Dendrites play a central role in the integration and flow of information in the nervous system. The morphogenesis and maturation of dendrites is hence an essential step in the establishment of neuronal connectivity. Recent studies have uncovered crucial functions for extrinsic cues in the development of dendrites. Here, we review the contribution of secreted polypeptide growth factors, contact-mediated proteins, and neuronal activity in distinct phases of dendrite development. We also highlight how extrinsic cues influence local and global intracellular mechanisms of dendrite morphogenesis. Finally, we discuss how these studies have advanced our understanding of neuronal connectivity and shed light on the pathogenesis of neurodevelopmental disorders.
Extrinsic cues regulate distinct steps in dendrite morphogenesis
To establish proper connectivity, dendrites transition through fundamental developmental stages from growth and guidance to branching and pruning to self-avoidance and tiling. The regulation of dendrite patterning can be broadly divided into cell-extrinsic and cell-intrinsic mechanisms. In the nervous system, cell-extrinsic cues consist of secreted or transmembrane signals as well as neuronal activity in response to trans-synaptic transmission. In contrast, cell-intrinsic pathways represent cell-autonomous mechanisms that are influenced by environmental cues but do not strictly depend on extrinsic cues to operate within neurons. These factors characteristically regulate intracellular neuronal responses to extrinsic cues [1-2].
Early studies of dendrite morphology were heavily focused on secreted cues such as neurotrophins and their effectors, the receptor tyrosine kinases (RTKs) [3]. However, additional cell-extrinsic cues and mechanisms of dendrite patterning have been identified (Table 1). For example, contact-mediated signaling through Down syndrome cell adhesion molecule (DSCAM) and similar molecules have provided significant insights into the targeting of dendrites, whereas ligand-gated and voltage-gated calcium channels and their respective downstream effectors have shed light on how neuronal activity regulates dendrite morphogenesis.
Table 1. Summary of cell-extrinsic molecular drivers of dendrite morphogenesis.
| Cell extrinsic regulators | |
|---|---|
| Secreted cues | Neurotrophins (NGF, BDNF, NT-3, NT-4) Receptors Tyrosine Kinases |
|
| |
| Semaphorins | |
|
| |
| Netrin/Frazzled/DCC and Slit/Robo | |
|
| |
| Wnts | |
|
| |
| Ephrins | |
|
| |
| Reelin | |
|
| |
| Bone Morphogenetic Proteins | |
|
| |
| Contact-mediated regulators | Cadherins and Protocadherins |
| Adhesion-G-protein-coupled receptors | |
| DSCAM | |
| Integrins | |
| Fusogens and other transmembrane proteins | |
|
| |
| Neuronal activity and calcium signaling | VGCCs |
|
| |
| NMDARs | |
The cell-intrinsic pathways driving dendrite patterning have been recently reviewed [2]. Here, we focus on the cell-extrinsic regulators of dendrite morphogenesis. We discuss the role of three major classes of extrinsic regulators: secreted cues, contact-mediated factors, and neuronal activity. The list of specific molecules driving these distinct forms of regulation continues to expand. A general concept emerging from these studies is that just as in the case of cell-intrinsic regulation of dendrite morphogenesis [2], extrinsic cues regulate diverse aspects of dendrite development from their growth and branching to pruning and maturation (Figures 1 and 2).
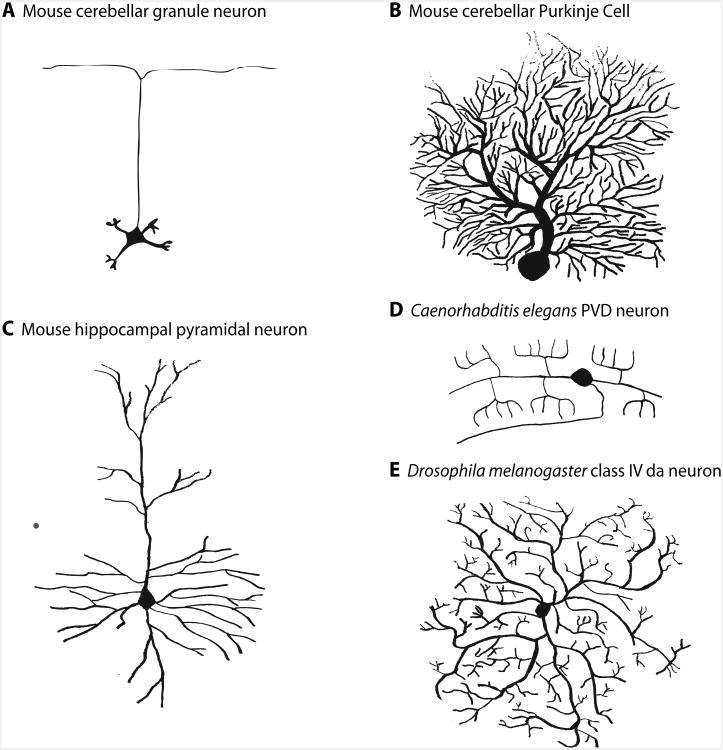
Figure 1. Diverse patterns of dendrite branching in different type of neurons.
(A) Mouse cerebellar granule neuron have only four to five dendrites, each of which ends with a dendritic claw that harbors postsynaptic dendritic specializations. (B) Elaborate dendritic tree in a mouse Purkinje cell. (C) Mouse hippocampal pyramidal neuron characterized by two distinct dendritic trees, the basal and apical dendrites. (D) Dendritic tree in Caenorhabditis elegans PVD neuron. (E) Multidendritic class IV da neuron in Drosophila melanogaster.
Figure 2. Cell-extrinsic regulators of dendrite morphogenesis.
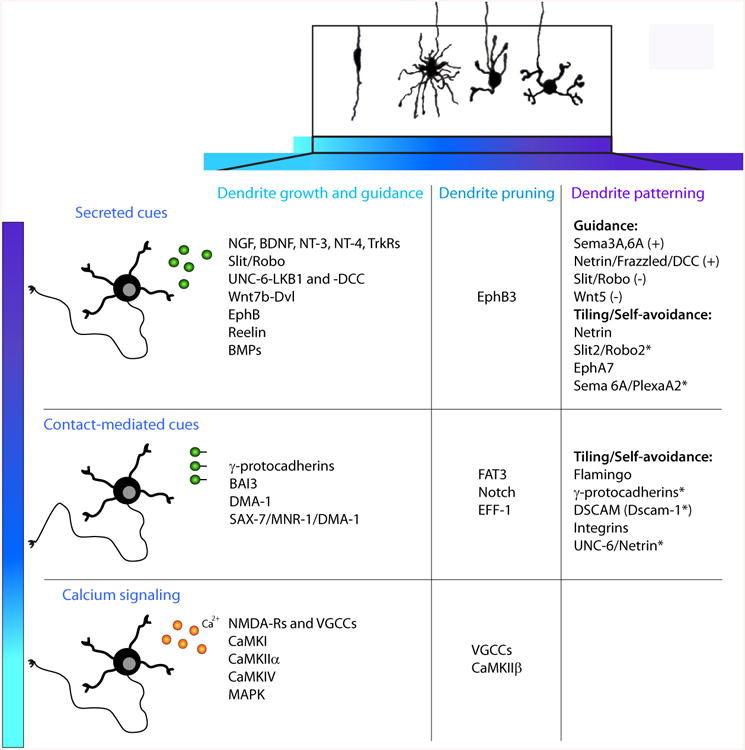
Summary of molecules regulating different stages of dendrite development, as described in the text. *Molecules that mediate repulsion between sister dendrites.
