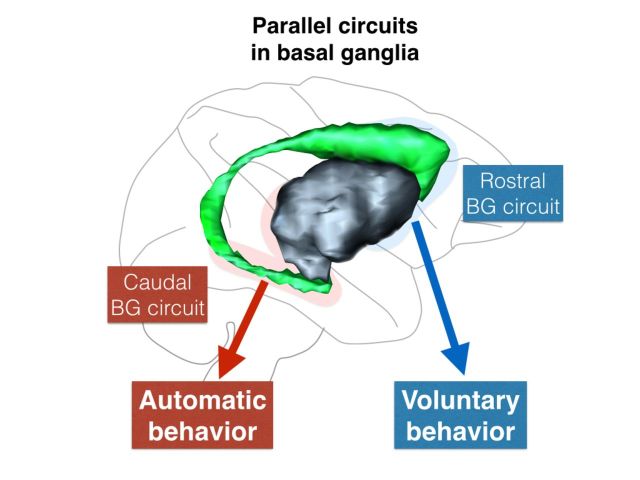
The contributions of topographically distinct basal ganglia circuits to reward-oriented behaviours are unclear. Kim and Hikosaka propose that parallel circuits support flexible voluntary behaviours required to obtain rewards, and stable automatic behaviours involved in manipulation of reward objects. This distinction may inform the diagnosis and treatment of basal ganglia disorders.
Keywords: basal ganglia, reward value, parallel circuit, voluntary behaviour, automatic behaviour
The contributions of topographically distinct basal ganglia circuits to reward-oriented behaviours are unclear. Kim and Hikosaka propose that parallel circuits support flexible voluntary behaviours required to obtain rewards, and stable automatic behaviours involved in manipulation of reward objects. This distinction may inform the diagnosis and treatment of basal ganglia disorders.
Abstract
The basal ganglia control body movements, value processing and decision-making. Many studies have shown that the inputs and outputs of each basal ganglia structure are topographically organized, which suggests that the basal ganglia consist of separate circuits that serve distinct functions. A notable example is the circuits that originate from the rostral (head) and caudal (tail) regions of the caudate nucleus, both of which target the superior colliculus. These two caudate regions encode the reward values of visual objects differently: flexible (short-term) values by the caudate head and stable (long-term) values by the caudate tail. These value signals in the caudate guide the orienting of gaze differently: voluntary saccades by the caudate head circuit and automatic saccades by the caudate tail circuit. Moreover, separate groups of dopamine neurons innervate the caudate head and tail and may selectively guide the flexible and stable learning/memory in the caudate regions. Studies focusing on manual handling of objects also suggest that rostrocaudally separated circuits in the basal ganglia control the action differently. These results suggest that the basal ganglia contain parallel circuits for two steps of goal-directed behaviour: finding valuable objects and manipulating the valuable objects. These parallel circuits may underlie voluntary behaviour and automatic skills, enabling animals (including humans) to adapt to both volatile and stable environments. This understanding of the functions and mechanisms of the basal ganglia parallel circuits may inform the differential diagnosis and treatment of basal ganglia disorders.
Introduction
The basal ganglia control body movements. This is a long-standing concept that has been confirmed repeatedly by animal experiments and human movement disorders. Experimental lesions of various parts of the basal ganglia impair the initiation, execution, and inhibition of spontaneous and planned body movements (Kennard, 1944; Crossman, 1987). Pathological lesions of the human basal ganglia are associated with a variety of movement disorders, including involuntary movements (Denny-Brown, 1968; Albin et al., 1989; Bhatia and Marsden, 1994). A number of neurodegenerative diseases involve the basal ganglia. The most common among them is Parkinson’s disease, which is typically associated with tremor, bradykinesia (akinesia), and rigidity (Crossman, 1987; Jankovic, 2008). Overall, basal ganglia dysfunctions lead to a remarkable set of movement disorders, suggesting that the basal ganglia contain multiple mechanisms for controlling body movements.
However, animals and humans with basal ganglia dysfunctions show deficits that may not simply be classified as movement disorders. For example, animals with large lesions in the striatum may ignore a moving object or obsessively follow it (Denny-Brown, 1962). Patients with Parkinson’s disease may have difficulty in performing two movements simultaneously (Schwab et al., 1954) or in learning of probabilistic classification (Knowlton et al., 1996). Dopamine deficiency in the striatum leads to severe contralateral hemi-neglect in monkeys (Miyashita et al., 1995). Humans with restricted lesions in the caudate nucleus or the globus pallidus may be unable to initiate everyday behaviours spontaneously (Laplane and Baulac, 1984; Caplan et al., 1990). Patients with Parkinson’s disease may be less motivated in achieving goals and may also show symptoms of depression (Pluck and Brown, 2002).
These observations, as well as many others not described here, suggest that the basal ganglia are involved in many mental processes, including motor, sensory, learning, memory, cognitive, executive, decision-making, motivational, and emotional functions.
Such diverse functionality makes it challenging to identify the essential principles of the basal ganglia function. One strategy for approaching this complexity in a tractable manner is to focus on one aspect of behaviour in which the basal ganglia are deeply involved. To this end, we chose ‘reward-oriented behaviour’ which is likely to require all the mental processes listed above. By discussing reward-oriented behaviour in view of diverse mental processes, we can cover a wide range of the diverse functionality, and do so in a logically connected manner. Conversely, by discussing each mental process in relation to reward-oriented behaviour, we may be able to reveal new aspects of the mental process.
To approach this general goal, we first discuss general features of basal ganglia circuits and functions for reward-oriented behaviour, and then discuss a new feature of basal ganglia function mostly by focusing on our recent studies. The last section is devoted to basal ganglia dysfunctions that are related to reward-oriented behaviour.
Because we will focus on reward-oriented behaviour, especially in relation to our study, this article may not cover some of the important concepts of the basal ganglia and related literature. Instead, we explore the literature widely in relation to reward-oriented behaviour, some of which have been rarely discussed in basal ganglia research. This exploratory approach may raise more questions and therefore our discussions may often be speculative. By doing so, however, we hope this article will trigger discussions among readers, potentially leading to novel concepts of the basal ganglia.
Basal ganglia circuits for selection of behaviour
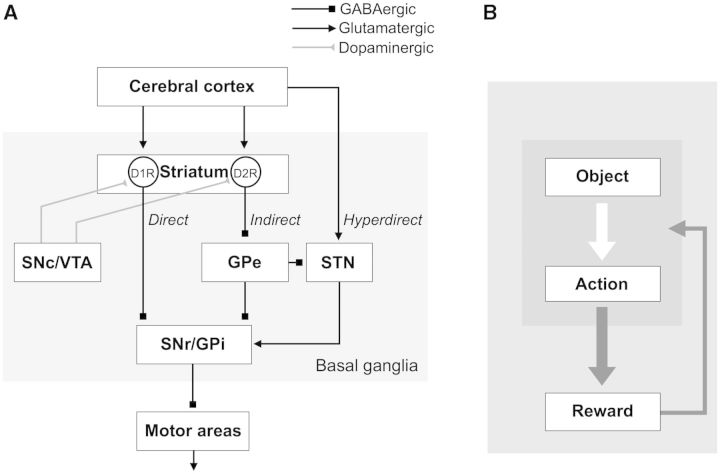
The basal ganglia play an important role in the selection of behaviour. This hypothesis is supported by their parallel organization of their component circuitry—direct, indirect, and hyperdirect pathways (Fig. 1A). We first discuss how these pathways may work.
Figure 1.
Basal ganglia circuits for reward-oriented learned behaviour. (A) Direct, indirect and hyperdirect pathways. The striatum receives inputs mainly from the cerebral cortex. D1R-expressing neurons in the striatum connect to SNr/GPi directly (direct pathway). D2R-expressing neurons connect to SNr/GPi indirectly through GPe and STN (indirect pathway). STN receives inputs directly from the cerebral cortex and send outputs to SNr/GPi (hyperdirect pathway). Dopaminergic neurons in SNc/VTA heavily innervate the striatum. (B) Two processes for skill behaviour. Finding a high-valued object among many objects requires object skill, and manipulating the high-valued object requires action skill. D1R = dopamine receptor D1; D2R = dopamine receptor D2.
Direct, indirect and hyperdirect pathways in basal ganglia
The brain contains many motor pattern generators, each of which is devoted to a particular body movement (Grillner et al., 1998) such as gaze orienting (Sparks, 2002), locomotion/posture (Takakusaki et al., 2004), vocalization (Hage and Jurgens, 2006), reaching/grasping (Kinoshita et al., 2012), and eating/drinking (Nakamura and Katakura, 1995). Triggered by particular sensory inputs or internal states, these mechanisms can work independently to generate adaptive movements (e.g. vestibulo-ocular reflex). However, the whole behaviour could become uncontrollable if these motor mechanisms are allowed to be active by simply following their own rules.
How then can the brain solve the uncontrollable situation? An efficient way would be to set up a mechanism to suppress all of the motor mechanisms. The basal ganglia appear to perform this function. Their final output neurons are all GABAergic and inhibitory, are highly active continuously (Hikosaka, 2007a), and are connected to these motor mechanisms (Takakusaki et al., 2004; Grillner et al., 2005). They are located in two structures: substantia nigra pars reticulata (SNr) and globus pallidus internal segment (GPi) (Hikosaka, 2007a) (Fig. 1A). Indeed, humans and monkeys with basal ganglia dysfunction often show involuntary movements (Crossman, 1987; DeLong and Wichmann, 2007), which may be caused by disruption of SNr/GPi-mediated inhibition (Hikosaka and Wurtz, 1985).
However, the SNr/GPi-induced inhibitions must be weakened in certain contexts. Otherwise, all movements would remain suppressed, which may be a cause of akinesia in patients with Parkinson’s disease (Wichmann and DeLong, 1996). The weakening occurs via GABAergic inhibitory connections from the striatum (direct pathway) (Chevalier and Deniau, 1990), and the net effect is a reduction of inhibition (i.e. disinhibition) (Hikosaka et al., 2000). In addition, SNr/GPi neurons receive indirect inputs from the striatum via the globus pallidus external segment (GPe) and possibly the subthalamic nucleus (STN) (indirect pathway) (Smith et al., 1998). As both striatal output neurons and GPe neurons are GABAergic and inhibitory, the net effect may be an enhancement of inhibition. The combination of the direct and indirect pathways can, theoretically, make a selection among a repertoire of body movements, which may be called ‘motor action’ (Mink, 1996; Hikosaka et al., 2000; Hikida et al., 2010; Kravitz et al., 2010).
The hyperdirect pathway (Nambu et al., 2002) appears to act as a prominent suppressor of ongoing body movements. It is mediated by the STN, which consists of glutamatergic excitatory neurons (unlike most neurons in the basal ganglia) (Robledo and Feger, 1990) and transmits signals quickly from the cerebral cortex to SNr/GPi (Nambu et al., 2000), thereby suppressing body movements. Its major function seems to be behavioural switching (Aron and Poldrack, 2006; Isoda and Hikosaka, 2008), in that it suppresses quick and automatic movements so that slow and voluntary movements can be initiated. Damage of STN thus leads to severe involuntary movements (hemiballismus) (Crossman et al., 1984).
Role of basal ganglia in reward-oriented behaviour
The evidence considered thus far indicates that the basal ganglia are equipped with machinery that is clearly suited to behavioural selection. However, a good mechanism requires a good motive, and a universal motive is reward (Dayan and Balleine, 2002). Indeed, activity of neurons in various parts of the basal ganglia is strongly modulated by reward, especially by the expectation of reward (Hikosaka et al., 1989a; Schultz et al., 1992; Bowman et al., 1996; Kawagoe et al., 1998; Lauwereyns et al., 2002; Sato and Hikosaka, 2002; Takikawa et al., 2002a). A general hypothesis is that the direct pathway mainly processes reward-predicting signals thereby facilitating reward-oriented movements, whereas the indirect pathway mainly processes non-reward-predicting signals thereby suppressing unrewarded movements. These two pathways together thus constitute a reward-oriented motor action (Frank, 2005; Hikosaka, 2007b; Hong and Hikosaka, 2011). Experiments using reward-biased behavioural tasks have yielded results that were consistent with this hypothesis (Nakamura and Hikosaka, 2006; Hikida et al., 2010).
Strong support for the role of the basal ganglia in reward-oriented behaviour derives from the prevalence of dopamine effects among many vertebrate species (Richfield et al., 1987). The striatum, in particular, receives dense projections from dopamine neurons located in the substantia nigra pars compacta (SNc), ventral tegmental area (VTA), and surrounding areas (Haber et al., 2000; Joel and Weiner, 2000; Ikemoto, 2007) (Fig. 1A). These dopamine neurons are sensitive to rewards and typically encoding reward prediction error (RPE), in that they are excited by increases in reward value and inhibited by decreases (Schultz, 1998). The reward-related signals of dopamine neurons may affect the activity and synaptic transmissions of striatal neurons (Nicola et al., 2000; Reynolds and Wickens, 2002; Calabresi et al., 2007; Surmeier et al., 2007; Kreitzer and Malenka, 2008). As individual striatal neurons encode sensorimotor, cognitive or emotional signals (Crutcher and DeLong, 1984; Nishino et al., 1984; Hikosaka et al., 1989b, c; Kimura, 1990; Kimura et al., 1992; Kermadi and Joseph, 1995), their outputs would be modified by the predicted change in reward outcome. This mechanism may underlie the reward-related activity changes in striatal neurons that have been repeatedly observed (Hikosaka et al., 1989a; Apicella et al., 1992; Schultz et al., 1992; Kawagoe et al., 1998).
Notably, dopamine neurons appear to differentially affect two groups of striatal neurons through different receptors: direct pathway neurons through D1 receptors and indirect pathway neurons through D2 receptors (Gerfen et al., 1990). As the effects of these receptors are mostly opposite (i.e. D1: facilitatory, D2: inhibitory) (West and Grace, 2002; Eyny and Horvitz, 2003; Mallet et al., 2006; Nakamura and Hikosaka, 2006; Shen et al., 2008), when reward is expected, the direct pathway neurons would be more active whereas the indirect pathway neurons would be less active (Hong and Hikosaka, 2011). These opposing actions of dopamine within the striatum are consistent with the general hypothesis described above. Accordingly, the loss of dopamine inputs to the striatum would then incapacitate the selection process controlled by the direct and indirect pathways, namely the execution of rewarded actions and suppression of unrewarded actions. This model may explain the pathological consequences of dopamine depletion in Parkinson’s disease, which include loss of control over various body movements including locomotion/posture (Morris, 2000; Jankovic, 2008), vocalization (Robbins et al., 1986), reaching/grasping (Bennett et al., 1995; Morris, 2000), manipulation (Fellows et al., 1998), eye movements (Chan et al., 2005), and eating/drinking (Robbins et al., 1986).
However, motor action by itself is not sufficient to obtain rewards. To obtain a reward, an animal must find the valuable object (e.g. ripe apple) before executing the action (e.g. reach and grasp) (Hikosaka et al., 2013) (Fig. 1B). Finding valuable objects requires sensory information associated with reward values, which may reflect common inputs of the basal ganglia from sensory cortical areas (Kemp and Powell, 1970; Saint-Cyr et al., 1990; Flaherty and Graybiel, 1991). We will focus on this issue in the later part of this article.
