Abstract
Growth mechanism of chemically-driven RuO2 nanowires is explored and used to fabricate three-dimensional RuO2 branched Au-TiO2 nanowire electrodes for the photostable solar water oxidation. For the real time structural evolution during the nanowire growth, the amorphous RuO2 precursors (Ru(OH)3·H2O) are heated at 180 °C, producing the RuO2 nanoparticles with the tetragonal crystallographic structure and Ru enriched amorphous phases, observed through the in-situ synchrotron x-ray diffraction and the high-resolution transmission electron microscope images. Growth then proceeds by Ru diffusion to the nanoparticles, followed by the diffusion to the growing surface of the nanowire in oxygen ambient, supported by the nucleation theory. The RuO2 branched Au-TiO2 nanowire arrays shows a remarkable enhancement in the photocurrent density by approximately 60% and 200%, in the UV-visible and Visible region, respectively, compared with pristine TiO2 nanowires. Furthermore, there is no significant decrease in the device’s photoconductance with UV-visible illumination during 1 day, making it possible to produce oxygen gas without the loss of the photoactvity.
Three-dimensional (3D) branched nanowire arrays have received much attention owing to their peculiar and interesting optical, electronic, and catalytic properties1,2,3. Because of their very high surface-to-volume ratios, the direct conduction pathway for charge transport, and the low light reflectance, the 3D nanostructures can in principle are used to create very practical nanophotonic devices such as photochemical cells, sensors, light-emitting diodes, and solar cells3,4,5.
Ruthenium dioxide (RuO2) are of great interest as a promising candidate as electrodes in electrochemical devices and active catalysts due to extremely low resistivity, excellent chemical and thermal stability, catalytic property6,7. In particular, it is well-known to be among the most efficient electrocatalysts for the oxygen evolution reaction (OER) in alkaline and acid electrolytes, promoting the photoelectrochemical (PEC) water oxidation in photochemical cells. The sluggish anodic OER as well as the predominant absorption in the UV region may be a main factor in determining the capability of the titanium dioxide (TiO2)-based photoelectrodes8. Recently, the IrO2/hemin-coated TiO2 nanowires showed much enhanced photocurrent with ~100% increase compared to the pristine TiO2 nanowires9. The photocatatytic activities of TiSi2/graphene nanoparticles and Nb/N-doped anatase TiO2 nanoparticles were reported to be improved by the loading of RuO2 nanoparticles10,11. Also, the RuO2-Au composites enhanced the catalytic property with ~30% than pure Au12. Thus, most of the catalysts are formed as a form of fine nanoparticles. The photocatalytic activity may be enhanced by the advantages imparted by the 3D branched nanostructure, as mentioned above. Furthermore, since 2012, it is also interesting that the price of Ru has decreased by approximately 40% on sales in Asia and U.S.13.
In spite of its great potential toward future real applications, on the other hand, the growth of highly single crystalline RuO2 nanowires has been recently reported by thermal oxidation or chemical vapor deposition (CVD) of appropriate Ru-based precursors and reactive sputtering using pure Ru metal targets7,14,15. However, it is still challenging to make RuO2 nanowires with well-defined crystal structures with desirable density in real applications. For the RuO2 nanowires grown by a CVD, a process referred to as vapor-solid (VS) growth model was suggested via the formation of gaseous RuO4 species which is a highly volatile and very low melting point intermediate14. A thermal conversion from amorphous oxides nanoparticle precursor was also suggested to synthesize single crystalline nanowires of oxide materials15. However, it is necessary to explore this mechanism in greater detail in order to fully demonstrate the preferential and unidirectional crystal growth of RuO2.
Here, we focus on the real-time structural evolution of chemically-driven RuO2 nanowires for the growth mechanism and report a facile method to fabricate RuO2 branched Au-TiO2 nanowire arrays for the photostable solar water oxidation. The in-situ synchrotron x-ray diffraction (in-situ SXRD) shows the direct transition of the amorphous RuO2 precursors (Ru(OH)3·H2O) to crystalline RuO2 nanoparticles with the tetragonal crystallographic structure at 180 °C, without the intermediate change formation. In the high-resolution transmission electron microscope (HRTEM), we find that most of the crystallized nanoparticles are in the vicinity of the nanowires and the amorphous region is Ru enriched by about 10% in comparison to the RuO2 nanowire. This means that the growth proceeds by Ru diffusion to the nanoparticles, increasing the size of the nanoparticles, eventually, forming the nanowires. At low temperature less than 250 °C, the diffusion is so slow, thereby, the nanowires are very short (less than 1 μm at the growth time of several hours). We believe that this process is supported by the nucleation theory. We also carefully explore the photocatalytic performance of the water splitting of the branched nanowires and the performance is compared with pristine TiO2 nanowires. We found that the photoactivity was effectively enhanced in the entire UV-visible region, especially more effective in visible light, due to the high catalytic properties of the RuO2 for the water oxidation and the efficient plasmonic absorption with efficient charge separation to TiO2 and Au interface, catalyzing the oxygen evolution of the 3D RuO2 branched structure on the Au surface. The electrodes were quite stable during long-term use (we believe that it may ascribe to the high-crystalline properties of the RuO2 nanowires).
