Abstract
To understand the efficacy of emamectin benzoate, avermectin, milbemectin, and thiacloprid on the reproduction and development of Bursaphelenchus xylophilus, seven parameters, namely population growth, fecundity, egg hatchability, larval lethality, percent larval development, body size, and sexual ratio, were investigated using sublethal (LC20) doses of these compounds in the laboratory. Emamectin benzoate treatment led to a significant suppression in population size, brood size, and percent larval development with 411, 3.50, and 49.63%, respectively, compared to 20850, 24.33, and 61.43% for the negative control. The embryonic and larval lethality increased obviously from 12.47% and 13.70% to 51.37% and 75.30%, respectively. In addition, the body length was also significantly reduced for both males and females in the emamectin benzoate treatment. Avermectin and milbemectin were also effective in suppressing population growth by increasing larval lethality and reducing larval development, although they did not affect either brood size or embryonic lethality. Body length for both male and female worms was increased by avermectin. Thiacloprid caused no adverse reproductive effects, although it suppressed larval development. Sexual ratio was not affected by any of these four nematicides. Our results indicate that emamectin benzoate, milbemectin, and avermectin are effective against the reproduction of B. xylophilus. We think these three nematicides can be useful for the control of pine wilt disease.
Keywords: avermectin, Bursaphelenchus xylophilus, emamectin benzoate, milbemectin, pine wilt disease, thiacloprid
Pinewood nematode, Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle, is the causal agent of pine wilt disease (PWD) (Mamiya and Kiyohara, 1972; Dropkin and Foudin, 1979). Since its first observation in Japan in 1905, B. xylophilus has been observed throughout the East Asian countries of China and Korea, as well as in North America and Europe (Fielding and Evans, 1996; Mota et al., 1999). In China, the first record of B. xylophilus was in Nanjing in 1982 (Sun, 1982). After that, it spread to Anhui, Guangdong, Zhejiang, Hongkong, Taiwan, and more than 10 other provinces, and became one of the most dangerous pest species, killing a large number of pine trees with huge economic losses (Sun, 2005). In the past decades, several different control measures against PWD have been developed, such as physical eradication by removing infected trees, aerial application of insecticide to kill the nematode’s vector, the pine sawyer (Monochamus spp.), and trunk injection of an antinematodal compound to control the nematode itself (Yang et al., 2003, pp. 90–133; Han et al., 2010).
Since trunk injection has a direct effect on the nematode and is environmentally friendly, this measure is widely used in China. One antinematodal compound, emamectin benzoate, is one of the most popular trunk-injection agents because of its persistent effect and application efficiency (Takai et al., 2000, 2003); however, little is known about how this compound acts against B. xylophilus. In addition, due to its extensive application, there are increasing concerns as to whether B. xylophilus is able to develop resistance to emamectin benzoate because several other pest insects (e.g., Frankliniella occidentalis, Lepeophtheirus salmonis, Plutella xylostella, and Thrips tabaci) have become resistant to this agent (Zhao et al., 2006; Wang et al., 2012; Espedal et al., 2013; Lebedev et al., 2013). Furthermore, there is a general need for more insight into the mode of action of nematicides on the reproduction and development of B. xylophilus so that more specific antinematodal compounds can be developed against this parasite.
Many plant extracts and essential oils have been reported as being active against B. xylophilus, such as trans-cinnamaldehyde, geraniol, malabaricones, isoeugenol, etc. (Kong et al., 2007a, 2007b; Park et al., 2007; Choi et al., 2008; Barbosa et al., 2010, 2012). In most of these studies, the nematicidal activity was defined according to the mortality of B. xylophilus after being treated with the active compound. However, very little work has been done to elucidate how these compounds act against B. xylophilus. To elucidate how different nematicides suppress the population growth and individual development of B. xylophilus, emamectin benzoate as well as three other kinds of widely used pesticides—avermectin, milbemectin, and thiacloprid—were used in our study. Emamectin benzoate, avermectin, and milbemectin are all gamma-aminobutyric acid (GABA) inhibitors. In contrast, thiacloprid is a nicotinoid insecticide that stimulates acetylcholine receptors. Seven parameters, namely population growth, fecundity, egg hatchability, larval lethality, percent larval development, sex ratio and body size of B. xylophilus, were investigated using sublethal (LC20) doses of these compounds in the laboratory. The objectives of this study were to obtain useful information for further studies on the toxic mechanisms of different chemical agents and for the development of a more specific and effective nematicide. Our results could also provide some suggestions for PWD control strategies using different nematicides alternatively or integratively instead of applying a single nematicidal agent for the control of PWD.
