Abstract
Objectives
To evaluate severe (grade 3/4) morbidity and mortality in HIV-exposed, uninfected infants.
Design
Secondary data analysis of The Breastfeeding, Antiretrovirals, and Nutrition (BAN) clinical trial.
Methods
BAN randomized 2369 mother–infant pairs to maternal, infant, or no extended antiretroviral prophylaxis during breastfeeding. Morbidity outcomes examined were pneumonia/serious febrile illness, diarrhea/growth faltering, and malaria. Infant death was defined as neonatal (≤ 30 days of life), and postneonatal (31 days to 48 weeks of life). Cox proportional hazards models were used to evaluate the effect of covariates on infant morbidity and mortality.
Results
The rate of pneumonia/serious febrile illness was highest in the first 12 weeks (0.83/100 person-weeks) before rapidly decreasing; rates of all morbidity outcomes increased after 24 weeks. Rates of pneumonia/serious febrile illness and diarrhea/growth faltering were higher during the rainy season. Prophylactic infant cotrimoxazole significantly decreased the rates of all morbidity outcomes. White blood cell (WBC) count less than 9000/μl at birth was associated with increased diarrhea/growth faltering [adjusted hazard ratio (aHR) 1.73, P = 0.04] and malaria (aHR 2.18, P = 0.02). Low birth weight (2000–2499 g) was associated with neonatal death (aHR 12.3, P <0.001). Factors associated with postneonatal death included rainy season (aHR 4.24, P = 0.002), infant cotrimoxazole (aHR 0.48, P = 0.03), and low infant WBC count at birth (aHR 2.53, P = 0.02).
Conclusion
Infant morbidity rates increased after 24 weeks, when BAN infants weaned. Introduction of prophylactic cotrimoxazole was associated with reduced rates of morbidity and mortality in HIV-exposed uninfected infants. Unexpectedly, a low WBC count at birth was significantly associated with later infant morbidity and mortality in this cohort.
Keywords: Africa, HIV, HIV-exposed, infant, morbidity, mortality, pediatric
Introduction
Infant morbidity and mortality has been greatly affected by the HIV epidemic in many resource-limited settings. Although the numbers of new infant HIV infections are expected to decrease as antiretroviral prophylaxis is now recommended by WHO during gestation, delivery, and breastfeeding for HIV-infected women [1], the number of infants who are HIV-exposed but uninfected will proportionately increase. Examining the determinants of these infants’ morbidity and mortality is, therefore, of increasing importance. Infant mortality is affected by many factors such as geographic and socioeconomic setting, feeding type, and the mother’s HIV status and disease stage. Pooled analyses of data from African studies indicate a mortality of HIV-exposed infants between 39.3 and 49 per 1000 [2,3]. Mortality of these infants is two to four times as high as that of HIV-unexposed infants in the same setting [4,5], and is even higher for infants of mothers who had advanced HIV disease or who died [3–6]. Morbidity is also higher among HIV-exposed infants compared to their unexposed counterparts. In the ZVITAMBO cohort, HIV-exposed uninfected infants made an average of 30% more sick clinic visits and had 20% more hospitalizations than unexposed infants [5]. Respiratory and gastrointestinal infections are the main causes of infant morbidity and mortality [6,7], with malaria also contributing substantially in endemic areas [8].
The Breastfeeding, Antiretrovirals, and Nutrition (BAN) study, conducted from 2004 to 2010 among 2369 mother–infant pairs in Lilongwe, Malawi, provides an opportunity to examine morbidity and mortality in a large cohort of HIV-exposed infants in a resource-limited setting, almost all of whom were weaned at about 6 months of age. We examined the effect of several prognostic factors on infant morbidity and mortality.
Methods
Study design and participants
All infants were enrolled in the BAN randomized controlled trial, which has been described in detail elsewhere [9,10]. Briefly, investigators recruited women who tested HIV-positive through a prevention of mother-to-child transmission program at antenatal clinics in Lilongwe, Malawi, from April 2004 to January 2010. Primary eligibility criteria included age of at least 14 years, gestation of 30 weeks or less, a CD4+ T-cell count of 250/μl or more (before 24 July 2006, count ≥200/μl; change in accordance with Malawi Ministry of Health guidelines for HIV treatment), and no history of antiretroviral drug use (including the HIVNET 012 regimen) [11]. Mothers or infants who had postnatal conditions that would preclude study interventions were excluded from the study, as were infants with birth weight less than 2000 g. All women provided written informed consent. The BAN study protocol was approved by the Malawi National Health Science Research Committee and by institutional review boards at the University of North Carolina (UNC) at Chapel Hill and the US Centers for Disease Control and Prevention (CDC).
Mother–infant pairs were randomized within 1 week of delivery to a two-group maternal nutritional intervention and to a three-group antiretroviral intervention consisting of a triple-drug antiretroviral regimen for the mother (maternal-regimen group), daily dose of nevirapine for the infant (infant-regimen group), or neither (control antiretroviral group). Irrespective of antiretroviral treatment assignment, all mothers in labor and their newborn infants were given single-dose nevirapine and zidovudine and lamivudine for 7 days. The interventions began after delivery and were continued until the cessation of breastfeeding, but no longer than 28 weeks. Infants found to be HIV-infected at birth or in the first 2 weeks of life were disenrolled from the BAN study and referred for care.
All mothers in the study were counseled to breastfeed exclusively for the first 24 weeks of life, and to wean over a 4-week period; infants were provided with a weaning supplement (a locally produced ready-to-use therapeutic food) [12]. Exclusive breastfeeding was defined as providing no other liquids or foods except breast milk.
Mother–infant pairs were followed at 1, 2, 4, 6, 8, 12, 18, 21, 24, 28, 32, 36, 42, and 48 weeks postpartum. The choice of a 48-week follow-up was made during the study design phase to most easily accommodate even spacing of study visits. Anthropometrics, vital signs, illnesses and hospitalizations since the last visit, current symptoms, and physical examination findings were collected at all follow-up visits. Participants were advised to return to the clinic between visits to receive treatment if the mother or child was ill. All infants received vaccines according to the Malawi immunization schedule, which included Bacillus Calmette–Guerin (BCG), and vaccines against tetanus, diphtheria, pertussis, Haemophilus influenzae, hepatitis B, poliomyelitis, and the conjugate pneumococcal vaccine.
