Summary
Background
Neuropathic pain is difficult to treat. New treatments, clinical trials and standards of quality for assessing evidence justify an update of evidence-based recommendations for its pharmacological treatment.
Methods
The Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain conducted a systematic review of randomised double-blind studies of oral and topical pharmacotherapy for neuropathic pain, including unpublished trials (retrieved from clinicaltrials.gov and pharmaceutical websites). Meta-analysis used Numbers Needed to Treat (NNT) for 50 % pain relief as primary measure and assessed publication bias. Recommendations used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE).
Findings
In total 229 studies were included. Analysis of publication bias suggested a 10% overstatement of treatment effects. Studies published in peer-review journals reported greater effects than online studies (R2=9·3%, p<0·01). Trial outcomes were generally modest even for effective drugs : in particular NNTs were 3·6 (95 % CI 3·0–4·4) for tricyclic antidepressants (TCAs), 6·4 (95 % CI 5·2–8·4) for serotonin- noradrenaline reuptake inbibitor (SNRI) antidepressants duloxetine and venlafaxine, 7·7 (95 % CI 6·5–9·4) for pregabalin and 6·3 (95 % CI 5·0–8·3) for gabapentin. NNTs were higher for gabapentin ER/enacarbil and capsaicin high concentration patches, lower for opioids and botulinum toxin A (BTX-A) and undetermined for lidocaine patches. Final quality of evidence was lower for lidocaine patches and BTX-A. Tolerability/safety and values/preferences were high for lidocaine patches and lower for opioids and TCAs. This permitted a strong GRADE recommendation for use and proposal as first line for TCAs, SNRIs, pregabalin, gabapentin and gabapentin ER/enacarbil in neuropathic pain, a weak recommendation for use and proposal as second line for lidocaine patches, capsaicin patches and tramadol, and a weak recommendations for use and proposal as third line for strong opioids (particularly oxycodone and morphine) and BTX-A. Data for cannabinoids, tapentadol, drug combinations, and several other antiepileptics, antidepressants and topical drugs were inconclusive.
Interpretation
Limited efficacy, large placebo responses, inadequate diagnostic criteria and poor phenotypic profiling probably account for modest trial outcomes and should be taken into account in future studies.
Funding
This study was funded by NeuPSIG.
Keywords: neuropathic pain, pharmacotherapy, systematic review, meta-analysis, evidence-based, recommendations
Introduction
Neuropathic pain, caused by a lesion or disease affecting the somatosensory nervous system,1 has a considerable impact on patients’ quality of life, and is associated with a high economic burden on the individual and society.2–4 It is now considered as a distinct clinical entity despite a large variety of aetiologies.5
Epidemiological surveys have shown that many patients with neuropathic pain do not receive appropriate treatment for their pain.2,6,7 This may be due to lack of diagnostic accuracy and relatively ineffective drugs, but also insufficient knowledge about effective drugs and their appropriate use in clinical practice.8 Evidence-based recommendations for the pharmacotherapy of neuropathic pain are therefore essential.
Over the past 10 years, a few recommendations have been proposed for pharmacotherapy of neuropathic pain9–11 or specific neuropathic pain conditions, such as painful diabetic neuropathies and postherpetic neuralgia.12–14 In the interim, new pharmacological therapies and high-quality clinical trials have appeared. Previously hidden and unpublished large trials can now be identified on the web (clinicaltrials.gov, pharmaceutical industry websites), which, together with analysis of publication bias, may limit the risk of bias in reporting data. Furthermore, prior recommendations sometimes came to discrepant conclusions because of inconsistencies in methods used to assess the quality of evidence (eg,13,15,16). In order to address these inconsistencies, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) was introduced in 200017,18 and has received widespread international acceptance. All these reasons justify an update of evidence-based recommendations for the pharmacotherapy of neuropathic pain.
The present work aimed to update the recommendations of the Special Interest Group on Neuropathic Pain (NeuPSIG) of the International Association for the Study of Pain (IASP) (www.neupsig.org) on the systemic and topical pharmacological treatments of neuropathic pain.19 Non-pharmacological management such as neurostimulation techniques were beyond the scope of this work (see20 for a recent systematic review) We conducted a systematic review and meta-analysis of randomised controlled trials (RCTs) of all drug treatments for neuropathic pain published since 1966 and of unpublished trials with available results, and assessed publication bias. We used GRADE to rate the quality of evidence and the strength of recommendations.17,18
Methods
The preparation of this article was conducted under the auspices of NeuPSIG. We followed the 23-item Appraisal of Guidelines for Research and Avaluation (AGREE II) Instrument for developing and reporting recommendations.21 Details of the working group, criteria for eligibility, search methods, reporting and statistical analysis are found in appendix 1.
Procedures
The systematic review of the literature compiled with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statements.22 We used a standardized review and data extraction protocol (unpublished, appendices 2 and 3). The full reports of randomised, controlled, double-blind studies published in peer-reviewed journals between 1966 and April 2013 were identified using searches of PubMed/Medline, the Cochrane Central Register of Controlled Trials, and Embase. Additional papers were identified from published reviews and the reference lists of selected papers.
The target population was patients of any age with neuropathic pain according to the IASP definition (ie, pain caused by a lesion or disease of the somatosensory nervous system),1; this included postherpetic neuralgia, diabetic and non-diabetic painful polyneuropathy, postamputation pain, post-traumatic/postsurgical neuropathic pain including plexus avulsion and complex regional pain syndrome type II (the latter was generally subsumed into post-traumatic/post-surgical neuropathic pain), central post-stroke pain, spinal cord injury pain, multiple sclerosis-associated pain. Neuropathic pains pertaining to multiple aetiologies were also considered. Neuropathic pain associated with nociceptive components (eg, cancer neuropathic pain and radiculopathy) was included provided that the primary outcome was neuropathic pain. Conditions such as complex regional pain syndrome type I, low back pain without radicular pain, fibromyalgia, and atypical facial pain were not included because they do not fulfill the current definition of neuropathic pain.1 Trigeminal neuralgia was considered separately because of generally distinct response to drug treatment.10,23
The interventions were systemic or topical treatments (oral, sublingual, oropharyngeal, intranasal, topical, subcutaneous, intradermal, and smoking) with at least 3 weeks duration of treatment. Single-administration treatments with long-term efficacy (high-concentration capsaicin patches and botulinum toxin) were included if there was a minimum follow-up of 3 weeks. Studies using intramuscular, intravenous, or neuraxial routes of administration and preemptive analgesia studies were excluded (see review20).
Randomised, double-blind, placebo-controlled studies with parallel group or crossover study designs that had at least 10 patients per arm were included. Enriched-enrolment, randomised withdrawal trials were summarised separately. Studies published only as abstracts were excluded. Double-blind active comparator trials of drugs generally proposed as first or second-line treatments24 were included. The study outcome (positive, negative) was based on the effect on the primary outcome measure, e.g. neuropathic pain intensity. Studies in which the primary outcome included a composite score of pain and paraesthesia or paraesthesia only were not included.
Studies were assessed for methodological quality using the five-point Oxford Quality Scale25 by two independent authors (appendix 1). Here, a minimum score of 2 out of 5 (randomised and double-blind study) was required for inclusion.25 We also assessed serious risk of bias relating to lack of allocation concealment, incomplete accounting of outcome events, selective outcome reporting, stopping early for benefit, use of invalidated outcome measures and carryover effects in crossover trials.
