Abstract
Proteolytic cleavage of amyloid β-protein precursor (AβPP) by β– and γ–secretases results in production of the amyloid β peptide (Aβ) that accumulates in the brains of sufferers of Alzheimer’s Disease (AD). We have developed a monoclonal antibody, 2B12, which binds in the vicinity of the β-secretase cleavage site on AβPP but does not bind within the Aβ region. We hypothesised that this antibody, directed against the substrate rather than the enzyme, could inhibit cleavage of AβPP by β-secretase via steric hindrance and thus reduce downstream production of Aβ. The antibody would enter cells by binding to AβPP when it is at the cell surface and then be internalised with the protein. We subsequently demonstrated that, after addition of 2B12 to standard growth media, this antibody was indeed capable of inhibiting Aβ40 production in neuroblastoma and astrocytoma cells expressing native AβPP, as measured by an ELISA. This inhibition was both concentration- and time-dependent and was specific to 2B12. We were only able to inhibit approximately 50% of Aβ40 production suggesting that not all APP is trafficked to the cell surface. We propose that this antibody could be used as a novel, putative therapy for the treatment of AD.
Keywords: Alzheimer’s disease, monoclonal antibodies, amyloid β, β-secretase, amyloid β-protein precursor, steric hindrance
1. Introduction
Alzheimer’s disease (AD) is characterised by two main neuropathological features: an over-accumulation in the brain of intracellular neurofibrillary tangles and extracellular deposits, termed senile plaques (SP) [1]. The main constituent of SP is amyloid β (Aβ), a 39-43 amino acid peptide that is cleaved from amyloid precursor protein (AβPP) [2]. Currently the amyloid hypothesis is one of the dominant theories of the aetiology of AD [3] where an increase in Aβ levels, especially Aβ42 [1], is thought to be crucial for the pathogenesis of AD.
AβPP can be processed by two proteolytic pathways. The non-amyloidogenic route involves cleavage of AβPP by α–secretase within the Aβ region to release sAβPPα [4]. In the amyloidogenic pathway β–secretase (BACE1) first cleaves AβPP to liberate sAβPPβ and C99; γ–secretase then cleaves C99 to produce Aβ and a C–terminal intracellular fragment [5]. A homologue of BACE1, BACE2, has also been identified [6,7]. Although there is still some debate regarding the co-localisation of the enzymes and substrates involved, it is generally considered that, after synthesis, a proportion of AβPP is transported to the cell membrane and then internalised for processing in the endosomal-lysosomal system [8,9].
The only licensed treatments currently available for AD are symptomatic, and target cholinergic and glutamatergic systems [10]. Recent therapeutic research has targeted Aβ aggregation [11], Aβ degradation [12], Aβ clearance [13], antioxidants, cholesterol-lowering drugs [10] and inhibitors of BACE1 and γ–secretase [5,14]. However, γ–secretase also cleaves Notch which has many crucial functions [14] and, while BACE1 knockout mice exhibit no major deleterious symptoms [15], this enzyme does have other substrates [16,17,18]. Furthermore, an inhibitor would have to be specific for BACE1 over BACE2 [19]. An alternative approach, Aβ vaccination, has been used successfully in transgenic mice to reduce Aβ deposition [20] and improve cognition [21,22,23]. A Phase 2 clinical trial was initiated based on these results but was terminated early when several of the patients developed meningoencephalitis [24].
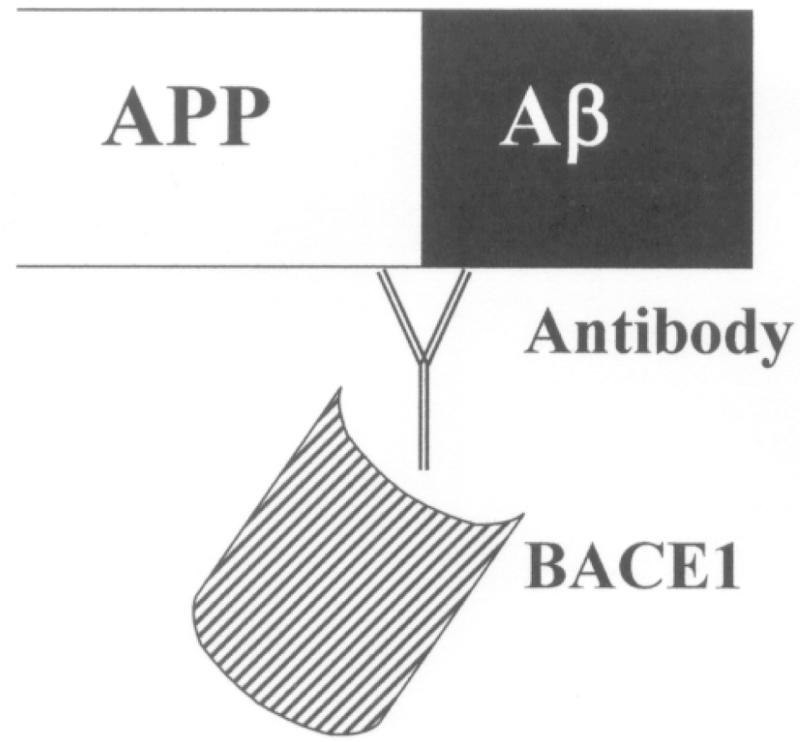
We have devised a different approach which could reduce the build-up of Aβ. We have produced a monoclonal antibody (MAb) which should inhibit BACE1 activity by steric hindrance as it binds in the vicinity of the enzyme’s cleavage site in AβPP (Figure 1). We hypothesised that the antibody would bind to AβPP when it is at the cell surface and then become internalised. We describe below the characterisation of this antibody and experiments detailing its interactions with AβPP. We subsequently demonstrated that the antibody was capable of inhibiting Aβ40 production by cells in culture and suggest that this could be a novel therapy for the treatment of AD.
Figure 1.
Potential action of 2B12. Antibody binding to the β–secretase cleavage site on AβPP blocking access of BACE1 to its substrate by steric hindrance
2. Materials and Methods
2.1 Materials
All peptides were synthesised by Severn Biotech LTD, Kidderminster, UK. Cell lines were purchased from ECACC, Porton Down, U.K. All chemicals and reagents were purchased from Sigma-Aldrich, Poole, U.K. or Fisher Scientific, Leicester, U.K. unless otherwise specified. The following primary antibodies were used: BAM401S (Autogen Bioclear, Calne, Wiltshire, U.K.), BAM401AP (Autogen Bioclear), 6E10 (Chemicon Europe, Chandlers Ford, Hampshire, U.K.) and nAβPP (Sigma-Aldrich). AβPP was detected in ELISAs with the APP DuoSet, (R & D Systems, Abingdon, Oxon., UK).