Anatomical segregation of basal ganglia
There are two steps to reach rewards: (i) find valuable objects using multiple sensory signals; and (ii) manipulate the objects using multiple motor signals. Furthermore, reward-oriented behaviour needs to be regulated by various factors such as attention, motivation, context, uncertainty, and assessment of risk (Dayan and Balleine, 2002; Doya, 2008; Gottlieb, 2012). All of these signals are relayed to the basal ganglia, primarily in the striatum, which could therefore process them either separately or integratively. A prominent source of these signals is the cerebral cortex, which may in turn give rise to the functional specialization within the striatum: limbic functions more medially versus sensorimotor functions more laterally (Parent, 1990; Brown et al., 1998; Haber et al., 2000). This medial-lateral functional topography is present in both rodents and primates (Yin and Knowlton, 2006; Balleine and O’Doherty, 2010).
The evolution of primates has resulted in a unique topographical organization along the rostral-caudal axis which may reflect further functional specialization: associative versus sensorimotor (Parent, 1990; Lehéricy et al., 2004a, b; Draganski et al., 2008). The rostral part of the putamen and caudate nucleus primarily serve associative (or cognitive) functions by receiving inputs mainly from the prefrontal cortical areas (Fig. 2A). In contrast, their caudal parts primarily serve sensorimotor functions, with the caudal putamen receiving inputs from the skeletal sensorimotor cortical areas (Lehéricy et al., 2004a). Particularly remarkable is the caudate nucleus of primates (monkeys and humans), which runs through the frontal and parietal lobes and finally reaches the temporal lobe. The shape of the caudate changes considerably along the rostral to caudal span of the structure as it progresses through three subregions including the head (caudate head), body (caudate body), and tail (caudate tail) (Fig. 2A). Following this topography, caudate head receives inputs mainly from the frontal cortex (Lehéricy et al., 2004b; Draganski et al., 2008; Haber and Knutson, 2010), whereas caudate tail receives inputs mainly from the temporal cortex (Kemp and Powell, 1970; Yeterian and Van Hoesen, 1978; Saint-Cyr et al., 1990).
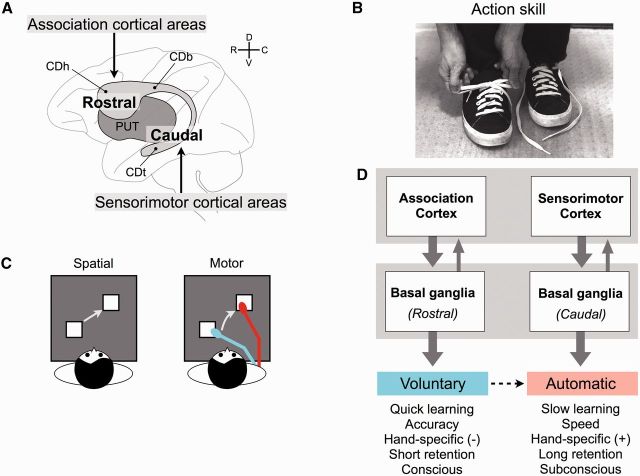
Figure 2.
Basal ganglia mechanisms for learning motor actions. (A) Differential cortical inputs to the striatum. The rostral striatum receives inputs mainly from the associative cortical areas. The caudal striatum receives inputs mainly from the sensorimotor cortical areas. The striatum includes the caudate nucleus (grey) and putamen (PUT) (dark grey). Caudate head (CDh), caudate body (CDb), and caudate tail (CDt) belong to the rostral, intermediate, and caudal striatum, respectively. R, C, D, V = rostral, caudal, dorsal, ventral. (B) An example of action skill (tying shoe laces) that consists of a sequence of many movements. (C) Two stages in learning of sequential movements. In the early learning stage, the movements are based on the spatial coordinates of target objects, and therefore are not specific to the learned hand and are performed consciously. In the late learning stage, the movements are based on the motor coordinates of the performing hand, and therefore are specific to the learned hand and are performed subconsciously. (D) Contrasting two separate mechanisms of action skill learning. Voluntary performance, which is learned quickly but is not retained for a long time, is controlled by the rostral basal ganglia circuit together with the association cortex. Automatic performance, which is learned slowly but is retained for a long time, is controlled by the caudal basal ganglia circuit together with the sensorimotor cortex.
These results need not imply that multiple signals are processed separately in the whole basal ganglia. They may converge in the downstream circuits (Fig. 1A) because the downstream areas (i.e. SNr/GPi, GPe, STN) are smaller and contain fewer neurons than the striatum (Percheron et al., 1984; Flaherty and Graybiel, 1993). However, anatomical studies have suggested that each of these areas (e.g. SNr) receives topographically organized inputs from the striatum (François et al., 1994) and likewise sends topographic outputs (Parent, 1990; Hoover and Strick, 1999; Middleton and Strick, 2002). The topographical separation may be maintained through the loop circuit mediated by the thalamus (e.g. basal ganglia – thalamus – cortex – basal ganglia) (Alexander et al., 1986; Middleton and Strick, 1996). As do the targets of the basal ganglia, the cerebral cortex contains diverse circuits that control learned body movements (e.g. fine finger movements) in the motor cortex (Jackson et al., 2006) and learned behavioural control (e.g. context-dependent decision) in the prefrontal cortex (Mansouri et al., 2009; Rushworth et al., 2011). In addition, the basal ganglia send their outputs through SNr/GPi to several brainstem regions (Hikosaka et al., 2000) that contain specific neural circuits devoted to innate body movements (pattern generators) (Grillner, 2003), as described above.
These findings suggest that different parts of the basal ganglia work independently as parallel circuits to control different behaviours. However, this hypothesis faces a basic question: how do these parallel circuits coordinate with each other? This question is important because everyday behaviour is composed of multiple motor and mental activities and yet is directed toward beneficial outcomes. In the rest of this article, we will discuss how the multiple circuits in the basal ganglia contribute to two fundamental behaviours—sequential motor learning and visual object-value learning—both of which are commonly used in our daily life.
Learning of sequential motor action
Action skill and basal ganglia
In daily life, we often physically manipulate objects. When you go out, you may change your shirt, button the shirt, wear shoes, tie shoelaces, open the door, walk out, close the door, lock the door, and walk to your car. Such a daily routine consists of a sequence of many movements (Fig. 2B). These behaviours may be called ‘action skills’ (Hikosaka et al., 2013), which have been acquired through long-term learning (Ericsson and Lehmann, 1996) and can be performed automatically (without conscious attention) (Seger, 1994; Destrebecqz and Cleeremans, 2001). In fact, our daily routines consist of a variety of action skills. Without such automatic action skills, we would spend an enormous amount of time and energy before approaching a goal. Animals also naturally develop a variety of action skills after long-term learning (Helton, 2008).
Action skills are acquired by repeated practice, during which performance changes drastically (Fitts, 1964; Anderson, 1982). In the early phase, the motor performance is done consciously and slowly with many errors (i.e. failure to reach the final goal). In the late phase, the motor performance is done automatically and quickly with few errors. With long-term learning, the time to complete one action becomes shorter, often up to 1/10 of the original time (Crossman, 1959; Newell and Rosenbloom, 1981). Once an action skill is acquired, it is hardly abolished (Ammons et al., 1958; Hikosaka et al., 2002). Therefore, we can acquire (or have acquired) many action skills, all of which together allow us to carry out daily routines smoothly. This suggests that the brains of humans and animals have the capacity to acquire and store long-term memories that are expressed as a variety of action skills.
Many studies on humans, monkeys, and rodents have suggested that the basal ganglia contribute to action skill performance and its learning (Whishaw et al., 1986; Hikosaka et al., 2002; Packard and Knowlton, 2002; Graybiel, 2008). A series of studies on monkeys and humans suggested that different parts of the basal ganglia contribute to action skill learning and performance in different manners (Hikosaka et al., 1999). In these studies, the subjects learned to press buttons sequentially in a spatially fixed order across many days using the left or right hand (Hikosaka et al., 1995). After experience with new sequences, they eventually acquired a repertoire of many learned sequences, half of which were learned with the left hand and the other half with the right hand. Both monkeys and humans learned to perform each sequence more accurately and faster with similar time courses across daily learning sessions: first, their performance became accurate (mostly <5 days), after which it continued to be faster (>10 days).
Detailed behavioural tests suggested that two separate learning mechanisms contribute to this action skill learning. The first mechanism that was not specific to the effector (i.e. hand) used for learning, whereas the second mechanism was specific to the effector (Seger and Spiering, 2011; Abrahamse et al., 2013). In experiments using monkeys, changing the hand with which they performed the task disrupted the learned performance only after extensive training (Rand et al., 2000). Thus, a non-hand-specific mechanism learned the sequence quickly, mainly to achieve the accurate performance, whereas a hand-specific mechanism learned the sequence slowly, mainly to achieve the fast performance (Fig. 2C). Moreover, the fast performance was retained for a long time with no further practice (6–19 months), but only when the same hand was used (Hikosaka et al., 2002). Human subjects also retained the well-learned performance for a long time (16 months), while retaining its speed more than accuracy, and yet had little awareness about their previous experiences (unlike the recently learned sequence) (Hikosaka et al., 2002). Therefore, these two mechanisms may be characterized as voluntary and automatic learning mechanisms (Fig. 2C and D). The voluntary mechanism would rely on signals encoding the spatial positions of the target buttons and thus can guide performance whichever hand is used, whereas the automatic mechanism would rely on signals encoding the movement of the performing hand and thus can guide the performance of one particular hand (Nakahara et al., 2001).
Different roles of rostral and caudal basal ganglia in action skill learning
The involvement of the basal ganglia in action skill learning was tested in two ways: reversible inactivation and single unit recording. The performance in the early learning phase was impaired by the inactivation of the rostral striatum (caudate head and rostral putamen), whereas the performance in the late learning phase was impaired by the inactivation of the caudal striatum (intermediate and caudal putamen) (Miyachi et al., 1997). Correspondingly, neurons in the rostral striatum tended to be more active in the early learning phase, whereas neurons in the caudal striatum tended to be more active in the late learning phase (Miyachi et al., 2002). These results suggest that the rostral striatum contributes to the voluntary learning mechanism, whereas the caudal striatum contributes to the automatic learning mechanism (Fig. 2D).
It is known that the rostral striatum receives inputs mainly from the associative region of the cerebral cortex (Selemon and Goldman-rakic, 1985; Parent, 1990; Haber et al., 2006) and may send signals back to the association cortex through a loop circuit (SNr/GPi – thalamus – cortex) (Alexander et al., 1986). In contrast, the caudal striatum is connected mainly with the sensorimotor region of the cortex (Kunzle, 1975; Flaherty and Graybiel, 1991; Takada et al., 1998). The rostral striatum processes visuospatial, attentional, working memory, and reward signals (Jueptner et al., 1997b; Lewis et al., 2004; Ding and Gold, 2010), together with the association cortex (Haber et al., 2006). The automatic signals in the caudal striatum may be related to sensorimotor signals derived from joints and muscles (Crutcher and DeLong, 1984; Alexander and DeLong, 1985; Kimura, 1986). The emergence of the effector (i.e. hand) selectivity during learning may be related to the emergence of automaticity or implicitness in action skill, because explicit control would not be affected by which hand is used.
Consistent with the experiments using monkeys described above, human functional MRI studies have shown that different regions in the basal ganglia and other brain areas become active depending on learning phases (Lehéricy et al., 2005; Jankowski et al., 2009; de Wit et al., 2012; Wunderlich et al., 2012; Wymbs et al., 2012): the association cortex (dorsolateral/dorsomedial prefrontal, parietal cortices) together with the rostral striatum is active in the early phase (Jueptner et al., 1997a; Tricomi et al., 2004; Poldrack et al., 2005; Monchi et al., 2006; Jankowski et al., 2009), while the sensorimotor cortex (M1/premotor cortex) together with the caudal striatum is active in the late phase (Jueptner et al., 1997a; Jankowski et al., 2009; Tricomi et al., 2009) when the performance becomes implicit (Rauch et al., 1997).
The basal ganglia are also essential for action learning in rodents (Koralek et al., 2012) and birds (Charlesworth et al., 2012), which in both cases are assisted by dopamine inputs (Faure et al., 2005). Equivalent functional regions for motor learning are found in rodents: dorsomedial striatum for the early phase of learning and the dorsolateral striatum for the late phase of learning (Yin et al., 2009). This may reflect the fact that the primate brain has been extended rostrocaudally compared with the rodent brain. If so, the dorsomedial striatum in rodents may correspond to caudate head in monkeys, while the dorsolateral striatum may correspond to the caudal putamen (Balleine and O’Doherty, 2010). These two striatal regions are often associated with two kinds of behaviour: goal-directed behaviour and habit (Yin and Knowlton, 2006; de Wit et al., 2009). In daily life, however, habit is often goal-directed (Aarts and Dijksterhuis, 2000; Wood and Neal, 2007) and may be better characterized as ‘skill’.
Gaze orienting to valuable objects
Object skill
The learning of motor actions is critical for all animals because it increases the chance of survival (Hikosaka et al., 2013). However, such learned motor actions are often guided by learned cognitive processes, and here also the basal ganglia play an important role (Graybiel, 2008). In most cases a reward is associated with an object (e.g. walnut). To reach the walnut-associated reward, an animal has to go through two processes (Fig. 1B): (i) find a walnut; and (ii) crack the walnut. Cracking a walnut requires an action skill (Takechi et al., 2009). Finding a walnut also requires another kind of skill (Pyke et al., 1977; Kamil and Roitblat, 1985), which may be called ‘object skill’ (Hikosaka et al., 2013). For primates, the finding process heavily depends on visual information and its behavioural outcome is gaze orienting. Among many objects, one is chosen at a time and gaze is oriented to it (with a saccadic eye movement) (Yarbus et al., 1967; Henderson, 2003; Land, 2006; Tatler et al., 2011). Furthermore, each object needs to be evaluated before gaze is settled on the most valuable object.
The fact that the basal ganglia may be involved in object skill is hinted at by their influence on the superior colliculus (SC) whose major function is to initiate orienting responses (Ingle, 1973; Carman and Schneider, 1992). Comparative anatomical studies suggest that superior colliculus is a major target of the basal ganglia among vertebrate species (Marín et al., 1998). In rats, a prominent effect of unilateral lesions or dysfunctions (including dopamine deficiency) of the striatum is spontaneous turning of the body and head, mostly to the ipsilateral side (Pycock, 1980). Inactivation of SNr causes involuntary saccades to the contralateral side in monkeys (Hikosaka and Wurtz, 1985) and rats (Sakamoto and Hikosaka, 1989). In monkeys, local dopamine deficiency, which is caused by injection of MPTP in the caudate head or caudate body, leads to contralateral hemineglect of gaze and attention (Kato et al., 1995; Kori et al., 1995; Miyashita et al., 1995). Interestingly, superior colliculus is targeted by SNr, but not GPi (Beckstead and Frankfurter, 1982). These observations imply that the control of orienting response is a major and distinctive function of the basal ganglia.
