosides IX and X. Phytother Res. 1989;C3:201. [Google Scholar]