Evidence summary and reporting
The GRADE classification was used to assess recommendations based on a group of RCTs pertaining to the same drug or drug class when relevant (ie TCAs)17,18 with final quality of evidence rated as strong or weak for the treatment, against the treatment, or inconclusive (the last category was added due to the large number of inconsistent results in RCTs). We did not conduct a new health economic analysis of costs,16 but estimated three levels of relative drug costs in various countries in relation to the average price (for oral drugs) for each country using price data for the daily dose as defined by WHO (appendix 1). The average of these percentages across countries was calculated, and the cost was rated as low if <67%, moderate if 67–300%, and high if >300% than the average.
Statistics
NNT for 50% pain intensity reduction (alternatively, 30% pain reduction or at least moderate pain relief) was the primary effect measure, and NNH was calculated as the number of patients that needed to be treated for one patient to drop out due to adverse effects. The 95% CI for NNT and NNH values was calculated as the reciprocal value of the 95% CI for the absolute risk difference using the normal approximation. In dose-finding studies, data from subgroups treated with low doses (eg, pregabalin 150 mg) were not included in the meta-analysis. Difference in pain intensity was a secondary outcome. Serious and common (>10% incidence) reported adverse events were also recorded in the data extraction form (appendix 3).
An assessment of publication bias was performed using funnel plots,26 Egger’s regression,27 and Duval and Tweedie’s non-parametric trim and fill approach.28 (appendix 1). Additionally, we estimated the susceptibility to bias for individual drug classes.29,30 The extent to which the observed variability (heterogeneity) in treatment effects is explained by publication in a peer-reviewed journal was assessed using meta-regression. Heterogeneity among trials was presented as a L’Abbé plot31 and with the use of the I2 statistic.
Results
Study selection and characteristics
The results of the database and registry search are shown in figure 1. In total, 191 published articles and 21 unpublished studies were included in the quantitative synthesis. Study characteristics are summarised in appendices 4 and 5. In addition, five published and 12 unpublished studies were retrieved between April 2013 and January 2014 (appendix 6). Thus, a total of 229 articles/studies were included. References are presented in appendix 7.
Figure 1.
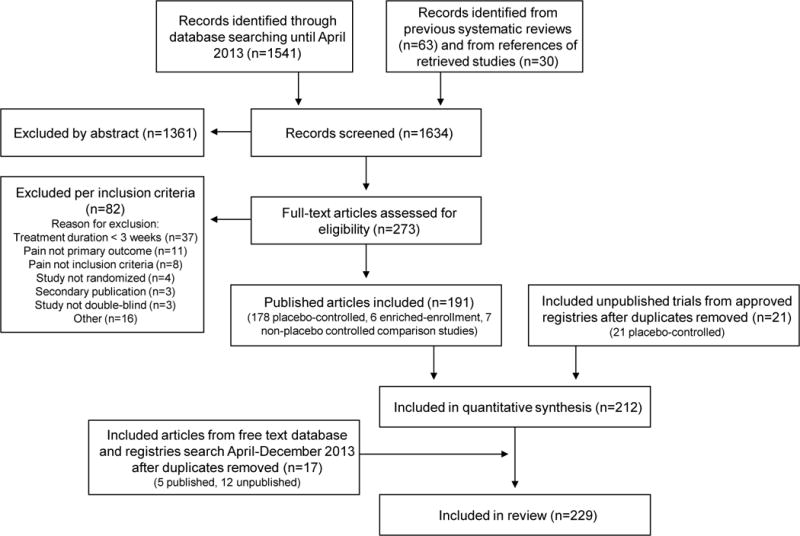
PRISMA flow chart
Eligible studies investigated tricyclic antidepressants (TCAs), serotonin- noradrenaline reuptake inbibitor (SNRI) antidepressants, other antidepressants, pregabalin, gabapentin/gabapentin extended release (ER) and enacarbil, other antiepileptics, tramadol, opioids, cannabinoids, lidocaine 5% patch, capsaicin 8% patch and cream, subcutaneous BTX-A, NMDA antagonists, mexiletine, miscellaneous topical, newer systemic drugs, and combination therapies. Fifty-five percent of the trials were conducted in diabetic painful polyneuropathy or postherpetic neuralgia. NNT and NNH could be calculated in 77% of published placebo-controlled trials.
Risk of bias in individual trials