Basal ganglia circuit for object skill
In the basal ganglia, the signal for gaze orienting (saccade) is first processed in the striatum including caudate nucleus and putamen (Hikosaka et al., 1989; Gerardin et al., 2003; Neggers et al., 2012; Phillips and Everling, 2012), possibly more dominantly in the caudate nucleus (CD) for monkeys (Hikosaka et al., 2000) and putamen for humans (Neggers et al., 2015). Studies in monkeys have mostly focused on the CD-SNr-SC circuit (Hikosaka et al., 2000). Before eye movements, a group of SNr neurons that project to superior colliculus pause in their tonic firing and thus cause a disinhibition of superior colliculus neurons (Hikosaka and Wurtz, 1983a). The cessation of SNr neuronal activity is mostly caused by phasic firing of caudate head/caudate body neurons, which project to SNr (Hikosaka et al., 1993). Importantly, saccades are heavily biased toward the spatial position where reward is expected (Hikosaka et al., 2006). When a saccade is followed by a reward in one direction and no reward in the other direction, its reaction time is shorter and its speed is higher for the reward-associated direction (Takikawa et al., 2002b). Many neurons in caudate head/caudate body, SNr, and superior colliculus respond more strongly to the reward-predicting visual cues (Kawagoe et al., 1998; Sato and Hikosaka, 2002; Ikeda and Hikosaka, 2003) or show a reward direction-selective anticipatory changes (Lauwereyns et al., 2002; Ikeda and Hikosaka, 2003). These behavioural and neuronal changes occur quickly after a couple of repetitions (Kawagoe et al., 1998). These results suggest that neurons in the CD-SNr-SC circuit contribute to reward-guided gaze orienting by changing their signals flexibly (Itoh et al., 2003). These data provide deeper understanding about how the basal ganglia circuits (Fig. 1A) contribute to reward-oriented behaviour. Specifically, the CD-SNr-SC circuit plays a key role in finding valuable positions.
Does the CD-SNr-SC circuit also contribute to finding valuable objects (i.e. object skill)? This was tested by associating multiple visual objects with different amounts of reward. In a first experiment using two visual objects (fractals) (e.g. A and B), the reward association was reversed after a block of 30–40 trials (i.e. A-large/B-small versus A-small/B-large) (Fig. 3B, left). Neurons responding to visual objects were found in the central part of caudate head. Most of them were sensitive to the immediate reward outcome, usually responding more strongly when the object was associated with a large reward (Fig. 3B, centre) (Kim and Hikosaka, 2013). As expected from the CD-SNr-SC circuit scheme, the monkey made a saccade more quickly when a large reward was expected.
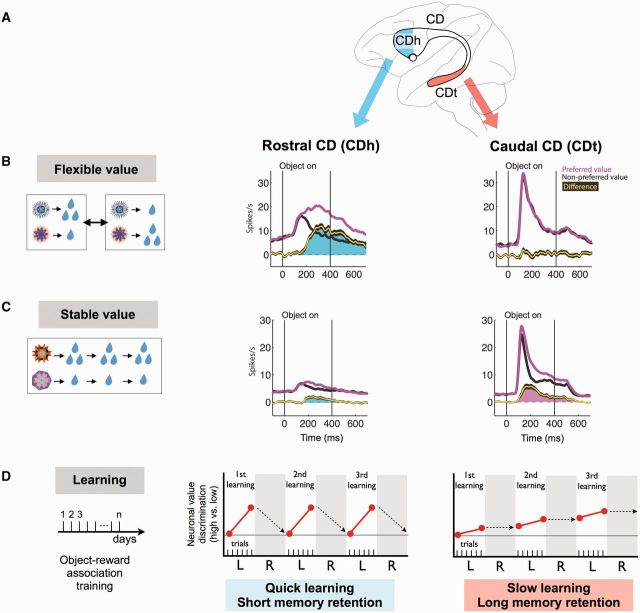
Figure 3.
Basal ganglia mechanisms for finding valuable objects. (A) Caudate nucleus (CD) of the macaque monkey. (B and C) Neuronal responses in the rostral caudate nucleus (CDh) and the caudal caudate nucleus (CDt) in response to visual objects with flexibly changing values (B) and stably fixed values (C). Neuronal responses were averaged for the neurons’ preferred (magenta) and non-preferred (black) values. The yellow line indicates the difference between the preferred and non-preferred responses [mean (yellow) ± SE (black)]. To test the neuronal responses to stable object values (C), the monkey had experienced each object consistently with a big or small reward or a long time (D), but during the test no reward was delivered after the object presentation. (D) Time course of object value learning for caudate head neurons (centre) and caudate tail neurons (right). The monkey experienced stable object-reward associations for several days of sessions (left). Neuronal value discrimination is shown schematically during learning (‘L’) and memory retention (‘R’). Reproduced with permission from Kim and Hikosaka (2013).
The values assigned to these objects can be called ‘flexible values’. In everyday life, however, values of many objects do not change (e.g. favourite foods since childhood), which can be called ‘stable values’. To simulate this everyday life situation, many fractal objects were presented repeatedly across many days, and each object was consistently associated with a large or small reward (Fig. 3C, left). When these objects were subsequently presented with no reward outcome, the caudate head neurons showed much weaker responses with little value bias (Fig. 3C, centre). Apparently, the lack of reward outcome caused the attenuation of the caudate head neuron’s response. According to the CD-SNr-SC circuit scheme, this would lead to the attenuation of the monkey’s gaze bias.
Surprisingly, however, the monkey’s gaze orienting changed dramatically, but slowly (i.e. over 5 days): when multiple objects were presented simultaneously, the monkey’s gaze was strongly attracted to the stably high-valued objects, even though no reward was delivered (Fig. 4A and B) (Yasuda et al., 2012; Kim and Hikosaka, 2013; Yamamoto et al., 2013). Such automatic gaze orienting emerged as the monkey experienced many more fractals (up to 400), and then remained intact for a long time (>100 days) during which the monkey never saw the fractals (Yasuda et al., 2012).
Figure 4.
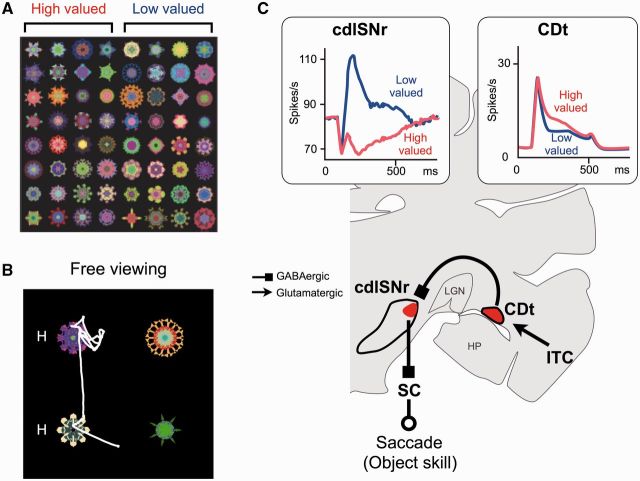
Stable object value coding in caudal basal ganglia circuit. (A) Fractal objects associated with stable reward values, half associated with a large reward (high-valued) and the other half associated with a small reward (low-valued). (B) Free-viewing procedure. Stably high- and low-valued objects were chosen randomly and presented simultaneously. The monkey’s gaze was strongly attracted to the previously learned high-valued objects (‘H’), even though no reward was given. (C) CDt–cdlSNr–SC circuit for object skill. Neurons in caudate tail (CDt) and cdlSNr (caudal-dorsal-lateral part of substantia nigra pars reticulata) encoded stable values of objects (in boxes). In response to high-valued objects (compared with low-valued objects), caudate tail neurons were more excited, cdlSNr neurons were more inhibited, and therefore superior colliculus (SC) neurons were more excited (disinhibited). This pattern of activity account for the facilitation of saccades to the high-valued objects. ITC = inferotemporal cortex; LGN = lateral geniculate nucleus; HP = hippocampus. Reproduced with permission from Yasuda et al. (2012) and Kim et al. (2014).
What causes the automatic gaze orienting with no reward outcome? One candidate is the caudate tail, which is a slender caudal extension of the caudate nucleus (Figs 2A and 3A) and is mostly unique to primates (Hjornevik et al., 2007). It receives inputs mainly from the inferior temporal cortex (Kemp and Powell, 1970; Yeterian and Van Hoesen, 1978; Saint-Cyr et al., 1990), which is specialized for processing of visual object information (Tanaka, 1996). Similarly to inferior temporal cortex neurons, a majority of neurons in monkey caudate tail respond to visual objects differentially (Caan et al., 1984; Brown et al., 1995; Yamamoto et al., 2012).
Importantly, caudate tail neurons responded differently from caudate head neurons in relation to object values. The visual responses of caudate tail neurons were not influenced by flexibly changing values (Fig. 3B, right), but were modulated by the stable value, usually responding more strongly to stably high-valued objects, even under conditions when no reward was expected (Kim and Hikosaka, 2013; Yamamoto et al., 2013) (Fig. 3C, right). The idea that caudate tail contributes to the automatic gaze orienting to stably high-valued objects was further supported by its robust oculomotor output (Fig. 4C).
Unlike inferior temporal cortex neurons, whose receptive fields usually include the fovea (Gross et al., 1969; Tanaka, 1996), many caudate tail neurons have eccentric receptive fields, mostly in the contralateral hemifield (Yamamoto et al., 2012). Furthermore, weak electrical stimulation of caudate tail induced saccades aimed at the nearby neurons’ receptive fields (Yamamoto et al., 2012). This effect was mediated by a cluster of neurons in the caudal-dorsal-lateral (cdl) part of SNr (Saint-Cyr et al., 1990; Kim et al., 2014; Yasuda and Hikosaka, 2015), many of which project to superior colliculus (Beckstead and Frankfurter, 1982; Francois et al., 1984). cdlSNr roughly corresponds to the region called ‘pars lateralis’, which is particularly prominent in primates (Francois et al., 1985) (Fig. 4C).
cdlSNr neurons showed categorical responses in that they were inhibited by stably high-valued objects and excited by stably low-valued objects (Fig. 4C), and did so for as many objects as the monkey experienced (e.g. 400) (Yasuda et al., 2012). They were not influenced by flexible values, similarly to caudate tail (CDt) neurons. Moreover, the categorical responses remained virtually unchanged for a long time, similarly to the automatic gaze orienting. Finally, many of the stable value-coding SNr neurons projected their axons to superior colliculus, as determined by antidromic activation (Yasuda et al., 2012).
These results suggest that the CDt-cdlSNr-SC circuit processes stable values, but not flexible values, of visual objects (Fig. 4C). This system evidently has a high capacity for long-term memory, and specifically encodes information about the association of visual objects and reward values. The object-value memory appears to be strengthened or purified along the caudate tail-cdlSNr connection. This mechanism would allow the animal to quickly find a valuable object among many others, without checking individual items. The ‘finding’ is expressed as gaze orienting through the cdlSNr-superior colliculus connection, so that the animal is ready to manipulate the valuable object. Such ‘object skill’ would be crucial for survival, and the responsible mechanism (CDt-cdlSNr-SC circuit) is prominently developed in primates (Hikosaka et al., 2013).
Automatic versus voluntary gaze orienting
However, the object skill mechanism has a flaw, namely slow learning (Fig. 3D, right). If you encounter a new object, or if the value of an object has changed, the CDt-cdlSNr-SC circuit is unable to judge their values. This weakness is compensated for by the caudate head (CDh) circuit, because neurons in the caudate head are sensitive to the immediate reward outcome and thus learn the values of new objects quickly (Fig. 3D, centre) (Kim and Hikosaka, 2013). Importantly, the downstream components are largely separate between caudate tail and caudate head circuits. The visually responsive part of the caudate head also has direct connections to SNr, but selectively targets the rostral-ventral-medial (rvm) part of SNr (Smith and Parent, 1986). This region roughly corresponds to the pars reticulata proper of the SNr, and is distinct from the pars lateralis that receives input from the caudate tail (Francois et al., 1985). Neurons in the rvmSNr are indeed sensitive to flexible values (Yasuda and Hikosaka, 2015), and some of them project to superior colliculus (Francois et al., 1984; Yasuda and Hikosaka, 2015). The caudate body, or intermediate region of the caudate nucleus, contains a mixture of stable value neurons and flexible value neurons (Kim and Hikosaka, 2013) and projects mainly to rvmSNr (Hikosaka et al., 1993).
These results suggest that the CD-SNr-SC circuit, as a whole, operates as a reward value-based gaze orienting mechanism, but it is roughly divided into two parallel circuits: CDt-cdlSNr-SC circuit for automatic finding of stably valuable objects and CDh-rvmSNr-SC circuit for voluntary finding of flexibly valuable objects. For more details, see above and Fig. 6.
Figure 6.
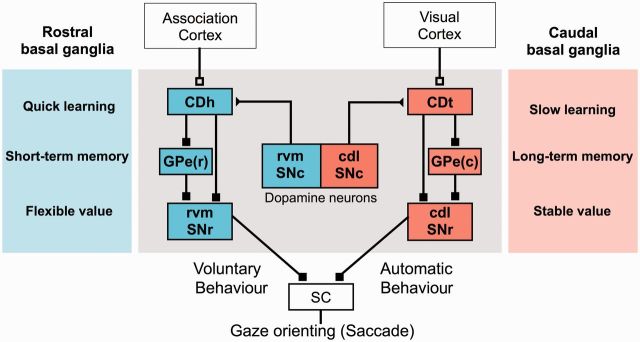
Parallel basal ganglia circuits for voluntary and automatic behaviour. Receiving inputs from different cortical areas, caudate head (CDh) and caudate tail (CDt) have separate downstream circuits, both targeting superior colliculus (SC). Both circuits process object–value memory and learning, but do so under different conditions. These two learning mechanisms lead to different outcomes, namely voluntary and automatic behaviour. Separate groups of dopamine neurons may contribute to the different kinds of memory processing. GPe(r) = rostral part of globus pallidus external segment; GPe(c) = caudal part of globus pallidus external segment.
Dopamine circuits for differential reward value coding
Functional heterogeneity of dopamine neurons
Many areas in the basal ganglia, especially the striatum, are heavily innervated by dopamine neurons (Richfield et al., 1987). It has repeatedly been shown that activity and synaptic transmission of striatal neurons are modulated by dopamine inputs (Nicola et al., 2000; Reynolds and Wickens, 2002; Wise, 2004; Surmeier et al., 2007), mostly through D1 or D2 receptors (Fig. 1). The finding that dopamine neurons encode RPE signals (Schultz, 1998) triggered a set of new theories on reward-based learning: the dopamine-RPE signal biases sensorimotor signals in the striatum stepwise until the gain of reward is maximized (Dayan and Balleine, 2002). According to this unified theory, dopamine neurons would transmit the unique signal (i.e. RPE) to all brain areas. However, earlier studies (Schultz and Romo, 1987) as well as more recent studies, showed heterogeneities among dopamine neurons (Bromberg-Martin et al., 2010). First, dopamine neurons in different regions of SNc and VTA receive inputs from and send outputs to different regions of the brain (Haber et al., 2000; Ikemoto, 2007). Second, different groups of dopamine neurons encode different signals. In particular, many dopamine neurons are excited by punishment or its predictor (Horvitz, 2000; Feenstra et al., 2001; Coizet et al., 2006; Brischoux et al., 2009; Matsumoto and Hikosaka, 2009; Lammel et al., 2011). Dopamine neurons in the dorsolateral part of the monkey SNc are excited by both reward-predicting and punishment-predicting stimuli (thus encoding ‘motivational salience’). This response pattern is clearly different from dopamine neurons in the ventromedial SNc, which are excited by reward-predicting stimuli but inhibited by punishment-predicting stimuli (thus encoding ‘motivational value’). RPE is clearly encoded by the value-coding dopamine neurons, but not the salience-coding dopamine neurons, partly due to differential inputs from the lateral habenula (Matsumoto and Hikosaka, 2007). These results raise the possibility that different groups of dopamine neurons serve heterogeneous functions through their connections from and to different basal ganglia circuits.