Materials and Methods
Nematode strain
Bursaphelenchus xylophilus NB-6 was originally isolated from chips of infected pinewood, Pinus massoniana, collected from the Ningbo area (in November 2008), Zhejiang province, China. The fungal food source, Botrytis cinerea Persoon, was obtained from the Research Institute of Forest Protection, Chinese Academy of Forestry, Beijing, China. The nematodes were cultured in the dark on B. cinerea mycelia in petri dishes with PDA media at 25°C. The larval and adult nematodes were isolated from the fungal cultures by the Baermann funnel technique.
Nematicides
The four nematicides used in this study were emamectin benzoate (purity ≥95%) and avermectin (purity ≥ 95%) (both from Zhejiang Shenghua Biok Biology Co. Ltd, Huzhou, China), milbemectin (purity ≥ 95%; Zhejiang Hisun Pharmaceutical Co. Ltd., Taizhou, China) and thiacloprid (purity ≥ 98%; Anhui Jiangnan Chemical Industry Co., Ltd, Huainan, China).
Sublethal toxicity test
Before undertaking the population inhibition test, a toxicity test was conducted to screen the 24-hr LC20 sublethal concentration for each of the different nematicides. Serial dilutions of each of the test compound solutions were prepared using distilled water containing Triton X-100 (5,000 ppm) giving six different concentrations in the range 0.1 to 8.0 mg/liter. After culturing B. xylophilus on B. cinerea for 24 hr, each of the different dilutions were uniformly sprayed onto petri dishes at a dosage of 10 µl/cm2. The plates were kept in the dark at 25°C. After being cultured for 24 hr, nematodes were isolated using the Baermann funnel technique. The dying nematodes were checked under a binocular microscope (Leica DMi1; Leica Microsystems, Germany) and considered to be dead if they did not respond to touching with a worm-picking wire. Dead nematodes on the plates were collected by putting the media under water upside down for at least 4 hr. The mortalities were calculated as the fraction of dead nematodes divided by the total number of nematodes. Four replicates were done for each concentration.
Population inhibition test
Each of the four nematicides was uniformly applied directly to mycelia of B. cinerea with the sublethal LC20 concentration at a dosage of 10 µl/cm2 once the mycelia had overgrown the petri dishes. The distilled water-Triton X-100 solution was used as a negative control. Twenty nematodes (10 males and 10 females) were inoculated to each petri dish and cultured in the dark with the nematicide-treated fungus at 25°C. Five petri dishes were used for every nematicidal treatment as replications.
Once it was seen that the fungal hyphae had been eaten up by the nematodes in one of the dishes, which normally occurred around the 9th d after inoculation, the nematodes from all the petri dishes were isolated separately using the Baermann funnel technique. Then the population numbers were counted as described previously (Wu et al., 2004).
Nematicidal effects on fecundity
To get enough male adults, about 1,000 fourth-stage larvae (before reaching maturity) from the above population inhibition test were selected and transferred separately to 24-well tissue plates. Hyphae of B. cinerea mould were added to wells to culture the J4 in the dark at 25°C. After 24 hr when the larvae had become mature adults, 20 nematodes (10 males and 10 females) were selected and mixed together for mating in small petri dishes (3 cm diam.) in the dark at 25°C. The resulting eggs were counted after 12 hr under a binocular microscope (Leica DMi1; Leica Microsystems, Germany). Due to the fluctuation of male numbers, treatments were replicated three to five times depending on the number of male nematodes that had survived the population inhibition test. The inhibitory effects of the four different nematicides on the egg-laying capacity of B. xylophilus were compared using the number of eggs laid per female.
Embryonic lethality test
Eggs collected from the nematodes in the population inhibition trials were used for the embryonic lethality test. To obtain synchronized new eggs, adult nematodes from the population inhibition trials were collected and then transferred to petri dishes containing shallow sterile distilled water (less than 5 mm in depth). After 0.5 to 1.0 hr, some eggs would already be laid and could be seen clearly. Most of them were stuck to the bottom of petri dish. The water together with the nematodes was then gently moved to another petri dish to obtain more new eggs. The synchronized eggs were collected by rinsing the petri dish several times with sterile distilled water.
The nematicide solutions in sublethal LC20 concentration were placed in the wells of 24-well tissue plates. Distilled water–Triton X-100 solution was used as a negative control. Subsequently, 100 eggs were inoculated into each well and reared in the dark at 25°C. An embryo was considered dead if the egg did not hatch after 36 hr. Embryonic lethality was calculated as the fraction of nonhatched embryos divided by the total number of eggs placed in the well (i.e., 100). All treatments were replicated five times.