Results and Discussion
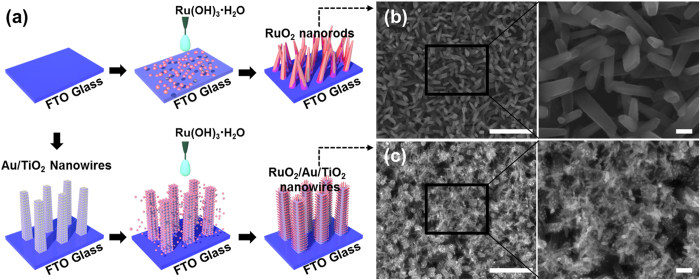
The schematic diagrams outlining the fabrication process of 1D RuO2 nanowires and 3D RuO2 branched Au-TiO2 nanowires are shown in Fig. 1a and detailed information described in Experimental section. Scanning electron microscopy (SEM) images in Fig. 1b shows the product (of a reaction carried out at 250 °C) to consist of long, randomly oriented RuO2 nanowires without any catalyst particles at the end of their tips. The nanowires (0.5 ~ 1 μm long and 130 ~ 170 nm in diameter) grow out of the plane of the substrate in the rectangular shape. Figure 1c shows RuO2 nanowires directly grown on hydrothermally-grown TiO2 nanowires with Au nanoparticles (AuNPs), producing 3D branched nanowires. The lengths of most of the nanowires fall in the range 60 ~ 80 nm. The mean nanowire width was determined to be 25 nm. The smaller RuO2 nanowires in the 3D branched structures ascribes to the decrease in the amount of the precursor, which is an important factor in determining the length and width of the nanowires.
Figure 1.
(a) Schematic diagram for the fabrication of RuO2 nanowires and 3D RuO2 branched Au-TiO2 nanostructure, (b,c) top-view SEM images of RuO2 nanowires and 3D branched nanostructures (scale bars in the left and right images, 1 μm and 100 nm, respectively).
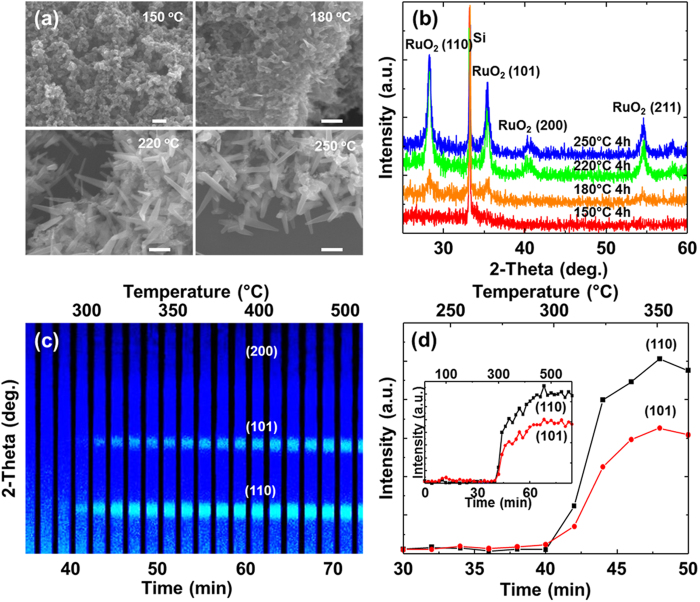
Figure 2a shows SEM images of the as-grown RuO2 nanowires on a Si substrate synthesized by heating the RuO2 precursors in air atmosphere in the range of 150 ~ 250 °C during 4 hrs. At low temperature less than 150 °C, only nanoparticles were observed and there were no nanowires on the substrates. In ex-situ x-ray diffraction (XRD), there are no peaks in the spectra, meaning that the nanoparticles are still amorphous. As the temperature increases to 180 °C, three peaks corresponding to the (110), (101), and (211) planes were clearly observed, confirming that the amorphous nanoparticles start to be crystallized at the temperature. In the SEM image, many straight nanowires with apparent tapering at the end and without any cluster at the tip were also seen. Further increase in the temperature enhances the growth of the nanowires, increasing the intensity of the three peaks, and produces additional peak corresponding to the (200) plane. At 250 °C, the length and diameter of the nanowires are in the range of 150 nm and 18 nm, respectively. The length and diameter of the nanowires increase as the temperature increases, while the aspect ratio decreases with the temperature (Supplementary Fig. S1).