Infants were tested for HIV-1 infection at birth and at 2, 12, 28, and 48 weeks with the use of the Roche Amplicor 1.5 DNA PCR assay (Roche Molecular Systems, Pleasanton, California, USA). Positive specimens were confirmed by testing a specimen obtained at the next visit. If an infant was lost to follow-up or died before a confirmatory test was obtained, then a second specimen from the same day was tested at the reference laboratory at UNC. For all positive infants, dried blood spots collected at all study visits were tested to narrow the window of seroconversion. Mother–infant pairs lost to follow-up were traced by a team of community nurses to determine HIV and vital status.
In accordance with the Malawi Ministry of Health and Population Guidelines and WHO guidelines, cotrimoxazole prophylaxis was initiated in the BAN study for eligible women and infants on 13 June 2006 [13]. Cotrimoxazole (240 mg once daily) was provided to all infants in the study beginning at 6 weeks of age through 36 weeks of age or until weaning occurred and HIV infection was ruled out. At the same time, cotrimoxazole was also initiated for mothers whose CD4+ T-cell count was less than 500 cells/μl, measured during pregnancy.
We examined severe morbidity outcomes and mortality among these infants during 48 weeks of follow-up. The analysis is limited to HIV-uninfected infants, by excluding infants HIV-infected by 2 weeks of life (thus, excluding perinatal infections), and by censoring infants who became infected during the study via breastfeeding at their last negative HIV test.
Morbidity and mortality outcomes
Morbidity and mortality data were derived from serious adverse events (SAEs) reported in the BAN study. SAEs were defined as any experience that was fatal or life-threatening, required in-patient hospitalization or prolongation of an existing hospitalization, or resulted in a persistent or significant disability or incapacity, and were graded according to toxicity tables from the National Institute of Allergy and Infectious Diseases, Division of AIDS (DAIDS) [14]. Infants were evaluated at all regular and interim sick visits for illnesses or clinical or laboratory abnormalities. If an illness met the criteria for an SAE, an SAE was reported. Growth faltering was defined by edema (due to malnutrition) or severe wasting (weight <70% expected for length) [15]. All diagnoses were based on clinical judgment and did not necessarily require microbiologic or other laboratory confirmation.
The following grade 3 or 4 infant morbidity outcomes were assessed between birth and 48 weeks of life: pneumonia/serious febrile illness (SFI; SFI included diagnoses of ‘sepsis’ and meningitis; as a result, this outcome included respiratory and other, nongastrointestinal, infectious morbidity), diarrhea with or without growth faltering, and malaria. These three combined outcomes comprised the great majority of all infant clinical SAEs in BAN [16]. Infant mortality was examined separately for the neonatal period, defined as occurring in the first 30 days of life, and for the postneonatal period, defined as occurring between 31 days and 48 weeks of life.
Statistical analysis
Morbidity rates were estimated using person-weeks of follow-up during four 12-week infant age intervals (0–12, 13–24, 25–36, and 37–48 weeks), and by potential risk factors. Hazard ratios and 95% confidence intervals for morbidity and mortality outcomes were estimated using the Cox proportional hazards model. Conditional gap time models were used for morbidity outcomes with recurrent events [17]. The extended Kaplan–Meier method was used to estimate the probability of death by time-dependent covariates. The cutpoints used to define low hemoglobin and platelets were for grade 1 toxicity, according to DAIDS tables [14]; for white blood cell (WBC) counts at birth, it was a value accepted as the lower limit of normal range [18]. For newborn absolute neutrophil count (ANC), the DAIDS cutpoints were modified to account for the high prevalence of ethnic neutropenia [19], so that the grade 4 DAIDS cutpoint was used. Rainy season (defined as November through March), and maternal and infant cotrimoxazole prophylaxis were modeled as time-dependent covariates. All multivariable models were adjusted for antiretroviral treatment assignment and stratified by randomization to the maternal nutritional supplement.
Role of the funding source
CDC representatives were part of the study team and were involved in the study design, coordination, data collection, data analysis, data interpretation, and writing of the report. The study group had full access to all study data and had final responsibility for the analysis, interpretation, and decision to submit for publication. The manufacturers of the study drugs had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Infant characteristics
Of 2369 mother–infant pairs enrolled in the BAN study, 119 (5.0%) infants tested HIV-positive between 0 and 2 weeks of life and were excluded, resulting in a total of 2250 infants contributing data to this analysis (Table 1). The mothers’ self-reported frequency of exclusive breastfeeding at 24 weeks postpartum was 89%, which decreased to 7% by 28 weeks postpartum. By 32 weeks postpartum, 94% of mothers reported no breastfeeding.
Table 1.
Baseline characteristics for 2250 infants enrolled in the Breastfeeding, Antiretrovirals, and Nutrition study who were HIV-uninfected at 2 weeks of age.