Separate groups of dopamine neurons projecting to caudate tail and caudate head
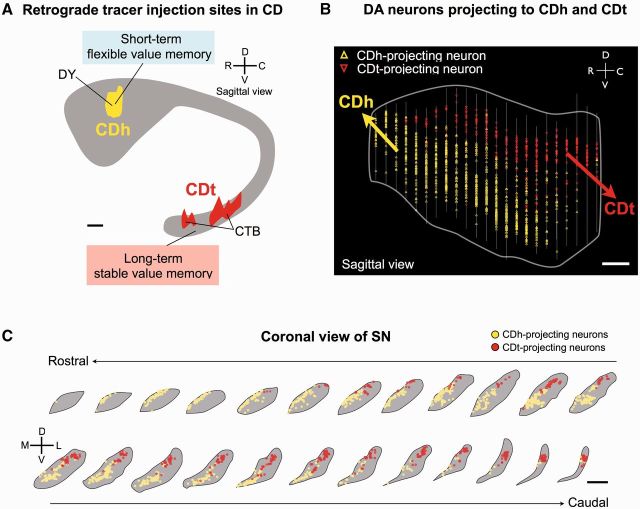
This hypothesis was tested for dopamine neurons projecting to caudate nucleus in monkeys, where neurons change their responses to visual objects depending on their reward values (Kim and Hikosaka, 2013). This neuronal learning might be guided by dopamine inputs, as all parts of monkey caudate nucleus are innervated by dopamine neurons (Richfield et al., 1987). However, the time course of the neuronal learning is different between caudate tail (i.e. slow) and caudate head (i.e. quick). The difference might be caused intrinsically (within caudate nucleus) or extrinsically (due to external inputs). Supporting the latter possibility (but not excluding the former possibility), a tracer study revealed that caudate tail and caudate head are innervated by different groups of dopamine neurons (Kim et al., 2014) (Fig. 5). Neurons in the cdl part of SNc projected to caudate tail, whereas neurons in the rvm part of SNc projected to caudate head (Fig. 5B and C); few neurons projected to both caudate tail and caudate head. These caudate tail and caudate head-projecting neurons were all dopaminergic (as far as examined). Intriguingly, caudate tail-projecting dopamine neurons in cdlSNc tend to have larger and less circular cell bodies than caudate head-projecting dopamine neurons in rvmSNc (Kim et al., 2014). These connections lead to an intriguing topographic relationship between the caudate nucleus-SNr connection and the SNc-caudate nucleus connection: caudate tail-projecting dopaminergic neurons and caudate tail-recipient GABAergic neurons are located adjacently (cdlSNc and cdlSNr); caudate head-projecting dopaminergic neurons and caudate head-recipient GABAergic neurons are located adjacently (rvmSNc and rvmSNr).
Figure 5.
Separate dopamine projections to caudate head and caudate tail. (A) Injection sites of retrograde tracers. Two different retrograde tracers, Diamidino yellow (DY) and cholera toxin subunit B (CTB), were injected in caudate head (CDh) and caudate tail (CDt) of the same monkey. (B and C) Retrogradely labelled neurons in the SN (B: sagittal view, C: coronal view). Yellow triangles: caudate head-projecting neurons; red triangles: caudate tail-projecting neurons in B. Scale bar = 1 mm. In C, coronal sections of SN are shown from the rostral to caudal levels. Scale bar = 2 mm. DA = dopamine. Reproduced with permission from Kim et al. (2014).
The topography of dopamine neurons described above may represent one of many mutual connections between dopamine neurons and the striatum in monkeys (Haber et al., 2000) and rodents (Ikemoto, 2007). In general, dopamine neurons located medially (in VTA/SNc) and laterally (in SNc) are connected with the medial and lateral parts of the striatum, respectively. The medial-lateral topography may reflect a functional specialization: goal-directed versus habitual (Yin and Knowlton, 2006). Primates have developed another functional topography in the rostral-caudal direction in the cerebral cortex as well as the striatum (Lehéricy et al., 2004b; Draganski et al., 2008), which may reflect another kind of functional specialization: associative versus sensorimotor (Parent, 1990; François et al., 1994) (Fig. 2). Anatomical studies have suggested that the rostral and caudal striatum receive inputs mainly from dopamine neurons in the medial and lateral parts of SNc, respectively (Szabo, 1980; Francois et al., 1984; Hedreen and DeLong, 1991). These findings raise the possibility that dopamine neurons in different subregions of SNc or VTA serve different functions by projecting to different subregions of the striatum, as observed in caudate tail- and caudate head-projecting dopamine neurons (Fig. 5).
Particularly relevant to this hypothesis is a finding from patients with Parkinson’s disease, in whom the degeneration of dopamine neurons occurs heterogeneously (Fearnley and Lees, 1991; Damier et al., 1999). A common finding is that the degeneration occurs earlier and dominantly in the lateral part of SNc (Goto et al., 1989; Rinne et al., 1989; Yamada et al., 1990; Fearnley and Lees, 1991; Gibb and Lees, 1991), although this tendency may not be universal (Pillon et al., 1986) partly due to the heterogeneity of Parkinson’s disease itself (Zetusky et al., 1985). According to the topographical schemes described above, patients with Parkinson’s disease would show impairments in sensorimotor functions more strongly than the emotional or cognitive impairments. This will be discussed more comprehensively below.
Other neuromodulators
Dopamine neurons are controlled by inputs from many brain areas (Haber and Knutson, 2010; Watabe-Uchida et al., 2012). Functionally significant among them are the input from the lateral habenula through the rostromedial tegmental nucleus (Hong et al., 2011; Barrot et al., 2012) and the input from the pedunculopontine nucleus (Scarnati et al., 1987; Hong and Hikosaka, 2014), which are organized topographically.
In addition to dopamine, there are other neuromodulators acting on basal ganglia circuits, including serotonin, acetylcholine, and norepinephrine (Doya, 2008). Both serotonin and dopamine neurons may encode reward values, but differently (Nakamura et al., 2008), thus influencing motivation and mood differently (Proulx et al., 2014). Acetylcholine is delivered both internally within the striatum (Lehmann and Langer, 1983) and externally from the pedunculopontine nucleus (Mena-Segovia et al., 2008), and may interact with dopamine (Di Chiara et al., 1994; Chuhma et al., 2014). Neuromodulators may work together with classical neurotransmitters (e.g. GABA, glutamate) as well as other neuromodulators (Charara et al., 1996). The interactions among the modulators and transmitters may be critical for the intricate function of the basal ganglia.
Parallel circuits for voluntary and automatic behaviour
Finding valuable objects is a critical step to obtaining rewards (Fig. 1B), and recent studies revealed that the basal ganglia play a crucial role in this process (Kim and Hikosaka, 2013; Hikosaka et al., 2014). To reach this goal, the basal ganglia are equipped with two parallel mechanisms (Fig. 6), each of which consists of the elementary circuits—direct pathway, indirect pathway, and dopamine input to striatum (Fig. 1A). These circuits share a common goal (i.e. gaze orienting) by targeting superior colliculus, but they process the values of visual objects differently: (i) stably as long-term memories; and (ii) flexibly as short-term memories. Before reaching superior colliculus, these signals are processed in the same basal ganglia nuclei (caudate nucleus and SNr), but in separate regions (caudate tail versus caudate head, cdlSNr versus rvmSNr). Both circuits receive dopamine inputs, but again from separate regions (cdlSNc versus rvmSNc). A main mediator of the indirect pathway, GPe, also seems separate: caudate tail projects to the caudal-ventral part of GPe (Saint-Cyr et al., 1990), whereas caudate head projects to the rostral-dorsal part of GPe (Cowan and Powell, 1966; Parent et al., 1984). The separation of the two mechanisms seems reasonable, because the flexible and stable values are often mutually conflicting (stability-flexibility dilemma) (Liljenström, 2003; Abraham and Robins, 2005; Anderson, 2007).
According to this scheme (Fig. 6), gaze orienting can rely more strongly either on the stable circuit or on the flexible circuit, which makes the system more adaptable. In a familiar and stable environment, you encounter many objects (e.g. foods), which you have previously seen many times and therefore can well predict their values (i.e. tastes) before experiencing them. In this scenario, the CDt-cdlSNr-SC circuit would inhibit or excite superior colliculus depending on the stably fixed values of the objects (Fig. 3C, right), but the CDh-rvmSNr-SC circuit would provide no biased signals (Fig. 3B, right). Gaze would then be oriented to the previously reward-associated object automatically (automatic saccade), because caudate tail neurons and cdlSNr neurons respond to the object even when there is no reward outcome.
Occasionally however, familiar objects change their values unpredictably (i.e. sweet to sour orange). Or, if you encounter novel objects, you cannot predict their values. In these cases, you need to pick some of the objects and check their values (e.g. by tasting them). Then, CDh-rvmSNr-SC circuit would inhibit or excite superior colliculus depending on the flexibly changing values of the objects (Fig. 3B, centre), but the CDt-cdlSNr-SC circuit would provide no biased signals (Fig. 3C, centre). Gaze would be oriented to the object only if it has recently been associated with reward (voluntary saccade), because caudate head neurons and rvmSNr neurons respond to the objects only when they are expected to be associated with reward.
Novel objects would gradually become familiar if you experience them repeatedly. For experiences that are associated with rewards, the flexible CDh-rvmSNr-SC circuit would be active initially, but activity within this circuit would gradually be replaced by the activation of the stable CDt-cdlSNr-SC circuit until ‘object skill’ is established. This shift of the active site in the basal ganglia is similar to what happens during motor learning that results in ‘action skill’ (Fig. 2). In both cases, the active site shifts from the rostral to caudal part of the basal ganglia. Also common to the action and object systems is cortical inputs: the flexible-early mechanism receives inputs mainly from the association cortex, whereas the stable-late mechanism receives inputs from the sensorimotor cortex (c.f. caudate tail mostly from the sensory cortex).
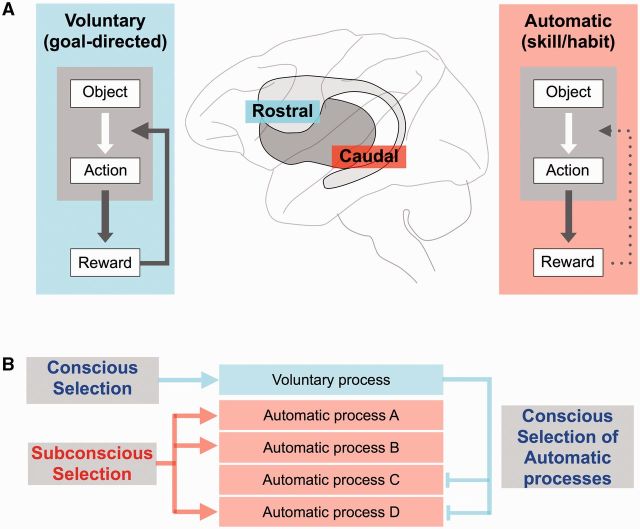
This model for separate parallel circuits mediating voluntary and automatic function (Figs 6 and 7A) is further supported by behavioural findings. Many kinds of motor learning occur equally with or without conscious control (Destrebecqz and Cleeremans, 2001; Maxwell et al., 2001). Conscious control could even worsen the performance, because the automatic learning occurs independently of the conscious control (Mazzoni and Krakauer, 2006). Attending to one’s own skilled performance often impairs that performance (Baumeister, 1984; Beilock and Carr, 2001). Performing another task simultaneously may even improve the skilled performance, potentially by directing conscious attention elsewhere (Beilock et al., 2002). These behavioural findings suggest that the automatic skilled process can operate independently of the voluntary conscious process, and moreover that independent operation is the optimal state of skilled performance. To this end, the automatic and voluntary mechanisms should be separated, as we propose (Fig. 7A).
Figure 7.
Interaction between voluntary and automatic behaviour—a hypothesis. (A) Voluntary and automatic mechanisms are separated between rostral and caudal basal ganglia circuits. Each mechanism controls behaviour in two steps: finding good objects; and manipulating the good objects, and is modulated by reward outcomes. Critically, the reward effect is strong but temporary for the voluntary mechanism and weak but cumulative for the automatic mechanism. (B) Separate versus interactive operations of voluntary and automatic mechanisms. These mechanisms may operate independently, with voluntary mechanism being guided consciously while multiple automatic mechanisms being guided subconsciously (left). Alternatively, they may interact with each other, with the automatic mechanisms consciously selected by the voluntary mechanism (right), or the voluntary mechanism triggered by automatic mechanisms (not shown).
However, the strict dichotomy of memory into short-term versus long-term systems may not be correct. The time course of memory might vary in a graded manner. If so, the scheme should be composed of multiple mechanisms with different memory time courses. Being located between caudate head and caudate tail, caudate body shows intermediate properties (i.e. between short-term and long-term) on average (Kim and Hikosaka, 2013). The dichotomy scheme may also not be completely correct if there are neuronal connections across the two mechanisms. This might occur through the indirect pathway (Yasuda and Hikosaka, 2015), but few data are available showing the exact connections through this polysynaptic pathway (i.e. through GPe and possibly STN). Such across-mechanism connections, if they exist, might provide a higher level of adaptability: utilize long-term memories in a flexible environment or short-term memories in a stable environment.
We so far have considered that the automatic and voluntary mechanisms aim at the same motor goal (e.g. gaze orienting). More generally, however, this may not be the case. For instance, when you cook, several automatic processes operate simultaneously (e.g. meat cutting, flour mixing, egg breaking, and pan tossing) while your active voluntary process aims at the final outcome of the cooked food (Fig. 7B). In this case, multiple automatic mechanisms and one voluntary mechanism operate in parallel, but aim at a higher level common goal that involves multiple motor and mental processes. Unlike the common motor goal scheme (Fig. 6), these multiple parallel mechanisms may not always operate together. Instead, different sets of the automatic mechanisms should be activated depending on different contexts, and this has been investigated using ‘set-shifting tasks’ (as described in the next section). This procedure requires functional interactions between the automatic and voluntary mechanisms: the voluntary mechanism would be responsible for the selection of the automatic mechanisms, whereas the automatic mechanisms would provide the voluntary mechanism with long-term memory-based information (which would be helpful for the selection) (Ericsson and Kintsch, 1995).
Behavioural deficits caused by basal ganglia dysfunctions
We have discussed how the parallel neural circuits in the basal ganglia are organized and how they operate to achieve beneficial behaviours. The discussion provides a good opportunity to examine and reinterpret the behavioural effects of basal ganglia disorders, as shown below. To this end, we mostly focus on Parkinson’s disease, for which a large amount of clinical and experimental data are available.
Deficits in action skill
The proposed scheme (Fig. 6) predicts that dysfunctions of the basal ganglia cause different behavioural impairments depending on which region is mainly affected: automatic behaviour by caudal dysfunctions versus voluntary behaviour by rostral dysfunctions (Fig. 7A). In Parkinson’s disease, cell degeneration tends to occur earlier and dominantly in dopamine neurons in the lateral part of SNc, which project mainly to the caudal striatum. As the caudal striatum, especially caudal putamen, primarily serves skeletal sensorimotor functions (Alexander and DeLong, 1985; Liles and Updyke, 1985; Kimura et al., 1996), patients with Parkinson’s disease often show skeletomotor dysfunctions, including akinesia, rigidity, and tremor (Crossman, 1987; Jankovic, 2008). Although the patients often have difficulty in initiating a movement (akinesia), the movement could be initiated in special contexts (Marsden, 1980; Glickstein and Stein, 1991), such as in response to abrupt sensory inputs (Schwab et al., 1959), planned sensory cues (Morris et al., 1996), or emotional events (Schwab and Zieper, 1965). These observations imply that the basic motor mechanisms located in the brainstem or spinal cord are largely intact in Parkinson’s disease.