2.2 Immunization procedures
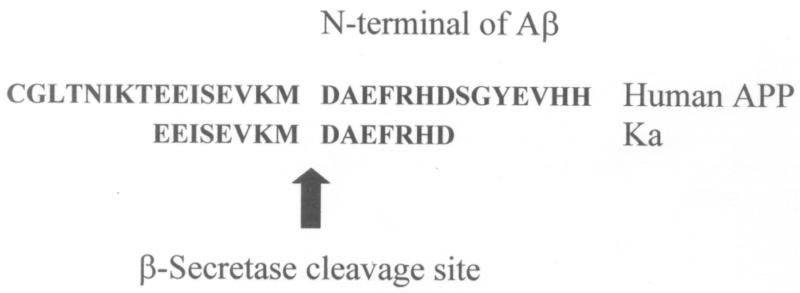
Monoclonal antibodies were raised against an immunogenic peptide, termed Ka, which represented the amino acid sequence spanning the β–secretase cleavage site in human AβPP (Figure 2). The peptide was synthesised using a poly-lysine, multiple antigenic peptide (MAP) core and then used to immunise female Balb/c mice (20-25g). All experiments on mice were performed in accordance with the Animals (Scientific Procedures) Act 1986 administered by the U.K. Government Home Office and with ethical approval from Cardiff University. Mice were immunized and hybridomas were generated by standard methods as first developed by Kohler and Milstein [25] and detailed elsewhere, including Liddell and Cryer [26]. The hybridoma supernatants were screened for high-affinity MAbs by indirect ELISA using the immunising peptide, prior to more complete cross-reactivity screenings. The 2B12-D2-F5 (2B12) clone was chosen for further screening based on preliminary experiments.
Figure 2.
The 30 amino acid sequence spanning the β-secretase cleavage site on human AβPP and the 15 amino acid sequence of the synthesised peptide spanning the cleavage site, Ka, used to immunise Balb/c mice to produce 2B12.
2.3 Cell Culture
Two human cell lines, astrocytoma MOG-G-UVW (MOG) and neuroblastoma SH-SY5Y (SY5Y) (e.g. [27], both of which constitutively express AβPP (Figure 4), were used to investigate the characteristics of 2B12. They were used as a source of AβPP and to characterise the binding properties of 2B12. They were also used as a model system to investigate whether 2B12 could reduce Aβ40 levels. MOG were grown in a 1:1 solution of Ham’s F10 and Dulbecco’s Modified Eagle’s Medium supplemented with 10% Foetal Bovine Serum (FBS) (Perbio Science U.K. LTD, Cramlington, Northumberland) and 2mM glutamine while SY5Y grew in a 1:1 solution of Ham’s F12 and Eagle’s Minimum Essential Medium supplemented with 10% FBS, 2mM glutamine and 1% non-essential amino acids. Cells lines were incubated at 37°C in 5% CO2 in air.
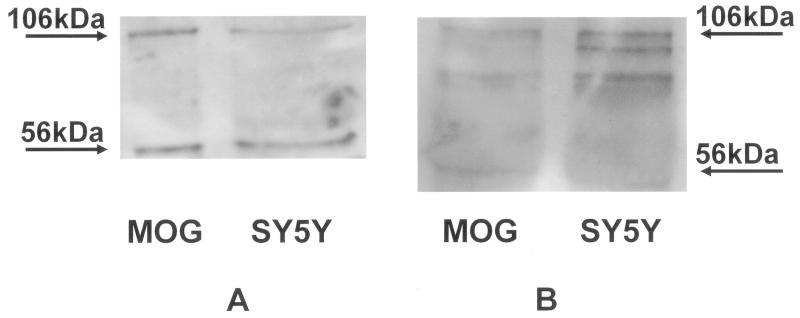
Figure 4.
Representative Western Blots comparing the labelling of AβPP in MOG and SY5Y cells obtained with (A) 2B12 and (B) a commercial anti-N-terminus AβPP antibody (Sigma-Aldrich). 50μg cell lysate samples were separated on a 10% SDS-PAGE gel, transferred to nitrocellulose membranes and probed with either 2B12 (2.7 μg/ml) followed by an anti-mouse IgG-HRP conjugate (1:30,000) or the n AβPP antibody (1:1000) followed by an anti-rabbit IgG-HRP conjugate (1:20,000) and the signal was detected using ECL. A 106kDa band corresponding to AβPP 695 and a 56kDa band corresponding to a thrombin cleavage product of AβPP were seen in both cell lines with both antibodies. n=3-6.
2.4 Antibody Processing and Characterisation
The 2B12 antibody was concentrated from culture medium using Amicon Centriplus YM-100 filters (Millipore, Billerica, Massachusetts, USA) with a nominal molecular weight cut off of 100kDa and the isotype determined using the Isostrip mouse monoclonal antibody isotyping kit (Serotec, Oxford, U.K.). Cross-reactivity studies were then performed with an indirect ELISA to test 2B12 against a number of peptides: Ka peptide, Aβ40, two unrelated peptides manufactured on a MAP core designated RAP and WYATT, C99 (generous gift from Prof. M. Ehrmann, Cardiff School of Biosciences, Cardiff University, U.K. [28]) and one other peptide also manufactured on a MAP core, Kb. Kb represented a portion of the amino acid sequence spanning the β–secretase cleavage site in human AβPP downstream from Ka, specifically the 5 residues before the cleavage site and the first 10 residues into the Aβ region (see Figure 2). 96-Well microplates (Falcon® Pro-BIND™ Flat Bottom, Becton Dickinson Labware, Oxford, UK) were standardised using a 10-point standard curve of the Ka peptide with the highest concentration at 10μg/ml. All test samples were plated at a protein concentration of 1 μg/ml in a carbonate / bicarbonate buffer (15mM Na2CO3, 35mM NaHCO3, pH9.8), and incubated overnight at 4°C. The plates were then aspirated and blocked with 0.1% (w/v) milk powder in phosphate-buffered saline (137mM NaCl, 1.5mM KH2PO4, 8mM Na2HPO4.12H2O, 2.5mM KCl, pH 7.4) with 0.05% Tween 20 (PBST) for 1 hour, followed by 2B12 at a concentration of 0.25μg/ml in PBST for 1 hour. 2B12 was detected with a secondary goat anti-mouse antibody conjugated to HRP, 1:3000 (Pierce Biotechnology Inc, Rockford, IL), incubated for 1 hour, and the enzyme substrate, o-phenylenediamine (OPD), in 0.1M citrate-phosphate buffer, pH 5.0, incubated for 20 minutes. The reaction was stopped with 1M H2SO4 and the absorbance determined at 492nm. All incubations were performed at room temperature (RT), unless otherwise stated and wells were aspirated and washed four times with PBST between each stage. All results were converted to Ka peptide equivalents using the Ka standard curve and expressed as a % of Ka at 1 μg/ml.
