Abstract
A growing body of evidence suggests that active oxygen is an important participant in the destruction of the pancreatic beta cell, which, in turn, leads to type I or insulin-dependent diabetes mellitus. Consequently, genetic factors predisposing susceptibility to insulin-dependent diabetes mellitus may include those that determine active oxygen metabolism. A direct test of this hypothesis is provided by a transgenic model for increased activity of Cu/Zn superoxide dismutase (EC 1.15.1.1), a principal radical scavenging enzyme. Here we demonstrate that elevated levels of this enzyme provided by a Cu/Zn superoxide dismutase transgene enhance the tolerance of pancreatic beta cells to oxidative stress-induced diabetogenesis. These results show that this transgenic approach holds promise for revealing the role of reactive oxygen in autoimmune models of diabetogenesis as well as in other models of disease pathology in which active oxygen has been implicated.
Full text
PDF
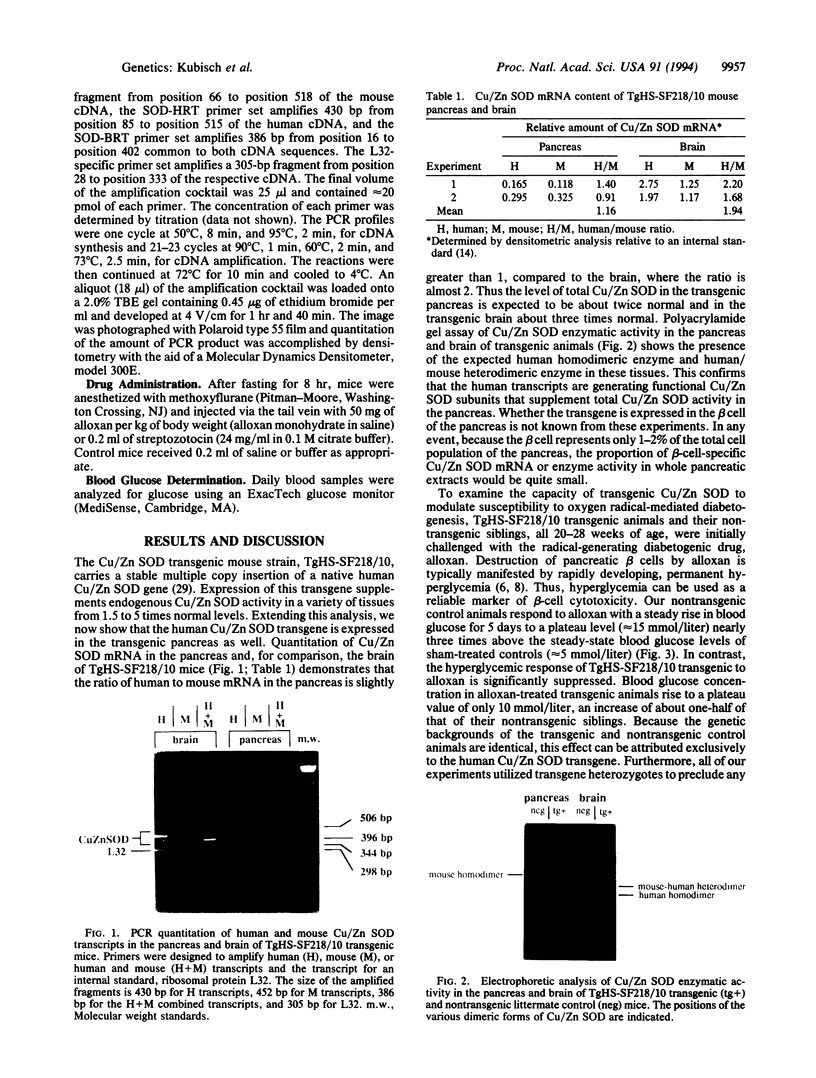
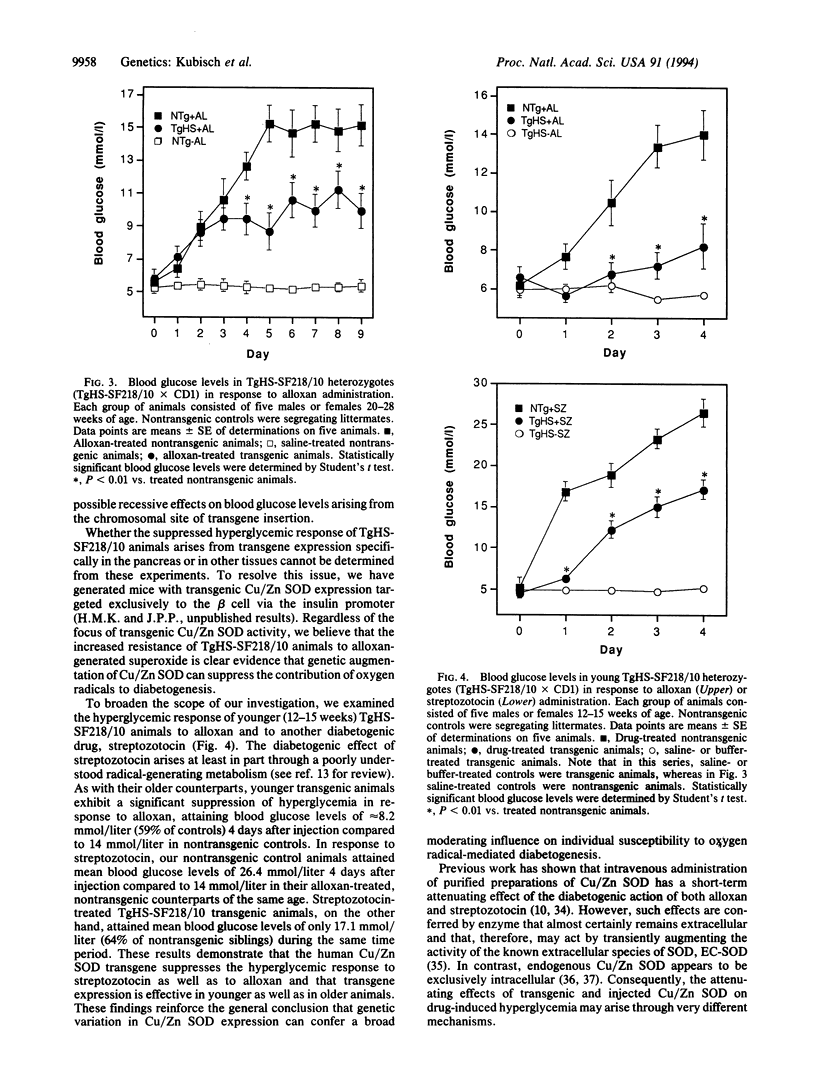

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beckman J. S. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991 Jan;15(1):53–59. [PubMed] [Google Scholar]
- Castaño L., Eisenbarth G. S. Type-I diabetes: a chronic autoimmune disease of human, mouse, and rat. Annu Rev Immunol. 1990;8:647–679. doi: 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- Chan P. H., Yang G. Y., Chen S. F., Carlson E., Epstein C. J. Cold-induced brain edema and infarction are reduced in transgenic mice overexpressing CuZn-superoxide dismutase. Ann Neurol. 1991 May;29(5):482–486. doi: 10.1002/ana.410290506. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., McDaniel M. L. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992 Aug;41(8):897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Mikhael A., Shimizu J., Frederick K., Misko T. P., McDaniel M. L., Kanagawa O., Unanue E. R. Nitric oxide production in islets from nonobese diabetic mice: aminoguanidine-sensitive and -resistant stages in the immunological diabetic process. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8992–8995. doi: 10.1073/pnas.90.19.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. A., Sweetland M. A., Wang J. L., Lancaster J. R., Jr, McDaniel M. L. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Oury T., Rabouille C., Slot J. W., Chang L. Y. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10405–10409. doi: 10.1073/pnas.89.21.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudov K. P., Perry R. P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984 Jun;37(2):457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986 May 22;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Avraham K. B., Lovett M., Smith S., Elroy-Stein O., Rotman G., Bry C., Groner Y. Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8044–8048. doi: 10.1073/pnas.84.22.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P. N., Boschero A. C., Atwater I., Cai X., Overbeek P. A. Expression of yeast hexokinase in pancreatic beta cells of transgenic mice reduces blood glucose, enhances insulin secretion, and decreases diabetes. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12038–12042. doi: 10.1073/pnas.89.24.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L. J., Hamburger S. A. Inhibition of alloxan action in isolated pancreatic islets by superoxide dismutase, catalase, and a metal chelator. Diabetes. 1980 Mar;29(3):213–216. doi: 10.2337/diab.29.3.213. [DOI] [PubMed] [Google Scholar]