Secreted cues
Neurotrophins
Neurotrophins represent a family of secreted proteins, consisting of nerve growth factor (NGF), brain-derived growth factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4 (NT-4), that act on neurons through members of the tyrosine receptor kinase (Trk) family [4]. In the rodent cerebral cortex, neurotrophins promote dendrite growth and arborization, but this effect varies depending on the specific neurotrophin, cortical layer, and location of dendrites [5].
Specific deletion of the BDNF receptor TrkB receptor in cortical pyramidal neurons, by crossing mice harboring a floxed allele of TrkB with mice expressing Cre downstream of the CaMKII driver, reduces dendrite complexity [6], and disruption of the TrkB dynein-mediated transporter Snapin decreases dendrite growth [7]. Interestingly, Trk receptors are also expressed as different splice variants, and these variants mediate distinct effects on dendrites [8], potentially offering another layer of control beyond the effects of specific cognate neurotrophin/Trk family members. Recent studies reveal that dendrite growth and branching is modulated by relative intracellular differences in NT-3/TrkC signaling. TrkC knockout mouse Purkinje neurons have reduced dendrite complexity, which is rescued by the removal of the TrkC ligand NT-3 from cerebellar granule neurons [9].
Although neurotrophins have essential roles in dendrite patterning, the precise downstream mechanisms remain to be identified. Neurotrophins may stimulate activity-dependent pathways to induce dendrite growth [10]. Recent studies link neurotrophin signaling to the activity-dependent phosphorylation of glycogen synthase kinase-3beta (GSK3β) at serine-9 in hippocampal neurons, which inhibits GSK3β activity and promotes dendrite growth, [11]. Interestingly, GSK3β activation appears to trigger phosphorylation of the scaffold protein gephyrin and thereby reduces GABAA receptor levels causing hyperexcitability and dendrite retraction [11].
Semaphorins
Several diffusible secreted factors that control axon guidance have also been implicated in dendrite patterning. Semaphorins 2A and 2B, and 3A (as well as the transmembrane Semaphorin 1A) regulate dendrite targeting and branching in mammalian cerebral cortical neurons and fly olfactory neurons [12-14]. Mouse genetics studies reveal a role for Sema3A in the regulating apical dendrite formation in hippocampal CA1 pyramidal neurons [15].
Notably, the Sema3A receptor neuropilin1 (NRP1) is uniformly expressed along axons and dendrites, suggesting that NRP1 expression may not explain differential effects of Sema3A on axons and dendrites. Recently, the protein kinase TAO kinase 2 (TAOK2) has been suggested to collaborate with NRP1 in directing Sema3A-induced basal dendrite growth [16]. Expression of TAOK2 stimulates Jun kinase (JNK) activity and thereby restores basal dendrite arborization in NRP1-deficient neurons. Substrates of JNK that mediate Sema3A-induced basal dendrite arborization remain to be identified. Interestingly, TAOK2 is an autism spectrum disorder susceptibility gene, and deficits in basal dendrite development uponTAOK2 downregulation may mimic the underdeveloped neuron morphology associated with autism [16].
In an alternate mechanism specifying the Sema3A response, guanylate cyclase and cyclic guanosine monophosphate (cGMP) have been observed to be localized in apical dendrites but not basal dendrites or axons [13]. These results have been extended in Xenopus spinal commissural interneurons, where Sema3A-induced cGMP stimulates Ca(V)2.3 channels and growth of dendrites [17]. Other studies suggest that Fyn and cyclin-dependent kinase 5 (Cdk5) operate downstream of Sema3A signaling [18], whereas in cultured hippocampal neurons Sema3A inhibits protein kinase A (PKA) signaling leading to reduced phosphorylation of the protein kinases liver kinase B1 (LKB1) and GSK3β, thereby inhibiting axon formation and triggering dendrite growth [19].
Semaphorins and their receptors, the plexins, are also involved in in directing lamina-specific neurite arborization in the developing mouse retina [20]. Recent studies suggest that semaphorin 6A (Sema6A) and its receptor plexinA2 (PlexaA2) control direction-selective responses to visual stimuli by regulating the dendrite morphology and stratification of the starburst amacrine cell in the mouse retina [21]. Semaphorins may also regulate dendrite development in neurons generated in the adult mouse hippocampus [22].
Netrins and Slits
The Drosophila midline is enriched with secreted guidance cues including Netrins and Slits, which act through Frazzled and Robo (Roundabout) receptors, respectively. Drosophila motoneuron dendrites make stereotyped guidance decisions based on these midline ligand-receptor interactions. Slits appear to drive motoneuron dendrites away from the central nervous system (CNS) midline [23]. In contrast, Netrin promotes midline crossing of dendrites in flies [24].
In the rodent cerebral cortex, Slit1/Robo interactions regulate the growth of pyramidal neuron apical dendrites [25]. The secreted repulsive guidance cue Slit2 and its cognate Robo receptor have also been implicated in self-avoidance of dendrites in Purkinje neurons in the mouse cerebellar cortex, where aberrant signaling of these pathways alters motor behavior in animal models [26]. In conditional knockout studies, Slit2 and its receptor Robo2 are required for cell autonomous self-avoidance of Purkinje neuron dendrites [26].
How a common pool of guidance molecules controls the morphogenesis of both axons and dendrites remains an important question in the field. Genetic studies in C. elegans provide some insight. The serine-threonine kinase Par4 (LKB1) and UNC-40 (DCC, deleted in colorectal cancer) promote dendrite growth in response to UNC-6 (Netrin), whereas the receptor UNC-5 repels axon growth downstream of UNC-6 [27]. Additional studies have shown that UNC-6 (Netrin) acts non-cell autonomously on neighboring dendrites via the receptor UNC-40 [28], offering a further layer of regulation. Thus, the effect of UNC-6 and other secreted cues may depend not only on the specific receptors and downstream signaling molecules but also on the surrounding cellular milieu.
Wnts
Wnts (wingless) bind to Frizzled receptors and signals through the scaffold protein Dishevelled (Dvl) [29]. Among the Wnt proteins, Wnt7b, which is expressed in the mouse hippocampus, appears to regulate dendrite growth and arborization [30]. Wnt7 and Dvl stimulate dendritic elaboration through the activation of the Rho family GTPase Rac and the protein kinase JNK (c-Jun N-terminal kinase). Wnt3a and Wnt5a may also regulate dendrite development in olfactory bulb interneurons [31]. These Wnt proteins act through canonical and non-canonical downstream signaling to exert opposing functions on dendrite growth [31]. Wnt5 acts as a repulsive guidance cue for the projection neurons (PN) dendrites in Drosophila. The spatially restricted expression pattern of Wnt5 orients the movements of the projection neuron dendrites, allowing PN dendrites expressing different levels of the Wnt5 receptor, Drl, to appropriately localize to their final glomerular positions [32].
Ephrins
Members of the ephrin ligand family and their cognate Eph receptor tyrosine kinase activate intracellular pathways that modulate both neuronal shape and contacts [33]. Among the EphA receptor proteins, EphA7 mediates the ability of the ligand ephrin-A5 to induce dendrite avoidance in cortical neurons via the signaling proteins Src and TSC1 [34]. Triple knockout of EphB1, EphB2, and EphB3 results in reduced dendrite number, length, and complexity in the mouse hippocampus [35], suggesting that EphB family receptors play key roles in dendrite development.
The ephrin receptor EphB3 has been implicated in dendrite pruning and synapse formation. In mouse hippocampal neurons, EphB3 operates at post-synaptic terminals where it is phosphorylated and subsequently binds to the SH2/SH3 adaptor protein growth-factor-receptor-bound protein 4 (GRB4) as well as the PDZ domain protein syntenin [36], emphasizing the need for additional studies to define the specific roles of individual EphB ligands and receptors.