| N | |
|---|---|
| Treatment arm | |
| Mother ARV | 803 (35.7%) |
| Infant ARV | 815 (36.2%) |
| No ARV | 632 (28.1%) |
| Maternal nutritional supplement | |
| Yes | 1133 (50.4%) |
| No | 1117 (49.6%) |
| Maternal marital status | |
| Married | 2083 (92.6%) |
| Other | 167 (7.4%) |
| Maternal education | |
| ≤Primary | 1457 (64.8%) |
| >Primary | 790 (35.2%) |
| Electricity in the homea | |
| Yes | 197 (19.1%) |
| No | 836 (80.9%) |
| Mother started on cotrimoxazole | |
| Yes | 956 (42.5%) |
| No | 1294 (57.5%) |
| Infant started on cotrimoxazole | |
| Yes | 1519 (67.5%) |
| No | 731 (32.5%) |
| Birth weight | |
| 2000–2499 g | 174 (7.7%) |
| ≥2500 g | 2072 (92.3%) |
| Maternal CD4+ T-cell count during pregnancy | |
| 200–350/μl | 676 (30.0%) |
| >350/μl | 1574 (70.0%) |
| Maternal hemoglobin during pregnancy | |
| <10 g/dl | 514 (22.9%) |
| ≥10 g/dl | 1732 (77.1%) |
| Infant WBC at delivery | |
| <9000 cells/μl | 149 (6.8%) |
| ≥9000 cells/μl | 2043 (93.2%) |
| Infant neutrophil count at delivery | |
| ≤1500 cells/μl | 19 (0.9%) |
| >1500 cells/μl | 2176 (99.1%) |
| Infant platelet count at delivery | |
| <85 000/μl | 38 (1.7%) |
| ≥85 000/μl | 2154 (98.3%) |
ARV, antiretroviral; WBC, white blood cell.
Variable only collected on 46% of Breastfeeding, Antiretrovirals, and Nutrition (BAN) study participants.
The majority of infants were born to married mothers (93%), mothers with only primary education or less (65%), and in homes without electricity (81%). Most infants (68%) were born after cotrimoxazole prophylaxis was introduced in the study. Nearly 8% of infants were born with low birth weight (2000–2499 g). Almost 7% of the newborns had a WBC count at birth less than 9000/dl.
Infant morbidity
The overall incidence rate for clinical grade 3/4 SAEs between 0 and 48 weeks of age was 0.744 per 100 person-weeks. The incidence rate for pneumonia/SFI was 0.430 per 100 person-weeks; for diarrhea/growth faltering, it was 0.170 per 100 person-weeks; and for malaria, 0.100 per 100 person-weeks. The rate of pneumonia/SFI was highest in the first 12 weeks of life, before rapidly decreasing (Table 2). After 24 weeks of age, an increase in pneumonia/SFI was observed for the rest of the infants’ first year of life. Diarrhea/growth faltering and malaria rates increased steadily from birth, and were highest between 37 and 48 weeks (Table 2).
Table 2.
Rates of HIV-uninfected infant morbidity outcomes by age interval.
| Pneumonia/serious febrile illness
|
Diarrhea/growth faltering
|
Malaria
|
||||
|---|---|---|---|---|---|---|
| Rate per 100 person-weeks (# of events) | P | Rate per 100 person-weeks (# of events) | P | Rate per 100 person-weeks (# of events | P | |
| Age interval | <0.001 | <0.001 | <0.001 | |||
| 0–12 weeks | 0.83 (206) | 0.03 (7) | 0.03 (7) | |||
| 13–24 weeks | 0.19 (42) | 0.07 (16) | 0.05 (12) | |||
| 25–36 weeks | 0.32 (66) | 0.30 (61) | 0.11 (22) | |||
| 37–48 weeks | 0.30 (57) | 0.33 (63) | 0.22 (42) | |||
The effects of several factors on each morbidity outcome were examined. Infant cotrimoxazole prophylaxis was associated with a significant decrease in the rate of morbidity for all three studied outcomes in adjusted models (Table 3), with the strongest protective effect observed for malaria [adjusted hazard ratio (aHR) = 0.36, P <0.001]. Other independent factors associated with pneumonia/SFI were rainy season (aHR = 1.53, P <0.001) and low infant ANC count at delivery (<1500/μl; aHR 2.32, P = 0.02). Rainy season was also significantly associated with diarrhea/growth faltering in adjusted models (aHR = 1.49, P = 0.02). Low maternal hemoglobin at delivery and low infant WBC count (<9000/μl) at delivery were independently associated with both diarrhea/growth faltering and malaria. Low platelet count at birth (<85 000/μl) was significantly associated with subsequent severe malaria (aHR = 3.00, P = 0.04).
Table 3.
Rates of HIV-uninfected infant morbidity outcomes by possible risk factors.
| Rate per 100 person-weeks (# of events) | Unadjusted
|
Adjusteda
|
|||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| Pneumonia/SFI | |||||
| Treatment arm | |||||
| Mother ARV | 0.44 (139) | 1.10 (0.84–1.45) | 0.78 | 1.12 (0.86–1.47) | 0.69 |
| Infant ARV | 0.42 (136) | 1.06 (0.80–1.40) | 1.06 (0.81–1.38) | ||
| No ARV | 0.42 (96) | 1.0 | 1.0 | ||
| Rainy seasonb | |||||
| Yes | 0.53 (192) | 1.56 (1.26–1.94) | <0.001 | 1.53 (1.20–1.95) | < 0.001 |