Figure 2.
(a) SEM images and (b) the corresponding XRD patterns (● : (110), ○ : (101), ■ : (200), and □ : (211)) of RuO2 nanowires as a function of temperature (scale bar, 100 nm). The peak at 33.2 ° is due to the substrate. (c) The in-situ synchrotron radiation diffraction from the growth of RuO2 nanowires as the temperature increases to 150 ~ 600 °C. Each vertical line is one XRD scan, with the intensity represented by the color. The first scan is at the left, and time progresses right. (d) The change in the intensity of (101) and (110) plane with time and inset image is full time.
In order to further investigate the synthetic process, such as intermediate phase formation over annealing time and temperature, in-situ SXRD experiments were carefully performed in the course of the growth process. In-situ SXRD data provides direct identification of the solids and a sequence of events in the formation of different objects and their growth. The ruthenium hydroxide precursors are put on the stages and then heated by external power sources up to 600 °C with the ramping speed of 7 °C/min. 42 data points for 2 minutes at each temperature interval were obtained. However, experience tells that the measured temperature is not equal to that of the nanoparticles, which will be lower. In the initial stage of the heating, there is no peak. As the temperature increases to about 300 °C, two peaks corresponding to the (110) and (101) planes are observed. However, we could observe any other peaks, indicating the spontaneous growth of the RuO2 phases from the amorphous phases without any intermediate phase formed.
The above spontaneous growth of the nanowires during the oxidation of precursors without the use of conventional templates or catalysts has received significant attention in the nanotechnology community. The VS model was usually proposed to understand the growth of ZnO and SnO2 nanowires based on the evaporation of the precursors16,17. However, the RuO2 synthesis is carried out at low temperature of ~180 °C, at which the equilibrium vapor pressures of the pure elements are negligibly small, therefore cannot be ascribed to the model. The growth of the nanowires may ascribe to the diffusion of the cations during the oxidation, forming the tip-like geometry of a single nanowire, in which the driving force for the diffusion is related to the internal stress associated with the phase formation at the CuO/Cu2O interface. Also, metal-oxides nanowires such as VO2, V2O5, MoO2, MoO3, and Fe3O4, can be grown via the diffusion and solidification of nanodroplets near the bulk melting point18,19,20,21,22,23.
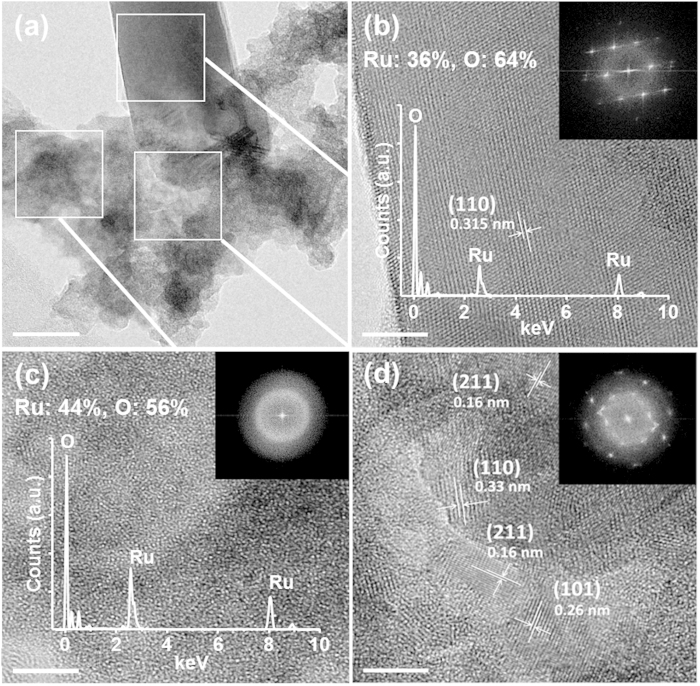
The detailed growth mechanism was characterized by bright-field TEM (BFTEM) and HRTEM images, as shown in Fig. 3. Figure 3a is a BFTEM image of a single nanowire grown at 190 °C with a diameter of approximately 30 nm. The HRTEM image and the corresponding fast-Fourier transformed (FFT) of the nanowire reveal highly ordered lattice fringes, demonstrating that the nanowire is a defect-free single crystal (Fig. 3b). The RuO2 nanowire has identified as a tetragonal crystalline (110) phase. In the vicinity of the region where the nanowire starts to be grown, there are some crystallized RuO2 nanoparticles that are ~4 nm in size are clearly seen in the vicinity of the nanowires, while most of the nanoparticles exist as a form of amorphous elsewhere (Fig. 3c,d). Any additional Ru-based crystalline phases except the RuO2 phase, tetragonal crystallographic structure, are not observed. This implies that the nanowires are grown from the amorphous nanoparticles by the direct recrystallization process.