Larval lethality test
Normally reared pinewood nematodes were synchronized at the L2 stage by hatching embryos in M9 buffer (22 mM KH2PO4, 42 mM Na2HPO4, 86 mM NaCl, 1 mM MgSO4) in the absence of food. The synchronized larvae were then transferred to petri dishes (roughly 1,000 per dish) containing a fresh fungal food source, B. cinerea. The four nematicides were applied as described in the population inhibition test. Distilled water–Triton X-100 solution was used as a negative control. After being cultured for 48 hr at 25°C in the dark, the J3- or J4-stage larvae were isolated using the Baermann funnel technique. Larval lethalities were determined as described above in the sublethal toxicity test. Five replicates were done for each treatment.
Nematicidal effects on larval development
Synchronized new eggs were obtained from normally reared nematodes using the method described above. About 500 eggs were inoculated into one petri dish on B. cinerea hyphae, which had been previously sprayed with one of the four nematicides at a sublethal concentration of LC20. Distilled water–Triton X-100 solution was used as a negative control. After being cultured for 92 hr in the dark at 25°C, the nematodes were collected and examined under a binocular microscope (Leica DMi1) to see if they had developed into adults with visible genitals. The percent larval development was defined as the fraction of progeny that had reached the adult stage divided by the total number of hatched embryos as described previously (Lee et al., 2008). All treatments were replicated four times.
Nematicidal effects on the sex ratio of the next generations and individual body size
Adult nematodes produced in the population inhibition experiment and that had been treated with one of the four different nematicides were randomly collected. The number of male and female worms was counted to determine the sex ratio (male versus female) of the different treatments. Before measuring the body size, nematodes were killed by warming up the water solution to 55°C. Individual body size (in length) was measured under a binocular microscope (Axio Observer A1; Zeiss, Germany). Forty males and 40 females were measured respectively for each treatment as repeats.
Statistical analysis
Population numbers and eggs laid per female were averaged. Embryonic lethality, percent larval development, sex ratio of next generation and individual body size were square root transformed to obtain a normally distributed data set with homogeneous variance among treatments before doing an analysis of variance (ANOVA; SAS OnlineDoc®, Version 8.01, Statistical Analysis System Institute, Cary, NC). Tukey’s honestly significant difference method and Bonferroni’s arranged P values were applied for multiple comparisons among pairs of means.
Results
Sublethal LC20 concentrations of the nematicides
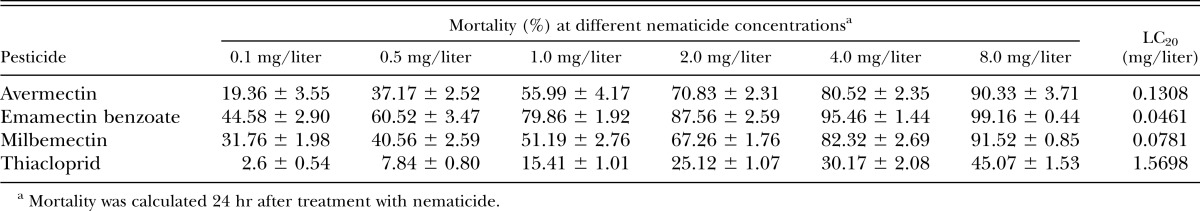
The LC20 of each of the four different nematicides was calculated (Table 1). For all four nematicides, the mortality of B. xylophilus tended to increase with concentration. Emamectin benzoate showed the best nematicidal activity with an LC20 value of 0.0461 mg/liter, followed by milbemectin and avermectin with 0.0781 and 0.1308 mg/liter, respectively. However, the nematicidal activity of thiacloprid was weak with an LC20 value of 1.5698 mg/liter, which was much higher than the others.
Table 1.
LC20 determination for four different nematicides against Bursaphelenchus xylophilus.
Population inhibition test
After 8 d, most of the B. cinerea mycelia in the control petri dishes had been eaten, but those treated with nematicides were not (Fig. 1A–E). The effects of the different nematicides on population numbers are summarized in Table 2. The population numbers of the emamectin benzoate, avermectin, and milbemectin treatments were significantly lower than that of the control (F = 26.48; df = 3; P < 0.0001). In contrast, no obvious difference was observed for the thiacloprid treatment (F = 0.77; df = 1; P = 0.4132). Only an average of 411 nematodes was isolated from the emamectin benzoate treatment, which is significantly lower than in the other three treatments (avermectin: 6,880; milbemectin: 12,080; thiacloprid 18,860). In contrast, the number of nematodes in the negative controls was 20,850.