| No | 0.35 (179) | 1.0 | 1.0 | ||
| Infant cotrimoxazoleb | |||||
| Yes | 0.39 (270) | 0.80 (0.63–1.02) | 0.07 | 0.78 (0.62–0.99) | 0.04 |
| No | 0.58 (101) | 1.0 | 1.0 | ||
| Birth weight | |||||
| 2000–2499 g | 0.58 (40) | 1.36 (0.96–1.91) | 0.08 | – | |
| ≥2500 g | 0.41 (328) | 1.0 | |||
| Maternal CD4+ T-cell count during pregnancy | |||||
| 200–350/μl | 0.45 (116) | 1.08 (0.87–1.34) | 0.51 | – | |
| >350/μl | 0.42 (255) | 1.0 | |||
| Maternal hemoglobin during pregnancy | |||||
| <10 g/dl | 0.40 (77) | 0.89 (0.68–1.16) | 0.39 | – | |
| ≥10 g/dl | 0.44 (293) | 1.0 | |||
| Infant WBC at delivery | |||||
| <9000 cells/μl | 0.53 (32) | 1.25 (0.88–1.77) | 0.22 | – | |
| ≥9000 cells/μl | 0.42 (328) | 1.0 | |||
| Infant neutrophil count at delivery | |||||
| ≤1500 cells/μl | 1.00 (6) | 2.35 (1.17–4.68) | 0.02 | 2.32 (1.15–4.67) | 0.02 |
| >1500 cells/μl | 0.42 (355) | 1.0 | 1.0 | ||
| Infant platelet count at delivery | |||||
| <85 000/μl | 0.75 (4) | 0.72 (0.28–1.88) | 0.50 | – | |
| ≥ 85 000/μl | 0.42 (356) | 1.0 | |||
| Diarrhea or growth faltering | |||||
| Treatment arm | |||||
| Mother ARV | 0.17 (53) | 0.92 (0.59–1.43) | 0.81 | 0.95 (0.64–1.42) | 0.92 |
| Infant ARV | 0.16 (52) | 0.86 (0.54–1.36) | 0.92 (0.61–1.39) | ||
| No ARV | 0.18 (42) | 1.0 | 1.0 | ||
| Rainy seasonb | |||||
| Yes | 0.22 (79) | 1.51 (1.09–2.09) | 0.01 | 1.49 (1.07–2.08) | 0.02 |
| No | 0.13 (68) | 1.0 | 1.0 | ||
| Infant cotrimoxazoleb | |||||
| Yes | 0.16 (113) | 0.64 (0.44–0.95) | 0.03 | 0.66 (0.45–0.98) | 0.04 |
| No | 0.19 (34) | 1.0 | 1.0 | ||
| Birth weight | |||||
| 2000–2499 g | 0.19 (13) | 1.05 (0.62–1.80) | 0.85 | – | |
| ≥2500 g | 0.17 (134) | 1.0 | |||
| Maternal CD4+ T-cell count during pregnancy | |||||
| 200–350/μl | 0.16 (41) | 0.96 (0.67–1.37) | 0.81 | – | |
| >350/μl | 0.17 (106) | 1.0 | |||
| Maternal hemoglobin during pregnancy | |||||
| <10 g/dl | 0.26 (50) | 1.77 (1.25–2.49) | 0.001 | 1.67 (1.18–2.35) | 0.004 |
| ≥10 g/dl | 0.14 (96) | 1.0 | 1.0 | ||
| Infant WBC at delivery | |||||
| <9000 cells/μl | 0.30 (18) | 1.81 (1.07–3.05) | 0.03 | 1.73 (1.04–2.88) | 0.04 |
| ≥9000 cells/μl | 0.16 (127) | 1.0 | 1.0 | ||
| Infant neutrophil count at delivery | |||||
| ≤1500 cells/μl | 0.67 (4) | 3.26 (0.96–11.0) | 0.06 | – | |
| >1500 cells/μl | 0.17 (141) | 1.0 | |||
| Infant platelet count at delivery | |||||
| <85 000/μl | 0.38 (2) | 0.96 (0.14–6.54) | 0.96 | – | |
| ≥85 000/μl | 0.17 (143) | 1.0 | |||
| Malaria | |||||
| Treatment arm | |||||
| Mother ARV | 0.07 (21) | 0.69 (0.37–1.28) | 0.08 | 0.73 (0.40–1.34) | 0.09 |
| Infant ARV | 0.12 (40) | 1.29 (0.75–2.21) | 1.35 (0.79–2.30) | ||
| No ARV | 0.10 (22) | 1.0 | 1.0 | ||
| Rainy seasonb | |||||
| Yes | 0.10 (36) | 1.10 (0.70–1.74) | 0.68 | – | |
| No | 0.09 (47) | 1.0 | |||
| Infant cotrimoxazoleb | |||||
| Yes | 0.08 (52) | 0.33 (0.21–0.52) | < 0.001 | 0.36 (0.23–0.58) | < 0.001 |
| No | 0.18 (31) | 1.0 | 1.0 | ||
| Birth weight | |||||
| 2000–2499 g | 0.15 (10) | 1.44 (0.69–3.00) | 0.33 | – | |
| ≥2500g | 0.09 (71) | 1.0 | |||
| Maternal CD4+ T-cell count during pregnancy | |||||
| 200–350/μl | 0.07 (17) | 0.65 (0.38–1.09) | 0.10 | – | |
| >350/μl | 0.11 (66) | 1.0 | |||
| Maternal hemoglobin during pregnancy | |||||
| <10 g/dl | 0.15 (29) | 1.84 (1.16–2.92) | 0.01 | 1.61 (1.01–2.55) | 0.04 |
| ≥10 g/dl | 0.08 (54) | 1.0 | 1.0 | ||
| Infant WBC at delivery | |||||
| <9000 cells/μl | 0.18 (11) | 2.18 (1.14–4.17) | 0.02 | 2.18 (1.14–4.17) | 0.02 |
| ≥9000 cells/μl | 0.09 (70) | 1.0 | 1.0 | ||
| Infant neutrophil count at delivery | |||||
| ≤1500 cells/μl | 0.17 (1) | 2.07 (0.28–15.0) | 0.47 | – | |
| >1500 cells/μl | 0.10 (80) | 1.0 | |||
| Infant platelet count at delivery | |||||
| <85 000/μl | 0.75 (4) | 3.27 (1.26–8.53) | 0.02 | 3.00 (1.07–8.40) | 0.04 |
| ≥85 000/μl | 0.09 (77) | 1.0 | 1.0 | ||
ARV, antiretroviral; CI, confidence interval; WBC, white blood cell.
Conditional gap time proportional hazards model adjusted for ARV treatment arm and all variables statistically significant at α = 0.05, stratified by maternal nutritional supplement.
Time-dependent variable.
Antiretroviral prophylaxis arm assignment (maternal or infant antiretrovirals or no extended antiretroviral prophylaxis during breastfeeding), antenatal maternal CD4+ T-cell count (200–350 or >350 cells/μl), and birth weight were not significantly associated with any of the morbidity outcomes examined.