Figure 3.
(a) BF- TEM image near the bottom of the RuO2 nanowire (scale bar 20 nm). (b) HRTEM image of RuO2 nanowire obtained after annealing at 190 °C for 1 hr. (c) Magnified HRTEM image of amorphous RuO2 region and (d) crystalline RuO2 nanoparticles at the interface between the region and nanowire (Fig. b, c and d scale bar 5 nm). Inset in (b,c) show the EDS spectra.
Previously, it was reported that the amorphous RuO2 precursors started to lose the water of the hydration to create the anhydrous RuO2 nanoparticles and Ru metals at 90 °C, as follows24;
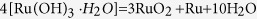 |
The rate of Ru/RuO2 content was approximately 0.3 in N2 ambient, meaning that the nanoparticles are amorphous, and increased with the annealing temperature. Actually, it was observed that the amorphous region in Fig. 3c, about 20 nm away from the nanowire, was Ru enriched by about 10% in comparison to the RuO2 nanowire in Fig. 3b, determined by energy dispersive spectroscopy (EDS). However, most of nanoparticles are seen to be crystalline in the vicinity of the nanowire. The size of the nanoparticles is in the range of 2 ~ 4 nm. According to the nucleation theory, the critical radius (r*) of the nucleation critically depends on the surface energy (γ), the latent heat of fusion (ΔHf), the melting temperature (Tm), and the growth temperature (Tg), which is given by,
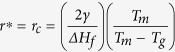 |
Here, the melting temperature and latent heat of fusion are 1200 °C and 1.66 × 107 J/m3, respectively. The surface free energy of (110) and (001) planes are 1.136 J/m2 and 1.398 J/m2, respectively24,25. In this case, the critical thickness of RuO2 is calculated to be about 2 nm at 190 °C, similar with those observed in the TEM images (Fig. 3d). The observation of the RuO2 crystalline nanoparticles between the amorphous phase and the nanowire may suggests that the amorphous phase is crystallized before the growth of the nanowires.
The growth rate of the nanowires is mainly limited by the rate of transport of mass, the diffusion rate of Ru and RuO2 materials produced by the heating process of RuO2 precursor. The activation energy (ΔE) for the migration of Ru and RuO2 of ΔE is supposed to be 384 kJ/mol and 3140 kJ/mol, respectively through latent heat of fusion (Ru: 24 kJ/mol, RuO2: 314 kJ/mol)26. This indicates that ΔE of RuO2 is about 8 times of magnitude of larger than that of Ru, meaning that the diffusion of Ru metals is the dominating process. The concentration gradient of Ru from the amorphous phase to the nanowires promotes the diffusion, enhancing the growth of the nanowires. We also believe that the Ru metals migrate from the bottom to top region of the nanowires through the surface because the bulk diffusion becomes significant at only high temperature. In O2 ambient, the Ru reacts with oxygen, producing the RuO2 on the surface27. Actually, at free oxygenless atmosphere such as ambient gases such as nitrogen or under vacuum conditions, there are no nanowires on the substrates.
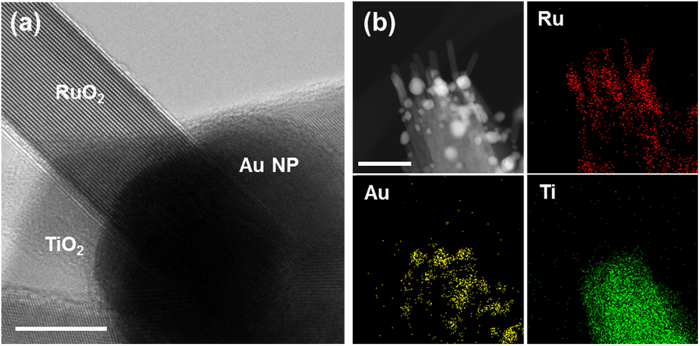
The RuO2 nanowires were successfully grown on the Au-decorated TiO2 nanowires, by precisely controlling the concentration of precursors and the growth temperature. HRTEM image in Fig. 4a shows that single-crystalline RuO2 nanowire is directly grown on the Au nanoparticle. EDS elemental mapping of Fig. 4b clearly also confirms each elemental distribution.
Figure 4.
(a) TEM image of RuO2 nanowire on Au nanoparticle-decorated TiO2 nanowire. It is clearly seen that the nanowire is grown on the Au nanoparticle (scale bar 20 nm). (b) High-angle annular dark field (HAADF) scanning TEM image of 3D branched nanowires. EDS elemental distribution map of Ru, Au, and Ti (scale bar 100 nm).