2.5 Western Blot
Western Blotting was performed using standard methods [29]. The samples and molecular weight marker (Precision Plus Protein Standards marker, Bio-Rad Laboratories, Hercules, California) were loaded onto 10% polyacrylamide gels and separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) in running buffer (25mM Tris base, 190mM glycine, 0.05% SDS, pH 8.3). The separated proteins were then blotted on to 0.2μm nitrocellulose membranes (Amersham Biosciences, Little Chalfont, UK), washed in Tris-buffered saline with Tween 20 (TBST, 2mM Tris, 15mM NaCl, 0.1% Tween-20, pH 7.5) and blocked for 1 hour, at RT, in TBST supplemented with 5% w/v fat-free dried milk (Blotto). Blots were then incubated with the relevant antibody in 1-5% Blotto at 4°C overnight. Membranes were washed five times in TBST and incubated with the relevant horseradish peroxidase-conjugated secondary antibody (1:20-30,000, Vector Laboratories, Burlingame, California) for 1 hour at RT. The membranes were washed as above in TBST, the bands visualised using enhanced chemiluminescent detection (Super Signal®, West Dura, Perbio Science U.K.) and exposed to high performance chemiluminescent X-ray film (Amersham Biosciences).
2.6 Immunocytochemistry
Immunocytochemistry (ICC) was performed as described in [29] with several modifications as detailed below. Both cell lines were grown on 10 μg/ml collagen-coated glass cover slips in 24 well cluster plates in the appropriate growth media. Cells were fixed in 2% formaldehyde in 0.1M phosphate buffered saline (PBS, 145mM NaCl, 96.4mM NaHPO4, 21.5mM NaH2PO4), pH 7.4, for 15 minutes at RT and subsequently washed three times in PBS (each 5 minutes). Cover slips were blocked for 30 minutes in 3% horse serum, 1% bovine serum albumin (BSA) and 0.1% Triton X-100 in PBS. The cover slips were incubated with 5μg/ml 2B12 in blocking buffer overnight at 4°C. The cover slips were washed with PBS as above before incubation with an anti-mouse secondary antibody conjugated to biotin (1:270, Vector Laboratories) for one hour at RT. After washing as above, coverslips were then incubated with avidin conjugated to fluorescein (FITC) (Vector Laboratories), 1:600, for 1 hour at RT. The coverslips were washed as above and finally dipped in distilled water to remove any residual buffer salts before being left to dry. Cover slips were mounted on ethanol-rinsed slides using VECTASHIELD Mounting Medium with propidium iodide (Vector Laboratories) and stored at 4°C. Slides were visualised using a Leica SP2 laser scanning confocal microscope system (Leica, Germany) and images were processed using Adobe Photoshop.
2.7 AβPP detection
The amount of mature AβPP expressed in either cell line was relatively low (reaching only 50 ng/ml after lysis and concentration through the Amicon Centriplus filter). It was therefore impossible to use this source of AβPP in its unpurifed form in an indirect ELISA in the above manner. To confirm that the 2B12 antibody detected AβPP, we therefore used a competition assay in conjunction with a sandwich ELISA which recognised an epitope in the region of the C-terminus of AβPP (APP DuoSet). Lysates were prepared from MOG cells using lysis buffer (50mM Tris, 5mM EDTA, 150mM NaCl, 1% Triton, 0.4mM NaVO4, 50mM NaF, 1mM PMSF, 20μM phenylarsine oxide, 10mM sodium molybdate, 10μg/ml leupeptin, 10μg/ml aprotinin) and then concentrated through Amicon Centriplus YM-100 centrifugal filters with a nominal molecular weight cut-off of 100 kDa. Recovered AβPP was quantified using a sandwich ELISA by comparing values with standard recombinant human AβPP from the APP DuoSet. The ELISA followed the manufacturer’s guidelines and methods detailed above unless otherwise stated. Briefly, the capture antibody was used at 4 μg/ml in PBS to coat 96-well microplates and incubated overnight at RT. Plates were blocked with 1% BSA and 5% sucrose in PBS for a minimum of 30 minutes. Samples and standards were prepared in 1% BSA in PBS and incubated on the plate for 2 hours. A six point standard curve was prepared with a highest concentration of 20 ng/mL. Biotinylated detection antibody, 300ng/ml in 1% BSA in PBS, was incubated for 1.5 hours and detected using streptavidin-HRP, diluted 1:200 in PBST for 20 minutes, followed by OPD substrate as above. The competition assay for AβPP followed the same protocol except for the following modifications. MOG cell lysate was used to provide AβPP at a concentration of 30ng/ml. Prior to incubating with the detection antibody, the samples were incubated with either 2B12 or an irrelevant mouse IgG antibody (Sigma-Aldrich) from 1.25 to 10 μg/ml for 1 hour. Binding of these antibodies was then inferred by a decrease in binding of the detection antibody compared to the PBST control alone.
2.8 Concentration-response curve for effects of 2B12 on Aβ40 levels
All experiments were performed in 24 well cluster plates. An equal volume of well-suspended MOG or SY5Y cells were distributed between experimental and control wells for each experimental run. Inter-plate differences were minimized as far as possible by plating the cells at a similar density for each experiment and including relevant controls on every plate. Concentrations and time points were done in triplicate on each plate. Cells were allowed to attach overnight then incubated in duplicate either with control media or 2B12 (0.0005 - 10 μg/ml) in relevant culture media for 4 days at 37°C. Controls with irrelevant mouse IgG were also included at 10 μg/ml. For subsequent analysis of Aβ40, 450 μl of media were collected from each well and subjected to combined immunoprecipitation and ELISA. Media was first immunoprecipitated with 1:2,000 BAM401S, specific to the C-terminus of human Aβ40, overnight at 4°C. Samples were then incubated with Protein A (Santa Cruz Biotechnology, Santa Cruz, California, USA) for 2 hours and then washed with CHAPS buffer (150mM NaCl, 50mMTris, 1mM EDTA, 10mM CHAPS) and centrifuged at 3275g, repeated 3 times. This was followed by a final wash in PBS. Samples were then boiled at 95°C for 5 minutes to dissociate the Protein A before being centrifuged for 1 minute at 3275g. The supernatant was subsequently removed and tested in an ELISA for Aβ40 following the methods above, except for the following. The ELISA employed the N-terminal MAb 6E10, as the capture antibody and affinity-purified BAM401AP, specific to the C-terminus of human Aβ40, as the detection antibody. 6E10, 5 μg/ml in carbonate/bicarbonate buffer, was incubated overnight at 4°C. Plates were then blocked with 0.1% (w/v) milk powder in PBS for 1 hour at RT. Samples and standards were added to the plates and incubated for 1.5 hours followed by the detection antibody, 0.45 μg/ml in PBST for 1 hour. The plates were then blocked for 30 minutes and a secondary anti-rabbit antibody conjugated to HRP, 1:4000 (Vector Laboratories), was incubated for 1 hour. This resulted in an ELISA with a lower limit of sensitivity of approximately 0.1 ng/ml giving good differentiation within the range of Aβ40 immunoprecipitated from the cell lines. The concentration of Aβ40 in samples from the cells was calculated from an Aβ40 standard curve (0-12.5ng/ml) run on the same plates and generated in Excel. Aβ40 concentrations were then expressed as a percentage of the relevant control values.