Reelin
Reeler mice have short dendrites with abnormal orientations in the hippocampus [37]. Defects in dendrite morphogenesis are not secondary to cellular ectopia, because heterozygous Reeler mice have reduced dendrite complexity with normal cellular organization [38]. Purkinje cells in Reeler mice have poorly developed dendrite arbors [39]. Recent studies reveal that in mice in which the Reelin adaptor proteins Crk and Crk-like (CrkL) are mutated, Purkinje cells that fail to migrate exhibit conical dendrites, whereas dendrites in properly positioned Purkinje cells display a classical planar morphology [40].
In other studies, the Reelin pathway appears to play a critical role in defining the molecular identity of the distal dendrite compartment in hippocampal CA1 and neocortical L5 pyramidal neurons. Reelin signaling is required for targeting HCN1 (hyperpolarization activated cyclic nucleotide-gated potassium channel 1) and GIRK1 (G-protein activated inwardly rectifying potassium channel 1) channels to the distal tuft, where the channels actively filter inputs targeted to these dendrite domains [41].
Bone Morphogenetic Proteins (BMP)
The Bone Morphogenetic Proteins (BMP) induce dendrite formation in cortical and hippocampal neurons [42]. BMPs interact with the cell surface receptors BMPR1 and BMPR2 [43]. Recent conditional knockout studies reveal that BMPR1A/1B participates in the control of dendrite growth in sympathetic neurons [44]. Inhibition of the protein kinase p21-activated protein kinase-1 (PAK1) blocks BMP7-induced cofilin phosphorylation, prevents remodeling of the actin cytoskeleton, and thereby blocks BMP7-induced dendrite formation in cerebral cortical neurons [42]. Another member of the bone morphogenetic protein subclass, GDF5 (growth differential factor 5) regulates the growth of pyramidal cell dendrites in the developing hippocampus via a high-affinity receptor complex consisting of BMPR1B and BMPR2, which activates SMAD (similar to mothers against decapentaplegic) signaling and regulates the expression of the transcription factor HES5 (hairy and enhancer of slit 5) [45]. The apical and basal dendrite arbors of hippocampal pyramidal cells in both homozygous and heterozygous Gdf5 null mutants are markedly stunted [45].
Contact-mediated regulators
Cadherins and Protocadherins
Cell adhesion molecules such as the cadherin Flamingo (Fmi) as well as the protocadherins and atypical cadherins are critical for dendrite tiling, self-avoidance, and arbor homeostasis [46-47]. In Drosophila, Fmi forms a complex with the LIM domain protein Espinas (Esn) in class IV da neurons [48]. Genetic evidence suggests that Fmi-Esn signal downstream to molecules regulating cell polarity such as Van Gogh (Vang) and Rho, two proteins essential for self-avoidance. However, the specific molecular links between Fmi, Esn, and cell polarity molecules remain unknown. Interestingly, studies in mammalian neurons suggest that the Fmi homologs Celsr2 (cadherin EGF LAG seven-pass G-type G-type receptor 2) and Celsr3 have conserved functions in the regulation of planar cell polarity and neuronal morphogenesis [49]. Knockdown of Celsr2 in organotypic cultures in pyramidal neurons and Purkinje cells induces simplification of dendrite arbors [50], whereas knockdown of Celsr3 in hippocampal slices leads to increased dendritic branching [49]. Determining whether these cell polarity genes function downstream of Fmi to regulate dendrite tiling will be an important line of future investigation.
In mammalian neurons, several protocadherins have been identified as regulators of dendrite morphogenesis. Conditional deletion of the γ-protocadherin gene cluster yields viable mice with deficits in dendrite arborization in cerebral cortical neurons [51]. γ-protocadherins appear to promote dendrite arborization during cortical development by negatively regulating the PKC/MARCKS (protein kinase C/Myristolated alanine-rich C-kinase substrate) signaling pathway. Interestingly, the atypical cadherin Fat3 appears to have the opposite effect on dendrite arbors in retinal amacrine cells, inducing the pruning of dendrites in the inner plexiform layer [52].
Genetic deletion of the γ-protocadherin cluster also impairs dendrite self-avoidance in retinal starburst amacrine cells and Purkinje neurons in cerebellum [53]. Strikingly, replacement of the 22 γ-protocadherin family members with a single isoform restores self-avoidance and also decreases dendrite-dendrite contacts between neighboring retinal starburst amacrine cells. Collectively, these studies highlight diverse and conserved functions for cadherin and cadherin-related proteins in dendrite patterning.
Adhesion-G protein-coupled receptors
The Brain Angiogenesis Inhibitors (BAIs) are adhesion-G protein-coupled receptors that are expressed in the brain and reside at postsynaptic densities in the forebrain and cerebellum [54-55]. Knockdown of BAI3 in the rodent cerebellar cortex in vivo leads to defects in dendrite arborization and misorientation of Purkinje cells. BAI3 regulates Purkinje cell dendritic arbor formation by reorganization of the actin cytoskeleton through activation of the RhoGTPase Rac1 and ELMO1, a key Rac1 regulator [56]. Interestingly, BAI proteins have been associated with schizophrenia [57] and bipolar disorder [58], raising the possibility that aberrant dendrite arborization and orientation contributes to disease pathogenesis.
DSCAM (Down Syndrome Cell Adhesion Molecule)
DSCAM is a homophilic cell adhesion molecule and has been implicated in different stages of the nervous system development [59]. DSCAM1 controls dendrite patterning [60] and dendrite self-avoidance [61-64]. In the avian retina, DSCAM directs lamina-specific synaptic connections [65], whereas in the mouse retina, DSCAM regulates neurite arborization, mosaic tiling, and dendrite self-avoidance [66].
Like γ-protocadherins, DSCAM regulates dendrite patterning through homophilic binding. Through alternative splicing, over 38,000 isoforms of DSCAM can be generated in Drosophila [67]. Drosophila neurons express a subset of DSCAM isoforms allowing individual dendrites to repel each other through isoform-specific homophilic interactions, thereby facilitating self-avoidance [68]. DSCAM is also dynamically expressed during cerebral cortical development and plays an important role in pyramidal neuron dendrite arborization and spine morphogenesis [69]. Mice carrying the spontaneous mutation DSCAM del 17 harbor morphological changes in brain size and shape, in addition to subtle changes in cerebral cortical organization, volume, and lamination [69].
Integrins
Although DSCAM and other transmembrane molecules offer a mechanism for contact-mediated self-avoidance, these observations do not explain how dendrites are retained in a single two-dimensional plane and thus subject to contact-mediated tiling. Independent research from two groups has implicated integrins in the patterning of Drosophila sensory neurons [70-71]. These studies show that upon mutation or knockdown of integrins or their epidermal ligands the laminins, class IV da neurons, which normally tile the larval body wall in a two-dimensional plane along the epidermis, have aberrant tiling with extension of dendrites into the overlying epidermis and non-contacting crossings [70-71]. In tiling mutants, overexpression of integrins restores normal tiling and dendrite patterning, suggesting that integrins promote arbor tiling by strengthening dendrite interactions with the underlying extracellular matrix [71]. Together, these studies demonstrate an essential role for integrins in directing the planar growth of Drosophila da neuron dendrites.
Fusogens and other transmembrane proteins
New data suggest that fusion-related proteins may play a role in dendrite morphogenesis. An essential role for the type 1 membrane protein EFF-1 (epithelial fusion failure 1) has been identified in the elaboration of dendrite arbors in mechanoreceptors PVD neurons in C.elegans [72]. Consistent with its role in cell-cell fusion, EFF-1 mediates pruning through neurite-neurite fusion as well as branch retraction. Using the same model system, a function for the leucine-rich repeat transmembrane protein DMA-1 (dendrite-morphogenesis-abnormal 1) has been observed [73]. In contrast to EFF-1, knockdown of DMA-1 results in reduced dendrite branching, while overexpression triggers exuberant arborization.