The PEC performance of the TiO2-based photoelectrodes was examined in a three-electrode configuration with Ag/AgCl as the reference electrode and a Pt wire as the counter electrode. Figure 5a shows the photocurrent-potential characteristics of the TiO2 nanowires, AuNPs decorated TiO2 nanowires, and RuO2 branched AuNPs-TiO2 nanowires recorded in 0.5 M Na2SO4 electrolyte (pH = 7.2) under AM 1.5G illumination of 100 mW/cm2. The dark scans revealed a small background current of ~2 × 10−3 mA/cm2, negligible compared to the photocurrent densities of all photoelectrodes. Upon illumination with white light, the pristine TiO2 nanowires electrodes show a photocurrent density of 0.673 mA/cm2 at 1.23 V vs. RHE (0.61 V vs. Ag/AgCl). As AuNPs are decorated onto the nanowires, the photocurrent density increases to 0.909 mA/cm2 at the same potential, associated with the surface plasmonic resonance effect of the AuNPs28. It was also observed that under visible-light illumination, the photocurrent density of AuNPs-TiO2 nanowires was increased by 1.93 times, in comparison with pristine TiO2 nanowires, obtained by adding a 420 nm long-pass filter to the white source, as shown in Fig. 5b. The formation of RuO2 nanowires on AuNPs-TiO2 nanowires increases the photocurrent density to 1.052 mA/cm2, around 56% enhancement, compared with pristine TiO2 nanowires. It is believed that the RuO2 promotes the electron transfer to the AuNPs due to the high catalytic properties for the water oxidation and the efficient hole injection to RuO2 to AuNPs, catalyzing the oxygen evolution (Supplementary Fig. S2). This process is prominent seen under visible light. We also observed that the photocurrent of the branched nanowires significantly increased over approximately 0.7 V vs. RHE, compared to that of the AuNPs-TiO2 nanowires, with a slight shift in the onset voltage. This may be related to the flat band voltage as 0.41 eV of the work function difference between Au and RuO2. The slight shift in the onset voltage may also be ascribed to the low overpotential on the Au-TiO2 nanowires associated with the oxygen reduction29,30.
Figure 5.
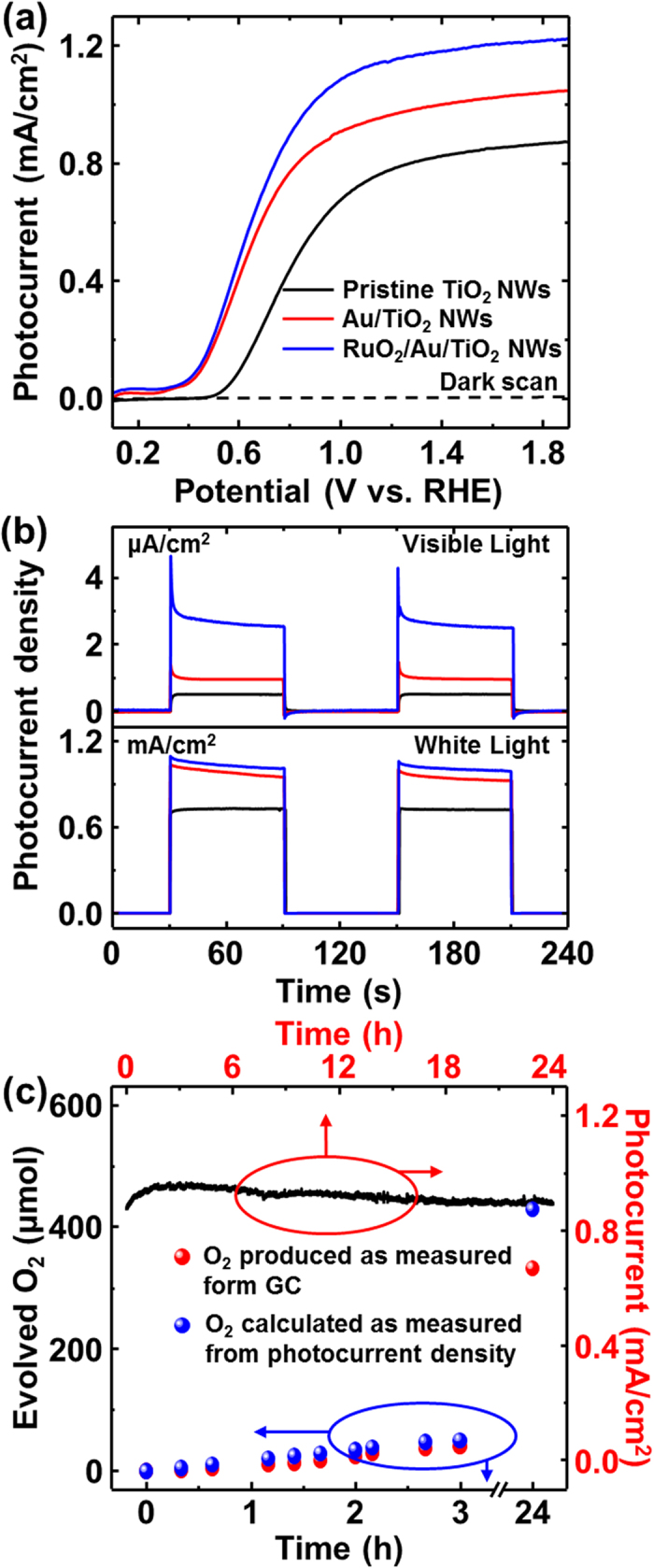
(a) Photocurrent versus potential characteristics of 0.5 M Na2SO4 (pH 7.2) electrolyte under AM 1.5 light illumination measured against the Ag/AgCl electrode with 30 mV/s scan rate. (b) Photocurrent density for chopped full spectrum (AM 1.5G) and visible illumination (λ > 420 nm long pass filter with AM 1.5G) at 1.62 V vs. RHE. (c) The quantity of evolved oxygen (red dots) measured (gas-chromatographically) as a function of time. The photocurrent simultaneously recorded with light illumination of white light (AM 1.5G) at 1.23 V vs. RHE.