Why then do patients with Parkinson’s disease struggle in execution of daily routines? Some hints have been suggested by behavioural tests. They have difficulty in generating ballistic actions accurately, which are characteristic of normal skilled movements (Flowers, 1976). Patients with Parkinson’s disease may perform a single movement fairly well, but often have difficulty in performing two movements simultaneously (Schwab et al., 1954; Benecke et al., 1986). For instance, the act of leaving the house could involve walking to the entrance while also being ready to grab the door knob, although these movements are usually performed automatically and subconsciously. The whole daily routine consists of so many automatic movements, some of which are performed simultaneously (Fig. 7B, also see section ‘Learning of sequential motor action’). These considerations suggest that, in Parkinson’s disease, the automatic processes do not work efficiently (Redgrave et al., 2010) and this deficit is caused by the dysfunction of the caudal basal ganglia (Fig. 7A).
It is now useful to consider how the automatic and voluntary processes would work together. Our hypothesis is illustrated in Fig. 7B. In daily routine, a single voluntary process and multiple automatic processes would work simultaneously and independently. Having seen an object of interest (e.g. the door knob), the voluntary process may be focused on how you drive to the store you are heading to, while automatic process A may prepare for grabbing the knob and automatic process B may generate walking. All of the automatic processes would work in a coordinated manner, due to coordinated long-term learning. In an extreme case of Parkinson’s disease, all of these processes may be no longer automatic, but instead need to be performed voluntarily with full attention. The voluntary process would then need to deal with all the multiple processes sequentially one at a time. Such a demand could be so high in patients with Parkinson’s disease (as suggested by functional MRI studies) (Wu and Hallett, 2005) that the main goal of the voluntary process (i.e. how to drive to the store) can scarcely be processed. The whole daily routine would thus be performed sequentially, and therefore slowed down (Koerts et al., 2011) and often with mental fatigue (Friedman et al., 2007).
Importantly, the automatic processes are acquired after long-term practice (which would be called ‘action skill’) (Hikosaka et al., 2013). Indeed, patients with Parkinson’s disease often have difficulty in reaching a normal level of automaticity. This was suggested by using various motor tasks (Doyon et al., 1997), including a serial reaction time task (Jackson et al., 1995; Stefanova et al., 2000) and a pursuit rotor task (Heindel et al., 1989). Even if patients with Parkinson’s disease do learn action skills, their skills are not retained for a long time, in contrast to patients with Alzheimer’s disease (Mochizuki-Kawai et al., 2004). These results suggest that the learning and retention mechanisms in the caudal basal ganglia are compromised in Parkinson’s disease, partly due to the lack of dopamine inputs to the caudal striatum (Redgrave et al., 2010).
In Huntington’s disease, on the other hand, cell loss occurs predominantly in the striatum (Vonsattel et al., 1985) and patients with Huntington’s disease show deficits in learning of action skills (Heindel et al., 1988; Gabrieli et al., 1997), especially learning of sequential movements (Knopman and Nissen, 1991; Willingham and Koroshetz, 1993; Willingham et al., 1997).
Deficits in object skill
Action skill is triggered by an object of interest (e.g. door knob) (i.e. stimulus-response theory) (de Wit et al., 2009). In other words, in order to initiate an automatic action, the relevant object must be found first. This is often demanding and time-consuming because we are surrounded by so many objects, as shown in visual search tasks (Treisman and Gelade, 1980). However, with repeated experiences in daily life, the object-finding task becomes easier and can be performed subconsciously (Shiffrin and Schneider, 1977; Chun and Jiang, 1998; Sigman and Gilbert, 2000). This learned ability can be called object skill (Hikosaka et al., 2013), as described above. Dysfunctions of the basal ganglia seem to disrupt object skill, in addition to action skill.
The basal ganglia are also involved in sensory processing. The striatum receives inputs from sensory cortices: somatosensory (Flaherty and Graybiel, 1991; Graziano and Gross, 1993), visual (Hikosaka et al., 1989c; Kimura, 1990; Graziano and Gross, 1993; Kermadi and Joseph, 1995; Ding and Hikosaka, 2006), and auditory (Hikosaka et al., 1989c). In primates, basal ganglia dysfunctions are sometimes associated with visual deficits (Bodis-Wollner, 1990; Lieb et al., 1999). Human patients with Parkinson’s disease often experience visual hallucinations (Diederich et al., 2009). Monkeys with local dopamine denervation of caudate head or caudate body (by local MPTP infusion) show strong contralateral hemi-neglect of gaze and attention (Kori et al., 1995; Miyashita et al., 1995). Electrical stimulation of caudate head and caudate tail impairs monkeys’ performance of a delayed visual discrimination task (Cohen, 1972). Patients with Parkinson’s disease occasionally have visual defects similar to hemi-neglect (Villardita et al., 1983). While scanning complex visual images, patients with Parkinson’s disease make fewer saccades with smaller amplitudes (Matsumoto et al., 2011; Archibald et al., 2013). They may identify fewer landmarks and traffic signs during driving (Uc et al., 2006), may not benefit from the repetition of a complex visual pattern (van Asselen et al., 2009), or may be slower in detecting visual images appearing out of noise (Meppelink et al., 2008). However, these deficits are different from loss of vision; they seem related to the visual memory-based behavioural control rather than visual perception, as shown below.
Amnesia is a loss of memory that is usually caused by dysfunctions of the hippocampus and adjacent structures (Mishkin, 1978). However, the loss of memory in amnesic patients is only partial, if memory is broadly defined as the information created and retained in the brain. In fact, amnesic patients can learn a variety of behaviours, although they may not consciously remember what they have done. For example, they can learn to perform the ‘mirror reading task’ (i.e. reading texts in which the orientation of letters is reversed) (Cohen and Squire, 1980) and the ‘weather forecast task’ (i.e. predicting the correct answer by viewing multiple pictures, each of which is associated with the answer probabilistically) (Knowlton et al., 1996), similarly to control subjects. These findings suggested that there is another kind of memory that is not controlled by the hippocampal area. A likely brain area responsible for this other kind of memory is the basal ganglia. This is supported by the findings that patients with Parkinson’s disease have difficulty in learning the mirror reading task (Roncacci et al., 1996; Yamadori et al., 1996) and the weather forecast task (Knowlton et al., 1996). These two kinds of memory are called declarative memory (mainly controlled by the hippocampal area) and procedural memory (mainly controlled by the basal ganglia).
Unlike declarative memory, procedural memory directly contributes to decision-making. Procedural memory tasks (e.g. mirror reading, weather forecast) typically require the transformation of visual signals to motor outputs. Of particular interest is a concurrent discrimination task developed by Mishkin and colleagues (Malamut et al., 1984) for experiments using monkeys. In this task, the monkeys learned which of two objects to choose in order to obtain a reward. The task involved learning multiple pairs of objects, which in normal monkeys occurred slowly across days. Importantly, the learning was impaired by caudate tail lesions (Fernandez-Ruiz et al., 2001), but not hippocampal lesions (Malamut et al., 1984). Equivalent results are found in human patients. Patients with hippocampal lesions are amnesic, but can normally learn the concurrent discrimination task (Bayley et al., 2005). In contrast, patients with Parkinson’s disease (but not control subjects) are impaired in the concurrent discrimination learning if they are unaware of the cue-reward relationships; both of them showed normal learning if they are aware of the relationships (Moody et al., 2010). These findings provide further evidence that declarative memory is processed by the hippocampal region and that procedural memory is processed in the basal ganglia. According to the recent study on monkeys (Hikosaka et al., 2013), the visually driven procedural memory (i.e. object skill) is processed by a specific mechanism (CDt-cdlSNr-SC circuit) in the basal ganglia, and this mechanism is likely impaired in Parkinson’s disease.
It has been reported that cell loss occurs first in caudate tail among striatal areas in Huntington’s disease (Vonsattel et al., 1985; Gomez-Tortosa et al., 2001). This raises the possibility that object skill is impaired in patients with Huntington’s disease. Indeed, patients with Huntington’s disease are severely impaired in the ‘weather forecast task’ (Knowlton et al., 1996). They are also slow in finding the target object in a visual search task (Lawrence et al., 2000). Possibly related to these findings, patients with Huntington’s disease show impairments in saccadic eye movements (Leigh et al., 1983; Lasker et al., 1987, 1988; Winograd-Gurvich et al., 2003).
Deficits in voluntary behaviour
The impairment of automatic behaviour in patients with Parkinson’s disease is consistent with the degeneration of dopamine neurons, primarily in the lateral SNc in Parkinson’s disease. However, there are signs that they may also be deficient in voluntary behaviours (Stern et al., 1983). Voluntary behaviour is flexible as it relies on short-term memory (Fig. 6), which is the key to many cognitive functions (e.g. cognitive flexibility) (Gathercole, 1999). Indeed, patients with Parkinson’s disease show deficits in visual short-term memory (especially working memory) (Zokaei et al., 2014) and in performing various cognitive tasks (Brown and Marsden, 1990). Working memory is often used to predict upcoming events, which allows one to prepare for the next action (Beauchamp et al., 2003; Lewis et al., 2004). Such predictive behaviour may be lost in patients with Parkinson’s disease (Flowers and Downing, 1978; Stern et al., 1983; Bronstein and Kennard, 1985), in which case they rely on sensory cues to trigger their behaviour (Cooke and Brown, 1979). These behavioural changes may be related to the dysfunctions of the rostral portions of the basal ganglia (Fig. 7A), which contain many neurons encoding the preparation of actions (Lauwereyns et al., 2002; Miyachi et al., 2002) or the prediction of events (particularly reward) (Hikosaka et al., 1989a).
Such a neuron-behaviour correlation is shown clearly using a memory-guided saccade task (Hikosaka and Wurtz, 1983b), in which a saccade is guided by spatial working memory. Some neurons in caudate head/caudate body (Hikosaka et al., 1989b) and SNr (Hikosaka and Wurtz, 1983b) change their activity with memory-guided saccades (but not visually guided saccades). Patients with Parkinson’s disease have more difficulty in making memory-guided saccades than visually-guided saccades (Crawford et al., 1989; Nakamura et al., 1994; Jackson et al., 1995; Chan et al., 2005; Terao et al., 2011). They are instead more likely distracted by visual stimuli (Terao et al., 2011). These results support the hypothesis that the rostral basal ganglia serve voluntary behaviour. They also suggest that the function of the rostral basal ganglia is also compromised in Parkinson’s disease (Fig. 7A).
Another critical role of cognitive flexibility based on short-term memory is new learning (Kehagia et al., 2010), in which the choice of behaviour must be modified flexibly when outcomes changes. Neurons in caudate head quickly encode value memory of objects at the onset of learning, but neurons in caudate tail do not (Fig. 3B) (Kim and Hikosaka, 2013). It is theorized that RPE encoded by dopamine neurons is used to repeat or avoid the behaviour (Schultz et al., 1997). As the basal ganglia nuclei are heavily innervated by dopamine neurons, RPE-related dopamine signals may help new learning through basal ganglia circuits by activating its rostral portion, particularly caudate head (Tricomi et al., 2004; Hikosaka et al., 2006; Wunderlich et al., 2012). The fact that patients with Parkinson’s disease are slow in new learning (Frith et al., 1986) may be due to the dysfunction of the rostral basal ganglia in Parkinson’s disease.
Patients with Parkinson’s disease are also deficient in switching and set shifting (Cools, 1980; Gauntlett-Gilbert et al., 1999; Cools et al., 2001), in which cognitive flexibility is critical (Cools et al., 2001). As discussed above, daily routine behaviour could be generated by simultaneous or sequential activation of multiple automatic processes (Fig. 7B). However, if the context changes unexpectedly, the automatic processes may need to be suppressed so that the voluntary process can switch behaviour. This kind of behavioural switching can be achieved by the activation of STN (Isoda and Hikosaka, 2008), which receives context change signals from the pre-supplementary motor area (pre-SMA) (Isoda and Hikosaka, 2007; Nachev et al., 2008; Chao et al., 2009) or other cortical areas (Aron and Poldrack, 2006). This mechanism may be deficient in Parkinson’s disease (Witt et al., 2004).
Switching as described above may reflect a conflict between automatic and voluntary processes, which has been tested using various tasks (e.g. pro- versus anti-saccade) (Cameron et al., 2010). However, there are different kinds of behavioural switching. Switching may occur between different voluntary processes, for example, in tasks in which each stimulus changes its value between two conditions (e.g. A-large/B-small versus A-small/B-large). This switching between two states for a given stimulus may be called intra-dimensional set-shifting (Owen et al., 1991). Alternatively, switching may occur between automatic processes and be guided by the voluntary process, for example in tasks in which each stimulus has two features (e.g. colour and shape) and the value of the stimulus is determined by its colour (Condition 1) or shape (Condition 2). The switching between the ‘colour’ and ‘shape’ conditions may be called extra-dimensional set-shifting (Owen et al., 1991).
Patients with Parkinson’s disease seem to have difficulty in any of these kinds of switching. However, it is unclear whether these deficits are caused by the dysfunction of the rostral basal ganglia. First, the responsible brain region might be the frontal cortex (Taylor et al., 1986), because Parkinson’s disease may affect the frontal cortex as well (Scatton et al., 1983) and dysfunctions of the frontal cortex are also associated with switching deficits (Owen et al., 1993). Second, dysfunctions of automatic processes (rather than voluntary process) could cause switching deficits if the switching involves an automatic process (Koerts et al., 2009; Redgrave et al., 2010).
Patients with Huntington’s disease also show a wide range of cognitive impairments including deficits in visuospatial memory, executive functions, and extra-dimensional set-shifting (Lawrence et al., 1996). These impairments may be related to deficits in inhibitory control mechanisms in the basal ganglia (Lawrence et al., 1998), which would be crucial for shifts of attention (Sprengelmeyer et al., 1995).
Future issues
We have proposed that the basal ganglia utilize short-term and long-term memories in separate circuits to control behaviour voluntarily as well as automatically, and that these functions are guided by separate groups of dopamine neurons. The voluntary and automatic circuits are separated within each of the basal ganglia, nuclei mainly in the rostral-caudal direction (Figs 6 and 7A). This model in turn raises many questions that may steer the direction of future research on the basal ganglia. We discuss some future issues below.
Time course of memory
The difference between the voluntary and automatic circuits could be explained by the time course of memory: quick learning and unlearning for the voluntary circuit, and slow learning and unlearning for the automatic circuit. What factors give rise to these different time courses? It has been hypothesized that reward-based learning is guided by dopamine inputs to the striatum that induce long-term plasticity of cortico-striatal synapses (Reynolds and Wickens, 2002). The fact that the voluntary and automatic circuits receive inputs from separate groups of dopamine neurons raises the possibility that the time course of the synaptic plasticity is determined uniquely by each group of dopamine neurons. However, it is not currently known whether the properties of synaptic plasticity in the striatum vary across different dopamine inputs. Moreover, synaptic plasticity has been investigated mostly in vitro, and consequently over time, scales too short to be comparable to the time course of memory formation in reward-seeking animals. Since learning under real world conditions can easily exceed 1 year for the automatic circuit that processes skill memory, this issue remains a challenging question for future research of memory.