2.9 Time course to determine effect of incubation period with 2B12 on Aβ40 levels
Experiments were performed as above except for the following. All cells were exposed to 2B12 at 10μg/ml and incubated in duplicate for a range of time periods from 3 hours to 6 days. Relevant controls (media alone) were included at each time point. At the end of the time period, media was collected and Aβ40 detected by combined immunoprecipitation and ELISA as described above.
2.10 Cell Counts
Viable cell counts were performed once conditioned media had been removed for analysis for Aβ40 levels. Cells were treated with 0.25% trypsin solution for 3 minutes at 37°C, then neutralised with the same volume of growth media. A well-suspended sample of cells was incubated with 0.2 % Trypan Blue for 5 minutes at 37°C and 100 μl of the cells were counted on a haemocytometer. Cell numbers were expressed as a percentage of control at the relevant concentration and time point.
2.11 Statistical Analyses
The results from the cross-reactivity indirect ELISA were converted to Ka equivalents (ng/ml), transformed (log(1 + Ka equivalents ng/ml)) and then analysed by ANOVA and LSD test to determine if any significant differences occurred between the responses of 2B12 to the peptides tested. The data generated in the ELISAs to measure Aβ40 levels were analysed using a Student’s t-test at the one-tailed significance level to determine if Aβ concentrations were significantly lower than media controls (100%). Viable cell counts were tested in the same manner (except two-tailed significance tests were used) to determine if the presence of antibody (2B12 or irrelevant IgG control) significantly altered this number from media control values.
3. Results
3.1 Antibody Characterisation
A number of monoclonal antibodies were raised following immunisation with the Ka peptide, representing the 15 amino acid sequence spanning the β-secretase cleavage site (Figure 2). One, 2B12-D2-F5 (2B12), isotype IgG2b, appeared to be the most promising in preliminary indirect ELISAs against the immunising peptide and was used in subsequent experiments.
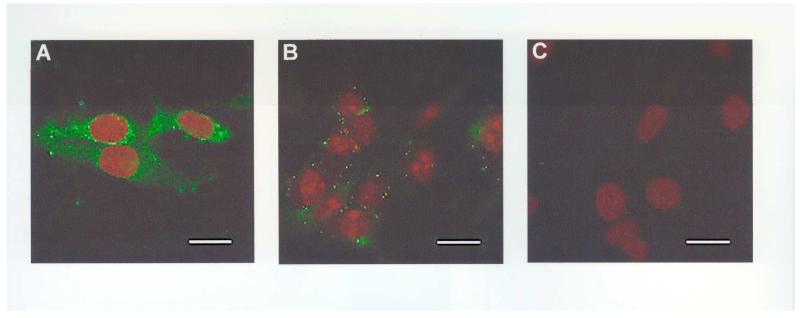
Binding to the immunising peptide was confirmed using ICC in the MOG cell line (Figure 3). 2B12 resulted in punctate labelling of the cytoplasm and perinuclear staining. This signal was blocked when cells were labelled with 2B12 previously incubated with the immunising peptide, Ka (10μg) for 24 hours (Figure 3, A-C). Similar results were also obtained with the SY5Y cell line (data not shown).
Figure 3.
Representative examples of the labelling of AβPP in MOG cells by 2B12 (A-C). (A) Punctuate labelling of MOG cytoplasm and perinuclear staining by 2B12 (5μg/ml) detected using a biotinylated anti-mouse antibody (1:270) and Avidin-FITC (1:600); (B) MOG incubated with 2B12 following pre-adsorption with Ka (10μg) and detected as above; (C) MOG incubated with the block solution alone, followed by detection as above. No labelling was detected in the absence of 2B12 or after pre-adsorption with Ka. Coverslips were mounted with VECTASHIELD mounting medium with propidium iodide to counter-stain the nuclei. Scale bar = 25μm, n=3.
We also confirmed that 2B12 bound to AβPP in lysates from both cell lines, MOG and SY5Y by Western blotting. Bands of 106kDa and 56kDa were seen for 2B12 corresponding to AβPP [30] and a thrombin cleavage fragment of AβPP [31]. A similar pattern was seen with a commercial antibody to the N-terminal of AβPP (Figure 4). We also confirmed that 2B12 bound to human AβPP in brain preparations from Tg2576 mice which over-express AβPP [32] (data not shown).
In a competition ELISA using MOG cell lysate as a source of protein, 2B12 interfered with the binding of the detection antibody from the AβPP DuoSet, in a concentration-dependent manner (Figure 5). This latter antibody was directed against an epitope in the C-terminal region of AβPP, some distance from the β-secretase cleavage site. The binding of this antibody was significantly reduced from control values at 2B12 concentrations of 5 and 10 μg/ml. No such reductions in absorbance from control levels were observed with an irrelevant mouse IgG (Figure 5). These data confirmed that 2B12 was binding to AβPP captured by the DuoSet antibody.
Figure 5.
Competition sandwich ELISA for AβPP from concentrated MOG cell lysate using anti-AβPP antibodies (capture and detection antibodies at 4 μg/ml and 300 ng/ml, respectively). Prior to incubation with the detection antibody, ELISA plates were coated with a range of concentrations of either 2B12 or an irrelevant mouse IgG antibody. Controls of PBST were also included and data are expressed as mean % of control absorbance (+/− 1 SEM). The irrelevant IgG had no effect on the ELISA. * Significantly different from control at p<0.05 in one-tailed Student’s t tests, n=3.
2B12 demonstrated significantly different responses to the peptides tested in an indirect ELISA as evidenced by an ANOVA (p<0.001) (Table 1). It strongly recognised the Ka peptide and produced significantly higher reactions when compared to the other peptides (LSD test). Reactions against Kb, although less than to Ka, were significantly greater than reactions to all the other peptides tested. 2B12 only very weakly recognised Aβ in the indirect ELISA and not at all in a Western blot (data not shown). The antibody did bind more strongly to C99, the C-terminal fragment of AβPP produced after cleavage by β–secretase, in a Western blot (data not shown). However, cross reactivity as measured in an ELISA was low with absorbance readings of only 2.53 (+/− 1.19) Ka equivalents. Reactions against the two other unrelated peptides, RAP and WYATT, in the ELISA were also low (Table 1). The binding of 2B12 to WYATT was significantly greater than that to Aβ and RAP but not C99. There was no significant difference in the binding of 2B12 to C99, Aβ40, and RAP.
Table 1.