The average Oxford quality scores for individual trials is presented in appendix 4. The mean score was 4·1 (SD 0·87, range 2–5). It was lower (average 3–4) for older studies of TCAs and capsaicin and higher (average >4) for more recent studies (pregabalin, gabapentin, SNRIs, opioids, capsaicin high-concentration patches). Detailed descriptions of study limitations of individual studies are available from the corresponding author per request.
Risk of bias across studies
Heterogeneity
Heterogeneity assessed with the I2 statistic is presented in figure 2 and appendix 8 and L’Abbé plot is presented in appendix 9. Heterogeneity, particularly heterogeneity that was not easily explained by differences in drug dose, diagnosis, and size of placebo response, was included in the GRADE recommendation.
Figure 2. Forest plot for data included in meta-analysis.
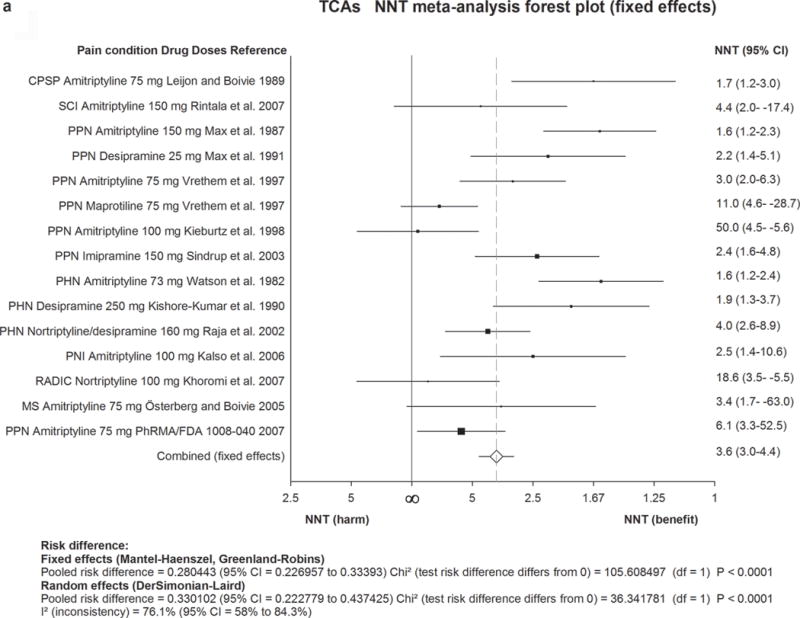
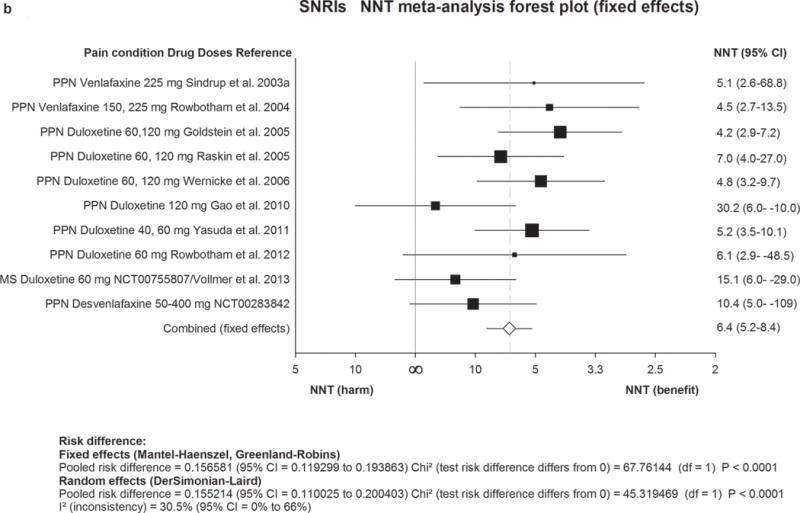
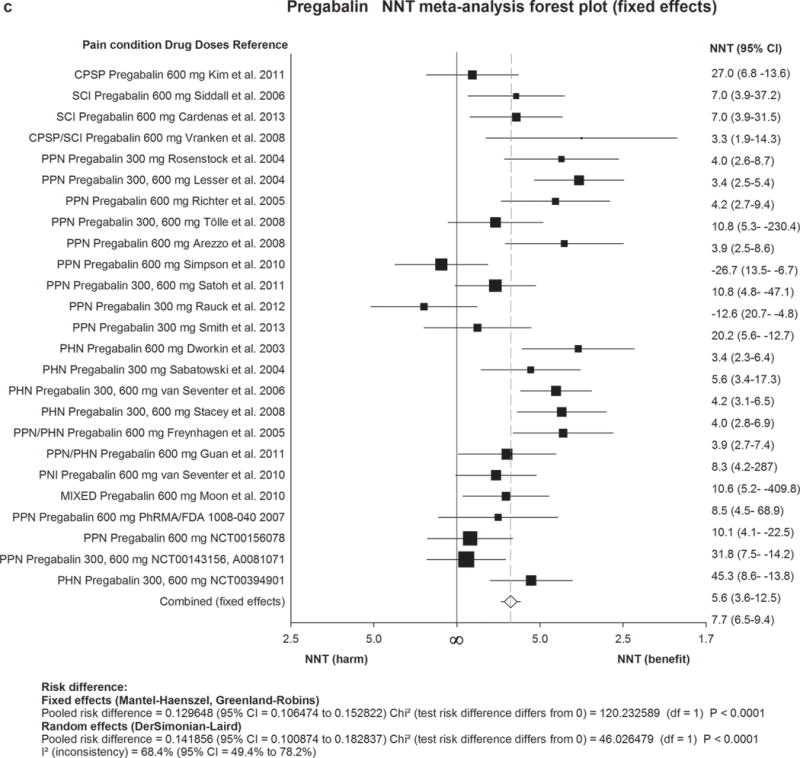
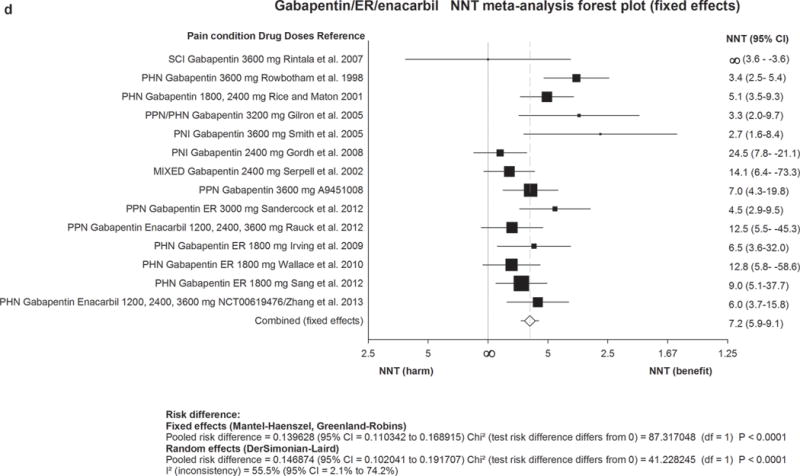
Numbers needed to treat (NNTs) with 95% confidence intervals (CI) are shown for each trial and for the overall estimate (fixed effects, Mantel-Haenszel) for first-line drugs. The size of the squares represents the Mantel-Haenszel weight that the corresponding study exerts in the meta-analysis. The full line indicates an NNT of ∞ corresponding to an absolute risk difference of 0, ie. no effect. Numbers to the right of the line indicate effect and numbers to the left harm. The dotted line represents the overall estimate.
2a) TCAs=Tricyclic antidepressants. 2b) SNRIs=serotonin noradrenaline reuptake inhibitors. 2c) Pregabalin. 2d) Gabapentin including gabapentin ER and Enacarbil.
MS=multiple sclerosis pain. PHN=postherpetic neuralgia. PNI=peripheral nerve injury. RADIC=painful radiculopathy. SCI=spinal cord injury pain. CPSP=Central poststroke pain. PPN=painful polyneuropathy.
Publication bias
A total of 165 published or unpublished trials with dichotomous data were analysed for publication bias. Visual inspection of the funnel plot (figure 3a) showed asymmetry, which was confirmed by Egger’s regression test (figure 3b). The trim and fill method suggested 34 theoretical missing studies (figure 3c) and adjusted our effect size from an OR of 1·8 (95% CI 1·7–1·9) to 1·6 (95% CI 1·5–1·7). This suggests a 10% relative overstatement of treatment effects. The analysis of susceptibility for publication bias amongst individual drug classes is summarised in table 1. Only the estimated effect size of capsaicin 8% patches demonstrated susceptibility to change to a clinical non-significant effect if studies with no effect were published. Using meta-regression, we identified that studies published in peer-review journals reported greater treatment effects (OR=2·2, 95% CI 1·5–3·0, n=153; adjusted R2=9·3%, p<0·01) than studies identified via online repositories (OR=1·4, 1·0–1·9, n=17).
Figure 3. Evidence of publication (reporting) bias.
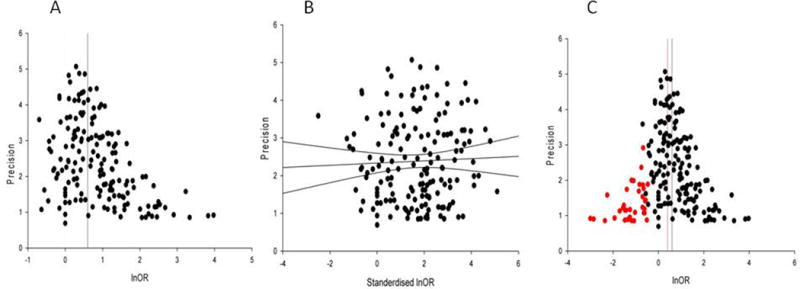
(a) Funnel plot showing the precision (inverse of the standard error) against the effect size; in the absence of bias the points should resemble a symmetrical inverted funnel, (b) Egger’s regression showing the precision plotted against the standardised effect size, the 95% confidence intervals of the regression line do not include the origin suggesting funnel plot asymmetry, and (c) Funnel plot showing the additional missing studies imputed by trim-and-fill in red. The red vertical line indicates the possible summary if the theoretical missing studies were to be included.
Table 1.