Recent studies suggest that a tripartite receptor ligand complex of cell-surface proteins plays an instructive role in directing growth of dendrite branches in PVD somatosensory neurons in C.elegans [74-75]. These studies demonstrate that SAX-7 (sensory axon guidance-7), a homolog of the vertebrate L1 cell adhesion molecule (L1CAM), forms a complex with MNR-1(menorin) in the hypodermis, acting on dendrite growth through the recently identified neuronal leucine-rich repeat containing transmembrane protein DMA-1 expressed in PVD neurons. All three molecules have homologs in vertebrate genomes, suggesting the function of these molecules might be conserved [75].
Neuronal activity and calcium signaling
In addition to secreted and contact-mediated regulators, neuronal activity represents a key cue in the regulation of dendrite development. The effects of neuronal activity on dendrite development are mediated by calcium signals [76-77]. Recent studies have revealed that calcium transients promote dendrite pruning in Drosophila sensory neurons. Voltage-gated calcium channels (VGCCs) are responsible for generating compartmentalized calcium transients, and the calcium-activated protease calpain operates downstream of calcium transients to trigger dendrite pruning [78]. Compartmentalized changes in dendrite branch excitability are also observed in mammalian neurons [79].
Calcium influx from voltage-gated calcium channels (VGCCs) or N-methyl-D-aspartate receptors (NMDA-Rs) triggers calcium binding to calmodulin (CaM), which activates calcium/calmodulin dependent protein kinases (CaMKs) (Figure 3). Calcium-dependent signaling through CaM and CaMKI, CaMKII, and CaMKIV positively and negatively regulates dendrite complexity [80-81].
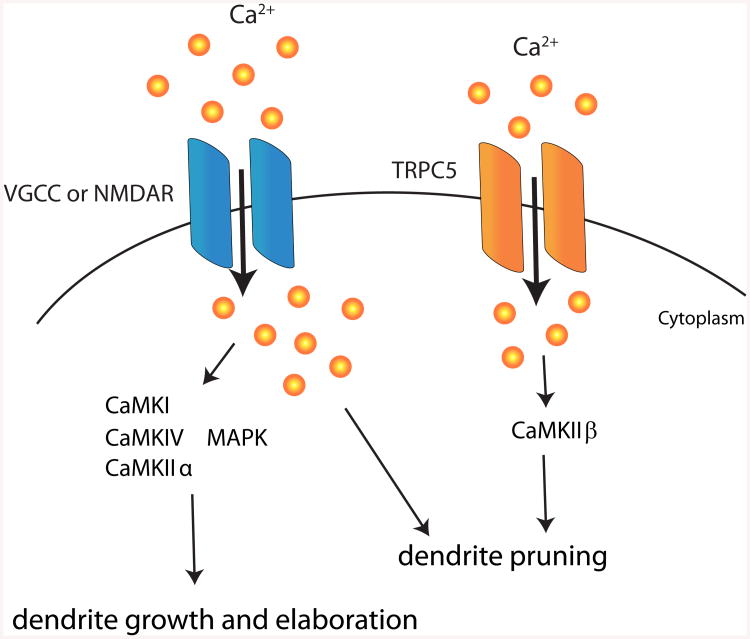
Figure 3. Effects of calcium signaling on dendrite morphogenesis.
Calcium influx from voltage-gated calcium channels VGCCs or NMDA receptors (NMDAR) activates several CaMK family members and MAPKs which in turn regulate dendrite growth and elaboration. VGCC are also responsible to generate compartmentalized calcium transients to trigger dendrite pruning. In later stage of dendrite development, CaMKIIβ activated by calcium influx from TRPC5 channel drives dendrite pruning.
Although brain CaMKII predominantly consists of the α and β isoforms, the vast majority of studies on CaMKII have focused on the functions of CaMKIIα including the regulation of neuronal morphogenesis, synapse development, and learning and memory [82]. CaMKIIα regulates the activity of transcription factors, whereas CaMKIIα and CaMKIIβ may work in concert to regulate actin dynamics [83-84]. VGCCs stimulate CaMKIIα activity, triggering the phosphorylation and activation of the bHLH transcription factor NeuroD at distinct sites, thereby inducing dendrite elaboration [84]. Likewise, in sympathetic neurons, calcium influx promotes dendrite growth in a CaMKIIα-dependent manner [10]. Several studies have focused on a redundant role for CaMKIIβ, working together with CaMKIIα, to direct dendritic arborization via its F-actin binding domain (FABD) [85-86]. However, this function does not depend on the catalytic activity of CaMKII [85, 87-88].
A specific catalytic function for CaMKIIβ has been identified in the mammalian brain [80, 89-90]. Strikingly, CaMKIIβ operates at the centrosome in a CaMKIIα-independent manner to drive dendrite retraction and pruning in the rodent cerebellar cortex [80]. The CaMKIIβ-induced phosphorylation of Cdc20 (cell-division cycle protein 20), the coactivator of the ubiquitin ligase APC (anaphase promoting complex), inhibits downstream centrosomal signaling cascades and triggers a transition from growth to retraction of dendrites [91]. These studies have also identified a role for the canonical calcium channel transient receptor potential channel 5 (TRPC5) as a key upstream activator of centrosomal CaMKIIβ signaling [89]. These results define a catalytic function for CaMKIIβ in the mammalian brain that couples calcium signaling to cell-intrinsic pathways operating at the centrosome, thereby orchestrating dendrite pruning and retraction. Consistent with these findings, CaMKIIβ knockout mice display cognitive deficits and motor impairment [92], highlighting a non-redundant role for CaMKIIβ in neuronal development.
Along with CaMKI and CaMKII, CaMKIV appears to be a critical effector of calcium influx via VGCCs. CaMKIV knockout mice have defects in dendrite development and locomotor behavioral deficits, consistent with altered cerebellar function [93]. Unlike other CaMKs, CaMKIV is localized primarily in the nucleus in neurons where it activates the transcription factor cyclic-AMP-responsive-element binding protein (CREB) to drive dendrite growth [94]. CaMKIV may also regulate CREB binding protein (CBP)-mediated expression of vascular endothelial growth factor D (VEGFD) to drive dendrite morphogenesis [95].
Interestingly, distinct CaMK signaling pathways may also interact and coordinately regulate dendrite patterning. For example, the GTPase Rem2 (Rad and Gem-like GTP-binding protein 2) is a substrate of CaMKII in a signaling cascade that negatively regulates dendrite growth. Upon membrane depolarization, Rem2 and activated CaMKII associate, allowing CaMKII to phosphorylate Rem2. The CaMKII-induced phosphorylation of Rem2 triggers its translocation to the nucleus, where it suppresses CaMKIV signaling and dendrite arborization [96].
VGCCs and NMDARs also trigger the activity of the protein kinase MAPK (mitogen-activated protein kinase). Multiple spaced stimuli in hippocampal neurons induce MAPK activation and promote the extension and stabilization of dendritic filopodia [97]. In sympathetic ganglion cells and cortical neurons, neuronal activity stimulates MAPK activity and dendrite growth [10, 94]. Thus, both CaMKs and MAPKs appear to be essential downstream effectors of intracellular calcium influx in the control of dendrite morphogenesis.