Fig. 1.
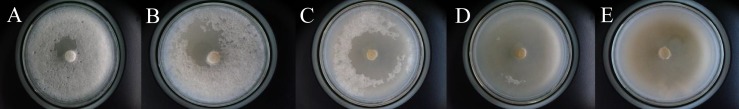
Area of gray mould mycelia eaten by Bursaphelenchus xylophilus after 8 d, showing the differences in population-inhibiting activity between the different nematicide treatments. (A) Emamectin benzoate, (B) avermectin, (C) milbemectin, (D) thiacloprid, (E) control.
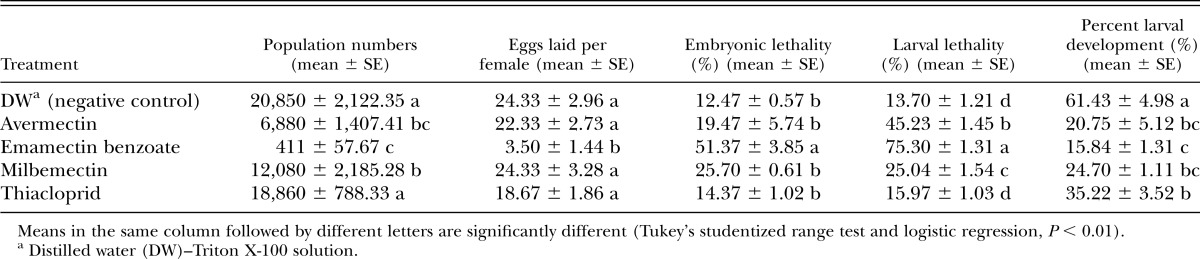
Table 2.
Effects of four different nematicides on the reproduction and development of Bursaphelenchus xylophilus.
Nematicidal effects on fecundity and embryonic lethality
The average number of eggs laid per B. xylophilus female from the emamectin benzoate treatment was only 3.33, which was significantly less than for the other nematicides (F = 12.009; df = 4; P = 0.0008) (Table 2). In contrast, there were no differences between the other three nematicidal treatments and the controls (F = 0.9380; df = 3; P = 0.4662).
Embryonic lethality induced by the nematicides was similar to their effect on fecundity. More than 50% of the embryos were dead after being treated with emamectin benzoate, which was significantly higher than with the other nematicides (F = 26.228; df = 4; P < 0.0001). No differences were observed between the controls and each of the other three nematicidal treatments (F = 3.31; df = 3; P = 0.0781).
Nematicidal effects on larval lethality and development
Larval lethality differed significantly between each of the nematicidal treatments (F = 381.52; df = 3; P < 0.0001). After being treated with emamectin benzoate, 75.3% of the larvae were dead, compared to 45.2%, 25.0%, and 16.0% for the avermectin, milbemectin, and thiacloprid treatments, respectively. There was no difference between thiacloprid and the control (F = 2.04; df = 1; P = 0.1909). These results suggest that emamectin benzoate is the most effective nematicide when applied to early larvae.
Only 15.8% of the hatched larvae developed into adults when they were treated with emamectin benzoate, followed by avermectin, milbemectin and thiacloprid with 20.7%, 24.7%, and 35.2%, respectively. These results all differed significantly from those of the controls (F = 22.78; df = 4; P < 0.0001) (Table 2).
Nematicidal effects on the sex ratio of the next generation and individual body size
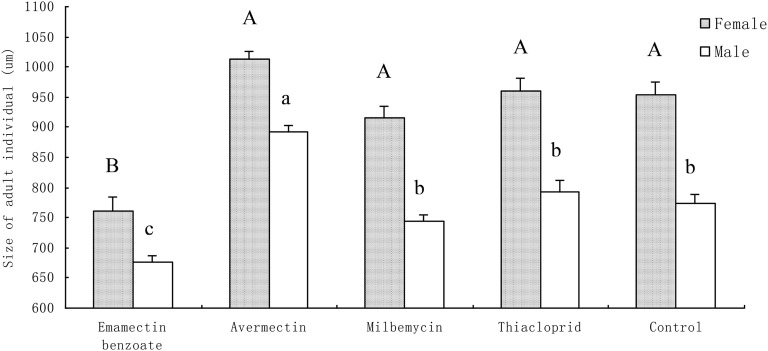
The ratio of male to female worms did not differ among the nematicide treatments (F = 1.31; df = 4; P = 0.3109), ranging from 0.58 for the milbemectin treatment to 0.64 for emamectin benzoate. These results show that the sex ratio of pinewood nematode was not affected by any of the nematicides. Nonetheless, after being treated with emamectin benzoate, the body length of both the males and females was shorter than in any of the other treatments (male: F = 35.99; df = 4; P < 0.0001 and female: F = 25.35; df = 4; P < 0.0001). In contrast, when the pinewood nematodes were treated with avermectin, the body length for both the male and female worms became longer than the controls (male: F = 43.07; df = 1; P < 0.0001 and female: F = 6.21; df = 1; P = 0.0172) (Fig. 2). No differences were observed between milbemectin, thiacloprid, and the control (male: F = 2.94; df = 2; P = 0.161 and female: F = 1.54; df = 2; P = 0.223).