Analysis of susceptibility to bias.
| Active drug class | Number of comparisons1 | Participants2 | Pain relief
|
NNT | Susceptibility to bias3 | |
|---|---|---|---|---|---|---|
| Active | Placebo | |||||
| TCAs | 15 | 948 | 217/473 | 85/475 | 3.57 (3.0–4.4) | 1973 |
| SNRIs | 10 | 2541 | 676/1559 | 278/982 | 6.40 (5.2–8.4) | 1826 |
| Pregabalin | 25 | 5940 | 1359/3530 | 578/2410 | 7.71 (6.5–9.4) | 2534 |
| Gabapentin4 | 14 | 3503 | 719/2073 | 291/1430 | 7.16 (5.9–9.1) | 1879 |
| Tramadol | 6 | 741 | 176/380 | 96/361 | 4.73 (3.6–6.7) | 982 |
| Strong opioids | 7 | 838 | 211/426 | 108/412 | 4.26 (3.4–5.8) | 1326 |
| Capsaicin 8% | 6 | 2073 | 466/1299 | 212/774 | 10.64 (7.4–19) | 70* |
| BTX-A | 4 | 137 | 42/70 | 4/67 | 1.85 (1.5–2.4) | 678 |
Number of comparisons with placebo in included published trials and unpublished trials with results from registries are included if the report number of responders.
Total number of patients treated with active and placebo, patients count twice if cross-over study
Number of patients in zero treatment effect trials that would make the NNT exceed 11, which is considered the cut-off for clinical relevance
Including gabapentin ER and Enacarbil.
Susceptible to publication bias, i.e. a new study with less than 400 participants with no effect could change the NNT to a level that is not clinical relevant.
Quantitative data for individual drugs or drug classes
Results of individual and combined NNT and NNH values for placebo-controlled studies are presented in appendix 4, other studies in appendices 5 and 6 and quality of evidence in appendix 10. Forest plots showing NNTs for all drug classes are presented in figure 2 or appendix 8. Risk differences, calculated using fixed effect and random effects models, are presented in appendix 8. There was no evidence showing different efficacy of most drugs in distinct neuropathic pain conditions, except otherwise specified. Long-term controlled studies were lacking and few lasted longer than 12 weeks, with the longest lasting 24 weeks.
TCA and SNRI antidepressants
In 18 placebo-controlled trials (20 comparisons with placebo of which seven used active placebos), evaluating mainly amitriptyline (25–150 mg/day), 16 were positive. The final quality of evidence was moderate (appendix 10). There was no evidence for a dose-response effect. Combined NNT (15 studies) was 3·6 (3·0–4·4) and NNH was 13·4 (9·3–24·4).
We identified 14 studies of SNRI antidepressants with results including notably nine with duloxetine (20–120 mg, seven positive) and four with venlafaxine (two positive, dosages 150–225 mg daily, two negative but with low dosages). The final quality of evidence was high. Combined NNT was 6·4 (5·2–8·4) and NNH was 11·8 (9·5–15·2).
Antiepileptics
A total of 18 out of 25 placebo-controlled RCTs of pregabalin (150–600 mg/day) were positive, with high final quality of evidence. There was a dose response gradient (higher response with 600 mg daily than 300 mg). Two trials of HIV-related painful polyneuropathy with high placebo responses (34 to 43% had 50% pain relief with placebo) were negative. Combined NNT was 7·7 (6·5–9·4) and NNH was 13·9 (11·6–17·4).
We identified 14 RCTs of gabapentin (900–3600 mg/day) (nine positive) and 6 RCTs of gabapentin ER or gabapentin enacarbil (1200–3600 mg/day) (four positive). Combined NNT was 6·3 (5·0–8·3) for gabapentin and 8·3 (6·2–13) for gabapentin ER/enacarbil. There was no evidence for a dose-response effect. Safety was good (NNH 25·6 (15·3–78·6) for gabapentin and 31·9 (17·1–230) for gabapentin ER).
Most studies using other antiepileptic drugs were negative. Topiramate, zonisamide, and oxcarbazepine/carbamazepine had the poorest safety profile, with combined NNH of 6·3 (5·1–8·0), 2·0 (1·3–4·6), and 5·5 (4·3–7·9), respectively.
Opioids
Tramadol is a weak opioid agonist and a serotonin and noradrenaline reuptake inhibitor. All seven studies of tramadol (mainly tramadol ER up to 400 mg daily) were positive, with moderate final quality of evidence. Combined NNT was 4·7 (3·6–6·7), with the highest NNT (6·4) in the largest study. Combined NNH was 12·6 (8·4–25·3). Tapentadol is an opioid with noradrenaline reuptake inhibition, with low affinity for the mu opioid receptor. We identified one negative study and one positive enrichment study of tapentadol ER: the latter had potential bias (probable unblinding of the patients enrolled in the double blind period) and high NNT (in 67 % of the patients responding to the open phase).
We identified 13 trials of strong opioids using mainly oxycodone (10–120 mg/day) and morphine (90–240mg/day) in peripheral neuropathic pain. The final quality of evidence was moderate. Ten trials were positive: combined NNT was 4·3 (3·4–5·8) and NNH was 11·7 (8·4–19·3). Maximum effectiveness seemed associated with 180 mg morphine or equivalent (no additional benefit for higher doses).
Oromucosal cannabinoids
Sativex is an oromucosally delivered spray prepared from extracts of the plant cannabis sativa with several active constituents (principally standardised 27 mg/ml delta-9-tetrahydrocannabinol and 25 mg/ml cannabidiol). We identified nine trials of Sativex in neuropathic pain, of which only two were positive. Of 2 studies in pain associated with multiple sclerosis, one was positive, while the larger one had a negative primary outcome.
Topical lidocaine
Based our inclusion criteria (at least 3 weeks’ duration), we identified only one small negative study of 5% lidocaine patches in postsurgical neuropathic pain and two enriched enrolment studies in postherpetic neuralgia. The smaller study (32 patients) was positive, the larger study (263 patients) was negative in the ITT population, but positive in the per protocol population. Of note, studies of less than 3 weeks’ duration found efficacy of 5% lidocaine patch.24 Safety and tolerability were excellent in all cases.
Capsaicin high-concentration patches
Five out of seven studies (in postherpetic neuralgia or HIV-related painful polyneuropathy) reported sustained efficacy of a single application of high-concentration capsaicin patch (8%) (better results for 60 minutes application in postherpetic neuralgia and 30 minutes in HIV neuropathy) compared with a low-concentration patch (0·04%) (aiming to minimize the risk of unblinding related to the burning sensation of capsaicin). The final quality of evidence was high. Combined NNT was 10·6 (7·4–19). Results on secondary outcomes were inconsistent.
Botulinum toxin type A
Six RCTs evaluated the efficacy of a single administration of BTX-A (50–200 units subcutaneously in the painful area) in peripheral neuropathic pain. The smaller studies had a positive primary outcome (NNT of 1·9 (1·5–2·4) for four studies) with a very low placebo effect, but one large unpublished study was negative. Safety was generally excellent.
Miscellaneous
Results concerning other drugs (SSRI antidepressants, capsaicin cream, NMDA antagonists, Δ-9-tetrahydrocannabinol, mexiletine, and newer topical or oral drugs), are in appendix 4. There were no RCTs with conventional nonopioid analgesics (NSAIDs, acetaminophen).
Combination therapy
Of seven RCTs of various combination therapy in neuropathic pain (appendices 4 and 6), two found that gabapentin combined to morphine or to nortriptyline was superior to monotherapy (and to placebo in one study) at reduced dosages and with no more side effects. However the largest study (not placebo controlled) showed no difference in efficacy or side effects between pregabalin combined to duloxetine at moderate dosages (300 mg pregabalin, 60 mg duloxetine daily) and monotherapy at high dosages (600 mg pregabalin, 120 mg duloxetine daily) in patients not responsive to monotherapy at moderate dosages.