Cross-reactivity profile of 2B12 (0.2 μg/ml) against peptides at 1 μg/ml. All values are expressed as % absorbance of Ka at 1 μg/ml after conversion to Ka equivalents (ng/ml). ANOVA followed by LSD tests were used to detect significant differences at p<0.05.
| Compound | Ka equivalents ng/ml (+/− 1 SEM) |
% Cross Reactivity (+/− 1 SEM) |
|---|---|---|
| Kaa | 1041.67 +/− 208.33 | - |
| Kbb | 30.95 +/− 3.28 | 3.48 +/− 1.26 |
| C99c,d | 2.53 +/− 1.19 | 0.32 +/− 0.20 |
| Aβd | 1.05 +/− 0.54 | 0.13 +/− 0.08 |
| WYATTc | 5.14 +/− 1.37 | 0.62 +/− 0.31 |
| RAPd | 0.96 +/− 0.63 | 0.13 +/− 0.09 |
The reaction of 2B12 with Ka was significantly greater than all other peptides.
The reaction of 2B12 with Kb was significantly greater than all others except Ka.
The reaction of 2B12 with WYATT was significantly greater than Aβ and RAP but not Ka, Kb or C99.
There were no significant differences between the cross-reactivity of 2B12 with RAP, Aβ or C99. (n=3).
3.2 Concentration-response curve for effects of 2B12 on Aβ40 levels
The effect of 2B12 on the concentration of Aβ40 in conditioned media was investigated in both cell lines, MOG and SY5Y (Figure 6). The presence of 2B12 significantly reduced the amount of Aβ40, expressed as % of control, in both cell lines in a concentration-dependent manner, as detected in a sandwich ELISA. Initially no effect was observed, however, at 0.05 μg/ml 2B12, Aβ40 was significantly reduced to 78.8+/−8.8% of control levels in the SY5Y cell line and at 0.5 μg/ml, to 75.5+/− 11.3% of control levels in the MOG cells. This trend continued and at 10μg/ml 2B12, Aβ40 levels were further reduced to 54.5+/−5.7% of controls in the SY5Y and 45.1+/−4.7% of controls in the MOG cells.
Figure 6.
Effect of 2B12 or an irrelevant mouse IgG on Aβ40 levels in MOG (A) and SY5Y (B) cells. Data are expressed as mean (+/− 1 SEM) % of control (media only) Aβ40 levels as detected in a sandwich ELISA. Cells were incubated with 2B12 or the irrelevant IgG for 4 days. Increasing concentrations of 2B12 significantly reduced Aβ40 levels from control in both cell lines (one-tailed Student’s t tests). The irrelevant IgG had no effect on Aβ40 levels. * Significantly different from control at p<0.05 in one-tailed Student’s t tests, n=3-4.
No such reductions in Aβ40 levels were observed when either cell line was incubated with the irrelevant mouse IgG antibody at 10μg/ml (Figure 6).
3.3 Time course to determine effect of incubation period with 2B12 on Aβ40 levels
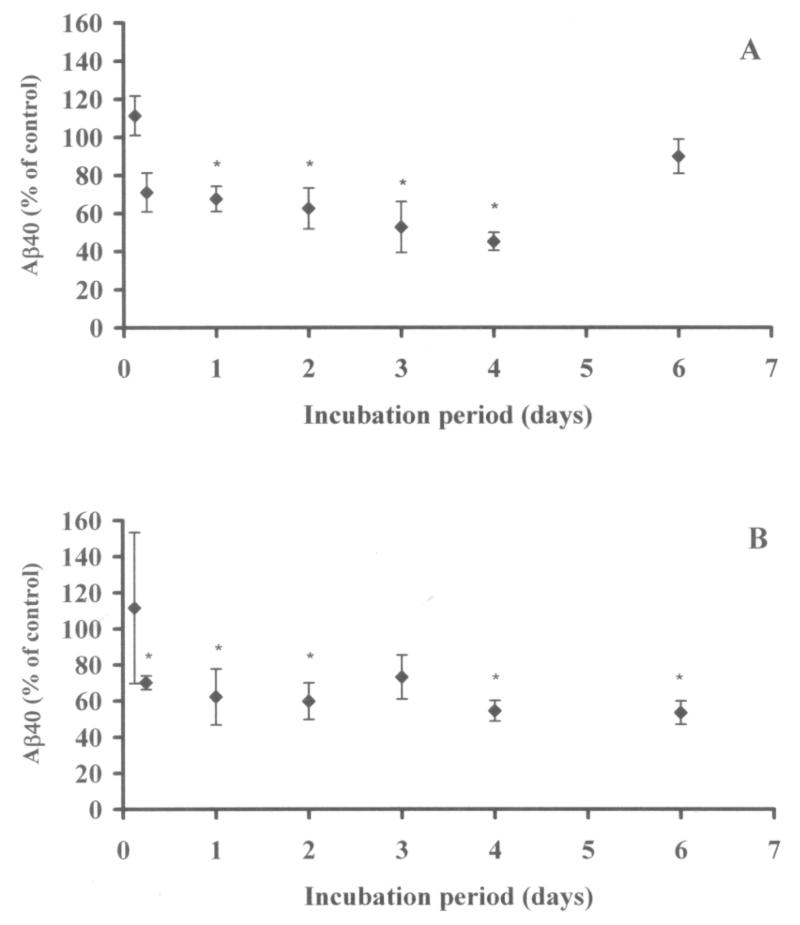
Altering the 2B12 incubation period was shown to have a significant effect on Aβ40 levels in conditioned media of both cell lines (Figure 7). No significant reduction in Aβ40 levels was observed when cells were incubated with the antibody for only 3 hours. A significant effect was, however, observable after 6 hours in the SY5Y when levels were reduced to 70.1+/−3.8% of control levels and after 1 day in the MOGs when Aβ40 was reduced to 67.6+/−6.7%. After this point Aβ40 was significantly reduced from control levels in both cell lines, for up to 6 days in the SY5Y line (with the exception of day 3) and for up to 4 days in the MOG cell line. There was an apparent increase in Aβ40 at day 6 in the MOG cells when compared to shorter incubation periods, probably due to increased cell death at this time point (seen in both control and antibody-treated cells) as MOG cells exhibited a higher growth rate than SY5Y cells and were over-confluent by day 6.
Figure 7.
Effect of period of incubation with 2B12 at 10 μg/ml on Aβ40 in MOG (A) and SY5Y (B) cells. Data are expressed as mean (+/− 1 SEM) % of control (media only) Aβ40 levels as detected in a sandwich ELISA. No effect was observed at 3 hours (MOG and SY5Y) or at 6 hours (MOG). The effect of the antibody was, however, significant at all other time points except 6 days (MOG) (one-tailed Student’s t tests). * Significantly different from control at p<0.05 in one-tailed Student’s t tests, n=3-4.