Fig. 2.
Body size (mean ± SE) of adult Bursaphelenchus xylophilus in relation to nematicides and sex. n = 40. Horizontal bar shows the standard error. Different letters indicate significant differences. Capital and small letters stand for female and male, respectively (P = 0.01, Bonferroni method).
Discussion
In this study, the LC20 was calculated according to the equation fitted by concentration and mortality of different nematicides. For emamection benzoate and milbemectin, however, their LC20 were outside the actual range of treatments and extrapolated by assuming a linear relationship. This might bring some deviation. Further sublethal toxicity test need to be conducted to get more precise LC20 of emamection benzoate and mibemectin. Our results show that the population numbers of B. xylophilus were suppressed by emamectin benzoate, avermectin, and milbemectin at their LC20 concentrations, but not by thiacloprid. This is not surprising as the latter is normally used as a synthetic pesticide for the control of the insect vector, Monochamus alternatus. This confirms the work of Takai et al. (2000), who reported that the GABA receptor agonists (such as avermectin, emamectin benzoate, and milbemectin) had a greater inhibitory effect against B. xylophilus than compounds influencing glutamate, β-adrenergic, dopamine, or acetylcholine receptors, or even those inhibiting acetylcholinesterase, monoamine oxidase, and ion channels. The propagation of B. xylophilus has also been shown to be inhibited by p-cymene, a compound extracted from plants (Kong et al., 2007b). However, in the same study, nine other plant compounds were shown to stimulate B. xylophilus propagation; namely, (−)-caryophyllene oxide, (+)-ledene, (+)- and (−)-limonene, linalool oxide, β-myrcene, (−)-α-phellandrene, (+)-α-pinene, and γ-terpinene. The mode of action of any compounds used in the control of this nematode should be analyzed carefully not only to elucidate the mechanisms in general but especially to pay attention to those compounds that are seen to stimulate B. xylophilus.
Very little work has been done to test the inhibitory activity of nematicides on the fecundity of B. xylophilus, although research has been done on other nematodes. Zhang et al. (2013) reported that acetochlor, a herbicide, could significantly inhibit the reproductive capacity of the diplogastrid nematode Pristionchus pacificus. Lee et al. (2008) demonstrated that the brood size of Caenorhabditis elegans was decreased about 20% by 0.1 to 1.0 mM flavone. In another study, however, serotonin and imipramine were found to be able to increase the egg-laying capacity of C. elegans (Trent et al., 1983). Our results proved that emamectin benzoate can effectively suppress the fecundity of B. xylophilus. We found that most of the females were not able to lay eggs successfully and died with eggs in their bodies after being treated with this compound. Conversely, avermectin, milbemectin, and thiacloprid showed no inhibitory activity. This result can explain why emamectin benzoate was more effective than the other three nematicides in inhibiting the population numbers of B. xylophilus.
Bolla and Boschert (1993) reported that B. xylophilus could reproduce gonochoristically (male and female) with a large brood size of more than 100 offspring, which is much higher than the numbers found in our present study. This difference might have been caused by the different strains of pinewood nematode used in the two studies. In addition, the egg-laying capacity of B. xylophilus in our study might also have been reduced because the gravid adults were tested in distilled water, where the O2 concentration was comparatively lower than that in the air environment used by Bolla and Boschert. Indeed, in a previous study, Miller and Roth (2009) found that the egg-laying capacity of C. elegans could be arrested in a hypoxic environment.
Egg hatchability is one of the most important factors affecting population growth. Chemicals with high embryonic lethality should be considered as prospective candidates for effective nematicides. Our results show that emamectin benzoate could effectively decrease the hatchability of B. xylophilus, whereas the other three nematicides evinced almost no potential embryonic lethality. This is possibly due to the low concentration used (LC20). Still, this result indicates the greater effectivity of emamectin benzoate.