- Gandy S. E., 3rd, Galbraith R. A., Crouch R. K., Buse M. G., Galbraith G. M. Superoxide dismutase in human islets of Langerhans. N Engl J Med. 1981 Jun 18;304(25):1547–1548. doi: 10.1056/NEJM198106183042518. [DOI] [PubMed] [Google Scholar]
- Gandy S. E., Buse M. G., Crouch R. K. Protective role of superoxide dismutase against diabetogenic drugs. J Clin Invest. 1982 Sep;70(3):650–658. doi: 10.1172/JCI110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankvist K., Marklund S. L., Täljedal I. B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981 Nov 1;199(2):393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankvist K., Marklund S., Täljedal I. B. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature. 1981 Nov 12;294(5837):158–160. doi: 10.1038/294158a0. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Tarui S. Immune pathogenesis of diabetes in the nonobese diabetic mouse: an overview. Curr Top Microbiol Immunol. 1990;156:15–25. doi: 10.1007/978-3-642-75239-1_2. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D. M., Bayney R. M., Li Y. W., Khosrovi H., Berger C. N., Epstein C. J., Mobley W. C. Dysregulation of gene expression in mouse trisomy 16, an animal model of Down syndrome. EMBO J. 1992 Feb;11(2):619–627. doi: 10.1002/j.1460-2075.1992.tb05094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Katz J. D., Wang B., Haskins K., Benoist C., Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993 Sep 24;74(6):1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- Keller G. A., Warner T. G., Steimer K. S., Hallewell R. A. Cu,Zn superoxide dismutase is a peroxisomal enzyme in human fibroblasts and hepatoma cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7381–7385. doi: 10.1073/pnas.88.16.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinouchi H., Epstein C. J., Mizui T., Carlson E., Chen S. F., Chan P. H. Attenuation of focal cerebral ischemic injury in transgenic mice overexpressing CuZn superoxide dismutase. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11158–11162. doi: 10.1073/pnas.88.24.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. Type 1 (insulin-dependent) diabetes mellitus and nitric oxide. Diabetologia. 1992 Aug;35(8):796–797. doi: 10.1007/BF00429103. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. The genetics of diabetes susceptibility in mice. FASEB J. 1989 Sep;3(11):2231–2241. doi: 10.1096/fasebj.3.11.2673897. [DOI] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Lukic M. L., Stosic-Grujicic S., Ostojic N., Chan W. L., Liew F. Y. Inhibition of nitric oxide generation affects the induction of diabetes by streptozocin in mice. Biochem Biophys Res Commun. 1991 Aug 15;178(3):913–920. doi: 10.1016/0006-291x(91)90978-g. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Sener A., Pipeleers D. G. Determinants of the selective toxicity of alloxan to the pancreatic B cell. Proc Natl Acad Sci U S A. 1982 Feb;79(3):927–930. doi: 10.1073/pnas.79.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos I. N., Wang Y., Lafferty K. J. Involvement of O2 radicals in 'autoimmune' diabetes. Immunol Cell Biol. 1989 Feb;67(Pt 1):85–87. doi: 10.1038/icb.1989.12. [DOI] [PubMed] [Google Scholar]