When viable cell counts were compared (Table 2), neither the presence of 2B12 nor the irrelevant mouse IgG antibody, both at 10μg/ml, significantly altered the number of viable cells at any time point compared to media controls.
Table 2.
Viable cell counts for cells treated with either 2B12 or an irrelevant IgG, both at 10μg/ml, expressed as a percentage of cell counts of controls (growth media alone). Counts were performed after incubations lasting from 3 hours to 6 days. No significant differences (p<0.05) from control were detected in two-tailed Student’s t tests, n=3-4.
| Incubation period (days) |
Antibody | % no. of cells (+/− 1 SEM) |
|---|---|---|
| MOG | ||
| 3 hrs | 2B12 | 81.2 +/− 7.1 |
| 6 hrs | 2B12 | 145.4 +/− 38.4 |
| 1 day | 2B12 | 78.1 +/− 15.8 |
| 2 days | 2B12 | 79.7 +/− 7.8 |
| 3 days | 2B12 | 81.3 +/− 11.2 |
| 6 days | 2B12 | 106.1 +/− 3.9 |
| 4 days | IgG | 94.8 +/− 21.9 |
|
| ||
| SY5Y | ||
| 3 hrs | 2B12 | 109.9 +/− 9.3 |
| 6 hrs | 2B12 | 86.7 +/− 16.4 |
| 1 day | 2B12 | 129.7 +/− 12.0 |
| 2 days | 2B12 | 96.1 +/− 12.2 |
| 3 days | 2B12 | 130.2 +/− 17.2 |
| 6 days | 2B12 | 103.0 +/− 13.7 |
| 4 days | IgG | 83.2 +/− 6.8 |
4. Discussion
2B12 strongly recognised the immunising Ka peptide, showed reduced binding to another β-secretase cleavage site peptide, Kb, but exhibited very limited cross reactivity with the other cleavage products of AβPP tested. It did, however, bind to AβPP as evidenced in both ELISA and Western blots. In the latter an additional band at 56kDa was also seen which is likely to be a fragment of AβPP arising from thrombin cleavage [31]. In the competition ELISA, 2B12 interfered with the binding of another antibody that also recognises an epitope on AβPP therefore strongly suggesting that both antibodies do indeed bind to the same protein. The presence of an irrelevant IgG at similar concentrations did not interfere with the binding of this antibody indicating that the effect was due to 2B12. Crucially, 2B12 exhibited virtually no cross-reactivity with Aβ as no detectable signal was obtained in Western blots and very low cross reactions were seen in the ELISA. In immunocytochemical studies the labelling pattern seen with 2B12 was similar to that previously show for antibodies binding to AβPP [9]. Interestingly, 2B12 very weakly recognised a C99 construct including an extra amino acid, a methionine before the N-terminus of Aβ (M. Ehrmann, personal communication). This small cross-reaction, together with the reaction against Kb and lack of binding to Aβ, suggests that 2B12 binds to an epitope spanning the β-secretase cleavage site on AβPP, or to one just upstream of this site (the C-terminus of sAβPPβ).
Importantly, the presence of 2B12 led to a significant reduction in concentrations of Aβ40 in the conditioned media of both cell lines. The irrelevant IgG had no effect on Aβ40 levels indicating again that the effect was specific to 2B12. 2B12 does not recognise Aβ and therefore cannot be reducing Aβ40 levels by binding to this peptide in cell media thereby making it unrecognisable to the immunoprecipitating antibodies. Viable cell counts made on the same cells in parallel to measurements of Aβ40 showed no significant alteration in cell numbers from controls indicating that 2B12 does not have a toxic effect. We therefore suggest that the reduction in Aβ40 is due to the presence of 2B12 interfering with the cleavage of AβPP by β-secretase by steric hindrance. A number of studies have suggested that BACE1 and BACE2 have distinct specificities, cleave AβPP at different residues and therefore recognise different sequences [33]. It is therefore unlikely that our antibody would interfere with the activity of BACE2. Similarly, other physiological processes should not be affected as the metabolism of other substrates by β-secretase would not be altered. It is also possible that 2B12 may enhance APP processing by the lysosomal degradation pathway by interfering with the internalisation of APP into endosomes. This would also produce the desirable end result of reducing Aβ40 levels. However, since a previous study showed that monoclonal antibodies bound to APP were rapidly internalized into endosomal compartments via coated pits and that the APP could be recycled to the cell surface [9], it seems unlikely that 2B12 should affect the normal processing of APP.
Such a reduction has obvious potential as a therapy. Vaccination with Aβ or passive immunisation with MAbs to Aβ have shown that antibodies generated in, or administered to, the periphery cross the blood-brain barrier (BBB) and enter the CNS, possibly via extracellular pathways [34]. These levels have been shown to be physiologically relevant and led to an improvement in AD pathology [35,36]. It would seem likely that 2B12 would cross the BBB in a similar manner. However, antibodies raised against Aβ might also stimulate a cellular response against the peptide and microglial activation. Since 2B12 does not recognise Aβ this response would be absent. Furthermore, IgG2b (the isotype of 2B12) is the least effective activator of the complement system and binds with the lowest affinity to cell-associated Fc receptors [37,38] so is less likely to cause inflammation. It is therefore unlikely that 2B12 would affect existing plaque load directly but would alter the generation of Aβ leading to a reduction in non-fibrillar forms. Other studies have shown behavioural improvements with no major change in overall amyloid plaque load, although diffuse fibrillar forms of Aβ (oligomers and protofibrils) were reduced [39]. There is also evidence to suggest that oligomers and protofibrils correlate better with AD pathology rather than overall plaque load suggesting that they are also toxic [40]. Indeed, the level of soluble Aβ assemblies in Tg2576 mice was shown to have a strong inverse correlation with memory and suggested to be the causative agent for the cognitive decline seen in this mouse model of AD [41].
The efficacy of 2B12 in reducing Aβ42 levels has not yet been tested, nor has its effects on levels of Aβ retained intracellularly been ascertained. Since the β-secretase cleavage site is the same for both peptides, we believe that it is probable that 2B12 will also reduce levels of Aβ42. However, there is still some debate regarding the exact subcellular cleavage locations of the relevant secretases. Although early studies showed that the cleavage events for Aβ took place in the endosomal/lysosomal system [42,43], later studies showed that the γ-secretase complex resided in other cellular compartments – the “spatial paradox” [44]. While many authors still regard the endosomal/lysosomal system as the most likely site of Aβ production [45], others support the idea that there are multiple intracellular sites for the production of Aβ. The trans-golgi network [46,47] has been suggested as a site for Aβ40 production whereas Aβ42 is thought to be produced in the endoplasmic/intermediate compartments [48,49], possibly even in the absence of PS-1 and PS-2 [50] and is primarily retained intracellularly. Our data support the existence of multiple intracellular processing sites for AβPP as the maximum inhibition of Aβ40 levels produced by 2B12 was approximately 50%. This implies that a significant proportion of AβPP does not come to the surface but is cleaved directly after synthesis. Alternatively, the 2B12 concentration may not have been high enough to bind all free epitopes. Higher concentrations than those tested were, however, judged to be physiologically unrepresentative and therefore not investigated. Since the efficacy of our antibody relies on AβPP trafficking to the cell membrane so that it can bind to that molecule prior to cleavage, any variation in the intracellular fate of AβPP prior to its cleavage may alter the efficacy of 2B12. Interestingly, the reduction in Aβ40 became apparent relatively quickly and could be detected at 6 hours after addition of the antibody. This would imply a rapid turnover of Aβ and a rapid cycling of AβPP to the cell membrane agreeing with the findings of Savage et al. [51] who detected a similarly rapid turnover of Aβ and a reduction in Aβ levels 6 hours after inducing protein kinase C activity.