Little work has been done to study embryonic lethality in B. xylophilus. One study found that blue light (465–470 nm) was able to strongly inhibit the hatchability of B. xylophilus: no eggs could be hatched out after being treated with blue light for 12 hr at 1,000 lux (Hu et al., unpubl. data). Egg hatchability has, however, been investigated in other nematodes using other chemicals. It has been reported that about 90% of C. elegans eggs were not able to hatch out after being treated with flavone at concentrations greater than 0.3 mM (Lee et al., 2008). In addition, Fabiyi et al. (2012) reported that over 90% of Meloidogyne incognita eggs could be prevented from hatching using the extracts of two plants, Alstonia boonei and Bridelia ferruginea. All these values are greater than the embryonic lethality caused by emamectin benzoate, although no direct comparisons can be made between those investigations and ours due to their different experimental designs. More research should be done on embryonic lethality in B. xylophilus as this could be an efficient method of controlling this species.
Many plant essential oils and phytochemicals cause larval lethality in B. xylophilus (Kong et al., 2007a, 2007b; Park et al., 2007; Faria et al., 2013). Our results show that high larval lethality could be induced by emamectin benzoate, avermectin, and milbemectin at LC20, but not by thiacloprid, although all four nematicides had a potent inhibitory effect on B. xylophilus development. The development of different larval stages, dauer larva DL3 and reproductive larva L3, are regulated by chemosensory neurons in both C. elegans and B. xylophilus (Ren et al., 1996; Kim et al., 2009; Ogawa and Sommer, 2009; Kikuchi et al., 2011). How emamectin benzoate, avermectin, and milbemectin cause the larval lethality in B. xylophilus needs to be investigated further.
The standard number of haploid chromosomes in B. xylophilus is six (Hasegawa and Miwa, 2008), and the sex-determining system of B. xylophilus consists of an XX female and an XY male (Hasegawa et al., 2006). Therefore, the sex ratio of B. xylophilus should, in general, be 1:1. However, in this study, the average male to female ratio was 0.57 (range: 0.53–0.67), and no difference in sex ratio among the different treatments was observed. Similar to our results, Liu (2006, pp. 35–36) found no difference in sex ratio among the different fungal-food rearing treatments when the author reared B. xylophilus on three different arboreal fungal strains, Pestalotiopsis microspora M32, Sphaeropsis sapinea E11, and S. sapinea MHS7.3. However, the average sex ratio was 0.35, much lower than that found in our study. A possibility for this discrepancy might be the different nematode strains used.
The evolution of body size may be driven by somatic polyploidisation and cellular proliferation simultaneously (Flemming et al., 2000), and tends to be reduced with spontaneous mutation in the nematode Caenorhabditis briggsae (Ostrow et al., 2007). In this study, emamectin benzoate was found to reduce the body length of B. xylophilus, while, in contrast, avermectin could enhance the body length. This is the first report on a body length stimulatory activity towards B. xylophilus. Interestingly, Zhang et al. (2013) reported that acetochlor could inhibit the body length of C. elegans and Pristionchus pacificus at higher concentrations, whereas enhancing it at lower concentrations. In addition, Hirose et al. (2003) reported that the body size of C. elegans could be controlled by cyclic guanosine monophosphate (cGMP)-dependent protein kinase EGL-4. The nematicidal effect on the growth of B. xylophilus seen in this study could be due to the nematicides interfering with the nematode’s physiological metabolism, like reducing or increasing certain kinases or inhibiting the functions of such enzymes. However, further work will be needed to investigate the mechanisms involved.
The results of this study indicate that emamectin benzoate is the most efficient nematicide against B. xylophilus, followed by avermectin and milbemectin. Emamectin benzoate was more active in suppressing the population numbers of B. xylophilus by both decreasing its fecundity and inhibiting its egg hatchability. Conversely, thiacloprid was found to be weak in inhibiting the reproduction of B. xylophilus. This latter result was to be expected as this compound is used for the control of the vector and not the nematode itself.
Considering their differing effects on the reproduction and development of B. xylophilus, and although they are all GABA inhibitors, we suggest using emamectin benzoate, milbemectin, and avermectin alternatively or integratively for the control of PWD. Further research will, therefore, be necessary on the toxicology and synergistic effects of these three compounds. In addition, studies on their nematicidal mechanisms against the reproduction and development of B. xylophilus will also be needed for developing more specific and effective nematicides.