Storage of memory
The striatal synaptic plasticity theory (above) implies that procedural memory is stored in the cortico-striatal synapses. To test this hypothesis, we need to identify the signal carried by individual neurons along the basal ganglia circuits. For the object skill learning, the signal carried by caudate tail neurons expressing object-value memory is further enhanced in the downstream structure—cdlSNr. This enhancement could be caused by the separation of caudate tail signals to the direct pathway and the GPe-mediated indirect pathway before converging to cdlSNr. It is thus possible that synaptic plasticity occurs in a dispersed manner across the caudate tail, GPe, and cdlSNr. The synaptic changes underlying memory may be more dispersed for action skill learning, because it involves the cerebellum and the motor cortex (in addition to the basal ganglia) through the cortex-basal ganglia and cortex-cerebellum loop circuits (Hikosaka et al., 1999). To summarize, how procedural memory is localized or distributed remains to be studied.
Interaction between voluntary and automatic processes
If the voluntary and automatic circuits operate independently, how can their signals be integrated to produce an appropriate behaviour? We raise three possibilities. First, these signals are integrated only in the common target region (e.g. superior colliculus): whichever signal is more robust is translated to the behaviour (if the two signals are incongruent). Second, these signals are integrated as a consequence of a behaviour: for example, if gaze is oriented automatically to a stably valuable object, the updated gaze may trigger voluntary processes or vice versa. Third, these signals are integrated by neuronal connections between the voluntary and automatic circuits, which is not shown in our scheme because clear evidence is lacking.
Funding
This research was supported by the Intramural Research Program at the National Institutes of Health, National Eye Institute.
Acknowledgements
We thank D. McMahon, A. Ghazizadeh, M. Yasuda, W. Grigg, and A. Hidetoshi for comments and discussions, and M.K. Smith, D. Parker, I. Bunea, G. Tansey, A.M. Nichols, T.W. Ruffner, J.W. McClurkin, and A.V. Hays for technical assistance.
Glossary
Abbreviations
- cdl
caudal-dorsal-lateral
- GPe
globus pallidus external segment
- GPi
globus pallidus internal segment
- SC
superior colliculus
- SMA
supplementary motor area
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- SNc
substantia nigra pars compacta
- VTA
ventral tegmental area
- RPE
reward prediction error
- rvm
rostral-ventral-medial
References
- Aarts H, Dijksterhuis A. Habits as knowledge structures: automaticity in goal-directed behavior. J Pers Soc Psychol 2000; 78: 53–63. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Robins A. Memory retention—the synaptic stability versus plasticity dilemma. Trends Neurosci 2005; 28: 73–8. [DOI] [PubMed] [Google Scholar]
- Abrahamse EL, Ruitenberg MFL, de Kleine E, Verwey WB. Control of automated behavior: insights from the discrete sequence production task. Front Hum Neurosci 2013; 7: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 1989; 12: 366–75. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol 1985; 53: 1417–30. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9: 357–81. [DOI] [PubMed] [Google Scholar]
- Ammons RB, Farr RG, Bloch E, Neumann E, Dey M, Marion R, et al. Long-term retention of perceptualmotor skills. J Exp Psychol 1958; 55: 318–28. [DOI] [PubMed] [Google Scholar]
- Anderson JR. Acquisition of cognitive skill. Psychol Rev 1982; 89: 369–406. [Google Scholar]
- Anderson P. Flexibility and stability in the innovating economy. Adm Sci Q 2007; 52: 333–5. [Google Scholar]
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol 1992; 68: 945–60. [DOI] [PubMed] [Google Scholar]
- Archibald NK, Hutton SB, Clarke MP, Mosimann UP, Burn DJ. Visual exploration in Parkinson’s disease and Parkinson's disease dementia. Brain 2013; 136: 739–50. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. Brain 2006; 26: 2424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Asselen M, Almeida I, Andre R, Januário C, Gonçalves AF, Castelo-Branco M. The role of the basal ganglia in implicit contextual learning: a study of Parkinson’s disease. Neuropsychologia 2009; 47: 1269–73. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 2010; 35: 48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC. Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neurosci 2012; 32: 14094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF. Choking under pressure: self-consciousness and paradoxical effects of incentives on skillful performance. J Pers Soc Psychol 1984; 46: 610–20. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature 2005; 436: 550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, Dagher A, Aston JA, Doyon J. Dynamic functional changes associated with cognitive skill learning of an adapted version of the Tower of London task. Neuroimage 2003; 20: 1649–60. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Frankfurter A. The distribution and some morphological features of substantia nigra neurons that project to the thalamus, superior colliculus and pedunculopontine nucleus in the monkey. Neuroscience 1982; 7: 2377–88. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Carr TH. On the fragility of skilled performance: what governs choking under pressure? J Exp Psychol 2001; 130: 701–25. [PubMed] [Google Scholar]
- Beilock SL, Carr TH, MacMahon C, Starkes JL. When paying attention becomes counterproductive: impact of divided versus skill-focused attention on novice and experienced performance of sensorimotor skills. J Exp Psychol 2002; 8: 6–16. [DOI] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD. Performance of simultaneous movements in patients with Parkinson’s disease. Brain 1986; 109: 739–57. [DOI] [PubMed] [Google Scholar]
- Bennett KM, Marchetti M, Iovine R, Castiello U. The drinking action of Parkinson’s disease subjects. Brain 1995; 118: 959–70. [DOI] [PubMed] [Google Scholar]
- Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994; 117: 859–76. [DOI] [PubMed] [Google Scholar]
- Bodis-Wollner I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson’s disease patients. Trends Neurosci 1990; 13: 296–302. [DOI] [PubMed] [Google Scholar]
- Bowman EM, Aigner TG, Richmond BJ. Neural signals in the monkey ventral striatum related to motivation for juice and cocaine rewards. J Neurophysiol 1996; 75: 1061–73. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci USA 2009; 106: 4894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 2010; 68: 815–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein AM, Kennard C. Predictive ocular motor control in Parkinson’s disease. Brain 1985; 108: 925–40. [DOI] [PubMed] [Google Scholar]
- Brown LL, Smith DM, Goldbloom LM. Organizing principles of cortical integration in the rat neostriatum: corticostriate map of the body surface is an ordered lattice of curved laminae and radial points. J Comp Neurol 1998; 392: 468–88. [PubMed] [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson’s disease: from description to theory. Trends Neurosci 1990; 13: 21–9. [DOI] [PubMed] [Google Scholar]
- Brown VJ, Desimone R, Mishkin M. Responses of cells in the tail of the caudate nucleus during visual discrimination learning. J Neurophysiol 1995; 74: 1083–94. [DOI] [PubMed] [Google Scholar]
- Caan W, Perrett DI, Rolls ET. Responses of striatal neurons in the behaving monkey. 2. Visual processing in the caudal neostriatum. Brain Res 1984; 290: 53–65. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Cogn Sci 2007; 30: 211–19. [DOI] [PubMed] [Google Scholar]
- Cameron IGM, Watanabe M, Pari G, Munoz DP. Executive impairment in Parkinson’s disease: response automaticity and task switching. Neuropsychologia 2010; 48: 1948–57. [DOI] [PubMed] [Google Scholar]
- Caplan LR, Schmahmann JD, Kase CS, Feldmann E, Baquis G, Greenberg JP, et al. Caudate infarcts. Arch Neurol 1990; 47: 133–43. [DOI] [PubMed] [Google Scholar]
- Carman LS, Schneider GE. Orienting behavior in hamsters with lesions of superior colliculus, pretectum, and visual cortex. Exp Brain Res 1992; 90: 79–91. [DOI] [PubMed] [Google Scholar]
- Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia 2005; 43: 784–96. [DOI] [PubMed] [Google Scholar]
- Chao HH, Luo X, Chang JL, Li CS. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time—an intra-subject analysis. BMC Neurosci 2009; 10: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Smith Y, Parent A. Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J Comp Neurol 1996; 364: 254–66. [DOI] [PubMed] [Google Scholar]
- Charlesworth JD, Warren TL, Brainard MS. Covert skill learning in a cortical-basal ganglia circuit. Nature 2012; 486: 251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier G, Deniau JM. Disinhibition as a basic process in the expression of striatal functions. Trends Neurosci 1990; 13: 277–80. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci 1994; 17: 228–33. [DOI] [PubMed] [Google Scholar]
- Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron 2014; 81: 901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Jiang Y. Contextual cueing: implicit learning and memory of visual context guides spatial attention. Cogn Psychol 1998; 36: 28–71. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science 1980; 210: 207–10. [DOI] [PubMed] [Google Scholar]
- Cohen SM. Electrical stimulation of cortical-caudate pairs during delayed successive visual discrimination in monkeys. Acta Neurobiol Exp 1972; 32: 211–33. [PubMed] [Google Scholar]
- Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience 2006; 139: 1479–93. [DOI] [PubMed] [Google Scholar]
- Cooke JD, Brown JD. Increased dependence on visual information for arm movement in patients with Parkinson’s disease. In: Poirier LJ, Sourkes TL, Bedard PJ, editors. Extrapyramidal system and its disorders. New York: Raven Press; 1979. p. 185–9. [Google Scholar]
- Cools AR. Role of the neostriatal dopaminergic activity in sequencing and selecting behavioural strategies: facilitation of processes involved in selecting the best strategy in a stressful situation. Behav Brain Res 1980; 1: 361–78. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain 2001; 124: 2503–12. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Powell TP. Strio-pallidal projection in the monkey. J Neurol Neurosurg Psychiatry 1966; 29: 426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford T, Goodrich S, Henderson L, Kennard C. Predictive responses in Parkinson’s disease: manual keypresses and saccadic eye movements to regular stimulus events. J Neurol Neurosurg Psychiatry 1989; 52: 1033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman AR. Primate models of dyskinesia: the experimental approach to the study of basal ganglia-related involuntary movement disorders. Neuroscience 1987; 21: 1–40. [DOI] [PubMed] [Google Scholar]
- Crossman AR, Sambrook MA, Jackson A. Experimental hemichorea/hemiballismus in the monkey. Studies on the intracerebral site of action in a drug-induced dyskinesia. Brain 1984; 107: 579–96. [DOI] [PubMed] [Google Scholar]
- Crossman ERFW. A theory of the acquisition of speed-skill. Ergonomics 1959; 2: 153–66. [Google Scholar]
- Crutcher MD, DeLong MR. Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res 1984; 53: 244–58. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999; 122: 1437–48. [DOI] [PubMed] [Google Scholar]
- Dayan P, Balleine B. Reward, motivation, and reinforcement learning. Neuron 2002; 36: 285–98. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol 2007; 64: 20–4. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D. The basal ganglia and their relation to disorders of movement. Amen House, London: Oxford University Press; 1962. [Google Scholar]
- Denny-Brown D. Clinical symptomatology of diseases of the basal ganglia. In: Vinken PJ, Bruyn GW, editors. Diseases of the Basal Ganglia. Amsterdam: North Holland; 1968. p. 133–72. [Google Scholar]
- Destrebecqz A, Cleeremans A. Can sequence learning be implicit? New evidence with the process dissociation procedure. Psychon Bull Rev 2001; 8: 343–50. [DOI] [PubMed] [Google Scholar]
- De Wit S, Corlett PR, Aitken MR, Dickinson A, Fletcher PC. Differential engagement of the ventromedial prefrontal cortex by goal-directed and habitual behavior toward food pictures in humans. J Neurosci 2009; 29: 11330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. J Neurosci 2012; 32: 12066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich NJ, Fénelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol 2009; 5: 331–42. [DOI] [PubMed] [Google Scholar]
- Ding L, Gold JI. Caudate encodes multiple computations for perceptual decisions. J Neurosci 2010; 30: 15747–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Hikosaka O. Comparison of reward modulation in the frontal eye field and caudate of the macaque. J Neurosci 2006; 26: 6695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. Modulators of decision making. Nat Neurosci 2008; 11: 410–16. [DOI] [PubMed] [Google Scholar]
- Doyon J, Gaudreau D, Laforce R, Jr., Castonguay M, Bedard PJ, Bedard F, et al. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain Cogn 1997; 34: 218–45. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloppel S, Cook PA, Alexander DC, Parker GJ, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci 2008; 28: 7143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson KA, Kintsch W. Long-term working memory. Psychol Rev 1995; 102: 211–45. [DOI] [PubMed] [Google Scholar]
- Ericsson KA, Lehmann AC. Expert and exceptional performance: evidence of maximal adaptation to task constraints. Annu Rev Psychol 1996; 47: 273–305. [DOI] [PubMed] [Google Scholar]
- Eyny YS, Horvitz JC. Opposing roles of D1 and D2 receptors in appetitive conditioning. J Neurosci 2003; 23: 1584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci 2005; 25: 2771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’S disease: substantia nigra regional selectivity. Brain 1991; 114: 2283–301. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Vogel M, Botterblom MH, Joosten RN, de Bruin JP. Dopamine and noradrenaline efflux in the rat prefrontal cortex after classical aversive conditioning to an auditory cue. Eur J Neurosci 2001; 13: 1051–4. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson’s disease. Brain 1998; 121: 1771–84. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wang J, Aigner TG, Mishkin M. Visual habit formation in monkeys with neurotoxic lesions of the ventrocaudal neostriatum. Proc Natl Acad Sci USA 2001; 98: 4196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts PM. Perceptual-motor skill learning. In: Melton AW, editor. Categories of human learning. New York: Academic Press; 1964. p. 243–85. [Google Scholar]
- Flaherty AW, Graybiel AM. Corticostriatal transformations in the primate somatosensory system. Projections from physiologically mapped body-part representations. J Neurophysiol 1991; 66: 1249–63. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Output architecture of the primate putamen. J Neurosci 1993; 13: 3222–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers KA. Visual “closed-loop” and “open-loop” characteristics of voluntary movement in patients with Parkinsonism and intention tremor. Brain 1976; 99: 269–310. [DOI] [PubMed] [Google Scholar]
- Flowers KA, Downing AC. Predictive control of eye movements in Parkinson disease. Ann Neurol 1978; 4: 63–6. [DOI] [PubMed] [Google Scholar]
- Francois C, Percheron G, Yelnik J. Localization of nigrostriatal, nigrothalamic and nigrotectal neurons in ventricular coordinates in macaques. Neuroscience 1984; 13: 61–76. [DOI] [PubMed] [Google Scholar]
- Francois C, Percheron G, Yelnik J, Heyner S. A histological atlas of the macaque (Macaca mulatta) substantia nigra in ventricular coordinates. Brain Res Bull 1985; 14: 349–67. [DOI] [PubMed] [Google Scholar]
- François C, Yelnik J, Percheron G, Fénelon G. Topographic distribution of the axonal endings from the sensorimotor and associative striatum in the macaque pallidum and substantia nigra. Exp Brain Res 1994; 102: 305–18. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci 2005; 17: 51–72. [DOI] [PubMed] [Google Scholar]
- Friedman JH, Brown RG, Comella C, Garber CE, Krupp LB, Lou JS, et al. Fatigue in Parkinson’s disease: a review. Mov Disord 2007; 22: 297–308. [DOI] [PubMed] [Google Scholar]
- Frith CD, Bloxham CA, Carpenter KN. Impairments in the learning and performance of a new manual skill in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatryurology 1986; 49: 661–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JD, Stebbins GT, Singh J, Willingham DB, Goetz CG. Intact mirror-tracing and impaired rotary-pursuit skill learning in patients with Huntington’s disease: evidence for dissociable memory systems in skill learning. Neuropsychology 1997; 11: 272–81. [DOI] [PubMed] [Google Scholar]
- Gathercole SE. Cognitive approaches to the development of short-term memory. Trends Cogn Sci 1999; 3: 410–19. [DOI] [PubMed] [Google Scholar]
- Gauntlett-Gilbert J, Roberts RC, Brown VJ. Mechanisms underlying attentional set-shifting in Parkinson’s disease. Neuropsychologia 1999; 37: 605–16. [DOI] [PubMed] [Google Scholar]