Perspectives
During the past two decades, investigations of dendrite development have uncovered an enormously complex set of extrinsic cues and associated signaling mechanisms that regulate dendrite morphogenesis. Here, we have discussed major categories of regulators including secreted factors, contact-mediated cues, and neuronal activity as major extrinsic drivers of dendrite morphogenesis. A key observation emerging from these studies is that extrinsic cues regulate far more than dendrite morphogenesis, driving diverse aspects of neuronal development. How does specificity of function arise? Temporal and spatial considerations may provide part of the answer. For example, in granule neurons of the cerebellar cortex, calcium influx early in dendrite morphogenesis appears to stimulate CaMKIIα activation, leading to NeuroD-dependent dendrite growth [84], whereas calcium influx at later developmental stages triggers dendrite pruning and retraction through CaMKIIβ [80]. Besides timing, the subcellular locale of extrinsic cue action appears to contribute to specificity. For example, whereas the semaphorin Sema3A acts a repellent guidance molecule on axons, it acts as a chemoattractant for dendrites [13]. As technologies to study cellular microdomains further improve, an increasing array of spatial specificity determinants will be identified that control key aspects of dendrite morphogenesis. Certainly, this line of research represents a major challenge within the field that will benefit greatly from the use of high resolution live microscopy and genetically encoded calcium indicator proteins (GECIs).
Intracellular signaling networks by which neurons respond to extrinsic cues may provide an additional means of biologically specifying responses. In this vein, it will be essential to determine how extrinsic cues influence cell-intrinsic mechanisms of dendrite morphogenesis. Likewise, how cell-intrinsic drivers of dendrite morphogenesis (for a recent review see [2]) regulate the responsiveness of neurons and dendrites to extrinsic cues will be critical to our understanding of signaling specificity.
Based on the vast array of molecules regulating dendrite morphogenesis that have been identified to date, there is little doubt that dendrite development requires the coordinated cooperation of various classes of proteins. In many cases, the downstream mechanisms of extrinsic cues remain ill defined. Cell-extrinsic cues may module cell intrinsic pathways ranging from transcription factors to cytoskeletal regulators and motor proteins to secretory and endocytic pathways to ubiquitin ligase pathways [2]. Although the vast majority of research has focused on how specific cell extrinsic cues regulates dendrite development, network analyses linking these cues to each other and their downstream effectors at a system biological level is lacking. However, a better understanding of coordinated signaling network of dendrite morphogenesis may need to await the discovery of additional cues and signals that control dendrite patterning.
Although early studies of cell-extrinsic cues regulating dendrite morphogenesis have been critical to the field, many of these efforts were limited by the approaches and models employed, such as primary neuron culture paradigms. More recent studies have increasingly employed in vivo paradigms of knockout and in vivo RNAi studies, which allow more rigorous and reliable characterization of the role and mechanisms of extrinsic regulation of dendrite development in the mammalian brain. As advances in imaging continue, it will be essential to study mechanisms of dendrite morphogenesis in the living animal to capture changes in real-time while maintaining the normal physiologic environment.
Because impairment of dendrite morphogenesis is thought to contribute to diverse neurological disorders associated with intellectual disabilities or cognitive deficits, including genetic disorders, such as autism spectrum disorder, Down syndrome or Rett syndrome [98] as well as neurodegenerative conditions including Alzheimer's disease [99], elucidation of the role and mechanisms of regulation of dendrite morphogenesis by extrinsic cues will advance our understanding of brain diseases. However, pathologic analyses to date suggest that only a subset of patients with autism spectrum disorder have dendrite abnormalities. Thus, it remains unknown whether changes in dendrite morphogenesis contribute to disease pathogenesis or simply a marker of aberrant neuronal connectivity. Regardless, exploring the correlation of abnormal dendritogenesis and human pathophysiology may offer additional insight into these currently untreatable human diseases.
Highlights.
Extrinsic cues regulate distinct phases of dendrite morphogenesis.
Dendrite development requires secreted proteins, contact-mediated regulators and neuronal activity.
Extrinsic cues influence local and global mechanisms of dendrite development.
Acknowledgments
This work was supported by the NIH grant NS084393 (to A.B.) and the European Molecular Biology Organization (EMBO: ALTF 889-2011 to P.V.). We thank members of the Bonni laboratory for helpful discussions and critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldberg JL. Intrinsic neuronal regulation of axon and dendrite growth. Curr Opin Neurobiol. 2004 Oct;14(5):551–7. doi: 10.1016/j.conb.2004.08.012. doi:S0959-4388(04)00126-6 [pii] 10.1016/j.conb.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Puram SV, Bonni A. Cell-intrinsic drivers of dendrite morphogenesis. Development. 2013 Dec;140(23):4657–71. doi: 10.1242/dev.087676. doi:140/23/4657 [pii] 10.1242/dev.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. doi:10.1146/annurev.neuro.24.1.67724/1/677 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–42. doi: 10.1146/annurev.biochem.72.121801.161629. doi:10.1146/annurev.biochem.72.121801.161629121801.161629 [pii] [DOI] [PubMed] [Google Scholar]
- 5.McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995 Oct;15(4):791–803. doi: 10.1016/0896-6273(95)90171-x. doi:0896-6273(95)90171-X [pii] [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, et al. Cortical degeneration in the absence of neurotrophin signaling: dendritic retraction and neuronal loss after removal of the receptor TrkB. Neuron. 2000 Apr;26(1):233–45. doi: 10.1016/s0896-6273(00)81153-8. doi:S0896-6273(00)81153-8 [pii] [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, Cai Q, Xie Y, Sheng ZH. Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons. Cell reports. 2012 Jul 26;2(1):42–51. doi: 10.1016/j.celrep.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000 Apr;3(4):342–9. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- 9.Joo W, Hippenmeyer S, Luo L. Neurodevelopment. Dendrite morphogenesis depends on relative levels of NT-3/TrkC signaling. Science. 2014 Oct 31;346(6209):626–9. doi: 10.1126/science.1258996. doi:346/6209/626 [pii] 10.1126/science.1258996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaillant AR, Zanassi P, Walsh GS, Aumont A, Alonso A, Miller FD. Signaling mechanisms underlying reversible, activity-dependent dendrite formation. Neuron. 2002 Jun 13;34(6):985–98. doi: 10.1016/s0896-6273(02)00717-1. doi:S0896627302007171 [pii] [DOI] [PubMed] [Google Scholar]