In a sensitivity analysis removing morbidity outcomes before infant cotrimoxazole prophylaxis was started at 6 weeks of life, infant cotrimoxazole remained significantly associated with all three morbidity outcomes. Electricity in the home was associated with a significant decrease in the rates of pneumonia/SFI (aHR = 0.57, P = 0.02) and diarrhea/growth faltering (aHR = 0.32, P = 0.01). However, as this variable was only collected on a subset of BAN participants (46%), it was not included in the main analysis.
Infant mortality in the first 48 weeks of life
There were a total of 56 deaths among HIV-uninfected infants between birth and 48 weeks of life, of which 10 were in the neonatal period. This represents a neonatal mortality rate of 0.056 per person-year and an overall infant mortality rate of 0.034 per person-year in HIV-uninfected infants (0.027 from 0 to 28 weeks and 0.046 per person-year from 28 to 48 weeks). The only significant factor associated with neonatal death in an adjusted model was low birth weight (2000–2499 g, aHR = 12.3, P <0.001; Table 4). Low infant ANC count at birth was significantly associated with neonatal death in an unadjusted model (hazard ratio = 13.2, P = 0.02), but this association did not remain after adjustment for other risk factors. The effects of maternal CD4+ T-cell count, maternal anemia, and rainy season on neonatal mortality did not reach statistical significance; similarly, a protective effect of maternal prophylaxis with cotrimoxazole was not statistically significant.
Table 4.
Association of risk factors with neonatal and postneonatal mortality in HIV-uninfected infants.
| Unadjusted
|
Adjusteda
|
|||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| Neonatal death (≤ 30 days; n = 10) | ||||
| Treatment arm | ||||
| Mother ARV | 0.79 (0.20–3.15) | 0.54 | 0.76 (0.19–3.05) | 0.56 |
| Infant ARV | 0.38 (0.07–2.10) | 0.39 (0.07–2.14) | ||
| No ARV | 1.0 | 1.0 | ||
| Rainy seasonb | ||||
| Yes | 3.60 (0.93–13.9) | 0.06 | – | |
| No | 1.0 | |||
| Maternal cotrimoxazoleb | ||||
| Yes | 0.33 (0.07–1.54) | 0.16 | – | |
| No | 1.0 | |||
| Birth weight | ||||
| 2000–2499 g | 12.3 (3.56–42.5) | < 0.001 | 12.3 (3.55–42.4) | <0.001 |
| ≥ 2500 g | 1.0 | 1.0 | ||
| Maternal CD4+ T-cell count during pregnancy | ||||
| 200–350/μl | 1.57 (0.44–5.55) | 0.49 | – | |
| >350/μl | 1.0 | |||
| Maternal hemoglobin during pregnancy | ||||
| <10 g/dl | 0.84 (0.18–3.96) | 0.83 | – | |
| ≥ 10 g/dl | 1.0 | |||
| Infant WBC at delivery | ||||
| <9000 cells/μl | 1.51 (0.19–11.9) | 0.70 | – | |
| ≥ 9000 cells/μl | 1.0 | |||
| Infant neutrophil count at delivery | ||||
| ≤ 1500 cells/μl | 13.2 (1.67–104) | 0.02 | – | |
| >1500 cells/μl | 1.0 | |||
| Infant platelet count at delivery | ||||
| <85 000/μl | 6.3 (0.80–50.0) | 0.08 | – | |
| ≥ 85 000/μl | 1.0 | |||
| Postneonatal death (31 days to 48 weeks; n = 46) | ||||
| Treatment arm | ||||
| Mother ARV | 0.90 (0.43–1.86) | 0.95 | 0.96 (0.46–2.00) | 0.99 |
| Infant ARV | 0.92 (0.45–1.89) | 0.98 (0.48–2.02) | ||
| No ARV | 1.0 | 1.0 | ||
| Rainy seasonb | ||||
| Yes | 4.02 (2.08–7.76) | < 0.001 | 4.24 (1.68–10.7) | 0.002 |
| No | 1.0 | 1.0 | ||
| Infant cotrimoxazoleb | ||||
| Yes | 0.50 (0.26–0.96) | 0.04 | 0.48 (0.25–0.93) | 0.03 |
| No | 1.0 | 1.0 | ||
| Birth weight | ||||
| 2000–2499 g | 0.90 (0.28–2.91) | 0.86 | – | |
| ≥ 2500 g | 1.0 | |||
| Maternal CD4+ T-cell count during pregnancy | ||||
| 200–350/μl | 0.59 (0.29–1.23) | 0.16 | – | |
| >350/μl | 1.0 | |||
| Maternal hemoglobin during pregnancy | ||||
| <10 g/dl | 1.15 (0.58–2.26) | 0.69 | – | |
| ≥ 10 g/dl | 1.0 | |||
| Infant WBC at delivery | ||||
| < 9000 cells/μl | 2.58 (1.15–5.76) | 0.02 | 2.53 (1.13–5.67) | 0.02 |
| ≥ 9000 cells/μl | 1.0 | 1.0 | ||
| Infant neutrophil count at delivery | ||||
| ≤ 1500 cells/μl | 3.34 (0.46–24.3) | 0.23 | – | |
| >1500 cells/μl | 1.0 | |||
| Infant platelet count at delivery | ||||
| <85 000/μl | 2.83 (0.69–11.7) | 0.15 | – | |
| ≥ 85 000/μl | 1.0 | |||
ARV, antiretroviral; CI, confidence interval; WBC, white blood cell.
Proportional hazards model adjusted for ARV treatment arm and all variables statistically significant at α = 0.05, stratified by maternal nutritional supplement.
Time-dependent variable.
Independent factors associated with postneonatal infant death included rainy season (aHR = 4.24, P = 0.002), infant cotrimoxazole prophylaxis (aHR = 0.48, P = 0.03), and low infant WBC count at birth (aHR = 2.53, P = 0.02). Maternal CD4+ T-cell count did not have a significant effect on postneonatal infant mortality.
Figure 1 depicts infant survival by receipt of cotrimoxazole prophylaxis and by low WBC count at birth using Kaplan–Meier or extended Kaplan–Meier analysis.