- Gerardin E, Lehéricy S, Pochon JB, Du Montcel ST, Mangin JF, Poupon F, et al. Foot, hand, face and eye representation in the human striatum. Cereb Cortex 2003; 13: 162–9. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 1990; 250: 1429–32. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson’s disease. J Neurol Neurosurg Psychiatryy 1991; 54: 388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickstein M, Stein J. Paradoxical movement in Parkinson’s disease. Trends Neurosci 1991; 14: 480–82. [DOI] [PubMed] [Google Scholar]
- Gomez-Tortosa E, MacDonald ME, Friend JC, Taylor SA, Weiler LJ, Cupples LA, et al. Quantitative neuropathological changes in presymptomatic Huntington’s disease. Ann Neurol 2001; 49: 29–34. [PubMed] [Google Scholar]
- Goto S, Hirano A, Matsumoto S. Subdivisional involvement of nigrostriatal loop in idiopathic Parkinson’s disease and striatonigral degeneration. Ann Neurol 1989; 26: 766–70. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. Attention, learning, and the value of information. Neuron 2012; 76: 281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci 2008; 31: 359–87. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Gross CG. A bimodal map of space: somatosensory receptive fields in the macaque putamen with corresponding visual receptive fields. Exp Brain Res 1993; 97: 96–109. [DOI] [PubMed] [Google Scholar]
- Grillner S. The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci 2003; 4: 573–86. [DOI] [PubMed] [Google Scholar]
- Grillner S, Ekeberg El, Manira A, Lansner A, Parker D, Tegner J, et al. Intrinsic function of a neuronal network - a vertebrate central pattern generator. Brain Res Rev 1998; 26: 184–97. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs-roles for the striatum and pallidum. Trends Neurosci 2005; 28: 364–70. [DOI] [PubMed] [Google Scholar]
- Gross C, Bender D, Rocha-Miranda C. Visual receptive fields of neurons in inferotemporal cortex of the monkey. Science 1969; 1303–6. [DOI] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 2000; 20: 2369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J Neurosci 2006; 26: 8368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 2010; 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage SR, Jurgens U. Localization of a vocal pattern generator in the pontine brainstem of the squirrel monkey. Eur J Neurosci 2006; 23: 840–4. [DOI] [PubMed] [Google Scholar]
- Hedreen JC, DeLong MR. Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol 1991; 304: 569–95. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Butters N, Salmon DP. Impaired learning of a motor skill in patients with Huntington’s disease. Behav Neurosci 1988; 102: 141–7. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer’s, Huntington's, and Parkinson's disease patients. J Neurosci 1989; 9: 582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton WS. Expertise acquisition as sustained learning in humans and other animals: commonalities across species. Anim Cogn 2008; 11: 99–107. [DOI] [PubMed] [Google Scholar]
- Henderson JM. Human gaze control during real-world scene perception. Trends Cogn Sci 2003; 7: 498–504. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 2010; 66: 896–907. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. GABAergic output of the basal ganglia. Prog Brain Res 2007a; 160: 209–26. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Basal ganglia mechanisms of reward-oriented eye movement. Ann N Y Acad Sci 2007b; 1104: 229–49. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Kim H, Yasuda M, Yamamoto S. Basal Ganglia circuits for reward value-guided behavior. Annu Rev Neurosci 2014; 37: 289–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, et al. Parallel neural networks for learning sequential procedures. Trends Neurosci 1999; 22: 464–71. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J. Neurophysiol 2006; 95: 567–84. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr Opin Neurobiol 2002; 12: 217–22. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Rand MK, Miyachi S, Miyashita K. Learning of sequential movements in the monkey: process of learning and retention of memory. J Neurophysiol 1995; 74: 1652–61. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Rand MK, Nakamura K, Miyachi S, Kitaguchi K, Sakai K, et al. Long-term retention of motor skill in macaque monkeys and humans. Exp Brain Res 2002; 147: 494–504. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Miyashita N. Effects of caudate nucleus stimulation on substantia nigra cell activity in monkey. Exp Brain Res 1993; 95: 457–72. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol 1989a; 61: 814–32. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. I. Activities related to saccadic eye movements. J Neurophysiol 1989b; 61: 780–98. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Sakamoto M, Usui S. Functional properties of monkey caudate neurons. II. Visual and auditory responses. J Neurophysiol 1989c; 61: 799–813. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 2000; 80: 953–78. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol 1983a; 49: 1285–301. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and coulomotor functions of the monkey substantia nigra pars retinulata. III. Memory-contingent visual and saccadic responses. J Neurophysiol 1983b; 49: 1268–84. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol 1985; 53: 292–308. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Yamamoto S, Yasuda M, Kim HF. Why skill matters. Trends Cogn Sci 2013; 17: 434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjornevik T, Leergaard TB, Darine D, Moldestad O, Dale AM, Willoch F, et al. Three-dimensional atlas system for mouse and rat brain imaging data. Front Neuroinform 2007; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Dopamine-mediated learning and switching in cortico-striatal circuit explain behavioral changes in reinforcement learning. Front Behav Neurosci 2011; 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons. Neuroscience 2014; 282C: 139–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci 2011; 31: 11457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci 1999; 19: 1446–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience 2000; 96: 651–6. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Hikosaka O. Reward-dependent gain and bias of visual responses in primate superior colliculus. Neuron 2003; 39: 693–700. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 2007; 56: 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle D. Evolutionary perspectives on the function of the optic tectum. Brain Behav Evol 1973; 8: 211–37. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Switching from automatic to controlled action by monkey medial frontal cortex. Nat Neurosci 2007; 10: 240–8. [DOI] [PubMed] [Google Scholar]
- Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci 2008; 28: 7209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Nakahara H, Hikosaka O, Kawagoe R, Takikawa Y, Aihara K. Correlation of primate caudate neural activity and saccade parameters in reward-oriented behavior. J Neurophysiol 2003; 89: 1774–83. [DOI] [PubMed] [Google Scholar]
- Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature 2006; 444: 56–60. [DOI] [PubMed] [Google Scholar]
- Jackson S, Jackson G, Harrison J, Henderson L, Kennard C. The internal control of action and Parkinson’s disease: a kinematic analysis of visually-guided and memory-guided prehension movements. Exp Brain Res 1995; 105: 147–62. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008; 79: 368–76. [DOI] [PubMed] [Google Scholar]
- Jankowski J, Scheef L, Huppe C, Boecker H. Distinct striatal regions for planning and executing novel and automated movement sequences. Neuroimage 2009; 44: 1369–79. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The connections of the dopaminergic system with the striatum in rats and primates: an analysis with respect to the functional and compartmental organization of the striatum. Neuroscience 2000; 96: 451–74. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RSJ, Passingham RE. Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 1997a; 77: 1325–37. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol 1997b; 77: 1313–24. [DOI] [PubMed] [Google Scholar]
- Kamil AC, Roitblat HL. The ecology of foraging behavior: implications for animal learning and memory. Annu Rev Psychol 1985; 36: 141–69. [DOI] [PubMed] [Google Scholar]
- Kato M, Miyashita N, Hikosaka O, Matsumura M, Usui S, Kori A. Eye movements in monkeys with local dopamine depletion in the caudate nucleus. I. Deficits in spontaneous saccades. J Neurosci 1995; 15: 912–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, Hikosaka O. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci 1998; 1: 411–16. [DOI] [PubMed] [Google Scholar]
- Kehagia A, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol 2010; 20: 199–204. [DOI] [PubMed] [Google Scholar]
- Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain 1970; 93: 525–46. [DOI] [PubMed] [Google Scholar]
- Kennard MA. Experimental analysis of the functions of the basal ganglia in monkeys and chimpanzees. J Neurophysiol 1944; 7: 127–48. [Google Scholar]
- Kermadi I, Joseph JP. Activity in the caudate nucleus of monkey during spatial sequencing. J. Neurophysiol 1995; 74: 911–33. [DOI] [PubMed] [Google Scholar]
- Kim HF, Ghazizadeh A, Hikosaka O. Separate groups of dopamine neurons innervate caudate head and tail encoding flexible and stable value memories. Front Neuroanat 2014; 8: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HF, Hikosaka O. Distinct Basal Ganglia circuits controlling behaviors guided by flexible and stable values. Neuron 2013; 79: 1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Aosaki T, Hu Y, Ishida A, Watanabe K. Activity of primate putamen neurons is selective to the mode of voluntary movement: visually guided, self-initiated or memory-guided. Exp Brain Res 1992; 89: 473–7. [DOI] [PubMed] [Google Scholar]
- Kimura M, Kato M, Shimazaki H, Watanabe K, Matsumoto N. Neural information transferred from the putamen to the globus pallidus during learned movement in the monkey. J Neurophysiol 1996; 76: 3771–86. [DOI] [PubMed] [Google Scholar]
- Kimura M. The role of primate putamen neurons in the association of sensory stimuli with movement. Neurosci Res 1986; 3: 436–43. [DOI] [PubMed] [Google Scholar]
- Kimura M. Behaviorally contingent property of movement-related activity of the primate putamen. J. Neurophysiol 1990; 63: 1277–96. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Matsui R, Kato S, Hasegawa T, Kasahara H, Isa K, et al. Genetic dissection of the circuit for hand dexterity in primates. Nature 2012; 487: 235–8. [DOI] [PubMed] [Google Scholar]
- Knopman D, Nissen MJ. Procedural learning is impaired in Huntington’s disease: evidence from the serial reaction time task. Neuropsychologia 1991; 29: 245–54. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science 1996; 273: 1399–402. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Paulse J, Swerdlow NR, Swenso M, Butters N. Dissociations within nondeclarative memory in Huntington’s disease. Neuropsychology 1996; 10: 538–48. [Google Scholar]
- Koerts J, Van Beilen M, Tucha O, Leenders KL, Brouwer WH. Executive functioning in daily life in Parkinson’s disease: initiative, planning and multi-task performance. PLoS One 2011; 6: e29254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerts J, Leenders KL, Brouwer WH. Cognitive dysfunction in non-demented Parkinson’s disease patients: controlled and automatic behavior. Cortex 2009; 45: 922–9. [DOI] [PubMed] [Google Scholar]
- Koralek AC, Jin X, Long JD, II, Costa RM, Carmena JM. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature 2012; 483: 331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kori A, Miyashita N, Kato M, Hikosaka O, Usui S, Matsumura M. Eye movements in monkeys with local dopamine depletion in the caudate nucleus. II. Deficits in voluntary saccades. J Neurosci 1995; 15: 928–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz A V, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010; 466: 622–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron 2008; 60: 543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 1975; 88: 195–209. [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 2011; 70: 855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land MF. Eye movements and the control of actions in everyday life. Prog Retin Eye Res 2006; 25: 296–324. [DOI] [PubMed] [Google Scholar]
- Laplane D, Baulac M. Pure psychic akinesia with bilateral lesions of basal ganglia. J Neurol Neurosurg Psychiatry 1984; 47: 377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS. Saccades in Huntington’s disease: initiation defects and distractibility. Neurology 1987; 37: 364–70. [DOI] [PubMed] [Google Scholar]
- Lasker AG, Zee DS, Hain TC, Folstein SE, Singer HS. Saccades in Huntington’s disease: slowing and dysmetria. Neurology 1988; 38: 427–31. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, Watanabe K, Coe B, Hikosaka O. A neural correlate of response bias in monkey caudate nucleus. Nature 2002; 418: 413–17. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Hodges JR, Rosser AE, Lange KW, Robbins TW. Executive and mnemonic functions in early Huntington’s disease. Brain 1996; 119: 1633–45. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Sahakian BJ, Robbins TW. Cognitive functions and corticostriatal circuits: insights from Huntington’s disease. Trends Cogn Sci 1998; 2: 379–88. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Watkins LH, Sahakian BJ, Hodges JR, Robbins TW. Visual object and visuospatial cognition in Huntington’s disease: implications for information processing in corticostriatal circuits. Brain 2000; 123: 1349–64. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Benali H, Van de Moortele P-F, Pélégrini-Issac M, Waechter T, Ugurbil K, et al. Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci USA 2005; 102: 12566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Krainik A, Francois C, Van De Moortele PF, Ugurbil K, et al. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex 2004a; 14: 1302–9. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van De Moortele PF, Francois C, Thivard L, Poupon C, et al. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 2004b; 55: 522–9. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Langer SZ. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neuroscience 1983; 10: 1105–20. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Newman SA, Folstein SE, Lasker AG, Jensen BA. Abnormal ocular motor control in Huntington’s disease. Neurology 1983; 33: 1268–75. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Striatal contributions to working memory: a functional magnetic resonance imaging study in humans. Eur J Neurosci 2004; 19: 755–60. [DOI] [PubMed] [Google Scholar]
- Lieb K, Brucker S, Bach M, Els T, Lücking CH, Greenlee MW. Impairment in preattentive visual processing in patients with Parkinson’s disease. Brain 1999; 122: 303–13. [DOI] [PubMed] [Google Scholar]
- Liles SL, Updyke B V. Projection of the digit and wrist area of precentral gyrus to the putamen: relation between topography and physiological properties of neurons in the putamen. Brain Res 1985; 339: 245–55. [DOI] [PubMed] [Google Scholar]
- Liljenström H. Neural stability and flexibility: a computational approach. Neuropsychopharmacology 2003; 28 (Suppl 1): S64–73. [DOI] [PubMed] [Google Scholar]
- Malamut BL, Saunders RC, Mishkin M. Monkeys with combined amygdalo-hippocampal lesions succeed in object discrimination learning despite 24-hour intertrial intervals. Behav Neurosci 1984; 98: 759–69. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci 2006; 26: 3875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci 2009; 10: 141–52. [DOI] [PubMed] [Google Scholar]
- Marín O, Smeets WJAJ, González A. Evolution of the basal ganglia in tetrapods: a new perspective based on recent studies in amphibians. Trends Neurosci 1998; 21: 487–94. [DOI] [PubMed] [Google Scholar]
- Marsden CD. The enigma of the basal ganglia and movement. Trends Neurosci 1980; 3: 284–7. [Google Scholar]
- Matsumoto H, Terao Y, Furubayashi T, Yugeta A, Fukuda H, Emoto M, et al. Small saccades restrict visual scanning area in Parkinson’s disease. Mov Disord 2011; 26: 1619–26. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 2007; 447: 1111–5. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci 2009; 12: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JP, Masters RS, Kerr E, Weedon E. The implicit benefit of learning without errors. Q J Exp Psychol A 2001; 54: 1049–68. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 2006; 26: 3642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena-Segovia J, Winn P, Bolam JP. Cholinergic modulation of midbrain dopaminergic systems. Brain Res Rev 2008; 58: 265–71. [DOI] [PubMed] [Google Scholar]