- 11.Rui Y, Myers KR, Yu K, Wise A, De Blas AL, Hartzell HC, et al. Activity-dependent regulation of dendritic growth and maintenance by glycogen synthase kinase 3beta. Nat Commun. 2013;4:2628. doi: 10.1038/ncomms3628. doi:ncomms3628 [pii] 10.1038/ncomms3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeney LB, Chou YH, Wu Z, Joo W, Komiyama T, Potter CJ, et al. Secreted semaphorins from degenerating larval ORN axons direct adult projection neuron dendrite targeting. Neuron. 2011 Dec 8;72(5):734–47. doi: 10.1016/j.neuron.2011.09.026. doi:S0896-6273(11)00877-4 [pii] 10.1016/j.neuron.2011.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polleux F, Morrow T, Ghosh A. Semaphorin 3A is a chemoattractant for cortical apicaldendrites. Nature. 2000 Apr 6;404(6778):567–73. doi: 10.1038/35007001. [DOI] [PubMed] [Google Scholar]
- 14.Komiyama T, Sweeney LB, Schuldiner O, Garcia KC, Luo L. Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell. 2007 Jan 26;128(2):399–410. doi: 10.1016/j.cell.2006.12.028. doi:S0092-8674(07)00042-6 [pii] 10.1016/j.cell.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura F, Ugajin K, Yamashita N, Okada T, Uchida Y, Taniguchi M, et al. Increased proximal bifurcation of CA1 pyramidal apical dendrites in sema3A mutant mice. J Comp Neurol. 2009 Oct 10;516(5):360–75. doi: 10.1002/cne.22125. [DOI] [PubMed] [Google Scholar]
- 16.de Anda FC, Rosario AL, Durak O, Tran T, Graff J, Meletis K, et al. Autism spectrum disorder susceptibility gene TAOK2 affects basal dendrite formation in the neocortex. Nat Neurosci. 2012 Jul;15(7):1022–31. doi: 10.1038/nn.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishiyama M, Togashi K, von Schimmelmann MJ, Lim CS, Maeda S, Yamashita N, et al. Semaphorin 3A induces CaV2.3 channel-dependent conversion of axons to dendrites. Nat Cell Biol. 2011 Jun;13(6):676–85. doi: 10.1038/ncb2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasaki Y, Cheng C, Uchida Y, Nakajima O, Ohshima T, Yagi T, et al. Fyn and Cdk5 mediate semaphorin-3A signaling, which is involved in regulation of dendrite orientation in cerebral cortex. Neuron. 2002 Aug 29;35(5):907–20. doi: 10.1016/s0896-6273(02)00857-7. doi:S0896627302008577 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Shelly M, Cancedda L, Lim BK, Popescu AT, Cheng PL, Gao H, et al. Semaphorin3A regulates neuronal polarization by suppressing axon formation and promoting dendrite growth. Neuron. 2011 Aug 11;71(3):433–46. doi: 10.1016/j.neuron.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011 Feb 10;470(7333):259–63. doi: 10.1038/nature09675. doi:nature09675 [pii] 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, Matsuoka RL, et al. On and off retinal circuit assembly by divergent molecular mechanisms. Science. 2013 Nov 1;342(6158):1241974. doi: 10.1126/science.1241974. doi:342/6158/1241974 [pii] 10.1126/science.1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng T, Ryu JR, Sohn JH, Tan T, Song H, Ming GL, et al. Class 3 semaphorin mediates dendrite growth in adult newborn neurons through Cdk5/FAK pathway. PLoS One. 2013;8(6):e65572. doi: 10.1371/journal.pone.0065572. doi:10.1371/journal.pone.0065572 PONE-D-13-03115 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godenschwege TA, Simpson JH, Shan X, Bashaw GJ, Goodman CS, Murphey RK. Ectopic expression in the giant fiber system of Drosophila reveals distinct roles for roundabout (Robo), Robo2, and Robo3 in dendritic guidance and synaptic connectivity. J Neurosci. 2002 Apr 15;22(8):3117–29. doi: 10.1523/JNEUROSCI.22-08-03117.2002. doi:20026291 22/8/3117 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furrer MP, Kim S, Wolf B, Chiba A. Robo and Frazzled/DCC mediate dendriticguidance at the CNS midline. Nat Neurosci. 2003 Mar;6(3):223–30. doi: 10.1038/nn1017. doi:10.1038/nn1017nn1017 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Whitford KL, Marillat V, Stein E, Goodman CS, Tessier-Lavigne M, Chedotal A, et al. Regulation of cortical dendrite development by Slit-Robo interactions. Neuron. 2002 Jan 3;33(1):47–61. doi: 10.1016/s0896-6273(01)00566-9. doi:S0896627301005669 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Gibson DA, Tymanskyj S, Yuan RC, Leung HC, Lefebvre JL, Sanes JR, et al. Dendrite self-avoidance requires cell-autonomous slit/robo signaling in cerebellar purkinje cells. Neuron. 2014 Mar 5;81(5):1040–56. doi: 10.1016/j.neuron.2014.01.009. doi:S0896-6273(14)00014-2 [pii] 10.1016/j.neuron.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teichmann HM, Shen K. UNC-6 and UNC-40 promote dendritic growth through PAR-4 in Caenorhabditis elegans neurons. Nat Neurosci. 2011 Feb;14(2):165–72. doi: 10.1038/nn.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith CJ, Watson JD, VanHoven MK, Colon-Ramos DA, Miller DM., 3rd Netrin (UNC-6) mediates dendritic self-avoidance. Nat Neurosci. 2012 May;15(5):731–7. doi: 10.1038/nn.3065. doi:nn.3065 [pii] 10.1038/nn.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003 Apr 10;422(6932):583–8. doi: 10.1038/nature01522. doi:10.1038/nature01522 nature01522 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005 Jan;8(1):34–42. doi: 10.1038/nn1374. doi:nn1374 [pii] 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- 31.Pino D, Choe Y, Pleasure SJ. Wnt5a controls neurite development in olfactory bulb interneurons. ASN neuro. 2011;3(3):e00059. doi: 10.1042/AN20100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Y, Helt JC, Wexler E, Petrova IM, Noordermeer JN, Fradkin LG, et al. Wnt5 and drl/ryk gradients pattern the Drosophila olfactory dendritic map. J Neurosci. 2014 Nov 5;34(45):14961–72. doi: 10.1523/JNEUROSCI.2676-14.2014. doi:34/45/14961 [pii] 10.1523/JNEUROSCI.2676-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005 Jun;6(6):462–75. doi: 10.1038/nrm1662. doi:nrm1662 [pii] 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 34.Clifford MA, Athar W, Leonard CE, Russo A, Sampognaro PJ, Van der Goes MS, et al. EphA7 signaling guides cortical dendritic development and spine maturation. Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):4994–9. doi: 10.1073/pnas.1323793111. doi:1323793111 [pii] 10.1073/pnas.1323793111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoogenraad CC, Milstein AD, Ethell IM, Henkemeyer M, Sheng M. GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat Neurosci. 2005 Jul;8(7):906–15. doi: 10.1038/nn1487. doi:nn1487 [pii] 10.1038/nn1487. [DOI] [PubMed] [Google Scholar]
- 36.Xu NJ, Sun S, Gibson JR, Henkemeyer M. A dual shaping mechanism for postsynaptic ephrin-B3 as a receptor that sculpts dendrites and synapses. Nat Neurosci. 2011 Nov;14(11):1421–9. doi: 10.1038/nn.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanfield BB, Caviness VS, Jr, Cowan WM. The organization of certain afferents to the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979 Jun 1;185(3):461–83. doi: 10.1002/cne.901850304. [DOI] [PubMed] [Google Scholar]