Similar results to ours have been obtained very recently by two other groups [52,53]. Paganetti et al. [52] co-expressed human AβPP695 and an ‘intrabody’ consisting of the variable region of an antibody which recognises Aβ3-6 in AβPP. They observed a stable association between AβPP and the intrabody which persisted throughout the maturation of AβPP and resulted in a reduction of intracellular Aβ. Arbel et al. [53] produced monoclonal antibodies using a peptide containing part of the Swedish mutation at the β-secretase cleavage site. They showed internalisation of their antibody and reduced both extracellular and intracellular Aβ levels. They also demonstrated that it was possible to produce these antibodies and reduce Aβ levels by vaccination of Tg2576 mice with the peptide. The approach of both these groups relied on using cell lines over expressing AβPP and Paganetti et al. [52] relied on directly transfecting HEK 293 cells with their intrabody which would not be a feasible therapeutic approach. As far as we are aware, we have demonstrated for the first time that it is possible to inhibit the action of β-secretase by blocking its cleavage site with a whole IgG molecule in cells that constitutively express AβPP. We believe that our use of model cell lines which do not over-express AβPP is very important as the majority of cases of AD occur in people with much lower levels of AβPP than those associated with transfected cells. Our antibody produced larger reductions in Aβ levels than that of Arbel and co-workers [53] and these were sustained for up to 6 days. Similarly to Arbel et al. [53], we consider that it is very important that 2B12 was internalised with AβPP without the need for transfection and does not recognise Aβ. This lack of binding to Aβ and the IgG2b isotype of 2B12 means that is should be less likely to trigger an undesirable immune response.
Our findings suggest that it may be possible to use 2B12 to prevent or slow the development of AD. In conjunction with other strategies to reduce plaque load, the use of such an antibody might potentially revolutionise the current treatments available for this neurodegenerative disease.
Acknowledgements
R.S. Thomas was funded by a grant from the Alzheimer’s Society, U.K. We would like to thank Professor Michael Ehrmann for comments on the manuscript and for the provision of the C99 fragment and Karen Wilhelm and Dewi Williams for technical assistance provided.
References
- [1].Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- [2].Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid β protein in Alzheimer’s disease. J. Biol. Chem. 1992;267:17082–17086. [PubMed] [Google Scholar]
- [3].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- [4].Allinson TM, Parkin ET, Turner AJ, Hooper NM. ADAMs family members as amyloid precursor protein alpha-secretases. J. Neurosci. Res. 2003;74:342–352. doi: 10.1002/jnr.10737. [DOI] [PubMed] [Google Scholar]
- [5].Citron M. Strategies for disease modification in Alzheimer’s disease. Nat. Rev. Neurosci. 2004a;5:677–685. doi: 10.1038/nrn1495. [DOI] [PubMed] [Google Scholar]
- [6].Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- [7].Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J. Cell Sci. 1996;109:991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- [9].Yamazaki T, Koo EH, Selkoe DJ. Trafficking of cell-surface amyloid beta-protein precursor. II. Endocytosis, recycling and lysosomal targeting detected by immunolocalization. J. Cell Sci. 1996;109:999–1008. doi: 10.1242/jcs.109.5.999. [DOI] [PubMed] [Google Scholar]
- [10].Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer’s disease: current status and new perspectives. Lancet Neurol. 2003;2:539–547. doi: 10.1016/s1474-4422(03)00502-7. [DOI] [PubMed] [Google Scholar]
- [11].De Felice FG, Ferreira ST. Beta-amyloid production, aggregation, and clearance as targets for therapy in Alzheimer’s disease. Cell. Mol. Neurobiol. 2002;22:545–563. doi: 10.1023/a:1021832302524. [DOI] [PubMed] [Google Scholar]
- [12].Carson JA, Turner AJ. Beta-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases? J. Neurochem. 2002;81:1–8. doi: 10.1046/j.1471-4159.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- [13].Song S, Jung YK. Alzheimer’s disease meets the ubiquitin-proteasome system. Trends Mol. Med. 2004;10:565–570. doi: 10.1016/j.molmed.2004.09.005. [DOI] [PubMed] [Google Scholar]
- [14].Josien H. Recent advances in the development of gamma-secretase inhibitors. Curr. Opin. Drug Discov. Devel. 2002;5:513–525. [PubMed] [Google Scholar]
- [15].Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nature Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- [16].Kitazume S, Tachida Y, Oka R, Shirotani K, Saido TC, Hashimoto Y. Alzheimer’s beta-secretase, beta-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13554–13559. doi: 10.1073/pnas.241509198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lichtenthaler SF, Dominguez DI, Westmeyer GG, Reiss K, Haass C, Saftig P, De Strooper B, Seed B. The cell adhesion protein P-selectin glycoprotein ligand-1 is a substrate for the aspartyl protease BACE1. J. Biol. Chem. 2003;278:48713–48719. doi: 10.1074/jbc.M303861200. [DOI] [PubMed] [Google Scholar]
- [18].Li Q, Sudhof TC. Cleavage of amyloid-beta precursor protein and amyloid-beta precursor-like protein by BACE 1. J. Biol. Chem. 2004;279:10542–10550. doi: 10.1074/jbc.M310001200. [DOI] [PubMed] [Google Scholar]
- [19].Citron M. Beta-secretase inhibition for the treatment of Alzheimer’s disease--promise and challenge. Trends Pharmacol. Sci. 2004b;25:92–97. doi: 10.1016/j.tips.2003.12.004. [DOI] [PubMed] [Google Scholar]
- [20].Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- [21].Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- [22].Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, Connor K, Hatcher J, Hope C, Gordon M, Arendash GW. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- [23].Kotilinek LA, Bacskai B, Westerman M, Kawarabayashi T, Younkin L, Hyman BT, Younkin S, Ashe KH. Reversible memory loss in a mouse transgenic model of Alzheimer’s disease. J. Neurosci. 2002;22:6331–6335. doi: 10.1523/JNEUROSCI.22-15-06331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nature Rev. Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- [25].Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- [26].Liddell JE, Cryer A. A Practical Guide to Monoclonal Antibodies. John Wiley & Sons Ltd; Chichester: 1991. [Google Scholar]