For the long photostability of the electrodes, the photocurrent was measured under illumination conditions of illuminated with white light at 1.23 V vs. RHE as a function of time up to 24 hrs. Figure 5c shows that the photocurrent decreases slowly up to 24 hrs after a little increase in photocurrent within 3 hrs, which may ascribe to the build-up of hydrogen concentration on Pt electrodes. The rapid decrease in the photocurrent of the efficient photoelectrodes as major limitation to commercialization was not observed here31, which may ascribe to the highly crystalline properties of the RuO2 nanowires and the good chemical stability of the TiO2. Gas chromatographic measurements under the illumination conditions described above also shows that there is no significant difference between the calculated oxygen amount from the photocurrent density and the oxygen evolution amount. This may imply that the RuO2 branched Au-TiO2 electrodes has stoichiometric amount reaction for H2 and O2 evolution and avoid the back reaction, showing quite stable performance during the gas evolution.
Conclusion
We report the chemically-driven RuO2 nanowires and a facile method to fabricate three-dimensional RuO2 branched Au-TiO2 nanowire arrays for the photostable electrodes in PEC water oxidation. The amorphous RuO2 precursors (Ru(OH)3·H2O) are heated at 180 °C, producing the RuO2 nanoparticles with the tetragonal crystallographic structure and Ru enriched amorphous phases, observed by the real time structural evolution during the growth process through the in-situ SXRD and the HRTEM images. Growth proceeds by Ru diffusion to the nanoparticles, followed by diffusion to the growing surface of the nanowire in oxygen ambient, supported by the nucleation theory. The RuO2 branched Au-TiO2 nanowire arrays shows a remarkable enhancement in the photocurrent density by approximately 60% and 200%, in the UV-visible and Visible region, respectively, compared with pristine TiO2 nanowires at the same potential. Furthermore, there is no significant change in the device’s photoconductance with UV-visible illumination during 1 day, making it possible to produce oxygen gas without the loss of the photoactivity. This may ascribe to the highly crystalline properties of the RuO2 nanowires and means the good photostability of the designed electrode.
Methods
Synthesis of RuO2 nanowires and Growth Mechanism
Highly-crystalline RuO2 nanowires were grown by a simple annealing process of the RuO2 precursor via a simple reaction between RuCl3·xH2O and NaOH by carefully controlling pH (~9.00) of the aqueous solution at room temperature, as described previously14. This was found to be so simple and facile method for the growth of the nanowires. During this process, the amorphous RuO2 precursor (Ru(OH)3·H2O) were washed several times with deionized (DI) water to remove the remaining chlorides and sodium hydroxide, followed by the redispersion in DI water. For the detailed growth mechanism, it was spread on a Si (001) wafer and after drying at room temperature for 2 hrs, the sample are heated at the temperature ranging from 150 to 250 °C for 4 hrs.
Growth of 3D Branch-Shaped Nanostructure with Au nanoparticles
For the efficient oxygen production, TiO2 nanowire arrays were first grown on fluorine-doped tin oxide (FTO) substrate by the hydrothermal method, reported elsewhere32. After washing with DI water, the sample was annealed in air at 450 °C for 4 hrs to improve the crystallinity of the nanowires. The AuNPs are formed by metal aggregation. The ~1 nm thick (mass thickness) Au film was then deposited on the TiO2 nanowires using e-beam evaporation at a very slow deposition rate of less 0.1 Å/s and annealed at the temperature 250 °C for 1 min in nitrogen gas. The base pressure was maintained at 2.0 × 10−6 Torr. It produced well-separated AuNPs covering the nanowire uniformly. Finally, the RuO2 precursor mixed in DI water (RuO2 precursor are well-controlled from 3 × 10−6 to 6 × 10−4 wt%) are dropped into the sample and dried at 2 hrs, followed by the annealing process in atmosphere.