- 38.Niu S, Renfro A, Quattrocchi CC, Sheldon M, D'Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004 Jan 8;41(1):71–84. doi: 10.1016/s0896-6273(03)00819-5. doi:S0896627303008195 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Kim J, Kwon N, Chang S, Kim KT, Lee D, Kim S, et al. Altered branching patterns of Purkinje cells in mouse model for cortical development disorder. Sci Rep. 2011;1:122. doi: 10.1038/srep00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Park TJ, Kwon N, Lee D, Kim S, Kohmura Y, et al. Dendritic planarity of Purkinje cells is independent of Reelin signaling. Brain Struct Funct. 2014 May 15; doi: 10.1007/s00429-014-0780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kupferman JV, Basu J, Russo MJ, Guevarra J, Cheung SK, Siegelbaum SA. Reelin signaling specifies the molecular identity of the pyramidal neuron distal dendritic compartment. Cell. 2014 Sep 11;158(6):1335–47. doi: 10.1016/j.cell.2014.07.035. doi:S0092-8674(14)00985-4 [pii] 10.1016/j.cell.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podkowa M, Christova T, Zhao X, Jian Y, Attisano L. p21-Activated kinase (PAK) is required for Bone Morphogenetic Protein (BMP)-induced dendritogenesis in cortical neurons. Mol Cell Neurosci. 2013 Nov;57:83–92. doi: 10.1016/j.mcn.2013.10.005. doi:S1044-7431(13)00098-5 [pii] 10.1016/j.mcn.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000 Apr 17;19(8):1745–54. doi: 10.1093/emboj/19.8.1745. doi:10775259. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majdazari A, Stubbusch J, Muller CM, Hennchen M, Weber M, Deng CX, et al. Dendrite complexity of sympathetic neurons is controlled during postnatal development by BMP signaling. J Neurosci. 2013 Sep 18;33(38):15132–44. doi: 10.1523/JNEUROSCI.4748-12.2013. doi:33/38/15132 [pii] 10.1523/JNEUROSCI.4748-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osorio C, Chacon PJ, Kisiswa L, White M, Wyatt S, Rodriguez-Tebar A, et al. Growth differentiation factor 5 is a key physiological regulator of dendrite growth during development. Development. 2013 Dec;140(23):4751–62. doi: 10.1242/dev.101378. doi:dev.101378 [pii] 10.1242/dev.101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao FB, Kohwi M, Brenman JE, Jan LY, Jan YN. Control of dendritic field formation in Drosophila: the roles of flamingo and competition between homologous neurons. Neuron. 2000 Oct;28(1):91–101. doi: 10.1016/s0896-6273(00)00088-x. doi:S0896-6273(00)00088-X [pii] [DOI] [PubMed] [Google Scholar]
- 47.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999 Sep 3;98(5):585–95. doi: 10.1016/s0092-8674(00)80046-x. doi:S0092-8674(00)80046-X [pii] [DOI] [PubMed] [Google Scholar]
- 48.Matsubara D, Horiuchi SY, Shimono K, Usui T, Uemura T. The seven-pass transmembrane cadherin Flamingo controls dendritic self-avoidance via its binding to a LIM domain protein, Espinas, in Drosophila sensory neurons. Genes Dev. 2011 Sep 15;25(18):1982–96. doi: 10.1101/gad.16531611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shima Y, Kawaguchi SY, Kosaka K, Nakayama M, Hoshino M, Nabeshima Y, et al. Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nat Neurosci. 2007 Aug;10(8):963–9. doi: 10.1038/nn1933. doi:nn1933 [pii] 10.1038/nn1933. [DOI] [PubMed] [Google Scholar]
- 50.Shima Y, Kengaku M, Hirano T, Takeichi M, Uemura T. Regulation of dendritic maintenance and growth by a mammalian 7-pass transmembrane cadherin. Dev Cell. 2004 Aug;7(2):205–16. doi: 10.1016/j.devcel.2004.07.007. doi:10.1016/j.devcel.2004.07.007 S1534580704002382 [pii] [DOI] [PubMed] [Google Scholar]
- 51.Garrett AM, Schreiner D, Lobas MA, Weiner JA. gamma-protocadherins control cortical dendrite arborization by regulating the activity of a FAK/PKC/MARCKS signaling pathway. Neuron. 2012 Apr 26;74(2):269–76. doi: 10.1016/j.neuron.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deans MR, Krol A, Abraira VE, Copley CO, Tucker AF, Goodrich LV. Control of neuronal morphology by the atypical cadherin Fat3. Neuron. 2011 Sep 8;71(5):820–32. doi: 10.1016/j.neuron.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012 Aug 23;488(7412):517–21. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selimi F, Cristea IM, Heller E, Chait BT, Heintz N. Proteomic studies of a single CNS synapse type: the parallel fiber/purkinje cell synapse. PLoS Biol. 2009 Apr 14;7(4):e83. doi: 10.1371/journal.pbio.1000083. doi:08-PLBI-RA-4899 [pii] 10.1371/journal.pbio.1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins MO, Husi H, Yu L, Brandon JM, Anderson CN, Blackstock WP, et al. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J Neurochem. 2006 Apr;97(Suppl 1):16–23. doi: 10.1111/j.1471-4159.2005.03507.x. doi:JNC3507 [pii] 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- 56.Lanoue V, Usardi A, Sigoillot SM, Talleur M, Iyer K, Mariani J, et al. The adhesion-GPCR BAI3, a gene linked to psychiatric disorders, regulates dendrite morphogenesis in neurons. Mol Psychiatry. 2013 Aug;18(8):943–50. doi: 10.1038/mp.2013.46. doi:mp201346 [pii] 10.1038/mp.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liao HM, Chao YL, Huang AL, Cheng MC, Chen YJ, Lee KF, et al. Identification and characterization of three inherited genomic copy number variations associated with familial schizophrenia. Schizophr Res. 2012 Aug;139(1-3):229–36. doi: 10.1016/j.schres.2012.05.015. doi:S0920-9964(12)00308-8 [pii] 10.1016/j.schres.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 58.McCarthy MJ, Nievergelt CM, Kelsoe JR, Welsh DK. A survey of genomic studies supports association of circadian clock genes with bipolar disorder spectrum illnesses and lithium response. PLoS One. 2012;7(2):e32091. doi: 10.1371/journal.pone.0032091. doi:10.1371/journal.pone.0032091 PONE-D-11-17192 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmucker D, Chen B. Dscam and DSCAM: complex genes in simple animals, complex animals yet simple genes. Genes Dev. 2009 Jan 15;23(2):147–56. doi: 10.1101/gad.1752909. doi:23/2/147 [pii] 10.1101/gad.1752909. [DOI] [PubMed] [Google Scholar]
- 60.Zhu H, Hummel T, Clemens JC, Berdnik D, Zipursky SL, Luo L. Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat Neurosci. 2006 Mar;9(3):349–55. doi: 10.1038/nn1652. doi:nn1652 [pii] 10.1038/nn1652. [DOI] [PubMed] [Google Scholar]
- 61.Hughes ME, Bortnick R, Tsubouchi A, Baumer P, Kondo M, Uemura T, et al. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007 May 3;54(3):417–27. doi: 10.1016/j.neuron.2007.04.013. doi:S0896-6273(07)00292-9 [pii] 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matthews BJ, Grueber WB. Dscam1-mediated self-avoidance counters netrin-dependent targeting of dendrites in Drosophila. Curr Biol. 2011 Sep 13;21(17):1480–7. doi: 10.1016/j.cub.2011.07.040. doi:S0960-9822(11)00841-4 [pii] 10.1016/j.cub.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, et al. Dendrite self-avoidance is controlled by Dscam. Cell. 2007 May 4;129(3):593–604. doi: 10.1016/j.cell.2007.04.013. doi:S0092-8674(07)00470-9 [pii] 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Soba P, Zhu S, Emoto K, Younger S, Yang SJ, Yu HH, et al. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007 May 3;54(3):403–16. doi: 10.1016/j.neuron.2007.03.029. doi:S0896-6273(07)00288-7 [pii] 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008 Jan 24;451(7177):465–9. doi: 10.1038/nature06469. doi:nature06469 [pii] 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- 66.Fuerst PG, Koizumi A, Masland RH, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008 Jan 24;451(7177):470–4. doi: 10.1038/nature06514. doi:nature06514 [pii] 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, et al. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000 Jun 9;101(6):671–84. doi: 10.1016/s0092-8674(00)80878-8. doi:S0092-8674(00)80878-8 [pii] [DOI] [PubMed] [Google Scholar]