Comparative drug trials
We identified seven comparative RCTs without placebo groups (appendices 4, 5). Neither individual studies nor their statistical combination demonstrated significant differences in efficacy or safety between drugs. Despite limited sample sizes and unknown assay sensitivity in the lack of placebo groups, results suggest comparable efficacy across first- and most second-line recommended treatments.
GRADE recommendations
There was generally no evidence for efficacy of particular drugs in specific conditions. Therefore these recommendations apply to neuropathic pain in general. However, they may not be applicable for trigeminal neuralgia, for which we could extract only one study complying with our inclusion criteria. We therefore recommend referring to previous specific guidelines regarding this condition.10,23 Few studies included cancer-related neuropathic pain; the recommendations for the use of opioids may be different in certain cancer populations. Similarly these recommendations do not apply to acute pain or acute pain exacerbation. Treatment of neuropathic pain in children is a neglected area.32 However, none of the studies assessed pediatric neuropathic pain, and the present guidelines therefore only apply to adults.
Details regarding GRADE recommendations and practical use are provided in tables 2, 3 and appendix 10. Few relevant trials appeared since our meta-analysis, but none affected the recommendations (appendix 11). TCAs, SNRI antidepressants duloxetine and venlafaxine, pregabalin, gabapentin and gabapentin ER/enacarbil have strong GRADE recommendations for use in neuropathic pain and are proposed as first-line, with caution regarding most TCAs (Table 2). Tramadol, lidocaine patches and high-concentration capsaicin patches have weak GRADE recommendations for use and are proposed as generally second-line. Topical treatments are recommended for peripheral neuropathic pain with presumed local pain generator. In select circumstances, e.g when there are concerns due to side effects or safety of first-line treatments, particularly in frail and elderly patients, lidocaine patches may be considered as first-line.
Table 2.
Recommendations for individual drugs or drug classes based on the GRADE classification and for first-, second-, and third-line drugs for neuropathic pain. Drugs pertaining to the same drug class are presented in alphabetical order.
| GRADE classification | Drugs | Daily dosages and dose regime | Recommendations |
|---|---|---|---|
| STRONG FOR | Gapabentin Gabapentin ER/enacarbil Pregabalin SNRIs duloxetine/venlafaxine TCAs |
1200–3600 mg TID 1200–3600 mg BID 300–600 mg BID 60–120 mg QD (duloxetine);150–225 mg QD (venlafaxine ER) 25–150 mg qd or BID |
First-line First-line First-line First-line First-line 1 |
| WEAK FOR | Capsaicin 8% patches Lidocaine patches Tramadol BTX- A (SC) Strong opioids |
1–4 patches to the painful area for 30–60 min every 3 months 1–3 patches to the painful area for up to 12 hours 200–400 mg BID (tramadol ER) or TID 50–200 units to the painful area every 3 months Individual titration |
Second-line (PNP) 2 Second-line (PNP) Second-line Third-line ; specialist use (PNP) Third line3 |
| INCONCLUSIVE | Combination therapy Capsaicin cream Carbamazepine Clonidine topical Lacosamide Lamotrigine NMDA antagonists Oxcarbazepine SSRI antidepressants Tapentadol Topiramate Zonisamide |
||
| WEAK AGAINST | Cannabinoids Valproate |
||
| STRONG AGAINST | Levetiracetam Mexiletine |
Abbreviations: SNRIs=serotonin noradrenaline reuptake inhibitors. TCAs=tricyclic antidepressants. ER= extended realease; BID : twice daily; QD : once daily. PNP=peripheral neuropathic pain.
TCAs generally have similar efficacy (appendix 4). Tertiary amine TCAs (amitriptyline, imipramine, clomipramine) are not recommended at dosages > 75 mg/day in older adults because of their major anticholinergic and sedative side effects and potential risk of falls.33 An increased risk of sudden cardiac death has been reported for doses > 100 mg daily.34
The long-term safety of repeated applications of high concentration capsaicin patches in patients has not been clearly established particularly with respect to degeneration of epidermal nerve fibres, which may be a concern in progressive neuropathy.
Sustained release oxycodone and morphine have been the most studied with maximal daily dosages of 120 mg and 240 mg respectively in clinical trials (appendix 4). Long-term opioid use may be associated with abuse particularly at high doses, cognitive impairment and endocrine and immunologic changes.35–37
Table 3.
Summary of GRADE recommendations. Drug classes with recommendation for use.
| FIRST LINE DRUGS | SECOND LINE DRUGS | THIRD LINE DRUGS | ||||||
|---|---|---|---|---|---|---|---|---|
| SNRIs duloxetine venlafaxine | TCAs | Pregabalin Gabapentin Gabapentin ER/enacarbil | Tramadol | Capsaicin 8% patches | Lidocaine patches* | Strong opioids | BTX-A | |
| Quality of evidence | ||||||||
| High | Moderate | High | Moderate | High | Low* | Moderate | Low | |
| Balance between desirable and undesirable effects | ||||||||
| Effect size | Moderate | Moderate | Moderate | Moderate | Low | Unknown | Moderate | Moderate |
| Tolerability and safety** | Moderate | Low -Moderate | Moderate-high | Low-moderate | Moderate-high | High | Low-moderate | High |
| Values and preferences | ||||||||
| Low-moderate | Low-moderate | Low-moderate | Low-moderate | High | High | Low-moderate | High | |
| Cost and resource allocation | ||||||||
| Low-moderate | Low | Low-moderate | Low | Moderate-high | Moderate-high | Low-moderate | Moderate-high | |
| Strength of recommendation | ||||||||
| Strong | Strong | Strong | Weak | Weak | Weak | Weak | Weak | |
| Neuropathic pain conditions | All | All | All | All | Peripheral | Peripheral | All | Peripheral |
FDA and EMEA approval for the treatment of postherpetic neuralgia
Common side effects : antidepressants : somnolence, constipation, dry mouth (particularly TCAs), nausea (particularly duloxetine) ; pregabalin/gabapentin : somnolence, dizziness, weight gain; opioids (including tramadol) : constipation, nausea, vomiting, tiredness, somnolence, dizziness, dry mouth, itch ; lidocaine patches : local irritation ; capsaicin patches : local pain, edema, erythema ; botulinum toxin A : local pain.
Abbreviations : SNRIs=serotonin noradrenaline reuptake inhibitors. TCAs=tricyclic antidepressants ; BTX-A : botulinum toxin type A ; ER= extended realease.
Strong opioids (particularly oxycodone and morphine) and BTX-A (specialist use for peripheral neuropathic pain and presumed local pain generator) have weak GRADE recommendations for use and are recommended as third-line. Prescription of opioids should be strictly monitored particularly for patients requiring high dosages (including tracking the dose in morphine equivalence, use of risk assessment tools and treatment agreements).38,39
Tapentadol, other antiepileptics, capsaicin cream, topical clonidine, SSRI antidepressants, NMDA antagonists and combination therapy40–42 have inconclusive GRADE recommendations. Combination of pregabalin/gabapentin and duloxetine/TCAs may be considered as an alternative to increasing dosages in monotherapy for patients unresponsive to monotherapy with moderate dosages (appendix 10 for details).
Cannabinoids and valproate have weak recommendations against their use in neuropathic pain and levetiracetam and mexiletine have strong recommendations against their use (appendix 10 for details).
Discussion
The present manuscript presents the revised NeuPSIG recommendations for the pharmacotherapy of neuropathic pain based on an updated systematic review and meta-analysis of systemic or topical drug treatments. We used the GRADE system17,18 to assess the quality of evidence for all treatments, and the recommendations comply with the AGREE II guidelines.