Literature Cited
- Barbosa P, Faria JMS, Mendes MD, Dias LS, Tinoco MT, Barroso JG, Pedro LG, Figueiredo AC, Mota MM. Bioassays against pinewood nematode: Assessment of suitable dilution agent and screening for bioactive essential oils. Molecules. 2012;17(10):12312–12329. doi: 10.3390/molecules171012312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa P, Lima AS, Vieira P, Dias LS, Tinoco MT, Barroso JG, Pedro LG, Figueiredo AC, Mota MM. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. Journal of Nematology. 2010;42:8–16. [PMC free article] [PubMed] [Google Scholar]
- Bolla RI, Boschert M. Pine wood nematode species complex: Interbreeding potential and chromosome number. Journal of Nematology. 1993;25:227–238. [PMC free article] [PubMed] [Google Scholar]
- Choi NH, Kwon HR, Son SW, Choi GJ, Choi YH, Jang KS, Lee SO, Choi JE, Ngoc LH, Kim JC. Nematicidal activity of malabaricones isolated from Myristica malabarica fruit rinds against Bursaphelenchus xylophilus. Nematology. 2008;10(6):801–807. [Google Scholar]
- Dropkin VH, Foudin AS. Report of the occurrence of Bursaphelenchus lignicolus-induced pine wilt disease in Missouri. Plant Disease. 1979;63:904–905. [Google Scholar]
- Espedal PG, Glover KA, Horsberg TE, Nilsen F. Emamectin benzoate resistance and fitness in laboratory reared salmon lice (Lepeophtheirus salmonis) Aquaculture. 2013;416–417:111–118. [Google Scholar]
- Fabiyi OA, Olatunji GA, Atolani O. Nematicidal activities of chromatographic fractions from Alstonia boonei and bridelia ferruginea on meloidogyne incognita. Pakistan Journal of Nematology. 2012;30:189–198. [Google Scholar]
- Faria JM, Barbosa P, Bennett RN, Mota M, Figueiredo AC. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry. 2013;94:220–228. doi: 10.1016/j.phytochem.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Fielding NJ, Evans HF. The pine wood nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle (= B. lignicolus Mamiya and Kiyohara): An assessment of the current position. Forestry. 1996;96(1):35–45. [Google Scholar]
- Flemming AJ, Shen ZZ, Cunha A, Emmons SW, Leroi AM. Somatic polyploidization and cellular proliferation drive body size evolution in nematodes. PNAS. 2000;97:5285–5290. doi: 10.1073/pnas.97.10.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han QF, Ling LL, Diao HS, Kuang ZY. The test of controlling pine wood nematode disease by method of inject trunk and treatment of root application of insecticide. Guangdong Forestry Science and Technology. 2010;26(5):72–74. (in Chinese). [Google Scholar]
- Hasegawa K, Miwa J. 2008. Embryology and cytology of Bursaphelenchus xylophilus. Pp. 81–104 in B. G. Zhao, K. Futai, J. R. Sutherland, and Y. Takeuchi, eds. Pine Wilt Disease, vol. 2. Tokyo: Springer.
- Hasegawa K, Mota MM, Futai K, Miwa J. Chromosome structure and behaviour in Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae) germ cells and early embryo. Nematology. 2006;8:425–434. [Google Scholar]
- Hirose T, Nakano Y, Nagamstsu Y, Misumi T, Ohta H, Ohshima Y. Cyclic GMP-dependent protein kinase EGL-4 controls body size and lifespan in C. elegans. Development. 2003;130:1089–1099. doi: 10.1242/dev.00330. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Cotton JA, Dalzell JJ, Hasegawa K, Kanzaki N, McVeigh P, Takanashi T, Tsai IJ, Assefa SA, Cock PJ, Otto TD, Hunt M, Reid AJ, Sanchez-Flores A, Tsuchihara K, Yokoi T, Larsson MC, Miwa J, Maule AG, Sahashi N, Jones JT, Berriman M. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathogens. 2011;7(9):1–17. doi: 10.1371/journal.ppat.1002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Sato K, Shibuya M, Zeiger DM, Butcher RA, Ragains JR, Clardy J, Touhara K, Sengupta P. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong JO, Lee SM, Moon YS, Lee SG, Ahn YJ. Nematicidal activity of cassia and cinnamon oil compounds and related compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae) Journal of Nematology. 2007a;39(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Kong JO, Park IK, Choi KC, Shin SC, Ahn YJ. Nematicidal and propagation activities of thyme red and white oil compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae) Journal of Nematology. 2007b;39(3):237–242. [PMC free article] [PubMed] [Google Scholar]
- Lebedev G, Abo-Moch F, Gafni G, Ben-Yakir D, Ghanim M. High-level of resistance to spinosad, emamectin benzoate and carbosulfan in populations of Thrips tabaci collected in Israel. Pest Management Science. 2013;69(2):274–277. doi: 10.1002/ps.3385. [DOI] [PubMed] [Google Scholar]
- Lee YU, Kawasaki I, Lim Y, Oh WS, Paik YK, Shim YH. Inhibition of developmental processes by flavone in Caenorhabditis elegans and its application to the pinewood nematode, Bursaphelenchus xylophilus. Molecules and Cells. 2008;26:171–174. [PubMed] [Google Scholar]
- Liu HM. 2006. Effect of fungi dominantly inhabiting Pinus massoniana and Monochamus alternatus on population dynamics and individual development difference of Bursaphelenchus xylophilus. Master dissertation, Chinese Academy of Forestry, Beijing.