Fig. 1. Cumulative rates of survival in HIV-uninfected infants from 0 to 48 weeks by infant white blood cell (WBC – in cells/μl) at delivery and *receipt of prophylactic infant cotrimoxazole started at 6 weeks of age.
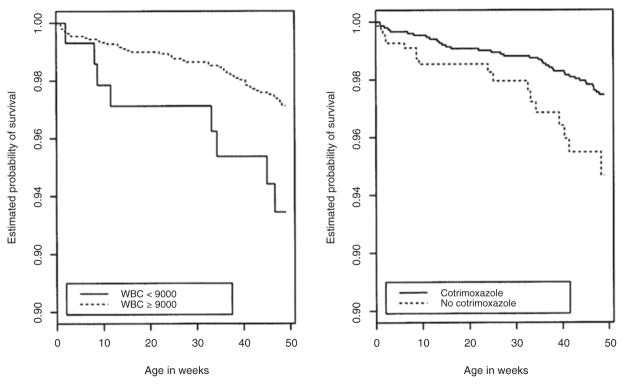
*Estimated using the extended Kaplan–Meier estimator.
Discussion
Our findings raise several points. First, and as would be expected for HIV-unexposed infants [5,8], the highest rates of respiratory and other infectious (with the exception of gastrointestinal) morbidity were seen in the neonatal and early infant life. In contrast, serious malaria morbidity peaked later in the first year of life, consistent with other studies that have shown an increase in malarial morbidity and mortality after 6 months of age in seasonal transmission areas [20,21]. As diagnoses were based on index of clinical suspicion, some degree of misclassification between malaria and other SFIs might have occurred.
Second, we observed increases in the rate of all three morbidity outcomes after 6 months of life (pneumonia/SFI increased over 50%, the rate of diarrhea/growth faltering increased to more than four times, and the rate of malaria more than doubled). Although this might be expected for malaria [20] and gastroenteritis [22,23], as the infant begins to crawl and get exposed to numerous environmental stimuli including new foods, increase in severe respiratory morbidity would not be expected at this stage in an HIV-uninfected population of infants [5]. The most likely explanation for this finding is weaning from breast milk at about 6 months of age. As almost all infants in this cohort stopped breastfeeding at that time (by maternal report), we cannot directly evaluate what proportion of the increased morbidity was due to weaning. However, other studies from African settings [22–24] have concluded that weaning from breast milk is, at least partially, responsible for postweaning increases in the risk of gastroenteritis-related morbidity and of infant mortality.
Approximately, 50% increases in both serious gastrointestinal and respiratory morbidity were seen during the rainy season in our study, highlighting the complex relationships of the social and physical environment and infectious diseases. Method of infant feeding is an important factor in this interaction. During the rainy season, no breastfeeding was associated with a more pronounced weight for age Z-score decline than during the dry season in the Zambia Exclusive Breastfeeding Study (ZEBS) [25]. Others have also reported an association between rainy season and malnutrition in African and other resource-limited settings [26,27]. Rainy season and flooding have been associated with diarrheal outbreaks and increased infant mortality, particularly among nonbreastfed infants, even those HIV-unexposed [28]. A study from Kenya showed a higher pneumonia-related mortality among children (age <5 years) residing in Nairobi’s slums in the rainy season [29], and a study from multiple African sites showed an all-cause mortality increase among children during the rainy season [8].
A surprising finding was the lack of correlation of rainy season with severe malaria morbidity. Other studies have also failed to confirm seasonal variation of deaths attributed to malaria [8].
Another unexpected, and, to our knowledge, not previously reported, finding was the association of low neutrophil counts at birth with infant morbidity and mortality beyond the immediate neonatal period. Although in a few (four of 19) cases, a low ANC at birth was due to a serious infection, most cases of low ANC counts at birth were seen in asymptomatic and seemingly healthy infants. This is attributed to ‘benign neutropenia’, which is prevalent among African populations [19]. Such neutropenia is largely believed to be without adverse health consequences [30]. However, the consistency of the observed association with all morbidity outcomes examined and with infant mortality strongly suggests that it is a marker of increased risk.
The introduction of cotrimoxazole prophylaxis midway through the trial provided a ‘natural experiment’ to evaluate its effects. Infant cotrimoxazole prophylaxis was significantly associated with lower frequency of severe respiratory/other infectious and gastrointestinal morbidity, and of severe malaria in uninfected infants in our study. This finding concurs with the findings of the Post-Exposure Prophylaxis in Infants (PEPI) Malawi study [24]. Reductions in hospitalizations, antibiotic prescriptions, and mortality among HIV-infected children have been reported from several African countries [31,32]. Not all studies have reported a benefit of cotrimoxazole for uninfected children, particularly with respect to bacterial infections. In a small (N = 185) randomized study in rural Uganda, cotrimoxazole prophylaxis yielded a 39% reduction in malaria incidence, but no significant differences in the incidence of complicated malaria, diarrhea, pneumonia, hospitalizations, or deaths after the first 6 months of life [33]. In a nonrandomized study of 363 children in Durban, South Africa, where malaria is not endemic [34], cotrimoxazole prophylaxis was associated with a nonsignificant increase in diarrheal illness, and no significant difference in lower respiratory infections [34]. These findings indicate the need for further study of the role of cotrimoxazole in HIV-exposed uninfected children, particularly given the concerns of microbial resistance with widespread use [35,36]. However, the evidence provided herein by the large BAN cohort provides reassurance as to the benefits of cotrimoxazole prophylaxis for the health and survival of HIV-exposed infants.
There was no effect of maternal CD4+ T-cell count on infant morbidity and mortality. However, all mothers in the BAN study had CD4+ T-cell counts greater than 200 cells/μl. Maternal CD4 cell count less than 200 cells/μl has, in other studies, been associated with increased risk of all-cause infant sick clinic visits in the first year of life [5], possibly due to the mother’s poorer health and inability to care for the infant, or other factors such as greater opportunity for infant exposure to mother’s pathogens or immunologic abnormalities in the HIV-exposed infants [6].