The present recommendations are driven by drug treatments rather than by the aetiology of pain, akin to prior NeuPSIG recommendations.19 Neuropathic pain is increasingly recognised as a specific multiaetiology entity across neuropathic syndromes.43 In accordance with previous reports24 results of our meta-analysis show that the efficacy of systemic drug treatments is generally not dependent on the aetiology of the underlying disorder (appendix 4). Side effects may, however, to some degree depend on the aetiology, eg, drugs with CNS-related side effects may be less tolerated in patients with CNS lesions.44 Pain due to HIV-related painful polyneuropathy and radiculopathy seems more refractory than other pain conditions in our meta-analysis. This may be due to large placebo responses in HIV-related neuropathy trials,45 a distinct clinical phenotype in subgroups of patients with radiculopathy,46 or psychological/psychosocial comorbidites, often neglected in large trials. Topical agents have no known relevance for use in central neuropathic pain, and this is clearly stated in our recommendations.
The strengths of this systematic review and meta-analysis are the analysis of publication bias29 and unpublished trials. Publication bias may be present if studies with positive results are published while those with no data or negative results are not.29 It may lead to major overestimation of efficacy in therapeutic studies.47 Our results showed that the effect sizes estimated from studies published in peer-reviewed journals were higher than those estimated from studies available in open databases. This finding emphasises the need for searching these databases in systematic reviews. Analysis of further publication bias (analysis of studies that are neither published nor reported with results in open trial registries) suggested a limited overstatement of overall efficacy of drug treatments (by 10%), although available methods to assess publication bias have limitations.48 Here, we found that high-concentration capsaicin patches were the most susceptible to publication bias, ie, a new study with less than 400 participants with no effect may increase the NNT to an unacceptable level. This supports the robustness of a meta-analysis taking into account unpublished trials, and suggests that effect sizes were overestimated in previous meta-analyses of pharmacotherapy for neuropathic pain.
Results of quantitative data for individual drugs, showing NNT for 50 % pain relief ranging from around 4 to 10 across most positive trials, emphasizes the overall modest study outcomes in neuropathic pain. Inadequate response of neuropathic pain to drug therapy constitutes a highly unmet need and may have substantial consequences in terms of psychological or social adjustment.49 However these results may also reflect insuffficient assay sensitivity of clinical trials of neuropathic pain50 (Table 4). One major issue is the placebo response which seems to have increased in recent trials of neuropathic pain and may lead to an underestimation of drug effects.51 Placebo response has been found to be higher in HIV-related neuropathies,45 and in patients with low or variable pain scores at inclusion.52 Conversely it seems to be lower in postherpetic neuralgia.45 Another issue concerns the lack of adequate diagnostic criteria for neuropathic pain (available on request for individual trials). The use of diagnostic algorithms for neuropathic pain53 and screening tools54 should contribute to reducing diagnostic heterogeneity (Table 4). Lastly, a largely debated issue concerns the heterogeneity of patient phenotypes in clinical trials, which may reflect various underlying mechanisms.55–57 Interestingly, the results of a number of very recent trials or posthoc analyses of recent trials suggest that some drugs might be differentially effective in patients classified based on their sensory phenotypes.58–60
Table 4.
Limitations of current clinical trials in neuropathic pain as outlined by the present meta-analysis and systematic review, and NeuPSIG recommendations for implementation of future clinical trials in neuropathic pain
| Issues raised by current drug clinical trials in neuropathic pain | NeuPSIG recommendation for future trials in neuropathic pain |
|---|---|
|
| |
| 1/Patients population | |
| All RCTs have been conducted in adults | Conduct more studies in peadiatric population |
| Lack of validated diagnostic tools/algorithms for neuropathic pain | Use IASP diagnostic criteria for probable or definite neuropathic pain and validated screening tools to confirm diagnosis1 |
| Classification of patients is generally based on aetiology | Classification should be based on sensory phenotypes rather than merely on aetiology2 |
| 2/ Characteristics of the trials | |
| Trial duration is 12 weeks or less in 81 % of the trials | Consider longer trial duration |
| High placebo response particularly in recent trials | Exclude patients with low pain intensity and high variability of pain at baseline45 |
Abbreviation: RCTs: randomized clinical trials
Criteria for neuropathic pain diagnosis were not available before the development of screening tools and of diagnostic algorithms for NP (2008).46,47 However less than 10 % of clinical trials conducted over the past decade have used screening tools or diagnostic algorithms for NP.
This recommendation tends to be confirmed by very recent clinical trials52,53 or posthoc analyses of recent clinical trials51 that could not be considered in the present meta-analysis. Some trials suggested in particular that drugs such as oxcarbazepine or topical clonidine may be significantly more effective in subgroups of patients with preserved nociceptive function as compared to those without such phenotype52, 53 However these individual trials need to be replicated and do not change the current level of recommendation for these drug treatments.
Our updated therapeutic algorithm for neuropathic pain based on GRADE differs in many ways from prior therapeutic recommendations. The latter generally proposed TCAs, pregabalin, gabapentin and lidocaine patches as first line for neuropathic pain9–13,15–16,19,61. We now also propose gabapentin ER/enacarbil, duloxetine and venlafaxine as first line based on strong GRADE recommendation for use. We no longer recommend lidocaine patches as first line because of weak final quality of evidence. However, owing to an excellent safety profile, high values and preferences, paucity of alternative well tolerated and safe medications, short term positive studies, we propose a weak GRADE recommendation for use as generally second line for peripheral neuropathic pain. Strong opioids are now recommended as third line, contrasting with several prior recommendations in which they were generally considered as first or second line17,61 This mainly stems from the consideration of potential risk of abuse, particularly with high doses35 and concerns about a recent increase in prescription opioid-associated overdose mortality, diversion, misuse and other opioid-related morbidity particularly in USA, Canada and UK.62–64 High-concentration capsaicin patches and cannabinoids are considered for the first time in therapeutic recommendations for neuropathic pain. Capsaicin patches are proposed as second-line for peripheral neuropathic pain because of high quality of evidence, but modest effect size, training requirements, and potential safety concerns on sensation with long-term use.65 We provide a weak recommendation against the use of cannabinoids in neuropathic pain, mainly because of negative results, potential misuse, abuse, diversion and long term mental health risks particularly in susceptible individuals.66–71
One important issue when proposing recommendations is to assess to what extent they are applied by practitioners and whether this may contribute to improving their practice. Few studies have investigated the real-life impact of evidence-based recommendations on physicians’ practices. It has recently been reported that the drug treatment of postherpetic neuralgia by primary care physicians was roughly consistent with the US recommendations issued some years before.6 In contrast, a recent large study of general practitioners’ adherence to current French recommendations observed a paucity of appropriate recall of first-line drugs.8 One important educational objective of the present guidelines will be to facilitate their dissemination and subsequently assess their real life implementation in various countries.7
Acknowledgments
The authors wish to express their thanks and gratitude for all IASP staff for the warm welcome and for providing a meeting room at their headquarters for the meeting.