- Meppelink AM, Koerts J, Borg M, Leenders KL, van Laar T. Visual object recognition and attention in Parkinson’s disease patients with visual hallucinations. Mov Disord 2008; 23: 1906–12. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The temporal lobe is a target of output from the basal ganglia. Proc Natl Acad Sci USA 1996; 93: 8683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal-ganglia “projections” to the prefrontal cortex of the primate. Cereb Cortex 2002; 12: 926. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 1996; 50: 381–425. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 1978; 273: 297–8. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res 2002; 146: 122–6. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Kárádi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res 1997; 115: 1–5. [DOI] [PubMed] [Google Scholar]
- Miyashita N, Hikosaka O, Kato M. Visual hemineglect induced by unilateral striatal dopamine deficiency in monkeys. Neuroreport 1995; 6: 1257–60. [DOI] [PubMed] [Google Scholar]
- Mochizuki-Kawai H, Kawamura M, Hasegawa Y, Mochizuki S, Oeda R, Yamanaka K, et al. Deficits in long-term retention of learned motor skills in patients with cortical or subcortical degeneration. Neuropsychologia 2004; 42: 1858–63. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol 2006; 59: 257–64. [DOI] [PubMed] [Google Scholar]
- Moody TD, Chang GY, Vanek ZF, Knowlton BJ. Concurrent discrimination learning in Parkinson’s disease. Behav Neurosci 2010; 124: 1–8. [DOI] [PubMed] [Google Scholar]
- Morris M, Iansek R, Matyas T, Summers J. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain 1996; 119: 551–68. [DOI] [PubMed] [Google Scholar]
- Morris ME. Movement disorders in people with Parkinson disease: a model for physical therapy. Phys Ther 2000; 80: 578–97. [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 2008; 9: 856–69. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Doya K, Hikosaka O. Parallel cortico-basal ganglia mechanisms for acquisition and execution of visuomotor sequences—a computational approach. J Cogn Neurosci 2001; 13: 626–47. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hikosaka O. Role of dopamine in the primate caudate nucleus in reward modulation of saccades. J Neurosci 2006; 26: 5360–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci 2008; 28: 5331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Bronstein AM, Lueck C, Marsden CD, Rudge P. Vestibular, cervical and visual remembered saccades in Parkinson’s disease. Brain 1994; 117: 1423–32. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neurosci Res 1995; 23: 1–19. [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, et al. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol 2000; 84: 289–300. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal “hyperdirect” pathway. Neurosci Res 2002; 43: 111–17. [DOI] [PubMed] [Google Scholar]
- Neggers SFW, van Diepen RM, Zandbelt BB, Vink M, Mandl RCW, Gutteling TP. A functional and structural investigation of the human fronto-basal volitional saccade network. PLoS One 2012; 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers SFW, Zandbelt BB, Schall MS, Schall JD. Comparative diffusion tractography of cortico-striatal motor pathways reveals differences between humans and macaques. J Neurophysiol 2015; 113: 2164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell A, Rosenbloom PS. Mechanisms of skill acquisition and the law of practice. Cogn Ski Their Acquis 1981; 17: 1–55. [Google Scholar]
- Nicola SM, Surmeier DJ, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 2000; 23: 185–215. [DOI] [PubMed] [Google Scholar]
- Nishino H, Ono T, Sasaki K, Fukuda M, Muramoto K. Caudate unit activity during operant feeding behavior in monkeys and modulation by cooling prefrontal cortex. Behav Brain Res 1984; 11: 21–33. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW. Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain 1993; 116: 1159–75. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 1991; 29: 993–1006. [DOI] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci 2002; 25: 563–93. [DOI] [PubMed] [Google Scholar]
- Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci 1990; 13: 254–8. [DOI] [PubMed] [Google Scholar]
- Parent A, Bouchard C, Smith Y. The striatopallidal and striatonigral projections: two distinct fiber systems in primate. Brain Res 1984; 303: 385–90. [DOI] [PubMed] [Google Scholar]
- Percheron G, Yelnik J, Francois C. A Golgi analysis of the primate globus pallidus. III. Spatial organization of the striato-pallidal complex. J Comp Neurol 1984; 227: 214–27. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Everling S. Neural activity in the macaque putamen associated with saccades and behavioral outcome. PLoS One 2012; 7: e51596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillon B, Dubois B, Lhermitte F, Agid Y. Heterogeneity of cognitive impairment in progressive supranuclear palsy, Parkinson’s disease, and Alzheimer's disease. Neurology 1986; 36: 1179–85. [DOI] [PubMed] [Google Scholar]
- Pluck GC, Brown RG. Apathy in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2002; 73: 636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, et al. The neural correlates of motor skill automaticity. J Neurosci 2005; 25: 5356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci 2014; 17: 1146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock CJ. Turning behaviour in animals. Neuroscience 1980; 5: 461–514. [DOI] [PubMed] [Google Scholar]
- Pyke GH, Pulliam HR, Charnov EL. Optimal foraging: a selective review of theory and tests. Q Rev Biol 1977; 52: 137–54. [Google Scholar]
- Rand MK, Hikosaka O, Miyachi S, Lu X, Nakamura K, Kitaguchi K, et al. Characteristics of sequential movements during early learning period in monkeys. Exp Brain Res 2000; 131: 293–304. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD, et al. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum Brain Mapp 1997; 5: 124–32. [PubMed] [Google Scholar]
- Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 2010; 11: 760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds J, Wickens J. Dopamine-dependent plasticity of corticostriatal synapses. Neural Networks 2002; 15: 507–21. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Young A, Penney JB. Comparative distribution of dopamine D-1 and D-2 receptors in the basal ganglia of turtles, pigeons, rats, cats, and monkeys. J Comp Neurol 1987; 262: 446–63. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Rummukainen J, Paljärvi L, Rinne UK. Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Ann Neurol 1989; 26: 47–50. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Logemann JA, Kirshner HS. Swallowing and speech production in Parkinson’s disease. Ann Neurol 1986; 19: 283–7. [DOI] [PubMed] [Google Scholar]
- Robledo P, Feger J. Excitatory influence of rat subthalamic nucleus to substantia nigra pars reticulata and the pallidal complex: electrophysiological data. Brain Res 1990; 518: 47–54. [DOI] [PubMed] [Google Scholar]
- Roncacci S, Troisi E, Carlesimo GA, Nocentini U, Caltagirone C. Implicit memory in parkinsonian patients: evidence for deficient skill learning. Eur Neurol 1996; 36: 154–9. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron 2011; 70: 1054–69. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr J, Ungerleider L, Desimone R. Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol 1990; 298: 129–56. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Hikosaka O. Eye movements induced by microinjection of GABA agonist in the rat substantia nigra pars reticulata. Neurosci Res 1989; 6: 216–33. [DOI] [PubMed] [Google Scholar]
- Sato M, Hikosaka O. Role of primate substantia nigra pars reticulata in reward-oriented saccadic eye movement. J Neurosci 2002; 22: 2363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarnati E, Proia A, Di Loreto S, Pacitti C. The reciprocal electrophysiological influence between the nucleus tegmenti pedunculopontinus and the substantia nigra in normal and decorticated rats. Brain Res 1987; 423: 116–24. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res 1983; 275: 321–8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 1998; 80: 1–27. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Scarnati E, Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 1992; 12: 4595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science 1997; 275: 1593–9. [DOI] [PubMed] [Google Scholar]
- Schultz W, Romo R. Responses of nigrostriatal dopamine neurons to high-intensity somatosensory stimulation in the anesthetized monkey. J Neurophysiol 1987; 57: 201–17. [DOI] [PubMed] [Google Scholar]
- Schwab R, Chafetz M, Walker S. Control of two simultaneous voluntary motor acts in normals and in parkinsonism. Arch Neurol Psychiatry 1954; 72: 591–8. [DOI] [PubMed] [Google Scholar]
- Schwab R, England A, Peterson E. Akinesia in Parkinson’s disease. Neurology 1959; 9: 65–72. [DOI] [PubMed] [Google Scholar]
- Schwab R, Zieper I. Effects of mood, motivation, stress and alertness on the performance in Parkinson’s disease. Psychiatr Neurol (Basel) 1965; 150: 345–57. [DOI] [PubMed] [Google Scholar]
- Seger CA. Implicit learning. Psychol Bull 1994; 115: 163–96. [DOI] [PubMed] [Google Scholar]
- Seger CA, Spiering BJ. A critical review of habit learning and the Basal Ganglia. Front Syst Neurosci 2011; 5: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L, Goldman-rakic P. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci 1985; 5: 776–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 2008; 321: 848–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing: II. Perceptual learning, automatic attending, and a general theory. Psychol Rev 1977; 84: 127–89. [Google Scholar]
- Sigman M, Gilbert CD. Learning to find a shape. Nat Neurosci 2000; 3: 264–9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 1998; 86: 353–87. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus). Neuroscience 1986; 18: 347–71. [DOI] [PubMed] [Google Scholar]
- Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci 2002; 3: 952–64. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Lange H, Homberg V. The pattern of attentional deficits in Huntington’s disease. Brain 1995; 118: 145–52. [DOI] [PubMed] [Google Scholar]
- Stefanova ED, Kostic VS, Ziropadja L, Markovic M, Ocic GG. Visuomotor skill learning on serial reaction time task in patients with early Parkinson’s disease. Mov Disord 2000; 15: 1095–103. [DOI] [PubMed] [Google Scholar]
- Stern Y, Mayeux R, Rosen J, Ilson J. Perceptual motor dysfunction in Parkinson’s disease: a deficit in sequential and predictive voluntary movement. J Neurol Neurosurg Psychiatry 1983; 46: 145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 2007; 30: 228–35. [DOI] [PubMed] [Google Scholar]
- Szabo J. Organization of the ascending striatal afferents in monkeys. J Comp Neurol 1980; 189: 307–21. [DOI] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal input zones from the supplementary motor area overlap those from the contra- rather than ipsilateral primary motor cortex. Brain Res 1998; 791: 335–40. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia-brainstem pathways in the control of motor behaviors. Neurosci Res 2004; 50: 137–51. [DOI] [PubMed] [Google Scholar]
- Takechi R, Tamura N, Hayashi F. Improved walnut-feeding skills with experience in wood mice, Apodemus speciosus. J Ethol 2009; 27: 83–9. [Google Scholar]
- Takikawa Y, Kawagoe R, Hikosaka O. Reward-dependent spatial selectivity of anticipatory activity in monkey caudate neurons. J Neurophysiol 2002a; 87: 508–15. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, Kawagoe R, Itoh H, Nakahara H, Hikosaka O. Modulation of saccadic eye movements by predicted reward outcome. Exp Brain Res 2002b; 142: 284–91. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Inferotemporal cortex and object vision. Annu Rev Neurosci 1996; 19: 109–39. [DOI] [PubMed] [Google Scholar]
- Tatler BW, Hayhoe MM, Land MF, Ballard DH. Eye guidance in natural vision: reinterpreting salience. J Vis 2011; 11: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AE, Saint-Cyr JA, Lang AE. Frontal lobe dysfunction in Parkinson’s disease. The cortical focus of neostriatal outflow. Brain 1986; 109: 845–83. [DOI] [PubMed] [Google Scholar]
- Terao Y, Fukuda H, Yugeta A, Hikosaka O, Nomura Y, Segawa M, et al. Initiation and inhibitory control of saccades with the progression of Parkinson’s disease - Changes in three major drives converging on the superior colliculus. Neuropsychologia 2011; 49: 1794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, Gelade G. A feature-integration theory of attention. Cogn Psychol 1980; 12: 97–136. [DOI] [PubMed] [Google Scholar]
- Tricomi EM, Balleine BW, O’Doherty JP. A specific role for posterior dorsolateral striatum in human habit learning. Eur J Neurosci 2009; 29: 2225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron 2004; 41: 281–92. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks J, Rodnitzky RL, Dawson JD. Impaired visual search in drivers with Parkinson’s disease. Ann Neurol 2006; 60: 407–13. [DOI] [PubMed] [Google Scholar]
- Villardita C, Smirni P, Zappala G. Visual neglect in Parkinson’s disease. Arch Neurol 1983; 40: 737–9. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP. Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol 1985; 44: 559–77. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 2012; 74: 858–73. [DOI] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci 2002; 22: 294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, O’Connor WT, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine and caudate-putamen to skilled forelimb use in the rat. Brain 1986; 109: 805–43. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 1996; 6: 751–8. [DOI] [PubMed] [Google Scholar]
- Willingham D, Peterson E, Manning C, Brashear H. Patients with Alzheimer’s disease who cannot perform some motor skills show normal learning of other motor skills. Neuropsychology 1997; 11: 261–71. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Koroshetz WJ. Evidence for dissociable motor skills in Huntington’s disease patients. Psychobiology 1993; 21: 173–82. [Google Scholar]
- Winograd-Gurvich CT, Georgiou-Karistianis N, Evans A, Millist L, Bradshaw JL, Churchyard A, et al. Hypometric primary saccades and increased variability in visually-guided saccades in Huntington’s disease. Neuropsychologia 2003; 41: 1683–92. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004; 5: 483–94. [DOI] [PubMed] [Google Scholar]
- Witt K, Pulkowski U, Herzog J, Lorenz D, Hamel W, Deuschl G, et al. Deep brain stimulation of the subthalamic nucleus improves cognitive flexibility but impairs response inhibition in Parkinson disease. Arch Neurol 2004; 61: 697–700. [DOI] [PubMed] [Google Scholar]
- Wood W, Neal DT. A new look at habits and the habit-goal interface. Psychol Rev 2007; 114: 843–63. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 2005; 128: 2250–9. [DOI] [PubMed] [Google Scholar]
- Wunderlich K, Dayan P, Dolan RJ. Mapping value based planning and extensively trained choice in the human brain. Nat Neurosci 2012; 15: 786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs NF, Bassett DS, Mucha PJ, Porter MA, Grafton ST. Differential recruitment of the sensorimotor putamen and frontoparietal cortex during motor chunking in humans. Neuron 2012; 74: 936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, Baimbridge KG, McGeer EG. Relative sparing in Parkinson’s disease of substantia nigra dopamine neurons containing calbindin-D28K. Brain Res 1990; 526: 303–7. [DOI] [PubMed] [Google Scholar]
- Yamadori A, Yoshida T, Mori E, Yamashita H. Neurological basis of skill learning. Brain Res. Cogn Brain Res 1996; 5: 49–54. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Kim HF, Hikosaka O. Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. J Neurosci 2013; 33: 11227–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Monosov IE, Yasuda M, Hikosaka O. What and where information in the caudate tail guides saccades to visual objects. J Neurosci 2012; 32: 11005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbus A, Haigh B, Rigss L. Eye movements and vision. New York: Plenum press; 1967. [Google Scholar]
- Yasuda M, Hikosaka O. Functional territories in primate substantia nigra pars reticulata separately signaling stable and flexible values. J Neurophysiol 2015; 113: 1181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Yamamoto S, Hikosaka O. Robust representation of stable object values in the oculomotor basal ganglia. J Neurosci 2012; 32: 16917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian E, Van Hoesen G. Cortico-striate projections in the rhesus monkey: the organization of certain cortico-caudate connections. Brain Res 1978; 139: 43–63. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci 2006; 7: 464–76. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci 2009; 12: 333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetusky WJ, Jankovic J, Pirozzolo FJ. The heterogeneity of Parkinson’s disease Clinical and prognostic implications. Neurology 1985; 35: 522–6. [DOI] [PubMed] [Google Scholar]
- Zokaei N, McNeill A, Proukakis C, Beavan M, Jarman P, Korlipara P, et al. Visual short-term memory deficits associated with GBA mutation and Parkinson’s disease. Brain 2014; 137: 2303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]