Microstructural analysis
The real time structural evolution during the growth process was done by the in-situ synchrotron radiation diffraction as the temperature increases to 150 ~ 600 °C, which was carried out at the 3D beamline at Pohang Accelerator Laboratory (PAL). For high-resolution XRD measurements, the wavelength of the incident X-ray was set at 1.488 Å by a double-bounce Si (111) monochromator. The SEM was done using a PHILIPS XL30S with an accelerating voltage of 5 kV. The HRTEM images were collected using a Cs-corrected JEM-2100 operated at 200 kV.
Photoelectrochemical Characterization
The PEC properties of the electrodes were studied in a three electrode cell with Ag/AgCl as the reference electrode and a Pt wire as the counter electrode. The working electrode area is in the range of ~0.2 cm2. 0.5 M Na2SO4 aqueous solution (pH = 7.2) was used as an electrolyte for PEC measurements. Linear sweeps and time-profiled current generation were obtained by an IviumStat electrochemical analyzer, with Ag/AgCl as reference and Pt wire as counter electrode under simulated sunlight with a 150 W xenon lamp coupled with an AM 1.5 global filters (100 mW/cm2). For visible light measurement, a long-wave pass filter (λ > 430 nm) was placed in front of the light source.
Additional Information
How to cite this article: Park, J. et al. Structural Evolution of Chemically-Driven RuO2 Nanowires and 3-Dimensional Design for Photo-Catalytic Applications. Sci. Rep. 5, 11933; doi: 10.1038/srep11933 (2015).
Supplementary Material
Acknowledgments
This work was supported the Pioneer Research Center Program (2013M3C1A3063602) of the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (MSIP), and by Basic Science Program through the National Research Foundation of Korea (NRF) funded by MEST (Nos. 2014R1A1A2059791 for MHK). This work was also financially supported by the KIST-UNIST partnership program (2V03870/2V03880) and equivalently by the 2014 Research Fund (1.140019.01) of UNIST (Ulsan National Institute of Science and Technology). We are very thankful to Prof. Suk-Bin Lee (UNIST) for great contribution in this paper.
Footnotes
Author Contributions J.P., J.W.L., B.U.Y. and S.H.C. performed the experiments and analyzed the data. H.Y.J. performed the TEM experiments. H.P., S.H.J. and H.L. revised the manuscript. J.M.B. and M.H.K. conceived of and supervised this study, and provided intellectual and technical guidance. All authors discussed the results, wrote and commented on the manuscript.
References
- Kargar A. et al. 3D Branched nanowire photoelectrochemical electrodes for efficient solar water splitting. ACS Nano 7, 9407–9415 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang X., Liu Y. & Kang Z. 3D branched ZnO nanowire arrays decorated with plasmonic Au nanoparticles for high-performance photoelectrochemical water splitting. ACS Appl. Mater. Interfaces. 6, 4480–4489 (2014). [DOI] [PubMed] [Google Scholar]
- Cho I. S. et al. Branched TiO2 nanorods for photoelectrochemical hydrogen production. Nano Lett. 11, 4978–4984 (2011). [DOI] [PubMed] [Google Scholar]
- Lee J.–H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuat. B 140, 319–336 (2009). [Google Scholar]
- Ye B. U. et al. Three-dimensional branched nanowire heterostructures as efficient light-extraction layer in light-emitting diodes. Adv. Funct. Mater. 24, 3384–3391 (2014). [Google Scholar]
- Kim M. H. et al. Growth direction determination of a single RuO2 nanowire by polarized Raman spectroscopy. Appl. Phys. Lett. 96, 213108 (2010). [Google Scholar]
- Chueh Y.–L. et al. RuO2 nanowires and RuO2–TiO2 core-shelled nanowires: from synthesis to mechanical, optical, electricaland photoconductive properties. Adv. Mater. 19, 143–149 (2007). [Google Scholar]
- Kudo A. & Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009). [DOI] [PubMed] [Google Scholar]
- Tang J. et al. Photoelectrochemical detection of glutathione by IrO2–Hemin–TiO2 nanowire arrays. Nano Lett. 13, 5350–5354 (2013). [DOI] [PubMed] [Google Scholar]
- Mou Z. et al. RuO2/TiSi2/graphene composite for enhanced photocatalytic hydrogen generation under visible light irradiation. Phys. Chem. Chem. Phys. 15, 2793–2799 (2013). [DOI] [PubMed] [Google Scholar]
- Breault T. M., Brancho J. J., Guo P. & Bartlett B. M. Visible light water oxidation using a co-catalyst loaded anatase–structured Ti1−(5x/4)NbxO2−y−δNy Compound. Inorg. Chem. 52, 9363–9368 (2013). [DOI] [PubMed] [Google Scholar]
- Jin J. et al. Exploration of the interaction of RuO2–Au composite nanoparticles formed by one-step synthesis within the mesopores. Chem. Mater. 25, 3921–3927 (2013). [Google Scholar]
- InvestmentMine, 5 Year Ruthenium Prices and Price Charts, http://www.infomine.com/investment/metal-prices/ruthenium/5-year/, accessed: August, 2014.