The HIV-exposed uninfected mortality rate in the first 48 weeks of life in this study was 34 per 1000 person-years (44.2 per 1000 person-years in the whole BAN cohort with inclusion of HIV-infected infants) [16]. The Malawi Demographic Health Survey estimated the infant mortality rate from 2005 to 2010 to be 66 per 1000 live births for the general infant population [37]; according to UNICEF 2009 data [38], the infant mortality rate in Malawi was 61 per 1000 person-years and according to the US Bureau of Census, 86 per 1000 person-years [39]. The lower rate of mortality in our study is likely due to close follow-up associated with clinical trial implementation and potentially improved access to health facilities in the urban setting where BAN was implemented. Also, we are limited when making comparisons with 12-month mortality rates from other studies, as BAN infants were only followed to 48 weeks of life. Mortality among HIV-uninfected infants rose after 28 weeks, the time of weaning for most infants in BAN. In the PEPI Malawi study, cumulative infant mortality for HIV-exposed uninfected infants at 12 months was 35 per 1000 among infants still breastfeeding, compared with 43 per 1000 for those previously weaned [24]. In the ZEBS study, a mortality rate of 94 per 1000 was observed among 749 HIV-uninfected infants by 12 months of age [40] and was substantially influenced by time of weaning.
Conclusion
In conclusion, this analysis of infant morbidity and mortality in the first 48 weeks of life from the large BAN study highlights the risks associated with stopping breastfeeding at 6 months in a resource-poor setting, underscoring the value of continued breastfeeding beyond 6 months of age (with appropriate antiretroviral prophylaxis), consistent with 2010 WHO guidelines [41]. It strengthens the evidence for a health and survival benefit of prophylactic cotrimoxazole in HIV-exposed uninfected infants in resource-poor settings. It also suggests that low WBC or ANC counts at birth may be a marker for higher infant morbidity and mortality, and implies that ethnic neutropenia may not be as benign as previously thought. Furthermore, it indicates that birth weight between 2000 and 2499 g, associated with 12 times the rate of neonatal mortality, may necessitate more frequent (such as weekly) follow-up of such infants in the first month of life.
Acknowledgments
The antiretrovirals used were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, and Bristol-Myers Squibb. The Call to Action for the Preventing Mother-to-Child Transmission program was supported by the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children’s Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson and Johnson, and the US Agency for International Development.
The authors would like to thank all the women and their infants who agreed to participate in the study. They thank Jane A. Zanca, for assistance with preparation of the article.
The BAN study was supported by grants from the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention (SIP 13–01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01); the National Institute of Allergy and Infectious Diseases; the University of North Carolina Center for AIDS Research (P30-AI50410); and the NIH Fogarty AIDS International Training and Research Program (DHHS/NIH/FIC 2-D43 TW01039-06 and R24 TW007988; the American Recovery and Reinvestment Act).
Footnotes
A.P.K., C.C., C.vd.H., and D.J.J. designed the trial. D.K., C.C., S.R.E., L.H., and M.H. collected data. J.W. analyzed data. A.P.K., J.W., S.R.E., C.vd.H., and D.J.J. interpreted data. A.P.K. wrote the report and had primary responsibility for final content. All authors reviewed versions of the report and contributed to the intellectual content of the article.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of interest
The University of North Carolina received grant support from Abbott Laboratories and GlaxoSmithKline. M.H. has received lecture fees from Abbott. All other authors declare that they have no conflicts of interest.
References
- 1.WHO. Rapid advice: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Geneva, Switzerland: WHO; 2009. [Google Scholar]
- 2.Becquet R, Marston M, Dabis F, Moulton LH, Gray G, Coovadia HM, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. doi: 10.1371/journal.pone.0028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 4.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, et al. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41:504–508. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 5.Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active anti-retroviral therapy. Pediatr Infect Dis J. 2011;30:45–51. doi: 10.1097/INF.0b013e3181ecbf7e. [DOI] [PubMed] [Google Scholar]
- 6.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41:1654–1661. doi: 10.1086/498029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venkatesh KK, de Bruyn G, Marinda E, Otwombe K, van Niekerk R, Urban M, et al. Morbidity and mortality among infants born to HIV-infected women in South Africa: implications for child health in resource-limited settings. J Trop Pediatr. 2011;57:109–119. doi: 10.1093/tropej/fmq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdullah S, Adazu K, Masanja H, Diallo D, Hodgson A, Ilbou-do-Sanogo E, et al. Patterns of age-specific mortality in children in endemic areas of sub-Saharan Africa. Am J Trop Med Hyg. 2007;77:99–105. [PubMed] [Google Scholar]
- 9.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. doi: 10.1016/j.cct.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 12.Manary MJ, Ndkeha MJ, Ashorn P, Maleta K, Briend A. Home based therapy for severe malnutrition with ready-to-use food. Arch Dis Child. 2004;89:557–561. doi: 10.1136/adc.2003.034306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults in resource-limited settings. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 14.Division of AIDS. Table for grading the severity of adult and pediatric adverse events, version 1.0 – December 2004. 2009. (Clarification dated August 2009) [Google Scholar]