Role of the funding source: The study was partially funded by NeuPSIG. NA, NF, PK, RB, AR, MH, BHS are members of NeuPSIG management committee. No author was paid to write this article by a pharmaceutical company or other agency. The corresponding author and all co-authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Conflicts of interest
NA has served on the advisory boards or speakers panels of Astellas Pharma, Adir Servier, Eli Lilly, Grunenthal, Johnson and Johnson, Sanofi Pasteur Merieux and Pfizer and has been investigator of studies sponsored by Astellas, Grunenthal and Astra Zeneca. RB has received grant/research support from Pfizer, Genzyme, Grünenthal, German Federal Ministry of Education and Research (BMBF): German Research Network on Neuropathic Pain, NoPain system biology and German Research Foundation (DFG). He has received speaker honorarium from Pfizer, Genzyme, Grünenthal, Mundipharma, Sanofi Pasteur, Medtronic, Eisai, Lilly, Boehringer Ingelheim, Astellas, Desitin, Teva Pharma, Bayer-Schering, MSD and served as consultant for Pfizer, Genzyme, Grünenthal, Mundipharma, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly, Boehringer Ingelheim, Astellas, Novartis, Bristol-Myers Squibb, Biogenidec, AstraZeneca, Merck, Abbvie. RHD has received research grants from US Food and Drug Administration and US National Institutes of Health, and compensation for activities involving clinical trial research methods from Acorda, Adynxx, Allergan, Analgesic Solutions, Anika, Astellas, AstraZeneca, Avanir, Axsome, Bayer, Biogen, Bioness, Bristol-Myers Squibb, Cardiome, Centrexion, Charleston, Chromocell, Collegium, Concert, Daiichi Sankyo, Depomed, Depuy, Eli Lilly, Epicept, Flexion, Genzyme, Glenmark, Inhibitex, Johnson & Johnson, Lpath, Medicinova, Merck, Metys, MMS Holdings, Nektar, Neura, NeurogesX, Olatec, Ono, Periphagen, Pfizer, Phillips, Phosphagenics, Prolong, Q-Med, QRx Pharma, Regenesis, Relmada, Sanofi-Aventis, Salix, Smith & Nephew, Sorrento, Spinifex, Takeda, Taris, Teva, Theravance, and Xenon. NBF has received speaker’s honorarium from Pfizer, Grunenthal, and Norpharma, research grant from Grünenthal, and consultancy fee from Astellas. MH has received honoraria from Eli Lilly, Janssen-Cilag, MSD, Mundipharma, Orion, Sanofi-Aventis for lecture, honoraria from Pfizer, Allergan, Astellas for lecture and consulting and honoraria from Abbvie for consulting TSJ have received honoraria from Pfizer, Grünenthal, Astellas, Orion and Sanofi Pasteur as speaker, advisory Board participant or grant. PK has served on advisory board for Reckitt Benckizer, and received speakers’ honoraria from Pfizer. KL has received travel grants from Pfizer and Astellas. EM reports grants from Richard Saltonstall Charitable Foundation, USA, during the conduct of the study. AM has received speaker’s honorarium from Pfizer, speaker’s honorarium and consultancy fees from Eli Lilly and Grünental and research grant from Grünenthal. SNR has served on the advisory boards of Purdue Pharma, QRx pharma, Salix Pharmaceuticals, and Shionogi. ASCR has share options in Spinifex Pharmaceuticals. He undertakes consulting for Imperial College Consultants, and has received fees from Spinifex Pharmaceuticals, Astellas, Servier, Allergan, Asahi Kasei, and Medivir. Through EuroPain, ASCR’s laboratory has received funding for research studentships from Pfizer and Astellas. Other recent or current grant/studentship funding for ASCR’s laboratory are: Wellcome Trust (London Pain Consortium), Dunhill Medical Trust, NC3Rs, Westminster Medical School Research Trust, International Association for the Study of Pain, National Institute of Academic Anaesthesia, Derek Butler Trust, Medical Research Council Industrial, Biotechnology and Biological Sciences Research Council and Pfizer/Christian-Albrechts University of Kiel (Neuropain). ASCR is a member of the England and Wales Joint Committee on Vaccination and Immunisation (varicella subgroup). MR reports personal fees and other from Afferent Pharmaceuticals, Centrexion, Nektar Therapeutics, Xenoport, ViroBay, Chromocell, Adynxx, Lilly, Zalicus, Biogen IDEC outside the submitted work. PS has a patent System and method for detecting pain and its components using magnetic resonance spectroscopy, US Patent 08755862 issued. BHS has consulted for Pfizer and Napp, and received unconditional educational grants from Pfizer to support epidemiological research. MW reports personal fees from Boston Scientific, Jazz Pharmaceutical, Spinal Modulations, Depomed and Inergetics. RB, NBF, KL, TSJ and ASCR are also members of the IMI “Europain” collaboration and industry members of this are: Astra Zeneca, Pfizer, Esteve, UCB-Pharma, Sanofi Aventis, Grünenthal, Eli Lilly, Boehringer Ingelheim, Astellas, Abbott and Lundbeck. The other authors have no conflicts of interest to disclose.
Contributors
NA, NF, SH, KL, and EM did the search and extracted data. NF performed the meta-analysis. ES did the analysis of publication bias. NA and NF drafted the manuscript and the tables. PH, MR, PS and MW were external reviewers for the manuscript. All panel members contributed to the guidelines in formulating the recommendations, revising and editing the final text. All panel members and external reviewers contributed to the final text version.
References
- 1.IASP taxonomy. 2012 http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698&navItemNumber=576. Assessed June 24, 2014.
- 2.Attal N, Lanteri-Minet M, Laurent B, Fermanian J, Bouhassira D. The specific disease burden of neuropathic pain: Results of a French nationwide survey. Pain. 2011;152:2836–43. doi: 10.1016/j.pain.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149:338–44. doi: 10.1016/j.pain.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 4.Langley PC, Van LC, Cappelleri JC, Carroll D. The burden associated with neuropathic pain in Western Europe. J Med Econ. 2013;16:85–95. doi: 10.3111/13696998.2012.729548. [DOI] [PubMed] [Google Scholar]
- 5.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–19. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 6.Dworkin RH, Panarites CJ, Armstrong EP, Malone DC, Pham SV. Is treatment of postherpetic neuralgia in the community consistent with evidence-based recommendations? Pain. 2012;153:869–75. doi: 10.1016/j.pain.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Torrance N, Ferguson JA, Afolabi E, et al. Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154:690–9. doi: 10.1016/j.pain.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez V, Attal N, Vanzo B, et al. Adherence of French GPs to Chronic Neuropathic Pain Clinical Guidelines: Results of a Cross-Sectional, Randomized, “e” Case-Vignette Survey. PLoS ONE. 2014;9:e93855. doi: 10.1371/journal.pone.0093855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attal N, Cruccu G, Haanpaa M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–69. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 10.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 11.Moulin DE, Clark AJ, Gilron I, et al. Pharmacological management of chronic neuropathic pain – consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12:13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubinsky RM, Kabbani H, El-Chami Z, Boutwell C, Ali H. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63:959–65. doi: 10.1212/01.wnl.0000140708.62856.72. [DOI] [PubMed] [Google Scholar]
- 13.Bril V, England J, Franklin GM, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–65. doi: 10.1212/WNL.0b013e3182166ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–67. doi: 10.1200/JCO.2013.54.0914. [DOI] [PubMed] [Google Scholar]
- 15.Tan T, Barry P, Reken S, Baker M. Pharmacological management of neuropathic pain in non-specialist settings: summary of NICE guidance. BMJ. 2010;340:c1079. doi: 10.1136/bmj.c1079. [DOI] [PubMed] [Google Scholar]
- 16.NICE clinical guideline. 173 Neuropathic pain – pharmacological management. http:/guidance.nice.org.uk/CG173 Assessed June 24, 2014 2013.