- 68.Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004 Sep 3;118(5):619–33. doi: 10.1016/j.cell.2004.08.021. doi:10.1016/j.cell.2004.08.021 S0092867404007962 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maynard KR, Stein E. DSCAM contributes to dendrite arborization and spine formation in the developing cerebral cortex. J Neurosci. 2012 Nov 21;32(47):16637–50. doi: 10.1523/JNEUROSCI.2811-12.2012. doi:32/47/16637 [pii] 10.1523/JNEUROSCI.2811-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Han C, Wang D, Soba P, Zhu S, Lin X, Jan LY, et al. Integrins regulate repulsion-mediated dendritic patterning of drosophila sensory neurons by restricting dendrites in a 2D space. Neuron. 2012 Jan 12;73(1):64–78. doi: 10.1016/j.neuron.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim ME, Shrestha BR, Blazeski R, Mason CA, Grueber WB. Integrins establish dendrite-substrate relationships that promote dendritic self-avoidance and patterning in drosophila sensory neurons. Neuron. 2012 Jan 12;73(1):79–91. doi: 10.1016/j.neuron.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oren-Suissa M, Hall DH, Treinin M, Shemer G, Podbilewicz B. The fusogen EFF-1 controls sculpting of mechanosensory dendrites. Science. 2010 Jun 4;328(5983):1285–8. doi: 10.1126/science.1189095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu OW, Shen K. The transmembrane LRR protein DMA-1 promotes dendrite branching and growth in C. elegans. Nat Neurosci. 2012 Jan;15(1):57–63. doi: 10.1038/nn.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salzberg Y, Diaz-Balzac CA, Ramirez-Suarez NJ, Attreed M, Tecle E, Desbois M, et al. Skin-derived cues control arborization of sensory dendrites in Caenorhabditis elegans. Cell. 2013 Oct 10;155(2):308–20. doi: 10.1016/j.cell.2013.08.058. doi:S0092-8674(13)01089-1 [pii] 10.1016/j.cell.2013.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dong X, Liu OW, Howell AS, Shen K. An extracellular adhesion molecule complex patterns dendritic branching and morphogenesis. Cell. 2013 Oct 10;155(2):296–307. doi: 10.1016/j.cell.2013.08.059. doi:S0092-8674(13)01090-8 [pii] 10.1016/j.cell.2013.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de la Torre-Ubieta L, Bonni A. Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron. 2011 Oct 6;72(1):22–40. doi: 10.1016/j.neuron.2011.09.018. doi:S0896-6273(11)00839-7 [pii] 10.1016/j.neuron.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002 Oct;3(10):803–12. doi: 10.1038/nrn941. doi:10.1038/nrn941 nrn941 [pii] [DOI] [PubMed] [Google Scholar]
- 78.Kanamori T, Kanai MI, Dairyo Y, Yasunaga K, Morikawa RK, Emoto K. Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science. 2013 Jun 21;340(6139):1475–8. doi: 10.1126/science.1234879. doi:science.1234879 [pii] 10.1126/science.1234879. [DOI] [PubMed] [Google Scholar]
- 79.Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008 Mar 27;452(7186):436–41. doi: 10.1038/nature06725. doi:nature06725 [pii] 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- 80.Puram SV, Kim AH, Ikeuchi Y, Wilson-Grady JT, Merdes A, Gygi SP, et al. A CaMKIIbeta signaling pathway at the centrosome regulates dendrite patterning in the brain. Nat Neurosci. 2011 Aug;14(8):973–83. doi: 10.1038/nn.2857. doi:nn.2857 [pii] 10.1038/nn.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008 Sep 25;59(6):914–31. doi: 10.1016/j.neuron.2008.08.021. doi:S0896-6273(08)00745-9 [pii] 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hell JW. CaMKII: claiming center stage in postsynaptic function and organization. Neuron. 2014 Jan 22;81(2):249–65. doi: 10.1016/j.neuron.2013.12.024. doi:S0896-6273(13)01185-9 [pii] 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sogawa Y, Yoshimura Y, Yamauchi T. Investigation of the Ca(2+)-independent form of Ca(2+)/calmodulin-dependent protein kinase II in neurite outgrowth. Brain Res Brain Res Protoc. 2001 Dec;8(3):159–69. doi: 10.1016/s1385-299x(01)00106-4. doi:S1385299X01001064 [pii] [DOI] [PubMed] [Google Scholar]
- 84.Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni AA. CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004 Jan 22;41(2):229–41. doi: 10.1016/s0896-6273(03)00841-9. doi:S0896627303008419 [pii] [DOI] [PubMed] [Google Scholar]
- 85.Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003 Jul 17;39(2):283–97. doi: 10.1016/s0896-6273(03)00428-8. doi:S0896627303004288 [pii] [DOI] [PubMed] [Google Scholar]
- 86.O'Leary H, Lasda E, Bayer KU. CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Mol Biol Cell. 2006 Nov;17(11):4656–65. doi: 10.1091/mbc.E06-03-0252. doi:E06-03-0252 [pii] 10.1091/mbc.E06-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okamoto K, Narayanan R, Lee SH, Murata K, Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc Natl Acad Sci U S A. 2007 Apr 10;104(15):6418–23. doi: 10.1073/pnas.0701656104. doi:0701656104 [pii] 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin YC, Redmond L. CaMKIIbeta binding to stable F-actin in vivo regulates F-actin filament stability. Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15791–6. doi: 10.1073/pnas.0804399105. doi:0804399105 [pii] 10.1073/pnas.0804399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Puram SV, Riccio A, Koirala S, Ikeuchi Y, Kim AH, Corfas G, et al. A TRPC5-regulated calcium signaling pathway controls dendrite patterning in the mammalian brain. Genes Dev. 2011 Dec 15;25(24):2659–73. doi: 10.1101/gad.174060.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Puram SV, Bonni A. Novel functions for the anaphase-promoting complex in neurobiology. Seminars in cell & developmental biology. 2011 Aug;22(6):586–94. doi: 10.1016/j.semcdb.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puram SV, Kim AH, Park HY, Anckar J, Bonni A. The ubiquitin receptor S5a/Rpn10 links centrosomal proteasomes with dendrite development in the mammalian brain. Cell Rep. 2013 Jul 11;4(1):19–30. doi: 10.1016/j.celrep.2013.06.006. doi:S2211-1247(13)00284-2 [pii] 10.1016/j.celrep.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bachstetter AD, Webster SJ, Tu T, Goulding DS, Haiech J, Watterson DM, et al. Generation and behavior characterization of CaMKIIbeta knockout mice. PLoS One. 2014;9(8):e105191. doi: 10.1371/journal.pone.0105191. doi:10.1371/journal.pone.0105191 PONE-D-14-19438 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ribar TJ, Rodriguiz RM, Khiroug L, Wetsel WC, Augustine GJ, Means AR. Cerebellar defects in Ca2+/calmodulin kinase IV-deficient mice. J Neurosci. 2000 Nov 15;20(22):RC107. doi: 10.1523/JNEUROSCI.20-22-j0004.2000. doi:20004718 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002 Jun 13;34(6):999–1010. doi: 10.1016/s0896-6273(02)00737-7. doi:S0896627302007377 [pii] [DOI] [PubMed] [Google Scholar]
- 95.Mauceri D, Freitag HE, Oliveira AM, Bengtson CP, Bading H. Nuclear calcium-VEGFD signaling controls maintenance of dendrite arborization necessary for memory formation. Neuron. 2011 Jul 14;71(1):117–30. doi: 10.1016/j.neuron.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 96.Ghiretti AE, Kenny K, Marr MT, 2nd, Paradis S. CaMKII-dependent phosphorylation of the GTPase Rem2 is required to restrict dendritic complexity. J Neurosci. 2013 Apr 10;33(15):6504–15. doi: 10.1523/JNEUROSCI.3861-12.2013. doi:33/15/6504 [pii] 10.1523/JNEUROSCI.3861-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001 Feb;4(2):151–8. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- 98.Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000 Oct;10(10):981–91. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 99.Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007 Jun;6(3):275–84. doi: 10.1111/j.1474-9726.2007.00289.x. doi:ACE289 [pii] 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]