- Oberley L. W. Free radicals and diabetes. Free Radic Biol Med. 1988;5(2):113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Oehen S., Buerki K., Pircher H., Ohashi C. T., Odermatt B., Malissen B., Zinkernagel R. M., Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991 Apr 19;65(2):305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Przedborski S., Kostic V., Jackson-Lewis V., Naini A. B., Simonetti S., Fahn S., Carlson E., Epstein C. J., Cadet J. L. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992 May;12(5):1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Robbins M. J., Sharp R. A., Slonim A. E., Burr I. M. Protection against streptozotocin-induced diabetes by superoxide dismutase. Diabetologia. 1980 Jan;18(1):55–58. doi: 10.1007/BF01228303. [DOI] [PubMed] [Google Scholar]
- Sandler S., Welsh M., Andersson A. Streptozotocin-induced impairment of islet B-cell metabolism and its prevention by a hydroxyl radical scavenger and inhibitors of poly(ADP-ribose) synthetase. Acta Pharmacol Toxicol (Copenh) 1983 Nov;53(5):392–400. doi: 10.1111/j.1600-0773.1983.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N., Liggitt D., Pitts S. L., Hansen S. E., Stewart T. A. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell. 1988 Mar 11;52(5):773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvetnick N., Shizuru J., Liggitt D., Martin L., McIntyre B., Gregory A., Parslow T., Stewart T. Loss of pancreatic islet tolerance induced by beta-cell expression of interferon-gamma. Nature. 1990 Aug 30;346(6287):844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Warner T. D., Ishii K., Sheng H., Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992 Feb 7;255(5045):721–723. doi: 10.1126/science.1371193. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J., Robinson M. O., Bellvé A. R., Simon M. I., Riggs A. D. Measurement by quantitative PCR of changes in HPRT, PGK-1, PGK-2, APRT, MTase, and Zfy gene transcripts during mouse spermatogenesis. Nucleic Acids Res. 1990 Mar 11;18(5):1255–1259. doi: 10.1093/nar/18.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T. A., Hultgren B., Huang X., Pitts-Meek S., Hully J., MacLachlan N. J. Induction of type I diabetes by interferon-alpha in transgenic mice. Science. 1993 Jun 25;260(5116):1942–1946. doi: 10.1126/science.8100367. [DOI] [PubMed] [Google Scholar]
- Sumoski W., Baquerizo H., Rabinovitch A. Oxygen free radical scavengers protect rat islet cells from damage by cytokines. Diabetologia. 1989 Nov;32(11):792–796. doi: 10.1007/BF00264909. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Warren J. S., Johnson K. J. Oxygen radicals, inflammation, and tissue injury. Free Radic Biol Med. 1988;5(5-6):403–408. doi: 10.1016/0891-5849(88)90114-1. [DOI] [PubMed] [Google Scholar]
- Yamada K., Nonaka K., Hanafusa T., Miyazaki A., Toyoshima H., Tarui S. Preventive and therapeutic effects of large-dose nicotinamide injections on diabetes associated with insulitis. An observation in nonobese diabetic (NOD) mice. Diabetes. 1982 Sep;31(9):749–753. doi: 10.2337/diab.31.9.749. [DOI] [PubMed] [Google Scholar]