- 17.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin RH, O’connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain. 2007;132:237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 20.Dworkin RH, O’connor AB, Kent J, et al. Interventional management of neuropathic pain: NeuPSIG recommendations. Pain. 2013;154:2249–61. doi: 10.1016/j.pain.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med. 2010;51:421–4. doi: 10.1016/j.ypmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruccu G, Gronseth G, Alksne J, et al. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15:1013–28. doi: 10.1111/j.1468-1331.2008.02185.x. [DOI] [PubMed] [Google Scholar]
- 24.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–81. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Light RJ, Pillemer DB. Summing up The science of reviewing research. Cambrige: Harvard University Press; 1984. [Google Scholar]
- 27.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 29.Moore RA, Kalso E, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors. Systematic reviews in pain research: methodology refined. Seattle: IASP Press; 2008. pp. 15–23. [Google Scholar]
- 30.Moore RA, Derry S, McQuay HJ, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults. Cochrane Database Syst Rev. 2011:CD008659. doi: 10.1002/14651858.CD008659.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.L’Abbe KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–33. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- 32.Howard RF, Wiener S, Walker SM. Neuropathic pain in children. Arch Dis Child. 2014;99:84–9. doi: 10.1136/archdischild-2013-304208. [DOI] [PubMed] [Google Scholar]
- 33.American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616–31. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2004;75:234–41. doi: 10.1016/j.clpt.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 35.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30:557–64. doi: 10.1097/AJP.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buss T, Leppert W. Opioid-induced endocrinopathy in cancer patients: an underestimated clinical problem. Adv Ther. 2014;31:153–67. doi: 10.1007/s12325-014-0096-x. [DOI] [PubMed] [Google Scholar]
- 37.Schiltenwolf M, Akbar M, Hug A, et al. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician. 2014;17:9–20. [PubMed] [Google Scholar]
- 38.Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furlan AD, Reardon R, Weppler C. Opioids for chronic noncancer pain: a new Canadian practice guideline. CMAJ. 2010;182:923–30. doi: 10.1503/cmaj.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol. 2013;12:1084–95. doi: 10.1016/S1474-4422(13)70193-5. [DOI] [PubMed] [Google Scholar]
- 42.Tesfaye S, Wilhelm S, Lledo A, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study” - a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154:2616–25. doi: 10.1016/j.pain.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 43.Attal N, Fermanian C, Fermanian J, Lanteri-Minet M, Alchaar H, Bouhassira D. Neuropathic pain: are there distinct subtypes depending on the aetiology or anatomical lesion? Pain. 2008;138:343–53. doi: 10.1016/j.pain.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ. 2013:f7339. doi: 10.1136/bmj.f7339. [DOI] [PubMed] [Google Scholar]
- 45.Cepeda MS, Berlin JA, Gao CY, Wiegand F, Wada DR. Placebo response changes depending on the neuropathic pain syndrome: results of a systematic review and meta-analysis. Pain Med. 2012;13:575–95. doi: 10.1111/j.1526-4637.2012.01340.x. [DOI] [PubMed] [Google Scholar]
- 46.Mahn F, Hullemann P, Gockel U, et al. Sensory symptom profiles and co-morbidities in painful radiculopathy. PLoS ONE. 2011;6:e18018. doi: 10.1371/journal.pone.0018018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8:e1000344. doi: 10.1371/journal.pbio.1000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207–16. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 49.Cherny NI. The treatment of neuropathic pain: from hubris to humility. Pain. 2007;132:225–6. doi: 10.1016/j.pain.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 50.Dworkin RH, Turk DC, Peirce-Sandner S, et al. Assay sensitivity and study features in neuropathic pain trials: an ACTTION meta-analysis. Neurology. 2013;81:67–75. doi: 10.1212/WNL.0b013e318297ee69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lund K, Vase L, Petersen GL, Jensen TS, Finnerup NB. Randomised controlled trials may underestimate drug effects: balanced placebo trial design. PLoS ONE. 2014;9:e84104. doi: 10.1371/journal.pone.0084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farrar JT, Troxel AB, Haynes K, et al. Effect of variability in the 7-day baseline pain diary on the assay sensitivity of neuropathic pain randomized clinical trials: an ACTTION study. Pain. 2014;155:1622–31. doi: 10.1016/j.pain.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–5. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 54.Haanpaa M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 55.Attal N, Bouhassira D, Baron R, et al. Assessing symptom profiles in neuropathic pain clinical trials: Can it improve outcome? Eur J Pain. 2011;15:441–3. doi: 10.1016/j.ejpain.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Baron R, Forster M, Binder A. Subgrouping of patients with neuropathic pain according to pain-related sensory abnormalities: a first step to a stratified treatment approach. Lancet Neurol. 2012;11:999–1005. doi: 10.1016/S1474-4422(12)70189-8. [DOI] [PubMed] [Google Scholar]
- 57.Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B. Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain. 2014;155:367–76. doi: 10.1016/j.pain.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Bouhassira D, Wilhelm S, Schacht A, et al. Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: Data from the randomized, double-blind, COMBO-DN study. Pain. 2014;155:2171–9. doi: 10.1016/j.pain.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 59.Demant DT, Lund K, Vollert J, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014 doi: 10.1016/j.pain.2014.08.014. Epub Aug 17. [DOI] [PubMed] [Google Scholar]
- 60.Campbell CM, Kipnes MD, Stouch BC, et al. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153:1815–23. doi: 10.1016/j.pain.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dworkin RH, Backonja M, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 62.Fischer B, Jones W, Urbanoski K, Skinner R, Rehm J. Correlations between prescription opioid analgesic dispensing levels and related mortality and morbidity in Ontario, Canada, 2005–2011. Drug Alcohol Rev. 2014;33:19–26. doi: 10.1111/dar.12089. [DOI] [PubMed] [Google Scholar]
- 63.Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and Regional Variation in Opioid Overdose Mortality Among Veterans Health Administration Patients, Fiscal Year 2001 to 2009. Clin J Pain. 2013;30:605–12. doi: 10.1097/AJP.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 64.Giraudon I, Lowitz K, Dargan PI, Wood DM, Dart RC. Prescription opioid abuse in the UK. Br J Clin Pharmacol. 2013;76:823–4. doi: 10.1111/bcp.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 1999;81:135–45. doi: 10.1016/s0304-3959(99)00007-x. [DOI] [PubMed] [Google Scholar]
- 66.Semple DM, McIntosh AM, Lawrie SM. Cannabis as a risk factor for psychosis: systematic review. J Psychopharmacol. 2005;19:187–94. doi: 10.1177/0269881105049040. [DOI] [PubMed] [Google Scholar]
- 67.Di FM, Morgan C, Dazzan P, et al. High-potency cannabis and the risk of psychosis. Br J Psychiatry. 2009;195:488–91. doi: 10.1192/bjp.bp.109.064220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kuepper R, van OJ, Lieb R, Wittchen HU, Hofler M, Henquet C. Continued cannabis use and risk of incidence and persistence of psychotic symptoms: 10 year follow-up cohort study. BMJ. 2011;342:d738. doi: 10.1136/bmj.d738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hall W, Degenhardt L. Cannabis and the increased incidence and persistence of psychosis. BMJ. 2011;342:d719. doi: 10.1136/bmj.d719. [DOI] [PubMed] [Google Scholar]
- 70.Davis GP, Compton MT, Wang S, Levin FR, Blanco C. Association between cannabis use, psychosis, and schizotypal personality disorder: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Schizophr Res. 2013;151:197–202. doi: 10.1016/j.schres.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilman JM, Kuster JK, Lee S, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34:5529–38. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]