- [27].Chen M, Fernandez HL. Stimulation of beta-amyloid precursor protein alpha-processing by phorbol ester involves calcium and calpain activation. Biochem. Biophys. Res. Commun. 2004;316:332–340. doi: 10.1016/j.bbrc.2004.02.052. [DOI] [PubMed] [Google Scholar]
- [28].Harnasch M, Grau S, Behrends C, Dove SL, Hochschild A, Iskandar MK, Xia W, Ehrmann M. Characterization of presenilin-amyloid precursor interaction using bacterial expression and two-hybrid systems for human membrane proteins. Mol. Membr. Biol. 2004;21:373–383. doi: 10.1080/09687860400008429. [DOI] [PubMed] [Google Scholar]
- [29].Kidd EJ, Miller KJ, Sansum AJ, Humphrey PP. Evidence for P2X3 receptors in the developing rat brain. Neuroscience. 1998;87:533–539. doi: 10.1016/s0306-4522(98)00294-2. [DOI] [PubMed] [Google Scholar]
- [30].Lim GP, Calon F, Morihara T, Yang F, Teter B, Ubeda O, Salem N, Frautschy SA, Cole GM. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chong YH, Jung JM, Choi W, Park CW, Choi KS, Suh Y-H. Bacterial expression, purification of full length and carboxyl terminal fragment of Alzheimer amyloid precursor protein and their proteolytic processing by thrombin. Life Sciences. 1994;54:1259–1268. doi: 10.1016/0024-3205(94)00853-1. [DOI] [PubMed] [Google Scholar]
- [32].Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good M, Bliss TVP, Hyman BT, Younkin SG, Hsaio KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- [33].Fluhrer R, Capell A, Westmeyer G, Willem M, Hartung B, Condron MM, Teplow DB, Haass C, Walter J. A non-amyloidogenic function of BACE-2 in the secretory pathway. J. Neurochem. 2002;81:1011–1020. doi: 10.1046/j.1471-4159.2002.00908.x. [DOI] [PubMed] [Google Scholar]
- [34].Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Passage of amyloid beta protein antibody across the blood-brain barrier in a mouse model of Alzheimer’s disease. Peptides. 2002;23:2223–2226. doi: 10.1016/s0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- [35].Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- [36].Lombardo JA, Stern EA, McLellan ME, Kajdasz ST, Hickey GA, Bacskai BJ, Hyman BT. Amyloid-beta antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J. Neurosci. 2003;23:10879–10883. doi: 10.1523/JNEUROSCI.23-34-10879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fridman WH, Sautes C. Cell-mediated effects of immunoglobulins. R.G. Landes; Austin, TX: 1997. [Google Scholar]
- [38].McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MHL, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HTJ, Przybylski M, St George-Hyslop P. Therapeutically effective antibodies against amyloid-peptide target amyloid-residues 4-10 and inhibit cytotoxicity and fibrillogenesis. Nature Med. 2002;8:1263–1269. doi: 10.1038/nm790. [DOI] [PubMed] [Google Scholar]
- [39].Lambert MP, Viola KL, Chromy BA, Chang L, Morgan TE, Yu J, Venton DL, Krafft GA, Finch CE, Klein WL. Vaccination with soluble Abeta oligomers generates toxicity-neutralizing antibodies. J. Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- [40].White AR, Hawke SH. Immunotherapy as a therapeutic treatment for neurodegenerative disorders. J. Neurochem. 2003;87:801–808. doi: 10.1046/j.1471-4159.2003.02064.x. [DOI] [PubMed] [Google Scholar]
- [41].Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 206;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- [42].Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357:500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- [43].Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J. Biol. Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- [44].Annaert WG, De Strooper B. Presenilins: molecular switches between proteolysis and signal transduction. Trends Neurosci. 1999;22:439–443. doi: 10.1016/s0166-2236(99)01455-1. [DOI] [PubMed] [Google Scholar]
- [45].Pasternak SH, Callahan JW, Mahuran DJ. The role of the endosomal/lysosomal system in amyloid-beta production and the pathophysiology of Alzheimer’s disease: reexamining the spatial paradox from a lysosomal perspective. J. Alzheimers Dis. 2004;6:53–65. doi: 10.3233/jad-2004-6107. [DOI] [PubMed] [Google Scholar]
- [46].Hartmann T, Bieger SC, Bruhl B, Tienari PJ, Ida N, Allsop D, Roberts GW, Masters CL, Dotti CG, Unsicker K, Beyreuther K. Distinct sites of intracellular production for Alzheimer’s disease A beta40/42 amyloid peptides. Nature Med. 1997;3:1016–1020. doi: 10.1038/nm0997-1016. [DOI] [PubMed] [Google Scholar]
- [47].Xu H, Sweeney D, Wang R, Thinakaran G, Lo AC, Sisodia SS, Greengard P, Gandy S. Generation of Alzheimer beta-amyloid protein in the trans-Golgi network in the apparent absence of vesicle formation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3748–3752. doi: 10.1073/pnas.94.8.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cook DG, Forman MS, Sung JC, Leight S, Kolson DL, Iwatsubo T, Lee VM, Doms RW. Alzheimer’s A beta(1-42) is generated in the endoplasmic reticulum/intermediate compartment of NT2N cells. Nature Med. 1997;3:1021–1023. doi: 10.1038/nm0997-1021. [DOI] [PubMed] [Google Scholar]
- [49].Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, Checler F, Sisodia SS, Greengard P, Xu H. Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer beta-amyloid peptides. Proc. Natl. Acad. Sci. U.S.A. 1999;96:742–747. doi: 10.1073/pnas.96.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wilson CA, Doms RW, Zheng H, Lee VM. Presenilins are not required for A beta 42 production in the early secretory pathway. Nature Neurosci. 2002;5:849–855. doi: 10.1038/nn898. [DOI] [PubMed] [Google Scholar]
- [51].Savage MJ, Trusko SP, Howland DS, Pinsker LR, Mistretta S, Reaume AG, Greenberg BD, Siman R, Scott RW. Turnover of amyloid beta-protein in mouse brain and acute reduction of its level by phorbol ester. J. Neurosci. 1998;18:1743–1752. doi: 10.1523/JNEUROSCI.18-05-01743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Paganetti P, Calanca V, Galli C, Stefani M, Molinari M. Beta-site specific intrabodies to decrease and prevent generation of Alzheimer’s Abeta peptide. J. Cell. Biol. 2005;168:863–868. doi: 10.1083/jcb.200410047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Arbel M, Yacoby I, Solomon B. Inhibition of amyloid precursor protein processing by β-secretase through site-directed antibodies. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7718–7723. doi: 10.1073/pnas.0502427102. [DOI] [PMC free article] [PubMed] [Google Scholar]