- Mamiya Y, Kiyohara T. Description of Bursaphelenchus lignicolus n. sp. (Nematoda: Aphelenchlididae) from pine wood and histopathology of nematode-infested trees. Nematologica. 1972;18:120–124. [Google Scholar]
- Miller DL, Roth MB. Caenorhabditis elegans are protected from lethal hypoxia by an embryonic diapause. Current Biology. 2009;19:1233–1237. doi: 10.1016/j.cub.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota MM, Braasch H, Bravo MA, Penas AC, Burgermeister W, Metge K, Sousa E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology. 1999;1:727–734. [Google Scholar]
- Ogawa A, Sommer RJ. Strategies to get arrested. Science. 2009;326:944–945. doi: 10.1126/science.1183272. [DOI] [PubMed] [Google Scholar]
- Ostrow D, Phillips N, Avalos A, Blanton D, Boggs A, Keller T, Levy L, Rosenbloom J, Baer CF. Mutational bias for body size in rhabditid nematodes. Genetics. 2007;176:1653–1661. doi: 10.1534/genetics.107.074666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IK, Kim J, Lee SG, Shin SC. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus) Journal of Nematology. 2007;39(3):275–279. [PMC free article] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Sun JH. 2005. Forest invasive species: Country report—P. R. China. Pp. 80–86 in M. Philip, B. Chris, J. H. Sun, and J. Wu, eds. The Unwelcome Guests, Proceedings of the Asia-Pacific Forest Invasive Species Conference. FAO Regional Office for Asia and Pacific, Thailand.
- Sun YC. Bursaphelenchus xylophilus was discovered in Sun Yet-sen’s mausoleum in Nanjing. Journal of Jiangsu Forestry Science and Technology. 1982;4:47. (in Chinese). [Google Scholar]
- Takai K, Soejima T, Suzuki T, Kawazu K. Emamectin benzoate as a candidate for a trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus. Pest Management Science. 2000;56:937–941. [Google Scholar]
- Takai K, Suzuki T, Kawazu K. Distribution and persistence of emamectin benzoate at efficacious concentrations in pine tissues after injection of a liquid formulation. Pest Management Science. 2003;60:42–48. doi: 10.1002/ps.777. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics Society of America. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SY, Yu Y, Liu YJ. Cross-resistance and biochemical resistance mechanisms of emamectin-benzoate resistant population of Frankliniella occidentalis. Acta Phytophylacica Sinica. 2012;39(2):159–165. (in Chinese). [Google Scholar]
- Wu GC, Wang Y, Song GX, Ma FL. Nematocides screening against pine wood nematode in lab. Forest Pest and Disease. 2004;23(5):10–13. (in Chinese). [Google Scholar]
- Yang BJ, Pan HY, Tang J, Wang YY, Wang LF. 2003. Bursaphelenchus xylophilus. Beijing: Chinese Forestry Press.
- Zhang J, Liang W, Wu X, Jiang S, Li Q. Toxic effects of acetochlor on mortality, reproduction and growth of Caenorhabditis elegans and Pristionchus pacificus. Bulletin of Environmental Contamination and Toxicology. 2013;90:364–368. doi: 10.1007/s00128-012-0915-1. [DOI] [PubMed] [Google Scholar]
- Zhao JZ, Collins HL, Li YX, Mau RF, Thompson GD, Hertlein M, Andaloro JT, Boykin R, Shelton AM. Monitoring of diamondback moth (Lepidoptera: Plutellidae) resistance to spinosad, indoxacarb, and emamectin benzoate. Journal of Economic Entomology. 2006;99(1):176–181. doi: 10.1603/0022-0493(2006)099[0176:MODMLP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]