- Lee Y. et al. Facile synthesis of single crystalline metallic RuO2 nanowires and electromigration-induced transport properties. J. Phys. Chem. C 115, 4611–4615 (2011). [Google Scholar]
- Chen Z. G., Pei F., Pei Y. T. & De Hosson J. T. M. A versatile route for the synthesis of single crystalline oxide nanorods: Growth behavior and field emission characteristics. Cryst. Growth Des. 10, 2585–2590 (2010). [Google Scholar]
- Yao B. D., Chan Y. F. & Wang N. Formation of ZnO nanostructures by a simple way of thermal evaporation. Appl. Phys. Lett. 81, 757–759 (2002). [Google Scholar]
- Dai Z. R., Pan Z. W. & Wang Z. L. Novel nanostructures of functional oxides synthesized by thermal evaporation. Adv. Funct. Mater. 13, 9–24 (2003). [Google Scholar]
- Shrestha K. M., Sorensen C. M. & Klabunde K. J. Synthesis of CuO nanorods, reduction of CuO into Cu nanorods, and diffuse reflectance measurements of CuO and Cu nanomaterials in the near infrared region. J. Phys. Chem. C 114, 14368–14376 (2010). [Google Scholar]
- Chen J. T. et al. CuO nanowires synthesized by thermal oxidation route. J. Alloy Comp. 454, 268–273 (2008). [Google Scholar]
- Kim M. H. et al. Growth of metal oxide nanowires from supercooled liquid nanodroplets. Nano Lett. 9, 4138–4146 (2009). [DOI] [PubMed] [Google Scholar]
- Takahashi K., Limmer S. J., Wang Y. & Cao G. Synthesis and electrochemical properties of single-crystal V2O5 nanorod arrays by template-based electrodeposition. J. Phys. Chem. B 108, 9795–9800 (2004). [Google Scholar]
- Liu J. et al. Enhanced field emission properties of MoO2 nanorods with controllable shape and orientation. Mater. Lett. 58, 3812–3815 (2004). [Google Scholar]
- Cai L., Rao P. M. & Zheng X. Morphology-controlled flame synthesis of single, branched, and flower-like α-MoO3 nanobelt arrays. Nano Lett. 11, 872–877 (2011). [DOI] [PubMed] [Google Scholar]
- Duvigneaud P. H. & Reinhard-Deire D. DTA study of RuO2 formation from the thermal decomposition of ruthenium(III) hydrate. Thermochim. Acta 51, 307–314 (1981). [Google Scholar]
-
Kim Y. D., Schwegmann S., Seitsonen A. P. & Over H.
Epitaxial growth of RuO2(100) on Ru(10
 0): Surface structure and other properties. J. Phys. Chem. B
105, 2205–2211 (2001). [Google Scholar]
0): Surface structure and other properties. J. Phys. Chem. B
105, 2205–2211 (2001). [Google Scholar] - O’Neill H. S. C. & Nell J. Gibbs free energies of formation of RuO2, lrO2, and OsO2: A high-temperature electrochemical and calorimetric study. Ceochim. Cosmochim. Acta 61, 5279–5293, (1997). [Google Scholar]
- Barin I. Thermochemical data of pure substances [1254] (Wiley, New York, 1989). [Google Scholar]
- Pu Y.–C. et al. Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting. Nano Lett. 13, 3817–3823 (2013). [DOI] [PubMed] [Google Scholar]
- Li J. et al. A three-dimensional hexagonalfluorine-doped tin oxide nanocone array: a superior light harvesting electrode for high performance photoelectrochemical water splitting. Energy Environ. Sci. 7, 3651–3658 (2014). [Google Scholar]
- Li X. Z., He C., Graham N. & Xiong Y. Photoelectrocatalytic degradation of bisphenol A in aqueous solution using a Au–TiO2/ITO film. J. Appl. Electrochem. 35, 741–750 (2005). [Google Scholar]
- Hisatomi T., Kubota J. & Domen K., Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting, Chem. Soc. Rev. 43, 7520–7535 (2014). [DOI] [PubMed] [Google Scholar]
- Liu B. & Aydil E. S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 131, 3985–3990 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.