- 15.Kerac M, Blencowe H, Grijalva-Eternod C, McGrath M, Shoham J, Cole TJ, et al. Prevalence of wasting among under 6-month-old infants in developing countries and implications of new case definitions using WHO growth standards: a secondary data analysis. Arch Dis Child. 2011;96:1008–1013. doi: 10.1136/adc.2010.191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamieson D, Chasela C, Hudgens M, King C, Kourtis A, Kayira D. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised, controlled trial. The Lancet. 2012;379:2249–2258. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice RL, Williams BJ, Peterson AV. On the regression analysis of multivariate failure time data. Biometrika. 1981;68:373–379. [Google Scholar]
- 18.Ohls R, Christensen R. Development of the hematopoietic system. In: Kliegman R, Jenson H, editors. Nelson textbook of pediatrics. 16. Philadelphia, PA: W.B. Saunders; 2000. pp. 1456–1460. [Google Scholar]
- 19.Kourtis AP, Bramson B, van der Horst C, Kazembe P, Ahmed Y, Chasela C, et al. Low absolute neutrophil counts in African infants. J Int Assoc Physicians AIDS Care (Chic) 2005;4:73–76. doi: 10.1177/1545109705282591. [DOI] [PubMed] [Google Scholar]
- 20.Snow RW, Nahlen B, Palmer A, Donnelly CA, Gupta S, Marsh K. Risk of severe malaria among African infants: direct evidence of clinical protection during early infancy. J Infect Dis. 1998;177:819–822. doi: 10.1086/517818. [DOI] [PubMed] [Google Scholar]
- 21.Hammer GP, Some F, Muller O, Kynast-Wolf G, Kouyate B, Becher H. Pattern of cause-specific childhood mortality in a malaria endemic area of Burkina Faso. Malar J. 2006;5:47. doi: 10.1186/1475-2875-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kafulafula G, Hoover DR, Taha TE, Thigpen M, Li Q, Fowler MG, et al. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 23.Onyango-Makumbi C, Bagenda D, Mwatha A, Omer SB, Musoke P, Mmiro F, et al. Early weaning of HIV-exposed uninfected infants and risk of serious gastroenteritis: findings from two perinatal HIV Prevention Trials in Kampala, Uganda. J Acquir Immune Defic Syndr. 2010;53:20–27. doi: 10.1097/QAI.0b013e3181bdf68e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taha TE, Hoover DR, Chen S, Kumwenda NI, Mipando L, Nkanaunena K, et al. Effects of cessation of breastfeeding in HIV-1-exposed, uninfected children in Malawi. Clin Infect Dis. 2011;53:388–395. doi: 10.1093/cid/cir413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arpadi S, Fawzy A, Aldrovandi GM, Kankasa C, Sinkala M, Mwiya M, et al. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009;90:344–353. doi: 10.3945/ajcn.2009.27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maleta K, Virtanen SM, Espo M, Kulmala T, Ashorn P. Childhood malnutrition and its predictors in rural Malawi. Paediatr Perinat Epidemiol. 2003;17:384–390. doi: 10.1046/j.1365-3016.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 27.Panter-Brick C. Seasonal growth patterns in rural Nepali children. Ann Hum Biol. 1997;24:1–18. doi: 10.1080/03014469700004732. [DOI] [PubMed] [Google Scholar]
- 28.Creek TL, Kim A, Lu L, Bowen A, Masunge J, Arvelo W, et al. Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr. 2010;53:14–19. doi: 10.1097/QAI.0b013e3181bdf676. [DOI] [PubMed] [Google Scholar]
- 29.Ye Y, Zulu E, Mutisya M, Orindi B, Emina J, Kyobutungi C. Seasonal pattern of pneumonia mortality among under-five children in Nairobi’s informal settlements. Am J Trop Med Hyg. 2009;81:770–775. doi: 10.4269/ajtmh.2009.09-0070. [DOI] [PubMed] [Google Scholar]
- 30.D’Angelo G. Ethnic and genetic causes of neutropenia: clinical and therapeutic implications. Lab Hematol. 2009;15:25–29. doi: 10.1532/LH96.09005. [DOI] [PubMed] [Google Scholar]
- 31.Mulenga V, Ford D, Walker AS, Mwenya D, Mwansa J, Sinyinza F, et al. Effect of cotrimoxazole on causes of death, hospital admissions and antibiotic use in HIV-infected children. AIDS. 2007;21:77–84. doi: 10.1097/QAD.0b013e3280114ed7. [DOI] [PubMed] [Google Scholar]
- 32.Walker AS, Mulenga V, Ford D, Kabamba D, Sinyinza F, Kankasa C, et al. The impact of daily cotrimoxazole prophylaxis and antiretroviral therapy on mortality and hospital admissions in HIV-infected Zambian children. Clin Infect Dis. 2007;44:1361–1367. doi: 10.1086/515396. [DOI] [PubMed] [Google Scholar]
- 33.Sandison TG, Homsy J, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, et al. Protective efficacy of cotrimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342:d1617. doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coutsoudis A, Pillay K, Spooner E, Coovadia HM, Pembrey L, Newell ML. Routinely available cotrimoxazole prophylaxis and occurrence of respiratory and diarrhoeal morbidity in infants born to HIV-infected mothers in South Africa. S Afr Med J. 2005;95:339–345. [PubMed] [Google Scholar]
- 35.Coutsoudis A, Coovadia HM, Kindra G. Time for new recommendations on cotrimoxazole prophylaxis for HIV-exposed infants in developing countries? Bull World Health Organ. 2010;88:949–950. doi: 10.2471/BLT.10.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill CJ, Mwanakasale V, Fox MP, Chilengi R, Tembo M, Nsofwa M, et al. Effect of presumptive cotrimoxazole prophylaxis on pneumococcal colonization rates, seroepidemiology and antibiotic resistance in Zambian infants: a longitudinal cohort study. Bull World Health Organ. 2008;86:929–938. doi: 10.2471/BLT.07.049668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malawi Demographic and Health Survey 2010: preliminary report. National Statistical Office; Zomba, Malawi: Measure DHS, ICF Macro Calverton; Maryland, USA: Feb, 2011. [Google Scholar]
- 38.UNICEF. Childinfo: monitoring the situation of children and women. [Accessed 4 April 2012];Trends in infant mortality rates; 1960–2010. Available at: http://www.childinfo.org/mortality_imrcountrydata.php.
- 39.United States Census Bureau. International programs. [Accessed 5 April 2012];International Data Base. Available at: http://www.census.gov/population/international/data/idb/country.php.
- 40.Kuhn L, Sinkala M, Semrau K, Kankasa C, Kasonde P, Mwiya M, et al. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;50:437–444. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHO. Guidelines on HIV and infant feeding: principles and recommendations for infant feeding in the context of HIV and a summary of evidence. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]


